Urban Air Pollution Exposure Impact on COVID-19 Transmission in a Few Metropolitan Regions
Abstract
:1. Introduction
2. Material and Method
2.1. Study Test Metropolitan Areas
- Berlin (52°31′ N 13°20′ E), the German capital located in northeastern Germany, with a surface of 1368 km2, has a predominantly flat topography, with a mean elevation of 35 m above sea level, and a temperate climate with precipitation in all seasons [52];
- London (51.33° N, 0.42° W), the largest European city and the second-largest economic center globally, with a surface of 1738 km2 and dense road traffic, placed in the southeastern part of England, mostly consisting of low-land terrain with a mean elevation of 42 m, has a temperate, oceanic climate with cool winters, warm summers, and precipitation fairly evenly distributed all year round [53];
- Madrid (40.42° N, 3.70° W), the largest metropolitan area in Spain, and the third largest city in the European Union, with a surface of 1365 km2, has a Mediterranean climate with continental influences, characterized by hot summers and cool winters. The urban area is settled on an uneven plain, approximately 700 m high, with the lowest altitudes of the basin located in the southeast, away from the mountains [54].
- Paris (48°51′24″ N 2°21′3″ E), the capital of France, located in a relatively flat area with a mean elevation of 62 m, has a surface of 2844 km2 and a typical Western European climate, affected by its proximity to the Atlantic Ocean, being mild and moderately wet [55] with cool winters with frequent rain and overcast skies and mild-to-warm summers.
2.2. Datasets
2.3. Methods
3. Results and Discussion
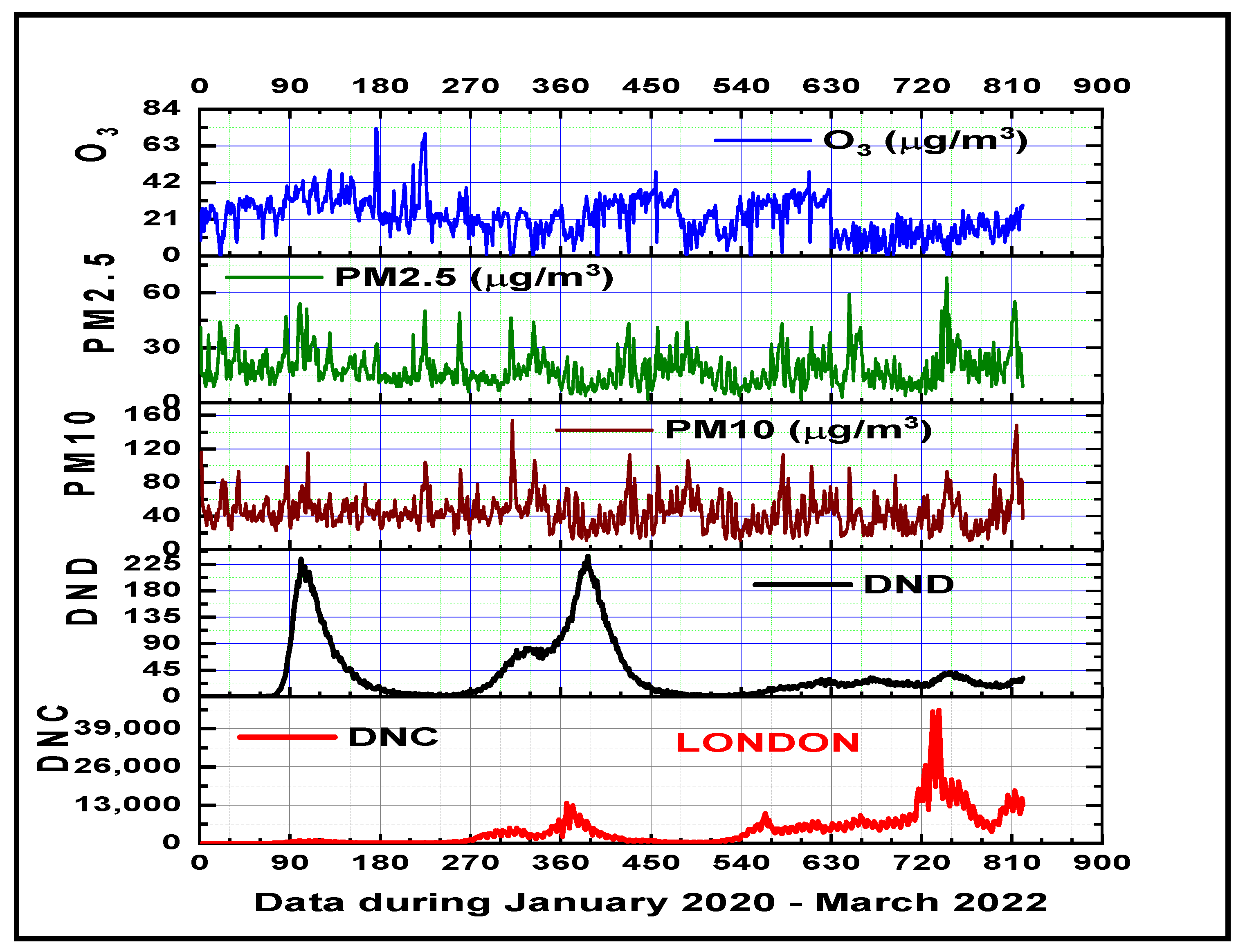
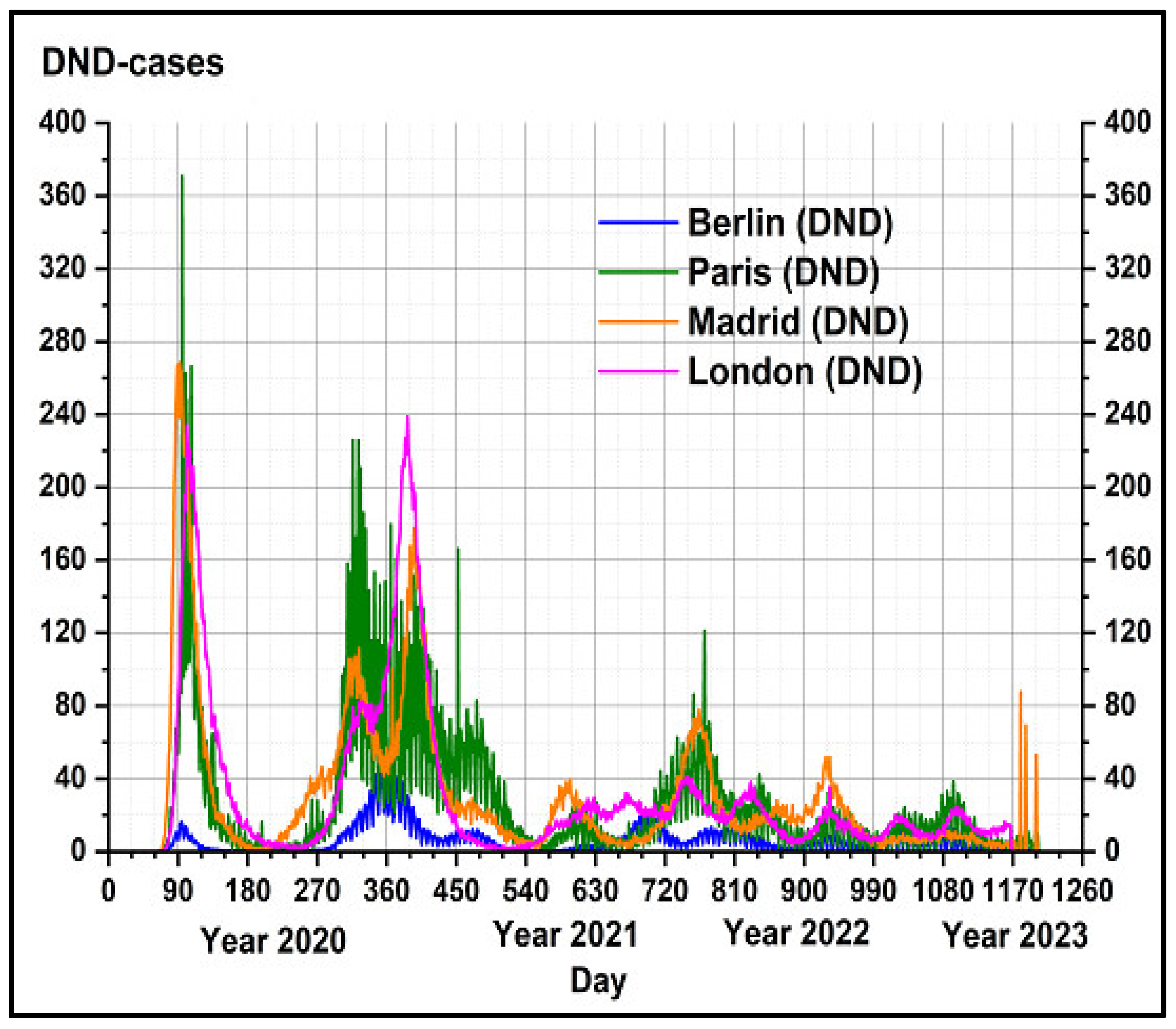
3.1. Air Pollutants and Climate Variability Impacts on COVID-19 Disease in the Metropolitan Areas
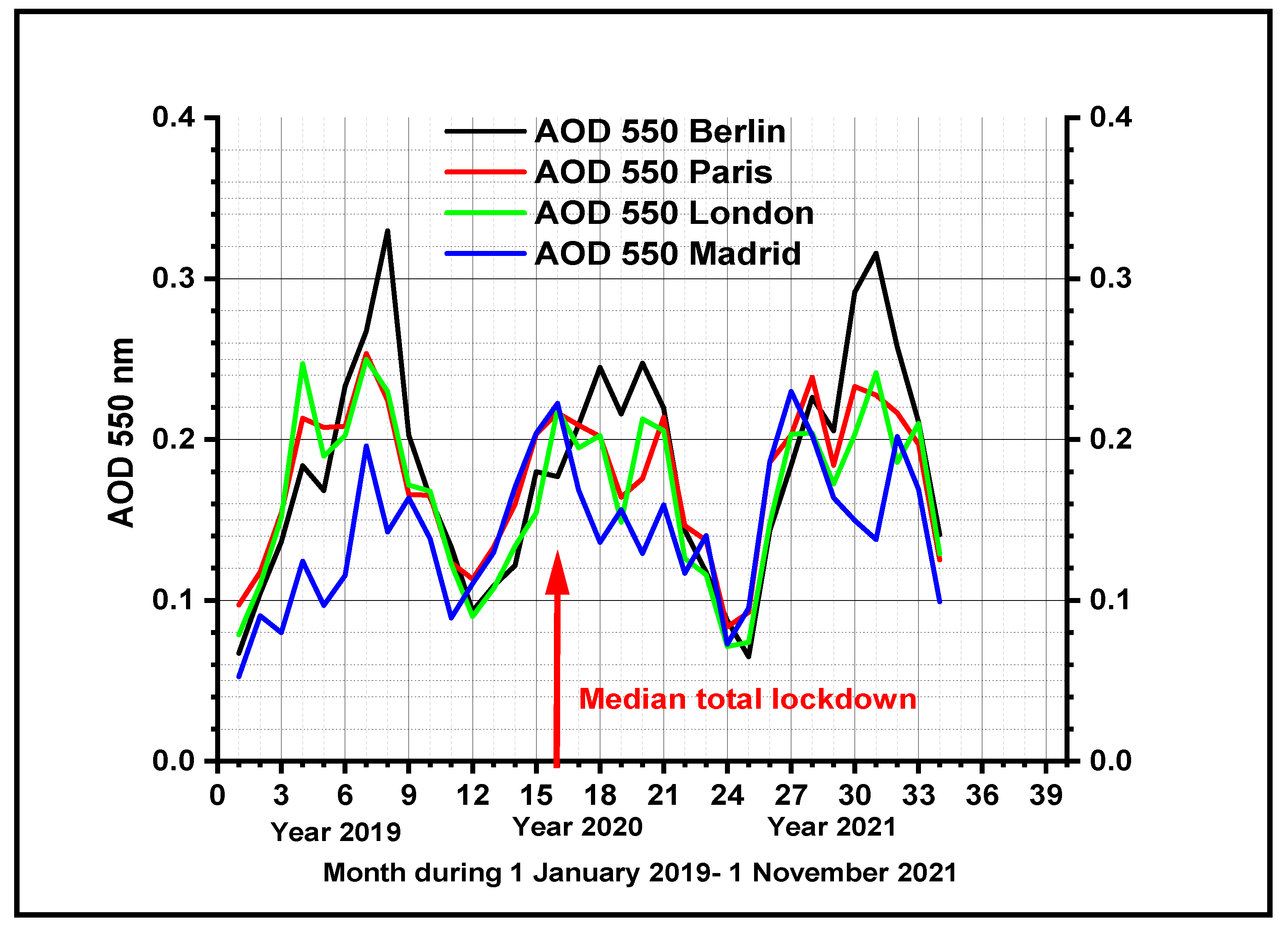
3.2. Evolution of Total Aerosol Optical Depth at 550 nm
