Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zeolite Synthesis
2.3. Catalytic Pyrolysis
3. Results
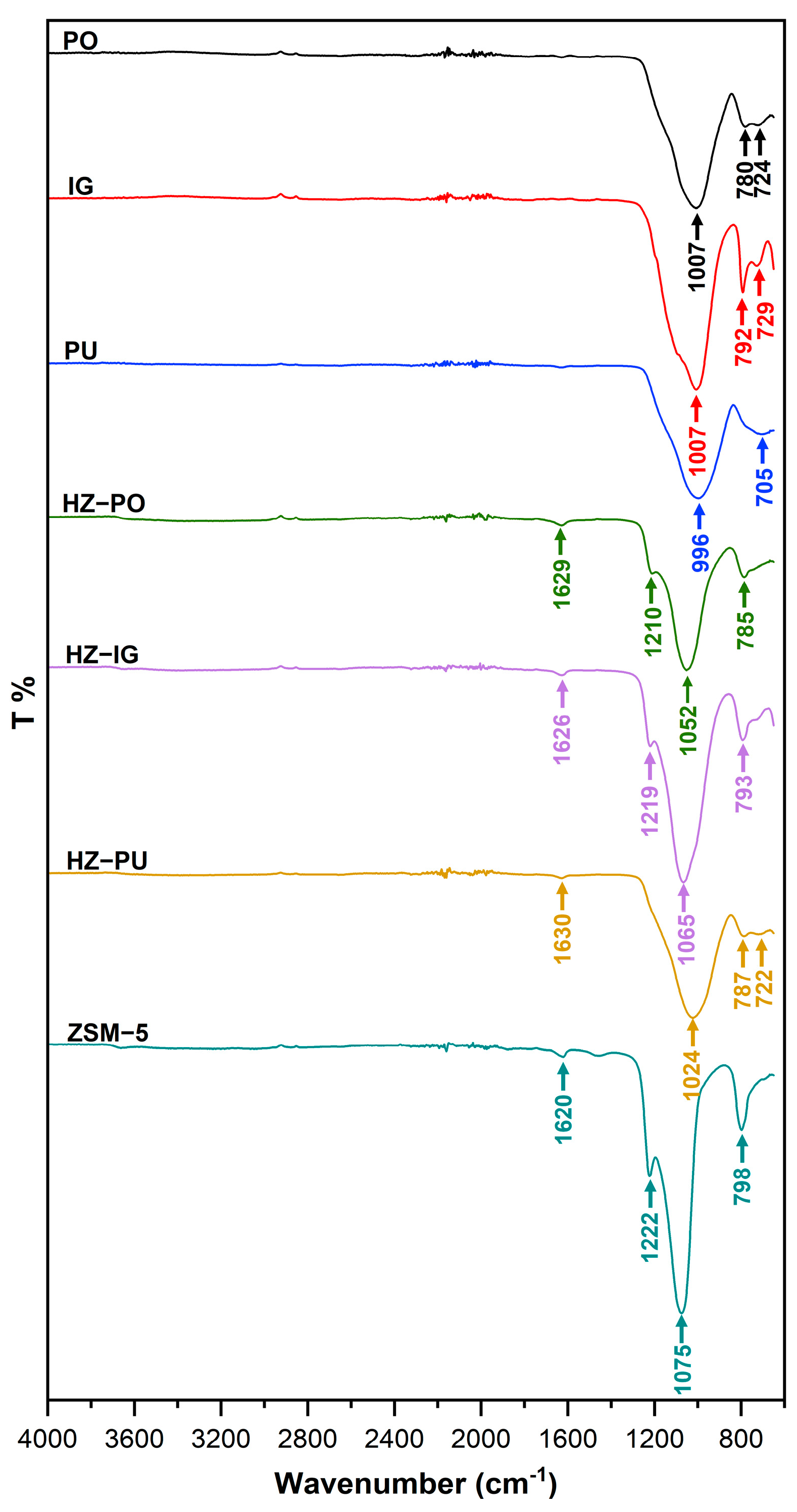
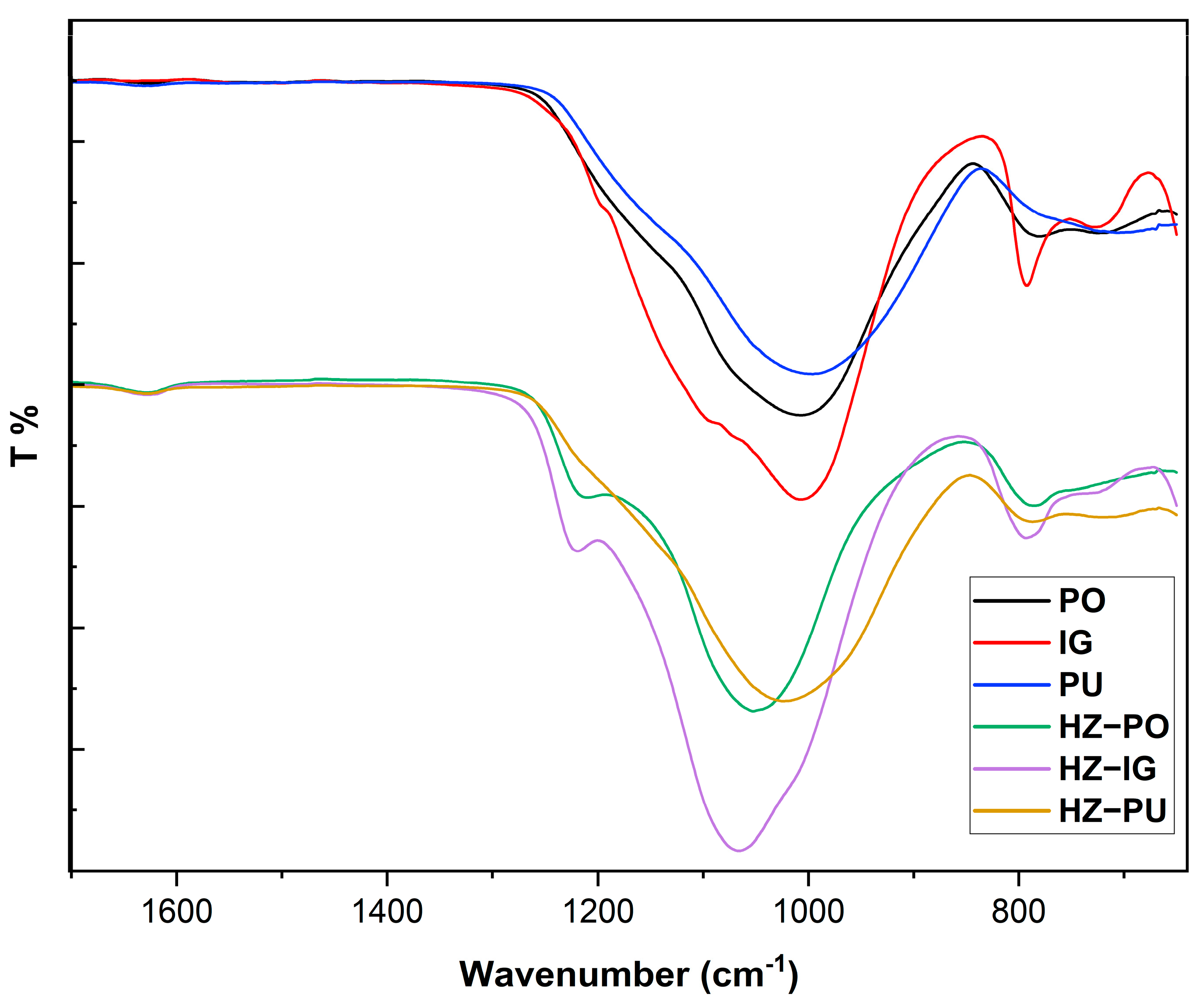
3.1. FTIR in Precursors and Synthesized Zeolites
3.2. XRD
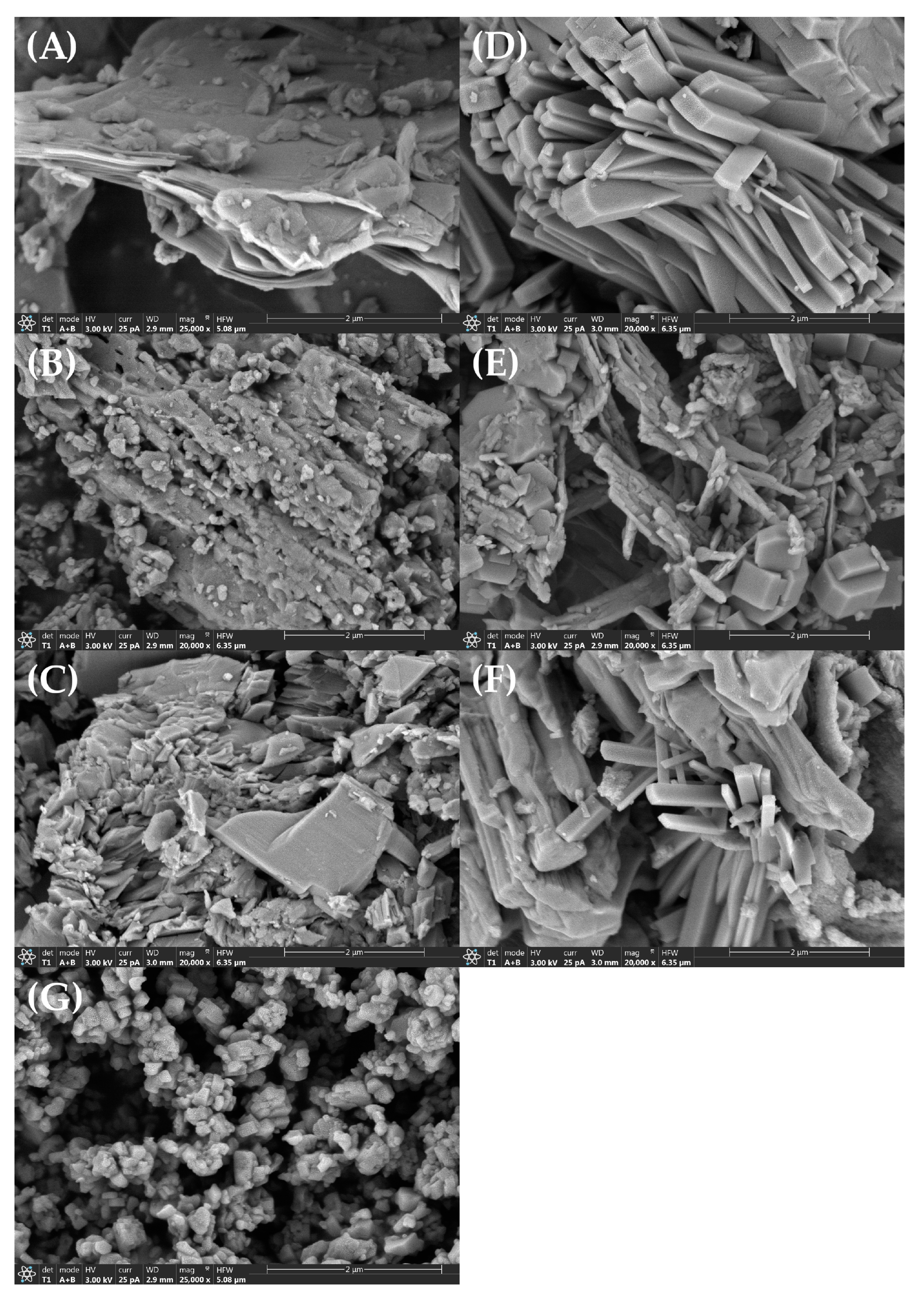
3.3. SEM Analysis
3.4. BET Analysis
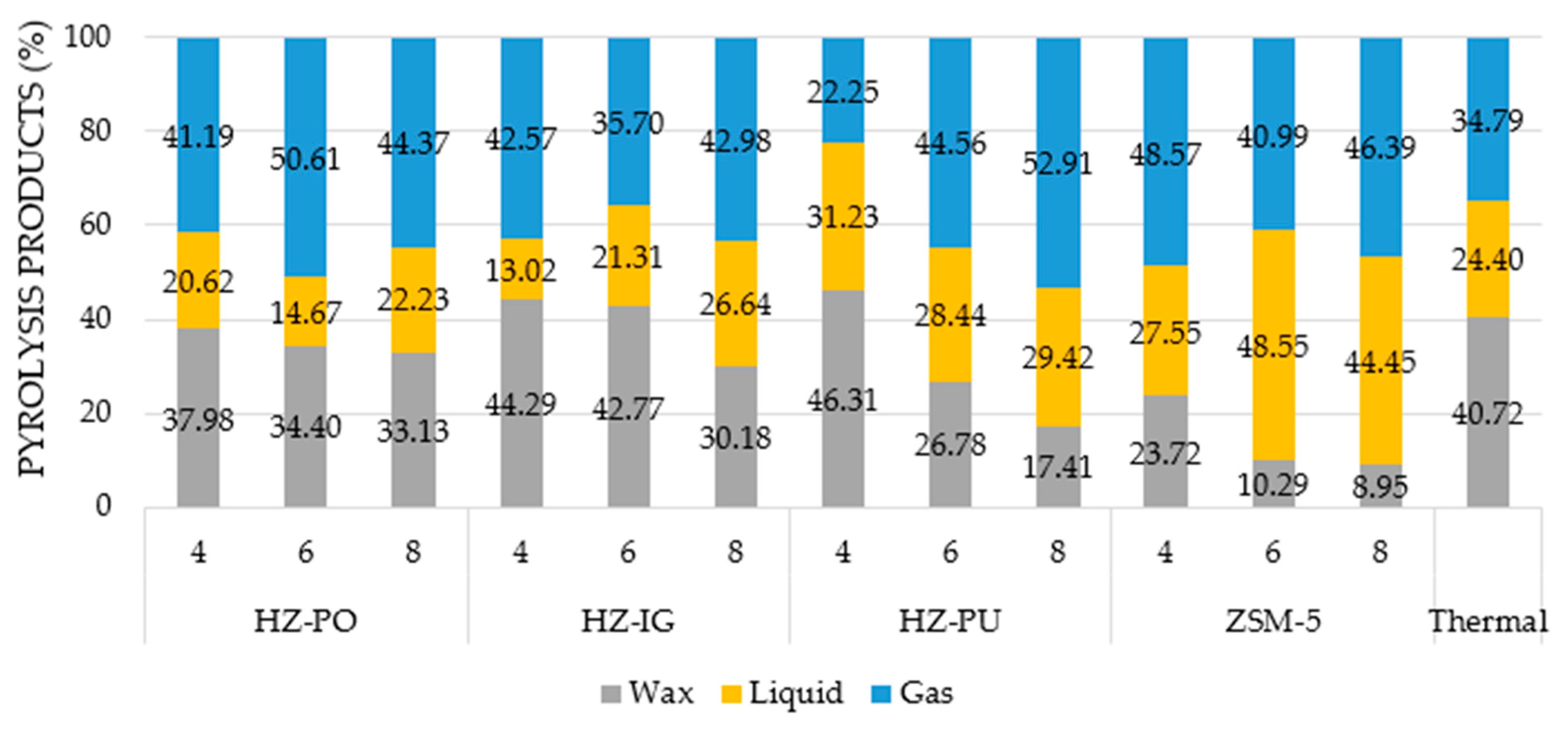
3.5. Pyrolysis Products
3.6. Analysis of Pyrolysis Liquid Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingemmet. ¿Por qué en Perú no son frecuentes las erupciones con flujos de lava? Ingemmet. Available online: https://www.gob.pe/institucion/ingemmet/noticias/494029-por-que-en-peru-no-son-frecuentes-las-erupciones-con-flujos-de-lava (accessed on 13 June 2023).
- Zeyad, A.M.; Almalki, A. Role of particle size of natural pozzolanic materials of volcanic pumice: Flow properties, strength, and permeability. Arab. J. Geosci. 2021, 14, 107. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- Wu, T.-L.; Chen, Y.-H.; Hsu, W.-D. Phase transition pathway of hydrothermal zeolite synthesis. Phys. Chem. Miner. 2021, 48, 1. [Google Scholar] [CrossRef]
- Lima, R.C.; Bieseki, L.; Melguizo, P.V.; Pergher, S.B.C. Zeolite Eco-friendly Synthesis. In Engineering Materials; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2019; pp. 65–91. [Google Scholar] [CrossRef]
- Research and Markets. Zeolite Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028. Research and Markets. Available online: https://www.researchandmarkets.com/reports/5820578/zeolite-market-global-industry-trends-share?utm_code=fxhbx3&utm_exec=joca220prd#product--toc (accessed on 13 June 2023).
- Yoldi, M.; Fuentes-Ordoñez, E.; Korili, S.; Gil, A. Zeolite synthesis from industrial wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Gao, S.; Peng, H.; Song, B.; Zhang, J.; Wu, W.; Vaughan, J.; Zardo, P.; Vogrin, J.; Tulloch, S.; Zhu, Z. Synthesis of zeolites from low-cost feeds and its sustainable environmental applications. J. Environ. Chem. Eng. 2023, 11, 108995. [Google Scholar] [CrossRef]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef]
