Abstract
Sea cucumbers have become a highly valuable fishery product, and therefore the determination of their proximate compositions and fatty acid profiles are useful indicators of their physiological status and nutritional value. Thus, various raw and processed products, such as raw, dried, frozen, boiled, and salted Holothuria polii and Holothuria tubulosa that were collected from the Aegean Sea were analyzed. Although there were some notable differences in the proximate and fatty acid profiles between the two species and among the various processing forms, both H. polii and H. tubulosa had a high nutritional value. They were rich in proteins (up to 68.5% in the dried form), and although their lipid fraction was low (0.3–3.9%), it was characterized by very high levels of arachidonic acid (19.1–30.9% of total fatty acids) and eicosapentaenoic acid (8.5–15.7) that play vital roles in human growth, development, and health. In addition, their n-3/n-6 fatty acid ratios were within the recommended ranges, while their low Atherogenic and Thrombogenic indices and high Hypocholesterolemic index denote their high protective role against coronary artery disease. The provided information sheds light on the high nutritional value of this important marine fishery resource and provides valuable information for its preservation and processing. This information could also help policy makers, stakeholders, and the public to recognize the importance of this valuable fishery resource for human nutrition and to adopt preventative measures toward its sustainable exploitation.
1. Introduction
Sea cucumbers (Echinodermata: Holothuroidea) are marine benthic invertebrates that play significant ecological roles such as sediment bioturbation and bioremediation, thus recycling nutrients and energy flows in benthic ecosystems [1,2,3,4,5]. The genus Holothuria includes 150 species, of which 13 species are found in the north-eastern Atlantic and the Mediterranean Sea region [6,7,8]. Holothuria polii (Delle Chiaje, 1824) and Holothuria tubulosa (Gmelin, 1791), though genetically undifferentiated [9], are two of the most common and marketed species in the Aegean Sea [10,11] having recently become a new fishing target [12,13,14].
Sea cucumbers have become an important and highly valuable fishery product in more than 90 countries worldwide, due to their increasing consumption and expanding market, being sold mainly, but not exclusively, as a luxury food item [15]. It is estimated that their annual world production is about 245,000 tons [16], although it is believed that this is much higher due to unreported catches [17]. The high demand has led to the overexploitation of the natural stocks in many regions, and thus intensified attempts are being made for their culture to provide future supplies [10,11,18]. They are marketed in various forms such as raw, live, frozen, cooked–dried, boiled, cooked–salted, and cooked–salted–dried products with the dried type called “bêche-de-mer” or “trepang” or “hai-san” being traded as a luxury seafood [15,19].
These marine organisms are rich in certain bioactive compounds such as peptides, collagens, triterpene glycosides, sulphated polysaccharides, and phenols that make them capable of pharmacological activities such as anti-angiogenic, anticancer, anticoagulant, anti-hypertensive, anti-inflammatory, antimicrobial, antioxidant, antithrombotic, antitumoral, and wound-healing [20,21]. Aside from being functional foods, sea cucumbers are also considered highly nutritious for humans and are comparable to other seafood. Their dried form may contain up to 83% protein that is rich in several essential amino acids, particularly arginine, threonine, and phenylalanine and that has a low lysine to arginine ratio that has hypocholesteremic effects [20,22,23,24]. In addition, they are good sources of vitamins, especially A, B1, B2, and B3, and minerals, especially sodium, magnesium, calcium, potassium, phosphorus, zinc, and selenium [20,24]. Although their lipid fraction is very low, usually below 1% of their wet weight, some Holothurian species and their processed products may contain notable levels of the essential n-3 polyunsaturated fatty acids [20,24] that have beneficial effects on numerous human health disorders [25,26].
The determination of the nutrient (proximate) composition and fatty acid profiles is an indicator of the nutritional value of seafood, denotes the physiological status of the organism, while also providing valuable information for its preservation and processing [27,28,29,30]. As in all seafood, the proximate composition and fatty acid profile of sea cucumbers vary to a wide extent depending on the species, feeding behavior, habitat, region, and season as well as post-harvest processing methods [22,24,31]. Therefore, the aim of the study was to determine the proximate compositions and fatty acid profiles of H. polii and H. tubolosa harvested from the Aegean Sea and to investigate the effects of various processing methods on them.
2. Materials and Methods
2.1. Holothuria Sampling
A total number of ninety raw and processed products of H. polii and H. tubulosa were provided by a processing plant in central Greece (Lamia, Greece) that had been previously collected from Aegean Sea (Cyclades islands) during summer of 2022 [32]. All provided samples were eviscerated and washed with clean seawater. Except the raw ones, all samples were boiled for 80 min and were allowed to cool in drinking water at ambient temperature. Samples with no further processing were classified as “boiled” and stored at 4 °C. Samples that were classified as “dried” were produced by drying the previously boiled product in an air-forced oven at 30 °C for two weeks. Samples that were classified as “frozen” were produced by freezing the previously boiled product in a blast chiller until the temperature dropped down to −18 °C. Samples that were classified as “salted” were produced by a second boiling for 2.5 h, cooling in drinking water, and salting and drying at 25 °C for 10 days. For each species, nine samples (including body wall and muscle) per processed form were chopped, minced, and homogenized for the analysis in triplicates of their proximate compositions and fatty acid profiles.
2.2. Proximate Composition
About 5 g of each homogenate was used for analysis. Proximate composition was conducted to determine the moisture/dry matter, crude protein, ash, and gross energy contents of the Holothurian samples. For moisture/dry matter contents, samples were dried to constant weight at 105 °C for 24 h in an oven. Kjeldahl analysis was performed through digestion and distillation (behr Labor-Technik, Düsseldorf, Germany), and total nitrogen was used to determine crude protein (N × 6.25). Ash content was determined by dry ashing at 600 °C for 5 h using a muffle furnace (Nabertherm L9/12/C6, Lilienthal, Germany), and gross energy content was determined adiabatically using an IKA oxygen bomb calorimeter (C5000, IKA Werke, Staufen, Germany).