4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsamadony, M.; Fujii, M.; Ryo, M.; Nerini, F.F.; Kakinuma, K.; Kanae, S. Preliminary quantitative assessment of the multidimensional impact of the COVID-19 pandemic on Sustainable Development Goals. J. Clean. Prod. 2022, 372, 133812. [Google Scholar] [CrossRef]
- Shuai, C.; Zhao, B.; Chen, X.; Liu, J.; Zheng, C.; Qu, S.; Zou, J.P.; Xu, M. Quantifying the impacts of COVID-19 on Sustainable Development Goals using machine learning models. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Hyman, S.; Zhang, J.; Andersen, Z.J.; Cruickshank, S.; Møller, P.; Daras, K.; Williams, R.; Topping, D.; Lim, Y.H. Long-term exposure to air pollution and COVID-19 severity: A cohort study in Greater Manchester, United Kingdom. Environ. Pollut. 2023, 327, 121594. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Bellazzi, S.; Caccamo, F.M.; Miino, M.C. Discussion about the Latest Findings on the Possible Relation between Air Particulate Matter and COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 5132. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, B.; Li, H.; Feng, F.; Zhang, Y.; Wang, W.; Qin, P. Air pollution and its associated health risks before and after COVID-19 in Shaanxi Province, China. Environ. Pollut. 2023, 320, 121090. [Google Scholar] [CrossRef]
- Kirwan, P.D.; Charlett, A.; Birrell, P.; Elgohari, S.; Hope, R.; Mandal, S.; De Angelis, D.; Presanis, A.M. Trends in COVID-19 hospital outcomes in England before and after vaccine introduction, a cohort study. Nat. Commun. 2022, 13, 4834. [Google Scholar] [CrossRef]
- Marchini, T. Redox and inflammatory mechanisms linking air pollution particulate matter with cardiometabolic derangements. Free. Radic. Biol. Med. 2023, 209 Pt 2, 320–341. [Google Scholar] [CrossRef]
