Biodegradable Spray Mulch Applications in Greenhouse Agroecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mulching Treatments
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Plant Reliefs
2.3. Soil Sampling and Pre-Treatment
2.4. Soil Laboratory Analyses
2.5. Statistical Analysis
3. Results
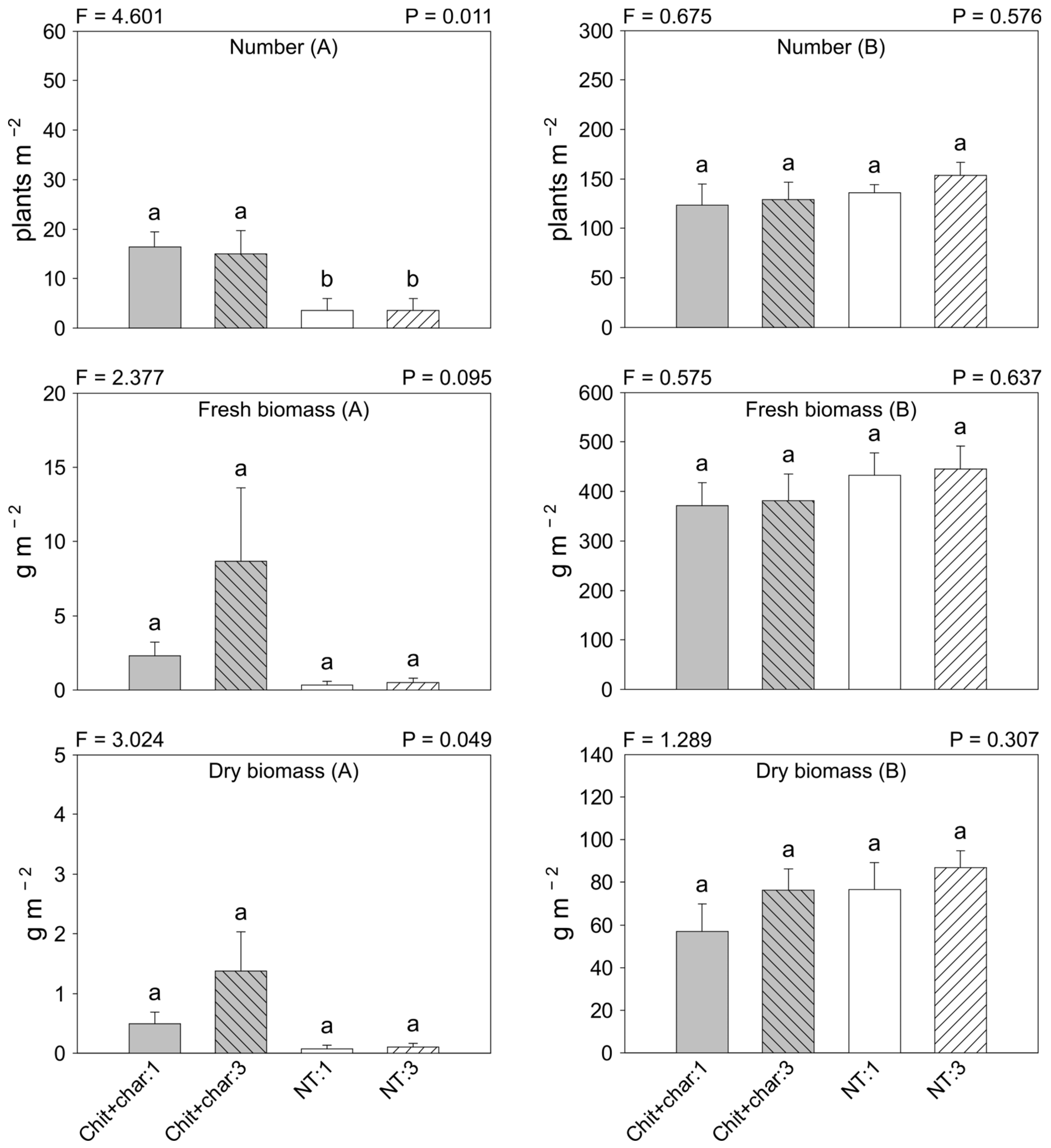
3.1. Experiment 1
3.2. Experiment 2