2.3. Total Lipid and Fatty Acid Profiles
Total lipid extraction was performed according to Folch et al. method. First, chloroform/methanol (C:M 2:1, v/v) was added to the homogenate, and then the sample was further homogenized using an Ultra-Turrax tissue disrupter (T 25 digital, IKA-Werke, Staufen, Germany) and a vortex shaker (MS3, IKA-Werke). Potassium chloride aqueous solution (0.88% KCl v/v) was added in order to induce phase separation, and the samples were left on ice for 4 h. The lipid phase was then filtered (Whatman paper, No 2) into pre-weighted glass tubes. Solvents were evaporated with a stream of nitrogen until dryness (N-EVAP Organomation, Berlin, NH, USA), and the total lipid content was determined gravimetrically. Extracted lipids were stored in C:M (2:1, v/v), containing butylated hydroxyltoluene (BHT) to prevent oxidation, at a final concentration of 20 mg lipid/mL.
Fatty acid methyl esters (FAMEs) were prepared by acid catalyzed transesterification using a sulfuric acid/methanol (1:99 v/v) methylating reagent. Lipid extract of 1 mg of total lipid was evaporated under a stream of nitrogen until dryness, and then 2 mL of the methylating reagent and 1 mL of toluene were added. The samples were incubated on a hot block (Kisker-Biotech, Steinfurt, Germany) at 50 °C for 16 h. Crude FAMEs were purified by thin-layer chromatography on 20 × 20 glass plates pre-coated with silica gel G (Macherey-Nagel, Sil G-25) in isohexane/diethyl ether/acetic acid (90:10:1, v/v) and then scraped and eluted with isohexane/diethyl ether (1:1, v/v). Purified FAMEs were re-dissolved in isohexane containing 0.01% BHT and stored under nitrogen at −80 °C until analysis.
Separation and quantification of FAMEs was conducted by gas–liquid chromatography with a Perkin Elmer Clarus 680 coupled with a Col-Elite FameWax capillary column (30 m × 0.25 mm id, film thickness 0.25 μm (Perkin Elmer, Waltham, MA, USA) and equipped with a flame ionization detector (FID). Hydrogen was used a carrier gas at flow rate of 1 mL/min. Injector temperature was set at 240 °C with a split ratio of 1:10 at a total flow rate of 5 mL/min. The temperature was programmed from 60 °C to 190 °C at a rate of 20 °C/min and maintained for 5 min and from 190 °C to 240 °C at a rate of 5 °C/min and maintained for 10 min. Identification of individual FAMEs was conducted by comparison to known standards (FAME MIX 37, Sigma-Aldrich, St. Louis, MO, USA), and peak areas were quantified with reference to the peak area of 17:0 internal standard, and chromatograms were analyzed using TotalChrom software (v. 6.3, Perkin Elmer).
The Atherogenic Index (AI), Thrombogenic Index (TI), and Hypocholesterolemic/Hypercholesterolemic ratio (H/H) were also measured according to the following formulae [33]:
Atherogenic Index (AI) = (12:0 + 4 × 14:0 + 16:0)/(MUFA + n − 3 PUFA + n − 6 PUFA).
Thrombogenic Index (TI) = (14:0 + 16:0 + 18:0)/[(0.5 × MUFA) + (0.5 × n − 6 PUFA) + (3 × n − 3 PUFA) + (n − 3 PUFA/n − 6 PUFA)].
Hypocholesterolemic/Hypercholesterolemic ratio (H/H) = (cis−C18:1+ ΣPUFA)/(12:0 + 14:0 + 16:0).
2.4. Statistical Analysis
Results are presented as means ± standard deviation. Percentages were arcsine-transformed prior to statistical analysis. Data were tested for normality by Shapiro–Wilk’s test and for homogeneity by Levene’s test and were transformed whenever required. Data for proximate composition among the different processed products were subjected to one-way analysis of variance (ANOVA), while data for proximate composition between the different species were subjected to independent t-test. Data for fatty acids were subjected to ANOVA. Tukey’s post hoc test was conducted to rank the groups using SPSS 26.0 (IBM SPSS Statistics 26). Differences were regarded as significant at p < 0.05.
3. Results
3.1. Proximate Compositions
The proximate compositions of the various processed products of H. polii and H. tubulosa are shown in Table 1. Depending on the processing method, the moisture content ranged 2.5–80.9% and 2.2–83.2%, the protein content ranged 10.8–68.5 and 10.5–66.0%, the lipid content ranged 0.3–3.9% and 0.3–2.1%, the ash content ranged 8.8–24.5% and 3.6–28.8%, and the energy content ranged 62.1–327.7 Kcal/100 g and 47.3–300.5 Kcal/100 g for H. polii and H. tubulosa, respectively. For both Holothurian species, as expected, the dried products had the lowest (p < 0.05) and the raw ones had the highest (p < 0.05) moisture content compared to the other processed products of the same species. For both species, the frozen products had a significantly lower moisture content than the raw ones, but this content was significantly higher compared to that of the boiled and salted products. Regardless of the species, the frozen, boiled, and salted products had a similar (p > 0.05) protein content, which was lower (p < 0.05) than that of the dried and higher (p < 0.05) than that of the raw forms. The frozen, boiled, and salted products had also a similar lipid content that was lower than that of the dried forms. In both species, the boiled and salted products exhibited a higher (p < 0.05) ash content compared to that of the raw and frozen products, while the dried products showed the highest (p < 0.05) values. The dried product had also the highest energy content, and the raw ones the lowest, while the frozen, boiled, and salted products had a similar energy content.

Table 1.
Proximate compositions (% as fed) of the different processed products of Holothuria polii and Holothuria tubulosa.