- Sofiev, M.; Sofieva, S.; Palamarchuk, J.; Šaulienė, I.; Kadantsev, E.; Atanasova, N.; Fatahi, Y.; Kouznetsov, R.; Kuula, J.; Noreikaite, A.; et al. Bioaerosols in the atmosphere at two sites in Northern Europe in spring 2021: Outline of an experimental campaign. Environ. Res. 2022, 214, 113798. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, Z.; Gao, X.; Yuan, Y.; Dong, S.; Li, X.; Liu, X. Investigating the cumulative lag effects of environmental exposure under urban differences on COVID-19. J. Infect. Public Health 2024, 17, 76–81. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020, 54, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Balyan, P.; Ghosh, C.; Das, S.; Banerjee, B.D. Spatio-temporal characterization of bioaerosols at diverse outdoor land-use sites in an urban environment. Aerobiologia 2020, 36, 77–81. [Google Scholar] [CrossRef]
- An, T.; Liang, Z.; Chen, Z.; Li, G. Recent progress in online detection methods of bioaerosols. Fundam. Res. 2024, 4, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, D.; Zhu, Y.; Yang, Z.; Yao, M.; Shi, X.; An, T.; Zhang, Q.; Huang, C.; Bi, X.; et al. An overview for monitoring and prediction of pathogenic microorganisms in the atmosphere. Fundam. Res. 2023, 4, 430–441. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, X.; Qiu, G.; Spillmann, M.; Ji, Z.; Wang, J. SARS-CoV-2 and other airborne respiratory viruses in outdoor aerosols in three Swiss cities before and during the first wave of the COVID-19 pandemic. Environ. Int. 2022, 164, 107266. [Google Scholar] [CrossRef]
- Robles-Romero, J.M.; Conde-Guillén, G.; Safont-Montes, J.C.; García-Padilla, F.M.; Romero-Martín, M. Behaviour of aerosols and their role in the transmission of SARS-CoV-2; a scoping review. Rev. Med. Virol. 2022, 32, e2297. [Google Scholar] [CrossRef]
- Nesmachnow, S.; Tchernykh, A. The Impact of theCOVID-19 Pandemic on the Public Transportation System of Montevideo, Uruguay: A Urban Data Analysis Approach. Urban Sci. 2023, 7, 113. [Google Scholar] [CrossRef]