- Jain, R.; Mallette, A.J.; Rimer, J.D. Controlling Nucleation Pathways in Zeolite Crystallization: Seeding Conceptual Methodologies for Advanced Materials Design. J. Am. Chem. Soc. 2021, 143, 21446–21460. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Kamimura, Y.; Iyoki, K.; Elangovan, S.P.; Itabashi, K.; Shimojima, A.; Okubo, T. OSDA-free synthesis of MTW-type zeolite from sodium aluminosilicate gels with zeolite beta seeds. Microporous Mesoporous Mater. 2012, 163, 282–290. [Google Scholar] [CrossRef]
- Williams, C.D. Application of Zeolites to Environmental Remediation. In Urban Pollution; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 249–258. [Google Scholar] [CrossRef]
- Scott, J.; Guang, D.; Naeramitmarnsuk, K.; Thabuot, M.; Amal, R. Zeolite synthesis from coal fly ash for the removal of lead ions from aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 63–69. [Google Scholar] [CrossRef]
- Wen, J.; Dong, H.; Zeng, G. Application of zeolite in removing salinity/sodicity from wastewater: A review of mechanisms, challenges and opportunities. J. Clean. Prod. 2018, 197, 1435–1446. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Jones, H.; Saffar, F.; Koutsos, V.; Ray, D. Polyolefins and Polyethylene Terephthalate Package Wastes: Recycling and Use in Composites. Energies 2021, 14, 7306. [Google Scholar] [CrossRef]
- Almirón, J.; Huaman, A.V.; Mamani, S.F.; De La Cruz, L.M. A review of the interaction of natural and synthetic zeolites in the pyrolysis of plastics. In Proceedings of the 20th LACCEI International Multi-Conference for Engineering, Education and Technology: “Education, Research and Leadership in Post-Pandemic Engineering: Resilient, Inclusive and Sustainable Actions, Boca Raton, FL, USA, 18–22 July 2022; Latin American and Caribbean Consortium of Engineering Institutions: Arequipa, Peru, 2022. [Google Scholar] [CrossRef]