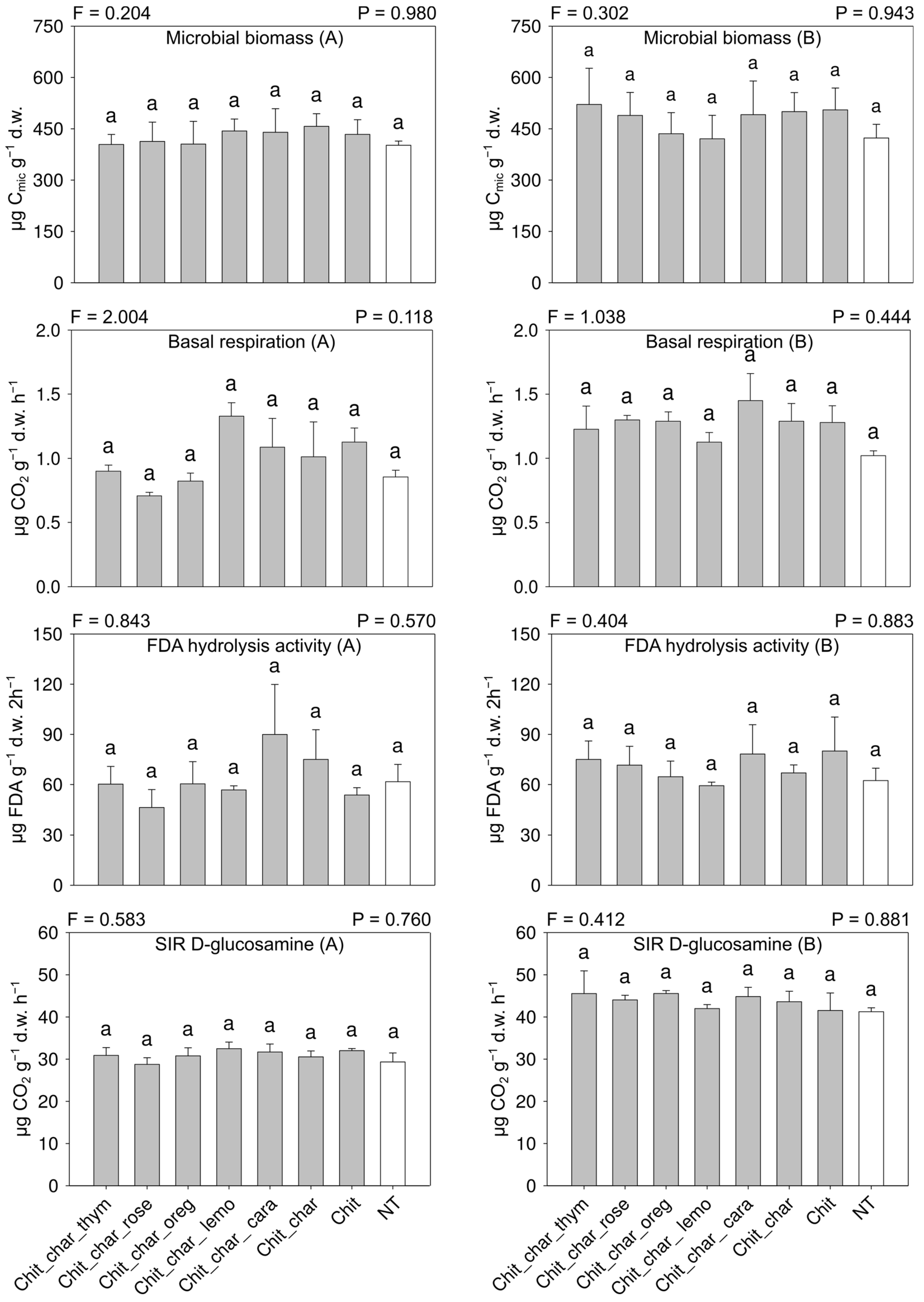
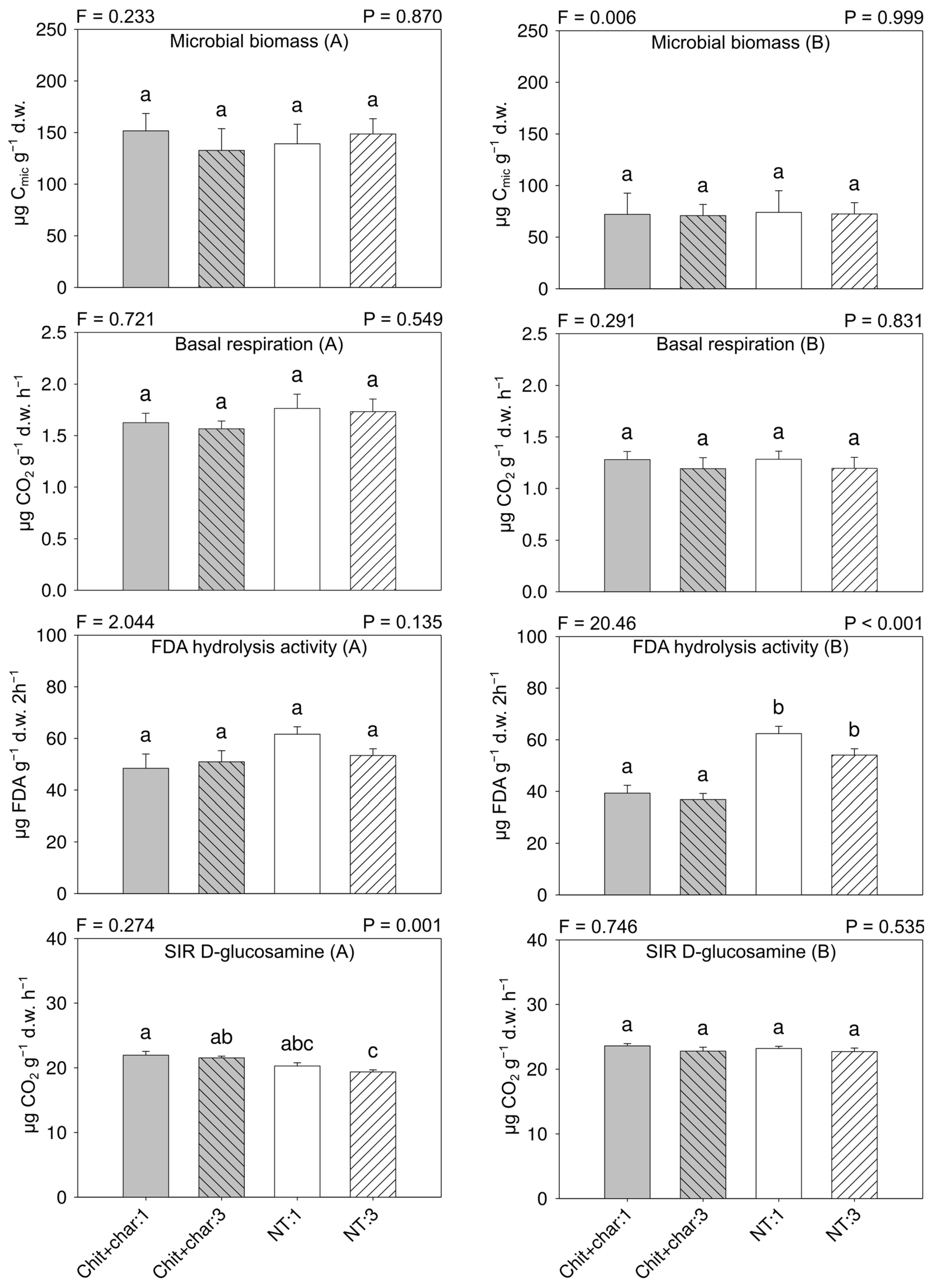
3.3. Soil Microbial Biomass and Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Portier, C.J.; Armstrong, B.K.; Baguley, B.C.; Baur, X.; Belyaev, I.; Bellé, R.; Belpoggi, F.; Biggeri, A.; Bosland, M.C.; Bruzzi, P.; et al. Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J. Epidemiol. Community Health 2016, 70, 741–745. [Google Scholar] [CrossRef]
- Dentzman, K.; Burke, I.C. Herbicide resistance, tillage, and community management in the Pacific Northwest. Sustainability 2021, 13, 1937. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kraehmer, H. Innovation: Changing trends in herbicide discovery. Outlooks Pest Man. 2012, 23, 115–118. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 5 June 2024).