As far as the differences in their proximate compositions between the two Holothurian species are concerned, some significant observations were found (Figure 1). Specifically, H. tubulosa had a higher moisture content in the raw product compared to H. polii, while the latter showed a higher lipid content in the dried product. The comparison between the two species in their protein content did not reveal any significant difference in any of the processed forms. However, the ash content of the raw, frozen, and salted H. polii was higher than that found in the corresponding processed forms of H. tubulosa, while the opposite was observed in the dried products. The raw and dried products of H. polii had a higher energy value compared to that of H. tubulosa, while the opposite was observed for the frozen and salted products.
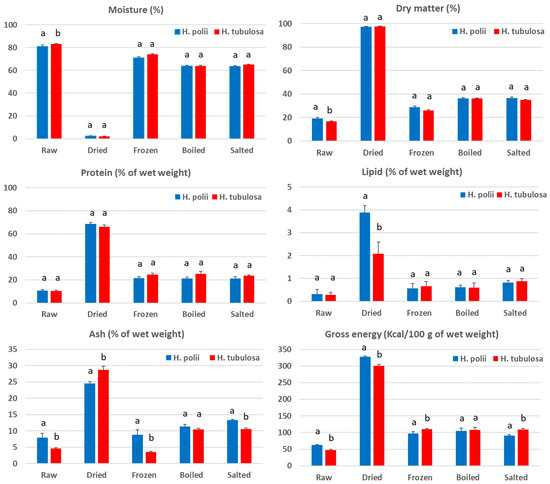
Figure 1.
Comparison of the proximate compositions of the various processed products between H. polii and H. tubulosa. Bars represent means ± st. deviation (n = 3). For each processed product, bars not sharing a common superscript are significantly different (p < 0.05) between the two species.
3.2. Fatty Acid Composition
A total number of thirty fatty acids of various chain lengths and saturation levels were identified by gas–liquid chromatography in both H. polii (Table 2) and H. tubulosa (Table 3). Of the saturated fatty acids (SFA), palmitic acid (16:0) and stearic acid (18:0) were found to be dominant in both species, irrespective of the processed form. Considerable levels of arachidic (20:0) and behenic (22:0) acids were present in both species, with lauric (12:0), myristic (14:0), pentadecanoic (15:0), and lignoceric (24:0) acids being minor components of Holothurian lipids. Of the monounsaturated fatty acids (MUFA), palmitoleic (16:1n-7), oleic (18:1n-9), vaccenic (18:1n-7), and nervonic (24:1n-9) acids were the most abundant in both Holothurian species. Gondoic acid (20:1n-9) was also found at high levels, especially in the salted form, while myristoleic (14:1), hypogeic (16:1n-9), and erucic (22:1n-9) acids were minor components. Of the polyunsaturated fatty acids (PUFA), the most abundant in Holothurian lipids were by far the arachidonic (20:4n-6, ARA) and eicosapentaenoic (20:5n-3, EPA) acids. Several n-6 PUFA accounted for 1–3% of the total fatty acids, such as linoleic (18:2n-6), eicosadienoic (20:2n-6), adrenic (22:4n-6), and n-6 docosapentaenoic (22:5n-6) acids, while the levels of docosahexaenoic acid (22:6n-3, DHA) accounted for 0.9–1.75%. Several other PUFA such as g-linolenic (18:3n-6), a-linolenic acid (18:3n-3), stearidonic (18:4n-3), dihomo-g-linolenic (20:3n-6), eicosatrienoic (20:3n-3), and n-3 docosapentaenoic (22:5n-3) acids were minor components.

Table 2.
Fatty acid composition (% of total fatty acids) of H. polii.

Table 3.
Fatty acid composition (% of total fatty acids) of H. tubulosa.
In H. polii lipids (Table 2), the SFA ranged 21.9–29.2%, MUFA ranged 28.9–33.8%, n-6 PUFA ranged 27.1–33.8%, and n-3 PUFA ranged 12.3–17.3% among the various processed products. The processing method exhibited a significant effect on the fatty acid profiles of the species, but without a clear trend. Thus, the frozen form had significantly higher SFA compared to the boiled form, due to its higher levels of 16:0 and 18:0. The dried form had significantly higher MUFA than the frozen and boiled products due mainly to its higher levels of 14:1, 16:1n-7, 18:1n-7, and 24:1n-9. On the other hand, the boiled products had significantly higher n-6 PUFA, mainly due to its higher levels of 20:4n-6 and 22:4n-6, compared to the raw, dried, and frozen products and significantly higher n-3 PUFA, due to its higher levels of 18:3n-3, 20:5n-3, and 22:6n-3, compared to the salted form. The lowest levels of n-3 PUFA were found in the salted form, which also had elevated levels of n-6 PUFA. As such, the salted products had a significantly lower n-3/n-6 ratio compared to the other products. Due to the highest level of n-3 PUFA and the lowest level of SFA, the boiled H. polii had the lowest Atherogenic Index (AI) and the highest Hypocholesterolemic/Hypercholesterolemic ratio (H/H), while the opposite was found in the frozen products. All forms of the products had a similar Thrombogenic Index (TI), though.
In H. tubulosa lipids (Table 3), the SFA ranged 20.1–29.1%, MUFA ranged 20.1–30.5%, n-6 PUFA ranged 29.6–38.6%, and n-3 PUFA ranged 9.9–18.8% among the various processed products. The processing method exhibited a significant effect on the fatty acid profiles of H. tubulosa too, but again without a clear trend. Thus, the raw form had significantly higher SFA compared to the dried, frozen, and salted forms, due mainly to its higher content of 16:0. The frozen products had a lower (p < 0.05) MUFA content compared to the rest but had higher (p < 0.05) n-3 PUFA and n-6 PUFA contents compared to the raw, boiled, and salted forms. The lowest n-3 PUFA was found in the dried form, which had also the highest n-6 PUFA. As such, the dried products had a significantly lower n-3/n-6 ratio compared to the other products. As far as the fatty acid indices are concerned (Table 4), the raw H. tubulosa had the highest AI and TI indices and the lowest H/H ratio, while the boiled products had an increased TI index and one of the lowest H/H ratios. On the other hand, the dried H. tubulosa had the highest H/H ratio and low AI and TI indices.