- Guan, Y.; Xiao, Y.; Chu, C.; Zhang, N.; Yu, L. Trends and characteristics of ozone and nitrogen dioxide related health impacts in Chinese cities. Ecotoxicol. Environ. Saf. 2022, 241, 113808. [Google Scholar] [CrossRef]
- De Angelis, E.; Renzetti, S.; Volta, M.; Donato, F.; Calza, S.; Placidi, D.; Lucchini, R.G.; Rota, M. COVID-19 incidence and mortality in Lombardy, Italy: An ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021, 195, 110777. [Google Scholar] [CrossRef]
- Eskandari, Z.; Maleki, H.; Neisi, A.; Riahi, A.; Hamid, V.; Goudarzi, G. Temporal fluctuations of PM2.5 and PM10, population exposure, and their health impacts in Dezful city, Iran. J. Environ. Health Sci. Eng. 2020, 18, 723–731. [Google Scholar] [CrossRef]
- Seposo, X.; Ueda, K.; Sugata, S.; Yoshino, A.; Takami, A. Short-term effects of air pollution on daily single- and co-morbidity cardiorespiratory outpatient visits. Sci. Total Environ. 2020, 29, 138934. [Google Scholar] [CrossRef] [PubMed]
- Bronte, O.; García-García, F.; Lee, D.-J.; Urrutia, I.; Uranga, A.; Nieves, M.; Martínez-Minaya, J.; Quintana, J.; Arostegui, I.; Zalacain, R.; et al. Impact of outdoor air pollution on severity and mortality in COVID-19 pneumonia. Sci. Total Environ. 2023, 894, 164877. [Google Scholar] [CrossRef] [PubMed]
- Aboura, S. The influence of climate factors and government interventions on the Covid-19 pandemic: Evidence from 134 countries. Environ. Res. 2022, 208, 112484. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.; Schuit, M.; Herzog, A.; Beck, K.; Wood, S.; Krause, M.; Miller, D.; Weaver, W.; Freeburger, D.; Hooper, I.; et al. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci. Technol. 2021, 55, 142–153. [Google Scholar] [CrossRef]