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crop Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]
- Nikolova, M.; Traykova, B.; Yankova-Tsvetkova, E.; Stefanova, T.; Dzhurmanski, A.; Aneva, I.; Berkov, S. Herbicide potential of selected essential oils from plants of Lamiaceae and Asteraceae families. Acta Agrobot. 2021, 74, 7411. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar] [CrossRef]
- Yang, N.; Sun, Z.X.; Feng, L.S.; Zheng, M.Z.; Dao-Cai, C.; Meng, W.Z.; Hou, Z.Y.; Bai, W.; Li, K.Y. Plastic film mulching for water-efficient agricultural applications and degradable films materials development research. Mat. Manuf. Proc. 2014, 30, 143–154. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Khandelwal, A.; Phour, M.; Sehrawat, A. Bioherbicidal potential of rhizosphere microorganisms for ecofriendly weed management. In Role of Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; Volume 1. [Google Scholar]
- Mas, M.T.; Pardo, G.; Pueyo, J.; Verdú, A.; Cirujeda, A. Can hydromulch reduce the emergence of perennial weeds? Agronomy 2021, 11, 393. [Google Scholar] [CrossRef]
- Mzabri, I.; Rimani, M.; Charif, K.; Kouddanne, N.; Bericchi, A. Study of the effect of mulching materials on weed control in Saffron cultivation in eastern Morocco. Sci. World J. 2021, 9727004. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- European Commission Proposal for a Directive of the European Parliament and of the Council on the Reduction of the Impact of Certain Plastic Products on the Environment. Brussels, 28 May 2018, COM 28 Final. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A52018PC0340 (accessed on 11 July 2024).
- UNEP. Single-Use Plastics: A Roadmap for Sustainability United Nations Environment Programme; UNEP: Nairobi, Kenya, 2018; Available online: www.unep.org/resources/report/single-use-plastics-roadmap-sustainability (accessed on 11 July 2024).
- Sartore, L.; Schettini, E.; De Palma, L.; Brunetti, G.; Cocozza, C.; Vox, G. Effect of hydrolyzed protein-based mulching coatings on the soil properties and productivity in a tunnel greenhouse crop system. Sci. Total Environ. 2018, 645, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Vox, G.; Santagata, G.; Malinconico, M.; Immirzi, B.; Mugnozza, G.S. Biodegradable films and spray coatings as eco-friendly alternative to petro-chemical derived mulching films. J. Agric. Eng. 2013, XLIV, e44. [Google Scholar] [CrossRef]
- Adhikari, R.; Bristow, K.L.; Casey, P.S.; Freischmidt, G.; Hornbuckle, J.W.; Adhikari, B. Preformed and sprayable polymeric mulch film to improve agricultural water use efficiency. Agric. Water Manag. 2016, 169, 1–13. [Google Scholar] [CrossRef]
- Malinconico, M.; Santagata, G.; Schettini, E. Sprayable polysaccharide-based fiber reinforced emulsions for environmentally sound plasticulture. Macromol. Symp. 2006, 245–246, 578–583. [Google Scholar] [CrossRef]
- Mormile, P.; Petti, L.; Rippa, M.; Immirzi, B.; Malinconico, M.; Santagata, G. Monitoring of the degradation dynamics of agricultural films by IR thermography. Polym. Degrad. Stab. 2007, 92, 777–784. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Reid, J.S.G.; Edwards, M.E. Galactomannans and other cell wall storage polysaccharides in seeds. In Food Polysaccharides and Their Application; Stephen, A.M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 155–186. [Google Scholar]