Table 4.
Fatty acid indices of Holothuria polii and Holothuria tubulosa.
4. Discussion
The proximate compositions of H. polii and H. tubulosa have not been studied extensively compared to that of other Holothurian species. The compositions determined in the present study were comparable to those reported for these species [34,35,36] and for other Holothurian species [20] and echinoderms [37] across the globe, with typically high moisture, high protein (in dry weight,) and low lipid contents. Comparing the two species, both exhibited similar levels of crude protein, regardless of the processing form, and this is in agreement with the findings of Aydın et al. [35] who reported similar protein levels for the raw form of the two species that were sampled in the Izmir area of the Aegean Sea. Sicuro et al. [23] also reported an identical protein content between dried H. polii and H. tubulosa from the southern Adriatic Sea, as well as similar levels of moisture, lipid, ash, and energy. However, we found notable differences between the two species, in the dried form, for their contents of lipid, energy, and ash. Aydın et al. [35] also observed significant differences between the two species for their ash and moisture contents. Certainly, the fact that proximate composition is affected by numerous factors such as feeding behavior, habitat, region, and season as well as stage of ontogenesis [22,24,31] can explain such discrepancies in the findings and therefore it is meaningless to compare absolute values among studies.
It is interesting, though, to determine the impact of processing methods on the proximate composition of Holothurian species, considering the various forms in which these are marketed. It should be pointed out that numerous processing methods and protocols are practiced around the globe based mainly on local traditional knowledge or ad hoc industrial processes and due to the lack of species-specific standards [38,39,40,41]. The production of commercially available dried sea cucumbers (bêche-de-mer) involves a sequencing process mainly of boiling, salting, and drying [39,40,41], and each process can exert an effect on the nutritional quality of the end product [22]. For this reason, the present study investigated the effects of each cooking method per se. The results reveal that the dried forms of both Holothurian species were the densest and the raw forms were the least dense in all nutrients and energy. This is reasonable as the proportion of moisture in the whole proximate composition acts as an indicator of the relative energy, protein, lipid, and mineral proportions in the biomass. The lower the moisture proportion, the higher the nutrient and energy contents.
Our results also show that the freezing process kept a substantial amount of moisture in the Holothurian body, though lower than that in the raw form, while boiling and salting significantly lowered the moisture levels. Boiling, as a hydrothermal treatment, is known to reduce the moisture content of raw seafood [42] as it aids the water evaporation in cells, while the salting process of seafood lowers the water activity and causes dehydration [43]. On the other hand, the freezing process usually does not have a significant effect on seafood’s moisture levels [44,45], but this was not observed in the present study. Interestingly, the freezing, boiling, and salting processes had a similar effect on the protein and lipid contents of both Holothurian species, thus resulting in these three product types having similar levels of these nutrients that were also elevated compared to the raw forms. Certainly, the moisture loss that occurred during freezing, boiling, and salting resulted in higher protein and lipid proportions. Moreover, it has been claimed that the increase in protein content of seafood during boiling could be due to the solubilization of some nitrogenous compounds [46,47,48]. The results also reveal that the boiling and salting processes increased the ash levels of both Holothurian species compared to the raw and frozen forms. This is mainly attributed to the increased dry matter of the former as it has been supported by other processed seafood studies [48], despite the fact that the ash content of seafood might be reduced after boiling due to the volatility of the mineral elements at high temperatures [49]. Certainly, the salting itself could also have increased the ash levels of Holothuria due to the addition of inorganic salt during the process [31,50], and this might explain the fact that the highest values of ash were observed in the salted forms of both Holothurian species.
Up until now, the effects of different processing methods on the proximate composition of Holothuria are extremely limited. Similarly to our findings, Çakli et al. [34] reported that the levels of moisture, protein, and lipid were intermediate in the boiled form compared to the raw and dried forms of H. tubulosa. Bilgin and Tanrikulu [36] working with the same species also reported that the boiled form contained intermediate levels of moisture and protein compared to the raw and dried forms, but their lipid and ash levels were similar to those of the raw form. Nishanthan et al. [31] studied the impacts of processing on the proximate compositions of several commercially available sea cucumber species, including Holothuria spinifera and Holothuria scabra. In fact, the authors compared the proximate compositions of a raw form versus dried products that were previously eviscerated, boiled, salted, boiled again, and dried with different protocols. They reported that the processing lowered the moisture levels of sea cucumbers, but its impact on the protein, lipid, and ash levels, although significant, was variable among species and between raw and processed forms. Nishanthan et al. [41] stressed the fact that the proximate composition of dried sea cucumbers is significantly affected by processing methods and can be altered even when a small deviation from the main processing steps is applied, such as changes in boiling medium and time, method for chalky material removal, salting medium, drying method, and time.
As far as the fatty acid profiles of H. polii and H. tubulosa are concerned, the present study reveals that the two species exhibited many similarities. Thus, and irrespective of the processed form, both species contained a higher PUFA fraction than that of SFA and MUFA and this is in agreement with the findings of other studies with these species sampled in the open sea [23,35,36,51]. On the other hand, Sadoul et al. [52] reported much higher values of SFA than PUFA and MUFA in H. tubulosa reared under fish cages and that there was an increase in the SFA content with increasing distance from the farm, which was attributed to the higher ingestion of SFA-rich microorganisms. The raw forms of the two species were characterized by very high amounts of ARA (19.1–19.4% of total fatty acids) that was by far the most dominant fatty acid. Such high levels of ARA have not been previously reported for these species, except those reported for H. polii [35] that were sampled during April from Ayvalik Bay (Aegean Sea). However, the richness of both H. tubulosa and H. polii in ARA has also been reported in other studies with these species [36,51,52] and it is characteristic in other sea cucumbers as well [22,24,53,54,55,56], especially in those from the tropics [57]. Arachidonic acid once was considered a harm-generating molecule due to its attributed proinflammatory/proagulatory effects, but recent studies revealed that this fatty acid and its metabolites play numerous fundamental roles in normal health and cell function in humans [58,59,60]. It is essential for the normal development and function of the nervous system, skeletal muscle, and immune system and in fact has protective effects against many health and metabolic disorders, including tumor development and cardiovascular complications [58,59,60].