- Al-Asadi, M.; Miskolczi, N.; Eller, Z. Pyrolysis-gasification of wastes plastics for syngas production using metal modified zeolite catalysts under different ratio of nitrogen/oxygen. J. Clean. Prod. 2020, 271, 122186. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Zang, Y.; Wang, J.; Gu, J.; Qu, J.; Gao, F.; Li, M. Cost-effective synthesis of hierarchical HZSM-5 with a high Si/TPA+ ratio for enhanced catalytic cracking of polyethylene. J. Solid State Chem. 2020, 291, 121643. [Google Scholar] [CrossRef]
- Lima, R.B.; Neto, M.M.; Oliveira, D.S.; Santos, A.G.; Souza, L.D.; Caldeira, V.P. Obtainment of hierarchical ZSM-5 zeolites by alkaline treatment for the polyethylene catalytic cracking. Adv. Powder Technol. 2021, 32, 515–523. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadistica Informatica (INEI). Industria del Plástico en el Perú. INEI. Available online: https://www.inei.gob.pe/media/MenuRecursivo/boletines/industria-plastico-peru.pdf (accessed on 4 April 2024).
- Santoso, A.; Sholikhah, A.; Sumari, S.; Asrori, M.R.; Wijaya, A.R.; Retnosari, R.; Rachman, I.B. Effect of Active Zeolite in the Pyrolysis of Polypropylene and Low Density Polyethylene Types of Plastic Waste. J. Renew. Mater. 2022, 10, 2781–2789. [Google Scholar] [CrossRef]
- Paula, T.P.; Marques, M.D.F.V.; Marques, M.R.D.C.; Oliveira, M.S.; Monteiro, S.N. Thermal and Catalytic Pyrolysis of Urban Plastic Waste: Modified Mordenite and ZSM-5 Zeolites. Chemistry 2022, 4, 297–315. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Zhou, Z.; Yang, J.; Qi, F.; Pan, Y. Online Study on the Pyrolysis of Polypropylene over the HZSM-5 Zeolite with Photoionization Time-of-Flight Mass Spectrometry. Energy Fuels 2015, 29, 1090–1098. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Characteristic of fly ash derived-zeolite and its catalytic performance for fast pyrolysis of Jatropha waste. Environ. Technol. 