- Anifantis, A.; Canzio, G.; Cristiano, G.; De Lucia, B.; Russo, G.; Vecchietti, L.; Immirzi, B.; Malinconico, M.; Santagata, G. Influence of the use of drip irrigation systems and different mulching materials on ornamental sunflowers in greenhouse cultivation. Acta Hortic. 2011, 952, 385–392. [Google Scholar] [CrossRef]
- Kirchinger, M.; Holzknecht, E.; Redl, M.; Steinkellner, S.; Emberger, P.; Remmele, E. A spray-on environmentally friendly degradable mulch material and its high efficiency in controlling above-ground biomass of weeds in greenhouse experiments. J. Plant Dis. Prot. 2024, 131, 1009–1020. [Google Scholar] [CrossRef]
- Immirzi, B.; Malinconico, M.; Santagata, G. Characterization of galactomannans and cellulose fibres based composites for new mulching spray technology. Acta Hortic. 2008, 801, 195–202. [Google Scholar] [CrossRef]
- Schettini, E.; Sartore, L.; Barbaglio, M.; Vox, G. Hydrolyzed protein based materials for biodegradable spray mulching coatings. Acta Hortic. 2012, 952, 359–366. [Google Scholar] [CrossRef]
- Giaccone, M.; Cirillo, C.; Scognamiglio, P.; Teobaldelli, M.; Mataffo, A.; Stinca, A.; Pannico, A.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. Biodegradable mulching spray for weed control in the cultivation of containerized ornamental shrubs. Biol. Technol. Agric. 2018, 5, 21. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, Y.H.; Jeon, M.S.; Kim, S.; E Choi, Y. Polyethylenimine linked with chitosan improves astaxanthin production in Haematococcus pluvialis. Appl. Microbiol. Biot. 2023, 107, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Marine Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quiñones, V.; Stockdale, E.A.; Banning, N.C.; Hoyle, F.C.; Sawada, Y.; Wherrett, A.D.; Jones, D.L.; Murphy, D.V. Soil microbial biomass. Interpretation and consideration for soil monitoring. Soil Res. 2011, 49, 287–304. [Google Scholar] [CrossRef]
- Wang, W.J.; Dalal, R.C.; Moody, P.W.; Smith, C.J. Relationship of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol. Biochem. 2003, 35, 273–284. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, R.W.; Hopkins, D.W. Mineralization of carbon from D- and L-amino acids and D-glucose in two contrasting soils. Soil Biol. Biochem. 1998, 30, 2009–2016. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Vojvodic-Vukovic, M. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Indurthi, S.; Ashoka, P.; Saikanth, D.R.K.; Das, H.; Kumar, V.; Pancholi, R. Application and impacts of mulch installation techniques on Indian horticulture: An in-depth review. Int. J. Plant Soil Sci. 2023, 35, 2135–2147. [Google Scholar] [CrossRef]
- Braunak, M.V.; Zaja, A.; Tam, K.; Filipovic, L.; Filipovic, V.; Wang, Y.; Bristow, K.L. A sprayable biodegradable polymer membrane (SBPM) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Young, S.L. Natural product herbicides for control of annual vegetation along roadsides. Weed Technol. 2004, 18, 580–587. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 6477–6497. [Google Scholar] [CrossRef]
- Abbate, C.; Scavo, A.; Pesce, G.R.; Fontanazza, S.; Restuccia, A.; Mauromicale, G. Soil bioplastic mulches for agroecosystem sustainability: A comprehensive review. Agriculture 2023, 13, 197. [Google Scholar] [CrossRef]
- Radics, L.; Bognar, E.S. Comparison of different mulching method for weed control in organic green bean and tomato. Acta Hortic. 2004, 638, 189–196. [Google Scholar] [CrossRef]
- Mas, M.T.; Verdú, A.M.C.; Pardo, G.; Pueyo, J.; Claramunt, J.; Cirujeda, A. Shoot and biomass reduction of perennial weeds using hydromulches and physical changes in the mulches. J. Plant Dis. Prot. 2024, 131, 433–443. [Google Scholar] [CrossRef]