- Manik, S.; Mandal, M.; Pal, S.; Patra, S.; Acharya, S. Impact of climate on COVID-19 transmission: A study over Indian states. Environ. Res. 2022, 211, 113110. [Google Scholar] [CrossRef] [PubMed]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between ground levels of ozone (O3) and nitrogen dioxide (NO2) with coronavirus (COVID-19) in Milan, Italy. Sci. Total Environ. 2020, 740, 140005. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.M. The simultaneous and cyclic appearance of epidemics of pneumonia, grip and enteritis on the Northern hemisphere and their synchronism with solar activity cycles. JAMA 1911, LVII, 1964–1967. [Google Scholar] [CrossRef]
- Bosch, B.J.; van der Zee, R.; de Hana, C.A.M.; Rottier, P.J.M. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef] [PubMed]
- Cáliz, J.; Triadó-Margarit, X.; Camarero, L.; Casamayor, E.O. A long-term survey unveils strong seasonal patterns in the airborne microbiome coupled to general and regional atmospheric circulations. Proc. Natl. Acad. Sci. USA 2018, 115, 12229–12234. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T. InhalableMicroorganisms in Beijing’s PM2.5 and PM10 Pollutants during a Severe Smog Event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hamid, A.H.; Bari, M.A.; Tajudin, A.B.A.; Latif, M.T.; Nadzir, M.S.M.; Sahani, M.; Wahab, M.I.A.; Yusup, Y.; Maulud, K.N.A.; et al. Airborne particles in he city center of Kuala Lumpur: Origin, potential driving factors, and deposition flux in human respiratory airways. Sci. Total Environ. 2019, 650 Pt 1, 1195–1206. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rovira, J.; Sierra, J.; Blanco, J.; Chen, S.-J.; Mai, B.-X.; Schuhmacher, M.; Domingo, J.L. Characterization of airborne particles and cytotoxicity to a human lung cancer cell line in Guangzhou, China. Environ. Res. 2021, 196, 110953. [Google Scholar] [CrossRef]
- Lepeule, J.; Bind, M.-A.C.; Baccarelli, A.A.; Koutrakis, P.; Tarantini, L.; Litonjua, A.; Sparrow, D.; Vokonas, P.; Schwartz, J.D. Epigenetic influences on associations between air pollutants and lung function in elderly men: The normative aging study. Environ. Health Perspect. 2014, 122, 566–572. [Google Scholar] [CrossRef]