2014, 35, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Pyrolysis of Millettia (Pongamia) pinnata waste for bio-oil production using a fly ash derived ZSM-5 catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 239–249. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Munagapati, V.S.; Wen, J.-C.; Shu, C.-M. Synthesis of novel ZSM-22 zeolite from Taiwanese coal fly ash for the selective separation of Rhodamine 6G. J. Mater. Res. Technol. 2020, 9, 15381–15393. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Yanti, F.M.; Murti, S.D.S. Synthesis of ZSM-5 zeolite from coal fly ash and rice husk: Characterization and application for partial oxidation of methane to methanol. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012031. [Google Scholar] [CrossRef]
- Wojciechowska, K.M.; Król, M.; Bajda, T.; Mozgawa, W. Sorption of Heavy Metal Cations on Mesoporous ZSM-5 and Mordenite Zeolites. Materials 2019, 12, 3271. [Google Scholar] [CrossRef] [PubMed]
- Frantz, T.S.; Ruiz, W.A.; da Rosa, C.A.; Mortola, V.B. Synthesis of ZSM-5 with high sodium content for CO2 adsorption. Microporous Mesoporous Mater. 2016, 222, 209–217. [Google Scholar] [CrossRef]
- Szostak, R. Molecular Sieves; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar] [CrossRef]
- Dey, K.P.; Ghosh, S.; Naskar, M.K. Organic template-free synthesis of ZSM-5 zeolite particles using rice husk ash as silica source. Ceram. Int. 2013, 39, 2153–2157. [Google Scholar] [CrossRef]
- Kim, D.J.; Chung, H.S. Synthesis and characterization of ZSM-5 zeolite from serpentine. Appl. Clay Sci. 2003, 24, 69–77. [Google Scholar] [CrossRef]
- Liang, G.; Li, Y.; Yang, C.; Hu, X.; Li, Q.; Zhao, W. Synthesis of ZSM-5 zeolites from biomass power plant ash for removal of ionic dyes from aqueous solution: Equilibrium isotherm, kinetic and thermodynamic analysis. RSC Adv. 2021, 11, 22365–22375. [Google Scholar] [CrossRef]