The levels of EPA in the lipids of raw H. polii and H. tubulosa were as high as 9.2–13.5% with the higher values coming from the former. These values are similar to those (10%) reported for H. tubulosa by Bilgin and Tanrikulu [36] and higher than those reported by Aydin et al. [35] for these two species (4.6–9.3% for H. polii and 5.1–7.5% H. tubulosa) sampled from other regions of the Aegean Sea. Prato et al. [51] reported a similar EPA level (8.8–8.9%) for the body wall of H. polii and H. tubulosa from the Taranto Gulf, while Sicuro et al. [23] reported EPA values at 12.9–15.4% for these two species sampled from the Adriatic Sea. The lowest reported EPA values (3–6%) for these two species were those found in specimens sampled from the Gulf of Valinco [52]. Sea cucumbers are known to be rich in EPA [22,24,53,54,56]. The vital roles of EPA, as well as DHA, for human nutrition are very well documented. These are essential fatty acids for normal growth and development playing also preventative roles in numerous health disorders related to inflammatory, cardiovascular, neurological, psychological, and cancerous diseases among many others [58,61,62]. It is worth noting that the EPA levels found in both Holothurian species were lower than their ARA levels. The fact that the levels of 18:2n-6 were found to be higher than those of 18:3n-3 in both Holothurian species might explain the higher ARA than EPA found in their tissues, as it is known that sea cucumbers are capable of synthesizing C20 PUFA from their C18 PUFA precursors [63].
Concerning the DHA levels of H. polii and H. tubulosa, these were found as low as 1.0–1.3% (of total fatty acids), which are much lower than those reported for these species in the Aegean Sea and other Mediterranean regions [23,35,36]. Prato et al. [51] also reported very low levels (below 1%) of DHA in the body wall of H. polii sampled from the Taranto Gulf during summer. Such very low DHA levels are common in Holothurians [31,54,56] and other sea cucumbers [24,31], while many studies did not even detect this fatty acid [22,55,64]. Certainly, the methodology of fatty acid analysis can exert a significant effect on the reported fatty acid values, and this fact has been stressed by other authors too [31,35,65]. It seems, however, that the primary n-3 PUFA in sea cucumbers is EPA, with the DHA levels being much lower even in specimens that are sampled from cold-water environments [66] that show a tendency to accumulate n-3 PUFA in their tissues.
Summarizing the effects of processing methods on the fatty acid profiles of H. polii and H. tubulosa, we could state that the processing methods exerted significant alterations, but the effects of each method were not similar to both species. For example, the drying process led to increased MUFA and decreased EPA levels in H. polii, compared to the raw form, but that was not detected in H. tubulosa in which the drying reduced the SFA and n-3 PUFA, and elevated the levels of ARA and n-6 PUFA. Another example was that the salting process reduced the EPA levels in H. polii, but the opposite was observed for H. tubulosa. Similarly, boiling exerted a significant effect on n-6 PUFA and an insignificant effect on the n-3 PUFA levels of H. polii, but the opposite was detected for H. tubulosa. Furthermore, the freezing process reduced the SFA and MUFA and elevated the ARA, EPA, DHA and PUFA in H. tubulosa, but did not have any effect on the profiles of H. polii. These vague effects cannot provide clear insights of how the various processing methods affect the fatty acid profiles of the Holothurians, and further research is needed to shed light on this field. Related studies on the effects of different processing/cooking methods on the fatty acid profiles of sea cucumbers are extremely limited. In agreement with our conclusions, Aydin et al. [35] reported a variable effect of drying on the fatty acid profiles of H. polii and H. tubulosa, and the authors attributed this to the regional origin of the individual specimens. Nishanthan et al. [31] observed that the processing (evisceration, first boiling, salting, and drying) resulted in a general trend of reduced SFA and MUFA, and increased PUFA in most of the sea cucumber species analyzed, but the opposite happened to some others.
The Atherogenic Index (AI), Thrombogenic Index (TI), and Hypocholesterolemic/Hypercholesterolemic ratio (H/H) are common indicators of the lipid nutritional value of food related to coronary heart disease [33]. Lower AI and TI values and higher H/H ratios are more beneficial for human health. In the present study, the values of AI (0.20–0.40) and TI (0.19–0.32) found in the lipids of all processed forms of H. polii and H. tubulosa are commonly found in seafood [33], which are lower than those found in meat and dairy products, denoting the high lipid nutritional value of these two Holothurian species. In addition, the fact that their H/H values (3.11–7.60) are much higher than those commonly found in most commercial fish species, as well as in meat and dairy products [33], strengthens their suitability even more as a healthy choice for the consumer. Similarly, Sales et al. [56] reported low values of AI (0.08–0.13) and TI (0.09–0.13) and high values for H/H (6.1–8.9) for Holothuria arguinensis and Holothuria forskali sampled in Portuguese waters denoting the protective potential of Holothurians for coronary artery disease. The different processing methods seemed to exert an influence on these lipid indices of the Holothurians, but again this was not clear. For example, all processing methods improved the lipid indicators in H. tubulosa, but that was not obvious for H. polii. As stated above, further research is needed to shed light on the influence of the various processing methods on the fatty acid profiles and lipid indices of the Holothurians.