- Orloff, N.; Mangold, J.; Miller, Z.; Menalled, F. A meta-analysis of field bindweed (Convolvulus arvesis L.) and Canada thistle (Cirsium arvense L.) management in organic agricultural systems. Agric. Ecosyst. Environ. 2018, 254, 264–272. [Google Scholar] [CrossRef]
- Feuilloley, P.; Cesar, G.; Benguigui, L.; Grohens, Y.; Pillin, I.; Bewa, H.; Lefaux, S.; Jamal, M. Degradation of polyethylene designed for agricultural purposes. J. Polym. Environ. 2005, 13, 349–355. [Google Scholar] [CrossRef]
- Santagata, G.; Schettini, E.; Vox, G.; Immirzi, B.; Mugnozza, S.; Malinconico, M. Biodegradable spray mulching and nursery pots: New frontiers for research. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture, Green Chemistry and Sustainable Technology; Malinconico, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 121–124. [Google Scholar]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; Bilotto, M.; Petriccione, M.; Ferrara, E.; Mori, M.; Morra, L. Assessing yield and quality of melon (Cucumis melo L.) improved by biodegradable mulching film. Plants 2023, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz-Walec, E.; Olszewska, M. Biostimulants in the production of forage grasses and turfgrasses. Agriculture 2023, 13, 1796. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.; dos Santos, C.A.; Lopes Alves, P.R.; de Paula, A.M.; Nakatani, A.S.; de Moraes Pereira, J.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]


| Biopolymer | Additive | Essential Oil |
|---|---|---|
| chitosan | charcoal | caraway |
| chitosan | charcoal | lemon balm |
| chitosan | charcoal | none |
| chitosan | charcoal | oregano |
| chitosan | charcoal | rosemary |
| chitosan | charcoal | thyme |
| galactomannan | charcoal | caraway |
| galactomannan | charcoal | lemon balm |
| galactomannan | charcoal | none |
| galactomannan | charcoal | oregano |
| galactomannan | charcoal | rosemary |
| galactomannan | charcoal | thyme |
| chitosan | none | none |
| galactomannan | none | none |
| none | none | none |
| Number | Fresh Biomass (g m−2) | Dry Biomass (g m−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||||||||
| T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | ||
| Annual plants | Gal + char + thyme e.o. | 85.0 a | 123 a | 101 a | 108 a | 1125 a | 1258 a | 259 a | 335 a | 113 a | 142 a | 42.0 a | 59.0 a |
| (0.44) | (0.51) | (0.08) | (0.21) | (1.03) | (0.66) | (0.07) | (0.48) | (0.94) | (0.38) | (0.13) | (0.53) | ||
| Gal + char + rosemary e.o. | 123 a | 128 a | 123 a | 123 a | 2284 a | 1897 a | 332 a | 280 a | 194 a | 203 a | 66.0 a | 49.0 a | |
| (0.30) | (0.21) | (0.17) | (0.30) | (0.38) | (0.32) | (0.40) | (0.38) | (0.31) | (0.38) | (0.40) | (0.31) | ||
| Gal + char + lemon balm e.o. | 145 a | 120 a | 118 a | 76.0 a | 2012 a | 1373 a | 327 a | 257 a | 200 a | 140 a | 41.0 a | 40.0 a | |
| (0.12) | (0.18) | (0.42) | (0.36) | (0.26) | (0.27) | (0.48) | (0.62) | (0.29) | (0.29) | (0.66) | (0.71) | ||
| Gal + char + oregano e.o. | 80.0 a | 75.0 a | 196 a | 111 a | 1066 a | 1009 a | 446 a | 371 a | 127 a | 124 a | 73.0 a | 59.0 a | |
| (0.60) | (0.61) | (0.72) | (0.34) | (0.51) | (0.45) | (0.30) | (0.38) | (0.46) | (0.18) | (0.12) | (0.40) | ||
| Gal + char + caraway e.o. | 100 a | 151 a | 100 a | 123 a | 1875 a | 1809 a | 344 a | 343 a | 202 a | 221 a | 50.0 a | 64.0 a | |
| (0.18) | (0.20) | (0.35) | (0.06) | (0.44) | (0.36) | (0.65) | (0.13) | (0.48) | (0.38) | (0.30) | (0.25) | ||