- Luo, W.; Yang, X.; Wang, Z.; Huang, W.; Chen, J.; Jiang, W.; Wang, L.; Cheng, X.; Deng, Y.; Zhao, D. Synthesis of ZSM-5 aggregates made of zeolite nanocrystals through a simple solvent-free method. Microporous Mesoporous Mater. 2017, 243, 112–118. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Adyaeva, L.V.; Ryabova, N.V. Effect of High-Temperature Steam Treatment of High-Silica Zeolites of the ZSM-5 Type on Their Acidity and Selectivity of Formation of Lower Olefins from Straight-Run Naphthas. Russ. J. Appl. Chem. 2003, 76, 95–98. [Google Scholar] [CrossRef]
- Klima, K.; Schollbach, K.; Brouwers, H.; Yu, Q. Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 2022, 314, 125574. [Google Scholar] [CrossRef]
- Cruz Sánchez, E.; Torres, M.E.; Diaz, C.; Saito, F. Effects of grinding of the feldspar in the sintering using a planetary ball mill. J. Am. Acad. Dermatol. 2004, 152, 284–290. [Google Scholar] [CrossRef]
- Rajakrishnamoorthy, P.; Karthikeyan, D.; Saravanan, C. Emission reduction technique applied in SI engines exhaust by using zsm5 zeolite as catalysts synthesized from coal fly ash. Mater. Today Proc. 2020, 22, 499–506. [Google Scholar] [CrossRef]
- Verrecchia, G.; Cafiero, L.; de Caprariis, B.; Dell’Era, A.; Pettiti, I.; Tuffi, R.; Scarsella, M. Study of the parameters of zeolites synthesis from coal fly ash in order to optimize their CO2 adsorption. Fuel 2020, 276, 118041. [Google Scholar] [CrossRef]
- Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Duquesne, S.; Roudet, F.; Silva-Vela, A. Influence of the Process of Synthesis of Zeolites from Volcanic Ash in Its Synergistic Action as a Flame-Retardant for Polypropylene Composites. Buildings 2021, 12, 24. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Ramadhani, A.N.; Abdullah, I.; Krisnandi, Y.K. Effect of Physicochemical Properties of Co and Mo Modified Natural Sourced Hierarchical ZSM-5 Zeolite Catalysts on Vanillin and Phenol Production from Diphenyl Ether. Bull. Chem. React. Eng. Catal. 2022, 17, 225–239. [Google Scholar] [CrossRef]