Overall, although there were some notable differences in the proximate and fatty acid profiles between the two species and among the various processing forms, it could be stated that H. polii and H. tubulosa have a high nutritional value. They are rich in proteins, and although their lipid fraction is low, it is characterized by high levels of ARA and EPA that play vital roles in human growth, development, and health. In addition, their n-3/n-6 ratios are within the range of 1:4–5, which is recommended by many international nutritional organizations [67,68]. This information could help policy makers, stakeholders, and the public to recognize the importance of this valuable fishery resource for human nutrition and to adopt preventative measures toward its sustainable exploitation. The provided information can be helpful to the food processing industry and could raise scientific and industrial interests for future applications. In order to assess the entire nutritional value of H. polii and H. tubulosa, future studies should focus on the more biologically active substances, such as polysaccharides, saponins, and functional lipids that act as nutraceuticals and pharmaceutical agents.
Author Contributions
Conceptualization, D.V. and I.T.K.; methodology, E.Z.G., C.A., K.V. and A.V.; validation, E.Z.G. and C.A.; formal analysis, E.Z.G. and C.A.; investigation, D.V., I.T.K., E.Z.G., C.A., K.V. and A.V.; resources, D.V. and I.T.K.; data curation, I.T.K. and E.Z.G.; writing—original draft preparation, I.T.K. and E.Z.G.; writing—review and editing, I.T.K. and D.V.; supervision, D.V. and I.T.K.; project administration, D.V.; funding acquisition, D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was implemented in the framework of the project titled “Exploitation and management of sea cucumber fisheries (Holothuria spp.): processing (food and biotech products) and safeguarding of stocks”, grant number MIS 5010720, co-funded by the “Operational Programme for Fisheries and Sea 2014–2020—Greece” and the “European Maritime and Fisheries Fund”.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the absence of a relevant framework for the usage of holothurians in marine research; Holothuria spp. is a fishery resource legally allowed to be collected from the wild, the collected specimens were immediately preserved without performing any experimental treatment that could torture the animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koukouras, S.A.; Sinis, I.A. Benthic fauna of the North Aegean Sea II. Crinoidea and Holothuroidea (Echinodermata). Vie Milieu 1981, 31, 271–281. [Google Scholar]
- Costa, V.; Mazzola, A.; Vizzini, S. Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Biol. Ecol. 2014, 461, 226–232. [Google Scholar] [CrossRef]
- Purcell, S.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. Oceanogr. Mar. Biol. 2016, 54, 367–386. [Google Scholar] [CrossRef]
- Ennas, C.; Pasquini, V.; Abyaba, H.; Addis, P.; Sarà, G.; Pusceddu, A. Sea cucumbers bioturbation potential outcomes on marine benthic trophic status under different temperature regimes. Sci. Rep. 2023, 13, 11558. [Google Scholar] [CrossRef]
- Skordas, K.; Georgiou, K.; Kinigopoulou, V.; Kelepertzis, E.; Apostologamvrou, C.; Lolas, A.; Petrotou, A.; Neofitou, N.; Vafidis, D. Potentially toxic element assessment and biological accumulation in two sea cucumbers species Holothuria poli and Holothuria tubulosa. Reg. Stud. Mar. Sci. 2024, 70, 103370. [Google Scholar] [CrossRef]
- Koukouras, A.; Sinis, A.I.; Bobori, D.; Kazantzidis, S.; Kitsos, M.S. The echinoderm (Deuterostomia) fauna of the Aegean Sea, and comparison with those of the neighbouring seas. J. Biol. Res. 2007, 7, 67–92. [Google Scholar]
- Antoniadou, C.; Vafidis, D. Population structure of the traditionally exploited holothurian Holothuria tubulosa in the south Aegean Sea. Cah. Biol. Mar. 2011, 52, 171–175. [Google Scholar]
- Kalthoumi, D.; Francisco, S.M.; Miladi, M.; Ruiz-Canales, A.; Azzouna, A.; Robalo, J.I. The first report of Holothuria (Thymiosycia) impatiens (Forsskål, 1775), (Holothuroidea: Holothuriidae) from Tunisia (Mediterranean Sea): Taxonomic, Morphological, and Molecular Data Compilation. Diversity 2023, 15, 542. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Sarantopoulou, J.; Apostologamvrou, C.; Antoniadou, C.; Exadactylos, A.; Fleris, G.; Vafidis, D. Admixture of Holothurian species in the Hellenic Seas (Eastern Mediterranean) as revealed by RADseq. Sustainability 2023, 15, 11493. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A.; Boncagni, P.; Lovatelli, A.; Scardi, M.; Cataudella, S. Spawning and rearing of Holothuria tubulosa: A new candidate for aquaculture in the Mediterranean region. Aquac. Res. 2018, 49, 557–568. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A.; Boncagni, P.; Scardi, M.; Cataudella, S. Artificial reproduction of Holothuria polii: A new candidate for aquaculture. Aquaculture 2019, 498, 444–453. [Google Scholar] [CrossRef]
- Kazanidis, G.; Antoniadou, C.; Lolas, A.P.; Neofitou, N.; Vafidis, D.; Chintiroglou, C.; Neofitou, C. Population dynamics and reproduction of Holothuria tubulosa (Holothuroidea: Echinodermata) in the Aegean Sea. J. Mar. Biol. Assoc. UK 2010, 90, 895–901. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Domínguez-Godino, J.A.; Cánovas, F. The fast development of sea cucumber fisheries in the Mediterranean and NE Atlantic waters: From a new marine resource to its over-exploitation. Ocean Coast. Manag. 2018, 151, 165–177. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C. Holothurian fisheries in the Hellenic Seas: Seeking for sustainability. Sustainability 2023, 15, 9799. [Google Scholar] [CrossRef]