| Gal + char | 128 a | 138 a | 120 a | 176 a | 1716 a | 1486 a | 362 a | 508 a | 169 a | 155 a | 46.0 a | 73.0 a | |
| (0.09) | (0.42) | (0.76) | (0.76) | (0.40) | (0.67) | (0.94) | (0.84) | (0.55) | (0.47) | (0.97) | (0.81) | ||
| Gal | 116 a | 158 a | 258 a | 125 a | 2246 a | 1759 a | 537 a | 469 a | 232 a | 205 a | 87.0 a | 78.0 a | |
| Perennial plants | (0.28) | (0.13) | (0.90) | (0.20) | (0.17) | (0.28) | (0.46) | (0.27) | (0.22) | (0.38) | (0.45) | (0.44) | |
| Gal + char + thyme e.o. | 0.00 a | 0.00 a | 105 a | 83.0 a | 0.00 a | 0.00 a | 160 a | 185 a | 0.00 a | 0.00 a | 43.0 a | 47.0 a | |
| (0.00) | (0.00) | (0.52) | (0.03) | (0.00) | (0.00) | (0.67) | (0.33) | (0.00) | (0.00) | (0.56) | (0.33) | ||
| Gal + char + rosemary e.o. | 0.00 a | 0.00 a | 131 a | 83.0 a | 0.00 a | 0.00 a | 156 a | 143 a | 0.00 a | 0.00 a | 43.0 a | 37.0 a | |
| (0.00) | (0.00) | (0.76) | (0.40) | (0.00) | (0.00) | (0.75) | (0.38) | (0.00) | (0.00) | (0.94) | (0.43) | ||
| Gal + char + lemon balm e.o. | 0.00 a | 0.00 a | 78.0 a | 101 a | 0.00 a | 0.00 a | 154 a | 233 a | 0.00 a | 0.00 a | 33.0 a | 44.0 a | |
| (0.00) | (0.00) | (0.48) | (0.16) | (0.00) | (0.00) | (0.52) | (0.33) | (0.00) | (0.00) | (0.54) | (0.19) | ||
| Gal + char + oregano e.o. | 0.00 a | 0.00 a | 88.0 a | 93.0 a | 0.00 a | 0.00 a | 149 a | 227 a | 0.00 a | 0.00 a | 37.0 a | 52.0 a | |
| (0.00) | (0.00) | (0.39) | (0.08) | (0.00) | (0.00) | (0.46) | (0.19) | (0.00) | (0.00) | (0.15) | (0.94) | ||
| Gal + char + caraway e.o. | 0.00 a | 0.00 a | 120 a | 75.0 a | 0.00 a | 0.00 a | 273 a | 160 a | 0.00 a | 0.00 a | 63.0 a | 43.0 a | |
| (0.00) | (0.00) | (0.15) | (0.35) | (0.00) | (0.00) | (0.14) | (0.82) | (0.00) | (0.00) | (0.15) | (0.94) | ||
| Gal + char | 0.00 a | 0.00 a | 75.0 a | 60.0 a | 0.00 a | 0.00 a | 164 a | 122 a | 0.00 a | 0.00 a | 33.0 a | 30.0 a | |
| (0.00) | (0.00) | (0.29) | (0.30) | (0.00) | (0.00) | (0.33) | (0.28) | (0.00) | (0.00) | (0.32) | (0.34) | ||
| Gal | 0.00 a | 0.00 a | 85.0 a | 66.0 a | 0.00 a | 0.00 a | 151 a | 305 a | 0.00 a | 0.00 a | 37.0 a | 42.0 a | |
| (0.00) | (0.00) | (0.52) | (0.24) | (0.00) | (0.00) | (0.21) | (0.34) | (0.00) | (0.00) | (0.31) | (0.02) | ||
| Number | Fresh Biomass (g m−2) | Dry Biomass (g m−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||||||||
| T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | ||
| Annual plants | Chit + char + thyme e.o. | 48.0 a | 138 a | 41.0 a | 186 a | 294 a | 1594 a | 89.0 a | 376 a | 28.0 a | 182 a | 8.00 a | 43.0 a |
| (0.75) | (0.42) | (1.09) | (0.75) | (0.94) | (0.81) | (1.51) | (0.85) | (1.07) | (0.69) | (1.55) | (0.61) | ||
| Chit + char + rosemary e.o. | 53.0 b * | 160 a | 50.0 a | 126 a | 575 a | 1286 a | 81.0 a | 267 a | 70.0 a | 140 a | 15.0 a | 41.0 a | |
| (0.05) | (0.19) | (0.44) | (0.61) | (0.53) | (0.59) | (0.77) | (0.58) | (0.43) | (0.51) | (0.88) | (0.44) | ||
| Chit + char + lemon balm e.o. | 38.0 b * | 168 a | 61.0 a | 120 a | 754 a | 2191 a | 77.0 a | 295 a | 93.0 a | 204 a | 10.0 a | 44.0 a | |
| (0.15) | (0.41) | (0.05) | (0.54) | (0.79) | (0.68) | (0.57) | (0.51) | (0.57) | (0.51) | (0.75) | (0.58) | ||
| Chit + char + oregano e.o. | 70.0 b * | 136 a | 85.0 a | 128 a | 985 a | 1555 a | 163 a | 440 a | 86.0 b * | 172 a | 11.0 b * | 78.0 a | |
| (0.26) | (0.13) | (0.39) | (0.21) | (0.53) | (0.12) | (1.20) | (0.53) | (0.54) | (0.13) | (0.65) | (0.18) | ||