- Yu, D.; Chen, W.; Zhang, S.; Chilivery, R.; Li, F.; Lu, X.; Song, Y.; Fang, Y. Synthesis of HZSM-5 zeolites with excellent DME aromatization properties by orthogonal test. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 464–473. [Google Scholar] [CrossRef]
- Rajaei, H.; Esmaeilzadeh, F.; Mowla, D. Synthesis and Characterization of Nano-Sized Pt/HZSM–5 Catalyst for Application in the Xylene Isomerization Process. Catal. Lett. 2022, 152, 139–150. [Google Scholar] [CrossRef]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Zhang, K.; Van Dyk, L.; He, D.; Deng, J.; Liu, S.; Zhao, H. Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater. Green Process. Synth. 2021, 10, 349–360. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Dai, S.; Tan, Y.; Yang, Y.; Zhu, L.; Liu, B.; Du, Y.; Cao, X. Organotemplate-free synthesis of Al-rich ZSM-35 and ZSM-22 zeolites with the addition of ZSM-57 zeolite seeds. CrystEngComm 2022, 24, 6987–6995. [Google Scholar] [CrossRef]
- Kocirik, M.; Kornatowski, J.; Masařík, V.; Novák, P.; Zikánová, A.; Maixner, J.; Kočřé, M. Investigation of sorption and transport of sorbate molecules in crystals of MFI structure type by iodine indicator technique. Microporous Mesoporous Mater. 1998, 23, 295–308. [Google Scholar] [CrossRef]
- Verboekend, D.; Chabaneix, A.M.; Thomas, K.; Gilson, J.-P.; Pérez-Ramírez, J. Mesoporous ZSM-22 zeolite obtained by desilication: Peculiarities associated with crystal morphology and aluminium distribution. CrystEngComm 2011, 13, 3408–3416. [Google Scholar] [CrossRef]
- Sousa, L.V.; Silva, A.O.; Silva, B.J.; Teixeira, C.M.; Arcanjo, A.P.; Frety, R.; Pacheco, J.G. Fast synthesis of ZSM-22 zeolite by the seed-assisted method of crystallization with methanol. Microporous Mesoporous Mater. 2017, 254, 192–200. [Google Scholar] [CrossRef]