- Purcell, S.W.; Lovatelli, A.; González-Wangüemert, M.; Solís-Marín, F.A.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World, 2nd ed.; FAO Species Catalogue for Fishery Purposes No. 6, Rev. 1; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture (SOFIA). In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Rahman, M.A.; Chowdhury, S.H.; Hasan, M.J.; Rahman, M.H.; Yeasmin, S.M.; Farjana, N.; Molla, M.H.R.; Parvez, M.S. Status, prospects and market potentials of the sea cucumber fisheries with special reference on their proper utilization and trade. Annu. Res. Rev. Biol. 2020, 35, 84–101. [Google Scholar] [CrossRef]
- Choo, P.S. Population status, fisheries and trade of sea cucumbers in Asia. In Sea Cucumbers. A Global Review of Fisheries and Trade; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; FAO Fisheries and Aquaculture Technical Paper No. 516; FAO: Rome, Italy, 2008; pp. 79–118. [Google Scholar]
- Vafidis, D.; Tsagridis, A.; Chintiroglou, C.; Stamatis, N.; Antoniadou, C. Fisheries, Processing and Trade of the South Aegean Holothurian Stocks; Operational Programme for Greek fisheries for the period 2000–2006, Final Technical Report; Ministry of Agricultural Development and Food: Volos, Greece, 2008; 83p. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Khotimchenko, Y. Pharmacological potential of sea cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef]
- Sicuro, B.; Piccinno, M.; Gai, F.; Abete, M.C.; Danieli, A.; Dapra, F.; Mioletti, S.; Vilella, S. Food quality and safety of Mediterranean Sea cucumbers Holothuria tubulosa and Holothuria polii in southern Adriatic Sea. Asian J. Anim. Vet. Adv. 2012, 7, 851–859. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S. The nutritional value of holothurians. Russ. J. Mar. Biol. 2015, 41, 409–423. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev. Int. 2004, 20, 77–90. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The benefits of fish consumption. Nutr. Bull. 2011, 36, 6–19. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical paper, No. 348; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 1995; p. 145. [Google Scholar]
- Karapanagiotidis, I.T. Nutrient profiles of tilapia. In Tilapia in Intensive Co-Culture, 1st ed.; Perschbacher, P.W., Stickney, R.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 261–305. [Google Scholar] [CrossRef]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of processing methods on nutritional and physico-chemical composition of fish: A review. MOJ Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Nishanthan, G.; Kumara, P.A.D.A.; de Croos, M.D.S.T.; Prasada, D.V.P.; Dissanayake, D.C.T. Effects of processing on proximate and fatty acid compositions of six commercial sea cucumber species of Sri Lanka. J. Food Sci. Technol. 2018, 55, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Boziaris, S.I.; Anagnostopoulos, D.A.; Parlapani, F.F.; Syropoulou, F.; Martsikalis, P.V.; Apostologamvrou, C.; Kokioumi, D.; Vafidis, D. Microbial and physicochemical status of raw and processed sea cucumbers from the Hellenic seawaters. Sustainability 2023, 15, 13467. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Çaklı, Ş.; Cadun, A.; Kışla, D.; Dinçer, T. Determination of quality characteristics of Holothuria tubulosa (Gmelin, 1788) in Turkish Sea (Aegean Region) depending on sun drying process step used in Turkey. J. Aquat. Food Prod. Technol. 2004, 13, 69–78. [Google Scholar] [CrossRef]
- Aydın, M.; Sevgili, H.; Tufan, B.; Emre, Y.; Köse, S. Proximate composition and fatty acid profile of three different fresh and dried commercial sea cucumbers from Turkey. Int. J. Food Sci. Technol. 2011, 46, 500–508. [Google Scholar] [CrossRef]
- Bilgin, S.; Tanrikulu, H. The changes in chemical composition of Holothuria tubulosa (Gmelin, 1788) with ambient-drying and oven-drying methods. Food Sci. Nutr. 2018, 6, 1456–1461. [Google Scholar] [CrossRef]
- Stamatis, N.; Vafidis, D. Effect of marinating and vacuum storage at 6 °C on the fate of chemical, microbial and sensory quality indices of echinoid gonads Paracentrotus lividus Lamark, 1816. Int. J. Food Sci. Technol. 2009, 44, 1626–1633. [Google Scholar] [CrossRef]
- Lavitra, T.; Rachelle, D.; Rasolofonirina, R.; Jangoux, M.; Eeckhaut, I. Processing and marketing of holothurians in the Toliara region, southwestern Madagascar. Beche-de-Mer Inf. Bul. 2008, 28, 24–33. [Google Scholar]
- Ram, R.; Chand, R.V.; Southgate, P.C. Effects of processing methods on the value of bêche-de-mer from the Fiji Islands. J. Mar. Sci. Res. Dev. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Purcell, S.W.; Ngaluafe, P.; Aram, K.T.; Lalavanua, W. Variation in postharvest processing of sea cucumbers by fishers and commercial processors among three Pacific Island countries. Beche-de-mer Inf. Bul. 2016, 36, 58–66. [Google Scholar]
- Nishanthan, G.; Kumara, P.A.D.A.; Navarathne, S.B.; Dissanayake, D.C.T. Impact of different combinations of processing steps on product quality and proximate composition of Bêche-de-mer: A case study from Sri Lanka. Int. J. Food Sci. 2022, 2022, 7877050. [Google Scholar] [CrossRef] [PubMed]