| Chit + char + caraway e.o. | 76.0 b ** | 153 a | 36.0 a | 115 a | 409 a | 1493 a | 45.0 a | 276 a | 68.0 b * | 214 a | 5.00 a | 44.0 a | |
| (0.16) | (0.16) | (0.31) | (0.69) | (0.87) | (0.27) | (0.50) | (0.93) | (0.64) | (0.32) | (0.23) | (0.81) | ||
| Chit + char | 56.0 a | 173 a | 55.0 a | 103 a | 434 b * | 1826 a | 55.0 a | 210 a | 69.0 a | 219 a | 6.00 b * | 46.07 a | |
| (0.33) | (0.42) | (0.24) | (0.47) | (1.07) | (0.29) | (0.29) | (0.56) | (0.73) | (0.42) | (0.57) | (0.58) | ||
| Chit | 55.0 b ** | 148 a | 170 a | 210 a | 554 a | 1590 a | 409 a | 720 a | 71.0 b * | 155 a | 45.0 a | 89.0 a | |
| Perennial plants | (0.16) | (0.19) | (1.02) | (0.95) | (0.30) | (0.41) | (1.00) | (0.43) | (0.40) | (0.28) | (0.68) | (0.39) | |
| Chit + char + thyme e.o. | 10.0 a | 0.00 a | 95.0 a | 103 a | 28.0 a | 0.00 a | 156 a | 195 a | 4.00 a | 0.00 a | 33.0 a | 50.0 a | |
| (0.86) | (0.00) | (0.97) | (0.77) | (0.86) | (0.00) | (0.65) | (0.84) | (0.86) | (0.00) | (0.65) | (0.79) | ||
| Chit + char + rosemary e.o. | 0.00 a | 8.00 a | 88.0 a | 65.0 a | 0.00 a | 28.0 a | 113 a | 133 a | 0.00 a | 5.00 a | 27.0 a | 28.0 a | |
| (0.00) | (0.91) | (0.66) | (0.61) | (0.00) | (0.91) | (0.91) | (0.51) | (0.00) | (0.91) | (0.97) | (0.60) | ||
| Chit + char + lemon balm e.o. | 9.00 a | 0.00 a | 95.0 a | 58.0 a | 47.0 a | 0.00 a | 156 a | 140 a | 8.00 a | 0.00 a | 31.0 a | 27.0 a | |
| (1.02) | (0.00) | (0.48) | (0.52) | (1.02) | (0.00) | (0.87) | (0.64) | (0.68) | (0.00) | (0.97) | (0.78) | ||
| Chit + char + oregano e.o. | 0.00 a | 6.00 a | 153 a | 73.0 a | 0.00 a | 32.0 a | 238 a | 145 a | 0.00 a | 4.00 a | 78.0 a | 37.0 a | |
| (0.00) | (1.07) | (0.41) | (0.38) | (0.00) | (1.07) | (0.39) | (0.42) | (0.00) | (0.91) | (0.53) | (0.66) | ||
| Chit + char + caraway e.o. | 9.00 a | 16.0 a | 145 a | 115 a | 26.0 a | 85.0 a | 199 a | 241 a | 5.00 a | 12.0 a | 45.0 a | 55.0 a | |
| (0.85) | (1.08) | (0.82) | (0.61) | (0.85) | (1.08) | (0.76) | (0.53) | (0.85) | (1.08) | (0.88) | (0.56) | ||
| Chit + char | 7.00 a | 0.00 a | 125 a | 70.0 a | 42.0 a | 0.00 a | 162 a | 155 a | 7.00 a | 0.00 a | 35.0 a | 38.0 a | |
| (1.06) | (0.00) | (0.59) | (0.14) | (1.06) | (0.00) | (0.88) | (0.57) | (1.06) | (0.00) | (0.82) | (0.62) | ||
| Chit | 15.0 a | 0.00 a | 88.0 a | 45.0 a | 29.0 a | 0.00 a | 116 a | 120 a | 6.00 a | 0.00 a | 28.0 a | 23.0 a | |
| (0.68) | (0.00) | (0.33) | (0.51) | (0.68) | (0.00) | (0.20) | (0.73) | (0.84) | (0.00) | (0.45) | (0.63) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, M.; Di Cesare, C.; Iovieno, P.; Immirzi, B.; Baldantoni, D.; Stipic, M.; Zaccardelli, M.; Venezia, A. Biodegradable Spray Mulch Applications in Greenhouse Agroecosystems. Sustainability 2024, 16, 5973. https://doi.org/10.3390/su16145973
Caputo M, Di Cesare C, Iovieno P, Immirzi B, Baldantoni D, Stipic M, Zaccardelli M, Venezia A. Biodegradable Spray Mulch Applications in Greenhouse Agroecosystems. Sustainability. 2024; 16(14):5973. https://doi.org/10.3390/su16145973
Chicago/Turabian StyleCaputo, Michele, Carlo Di Cesare, Paola Iovieno, Barbara Immirzi, Daniela Baldantoni, Marija Stipic, Massimo Zaccardelli, and Accursio Venezia. 2024. "Biodegradable Spray Mulch Applications in Greenhouse Agroecosystems" Sustainability 16, no. 14: 5973. https://doi.org/10.3390/su16145973
APA StyleCaputo, M., Di Cesare, C., Iovieno, P., Immirzi, B., Baldantoni, D., Stipic, M., Zaccardelli, M., & Venezia, A. (2024). Biodegradable Spray Mulch Applications in Greenhouse Agroecosystems. Sustainability, 16(14), 5973. https://doi.org/10.3390/su16145973