- Munusamy, K.; Das, R.; Ghosh, S.; Kumar, S.K.; Pai, S.; Newalkar, B. Synthesis, characterization and hydroisomerization activity of ZSM-22/23 intergrowth zeolite. Microporous Mesoporous Mater. 2018, 266, 141–148. [Google Scholar] [CrossRef]
- Song, C.-Y.; Meng, J.-P.; Li, C.; Zeyaodong, P.; Liang, C.-H. Synthesis of ZSM-22/ZSM-23 intergrowth zeolite as the catalyst support for hydroisomerization of n-hexadecane. J. Fuel Chem. Technol. 2021, 49, 712–725. [Google Scholar] [CrossRef]
- Wong, S.L.; Armenise, S.; Nyakuma, B.B.; Bogush, A.; Towers, S.; Lee, C.H.; Wong, K.Y.; Lee, T.H.; Rebrov, E.; Muñoz, M. Plastic pyrolysis over HZSM-5 zeolite and fluid catalytic cracking catalyst under ultra-fast heating. J. Anal. Appl. Pyrolysis 2023, 169, 105793. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K. Experimental Optimization of Process for the Thermo-catalytic Degradation of Waste Polypropylene to Liquid Fuel. Adv. Energy Eng. 2013, 1, 74–84. [Google Scholar]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.; Nizami, A. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Tekin, K.; Akalın, M.K.; Kadı, Ç.; Karagöz, S. Catalytic degradation of waste polypropylene by pyrolysis. J. Energy Inst. 2012, 85, 150–155. [Google Scholar] [CrossRef]


| Oxide | PO (%) | PU (%) | IG (%) |
|---|---|---|---|
| SiO2 | 77.90 | 78.60 | 67.60 |
| Al2O3 | 15.10 | 14.80 | 20.30 |
| K2O | 3.65 | 3.49 | 2.29 |
| Fe2O3 | 1.33 | 1.35 | 4.43 |
| CaO | 1.30 | 1.14 | 4.22 |
| TiO2 | 0.25 | 0.23 | 0.60 |
| Others | 0.47 | 0.39 | 0.56 |
| Material/Zeolite | Bending Stretching H-O-H | Asymmetric Stretching Si-O-T | Symmetric Stretching Si-O-T | |
|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | cm−1 | |
| PO | - | 1007 | 780 | 724 |
| IG | - | 1007 | 792 | 729 |
| PU | - | 996 | - | 705 |
| HZ-PO | 1629 | 1052 | 785 | - |
| HZ-IG | 1626 | 1065 | 793 | - |
| HZ-PU | 1630 | 1024 | 787 | 722 |
| ZSM-5 | 1620 | 1075 | 798 | - |