- Okomoda, V.T.; Tiamiyu, L.O.; Ricketts, A.O.; Ricketts, A.O.; Oladimeji, S.A.; Agbara, A.; Ikhwanuddin, M.; Alabi, K.I.; Munafi, A.B.A. Hydrothermal processing of Clarias gariepinus (Burchell, 1822) filets: Insights on the nutritive value and organoleptic parameters. Vet. Sci. 2020, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Fitri, N.; Chan, S.X.Y.; Che Lah, N.H.; Jam, F.A.; Misnan, N.M.; Kamal, N.; Sarian, M.N.; Mohd Lazaldin, M.A.; Low, C.F.; Hamezah, H.S.; et al. A comprehensive review on the processing of dried fish and the associated chemical and nutritional changes. Foods 2022, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Foruzani, S.; Maghsoudloo, T.; Noorbakhsh, H.Z. The effect of freezing at the temperature of −18 °C on chemical compositions of the body of Lutjanus johnii. AACL Bioflux 2015, 8, 431–437. [Google Scholar]
- Shafi, J.; Waheed, K.N.; Zafarullah, M.; Mirza, Z.S.; Yaqoob, S.S. Effect of icing on quality of silver carp during frozen storage. J. Food Process. Preserv. 2020, 44, e14654. [Google Scholar] [CrossRef]
- Ninawe, A.S.; Rathnakumar, K. Fish Processing Technology and Product Development, Impact of Curing, 1st ed.; Narendra Publishing House: New Delhi, India, 2008; p. 572. [Google Scholar]
- Mustafa, M.G.; Begum, S.R.; Abdul Khaleque, M.D.; Jannat, M.; Ahsan, D.A. Nutritional qualities of hilsha and sarpunti in different salt curing methods. Dhaka Univ. J. Biol. Sci. 2012, 21, 97–104. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, K. Comparison of the effects of cooking methods on nutritional composition of fresh and salted Tenualosa Ilisha. Aquac. Fish. 2020, 5, 294–299. [Google Scholar] [CrossRef]
- Aberoumand, A. Preliminary studies on nutritive and organoleptic properties in processed fish fillets obtained from Iran. J. Food Sci. Technol. 2014, 34, 287–291. [Google Scholar] [CrossRef]
- Clucas, I.J.; Ward, A.R. Post Harvest Fisheries Development. A Guide to Handling, Preservation, Processing and Quality; Natural Resources Institute: Chatham, UK, 1996; p. 453. [Google Scholar]
- Prato, E.; Fanelli, G.; Parlapiano, I.; Biandolino, F. Bioactive fatty acids in seafood from Ionian Sea and relation to dietary recommendations. Int. J. Food Sci. Nutr. 2020, 71, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Caprioli, J.P.; Barrier-Loiseau, C.; Cimiterra, N.; Laugier, T.; Lagarde, F.; Chary, K.; Callier, M.D. Is Holothuria tubulosa the golden goose of ecological aquaculture in the Mediterranean Sea? Aquaculture 2022, 554, 738149. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Qi, Y.; Guo, Z.; Lin, Y.; Li, W.; Hu, Y.; Zhao, Q. Proximate composition and nutritional quality of deep sea growth sea cucumbers (Stichopus japonicus) from different origins. J. Sci. Food Agric. 2016, 96, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Dias, S.; Pinteus, S.; Silva, J.; Alves, C.; Tecelão, C.; Pedrosa, R.; Pombo, A. Sea cucumber Holothuria forskali, a new resource for aquaculture? Reproductive biology and nutraceutical approach. Aquac. Res. 2015, 47, 2307–2323. [Google Scholar] [CrossRef]
- Al Azad, S.; Shaleh, S.R.M.; Siddiquee, S. Comparison of fatty acid and proximate composition between Holothuria edulis and Holothuria scabra collected from coastal water of Sabah, Malaysia. Adv. Biosci. Biotechnol. 2017, 8, 91–103. [Google Scholar] [CrossRef]
- Sales, S.; Lourenço, H.M.; Pessoa, M.F.; Pombo, A.; Félix, P.M.; Bandarra, N.M. Chemical composition and omega 3 human health benefits of two sea cucumber species of north Atlantic. J. Aquat. Food Prod. Technol. 2021, 30, 596–614. [Google Scholar] [CrossRef]
- Svetashev, V.I.; Levin, V.S.; Lam, C.N.; Nga, D.T. Lipid and fatty acid composition of holothurians from tropical and temperate waters. Comp. Biochem. Physiol. 1991, 4, 489–494. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of arachidonic acid metabolites on cardiovascular health and disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Zárate, R.; el Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Trans. Med. 2017, 6, e25. [Google Scholar] [CrossRef]
- Crupi, R.; Cuzzocrea, S. Role of EPA in inflammation: Mechanisms, effects, and clinical relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef]
- Li, W.X.; Feng, Z.F.; Song, X.J.; Zhu, W.; Hu, Y.J. Cloning, expression and functional characterization of the polyunsaturated fatty acid elongase (ELOVL5) gene from sea cucumber (Apostichopus japonicus). Gene 2016, 593, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.O.; Mahdy, A.; Nasser, S.A.M.; Abd El-Wakeil, K.F.; Obuid-Allah, A.H.; Hassan, M.M. Biochemical composition of some Echinodermata (Holothuroidea, Echinoidea) from the Red Sea, Egypt. Braz. J. Biol. 2022, 82, e246309. [Google Scholar] [CrossRef]
- Fredalina, B.D.; Ridzwan, B.H.; Abidin, A.A.Z.; Kaswandi, M.A.; Zaiton, H.; Zali, I.; Kittakoop, P.; Mat Jaise, A.M. Fatty acid composition in local sea cucumber, Stichopus chloronotus, forwound healing. Gen. Pharmacol. 1999, 33, 337–340. [Google Scholar] [CrossRef]
- Zhong, Y.; Ahmad, K.M.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef]
- European Commission- Scientific Committee on Food. Report of the Scientific Committee on Food on Composition and Specification of Food Intended to Meet the Expenditure of Intense Muscular Effort, Especially for Sportsmen. SCF/CS/NUT/SPORT/5 Final 2001. Available online: https://food.ec.europa.eu/system/files/2020-12/sci-com_scf_out64_en.pdf (accessed on 15 April 2024).
- World Health Organization (WHO). Diet, Nutrition and the Prevention of Chronic Diseases; Report of a joint WHO/FAO expert consultation; WHO technical report series 916; WHO: Geneva, Switzerland, 2003. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).