| Zeolite | Surface Specific Area | Micropore Area | Micropore Volume | Pore Diameter |
|---|---|---|---|---|
| m2/g | m2/g | cm3/g | nm | |
| HZ-PO | 229 | 144 | 0.076 | 3.8 |
| HZ-IG | 205 | 147 | 0.076 | 4.8 |
| HZ-PU | 156 | 101 | 0.053 | 5.8 |
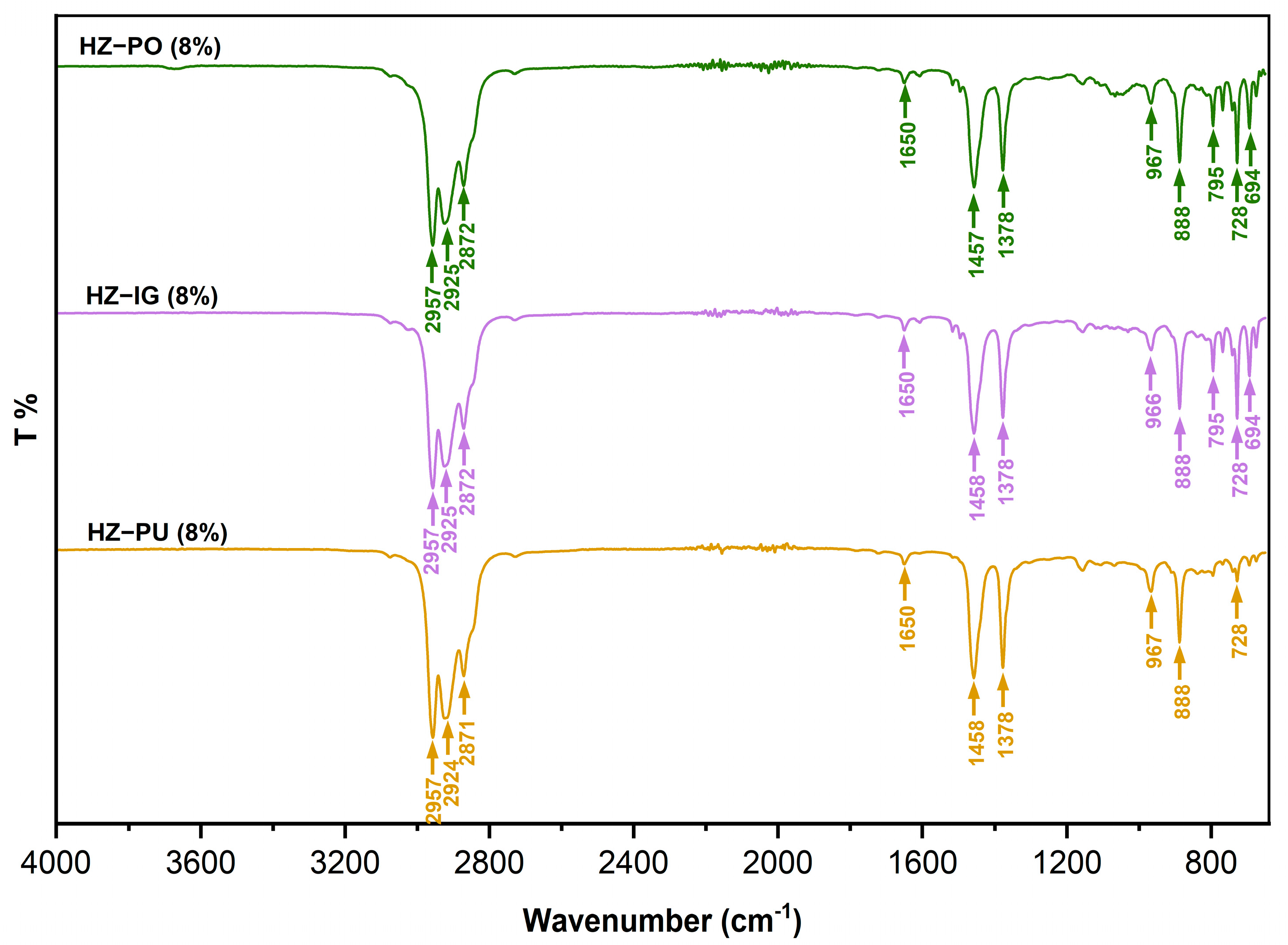
| Bands (cm−1) | Bond | Functional Group |
|---|---|---|
| 2957 | Asymmetric C-H stretching of CH3 | Methyl-alkanes |
| 2925, 2924 | Asymmetric C-H stretching of CH3 | Methyl-alkanes |
| 2872, 2871 | Symmetric stretching C-H of CH3 | Methyl-alkanes |
| 1650 | Stretching C=C | Alkenes |
| 1458, 1457 | Asymmetric bending C-H of CH3; flexion in the plane C-H (scissoring) of CH2 | Methyl and methylene Alkanes |
| 1378 | Symmetric C-H bending of CH3 | Methyl-alkanes |
| 967, 966 | C-H bending | Alkenes |
| 888 | Flexion out of the C-H plane | Alkenes |
| 795 | Flexion out of the C-H plane | Aromatics |
| 728 | Bending in the plane C-H (rolling) of CH2 | Methyl-alkanes |
| 694 | Flexion out of the C-H plane | Aromatics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Huaman, A.G.; Fuentes-Mamani, S.H.; Mamani-De La Cruz, L.F.; Velasco, F.; Churata, R.; Silva-Vela, A.; Mamani-Quispe, J.; Almirón, J. Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers. Sustainability 2024, 16, 5910. https://doi.org/10.3390/su16145910
Valencia-Huaman AG, Fuentes-Mamani SH, Mamani-De La Cruz LF, Velasco F, Churata R, Silva-Vela A, Mamani-Quispe J, Almirón J. Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers. Sustainability. 2024; 16(14):5910. https://doi.org/10.3390/su16145910
Chicago/Turabian StyleValencia-Huaman, Angel Gabriel, Sandro Henry Fuentes-Mamani, Luis Fernando Mamani-De La Cruz, Francisco Velasco, Rossibel Churata, Alejandro Silva-Vela, Jose Mamani-Quispe, and Jonathan Almirón. 2024. "Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers" Sustainability 16, no. 14: 5910. https://doi.org/10.3390/su16145910