- Ciencewicki, J.; Jaspers, I. Air Pollution and Respiratory Viral Infection. Inhal Toxicol. 2007, 19, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Z.-F.; Froines, J.; Zhao, J.; Wang, H.; Yu, S.-Z.; Detels, R. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ. Health 2003, 2, 15. [Google Scholar] [CrossRef]
- Innocente, E.; Squizzato, S.; Visin, F.; Facca, C.; Rampazzo, G.; Bertolini, V.; Gandolfi, I.; Franzetti, A.; Ambrosini, R.; Bestetti, G. Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci. Total Environ. 2017, 593–594, 677–687. [Google Scholar] [CrossRef]
- Herman, J.; Biegel, B.; Huang, L. Inactivation times from 290 to 315 nm UVB in sunlight for SARS coronaviruses CoV and CoV-2 using OMI satellite data for the sunlit Earth. Air Qual. Atmos. Health. 2020, 14, 217–233. [Google Scholar] [CrossRef]
- Giese, A.C. Living with Our Sun’s Ultraviolet Rays; Plenum Press: New York, NY, USA, 1976; Chapter 3; pp. 33–34. [Google Scholar]
- Heßling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 14, Doc08. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Lytle, C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID-19. Photochem. Photobiol. 2020, 96, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.E.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcala Díaz, J.F.; Lopez Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar]
- Schuit, M.; Ratnesar-Shumate, S.; Yolitz, J.; Williams, G.; Weaver, W.; Green, B.; Miller, D.; Krause, M.; Beck, K.; Wood, S.; et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020, 222, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Jeyakumar, D.T.; Francis, T.V.; Misra, A. Impact of the vitamin D deficiency on COVID-19 infection and mortality in Asian countries. Diabetes, Metab. Syndr. Clin. Res. Rev. 2021, 15, 757–764. [Google Scholar]
- Zhang, H.; Wang, J.; Liang, Z.; Wu, Y. Non-linear effects of meteorological factors on COVID-19: An analysis of 440 counties in the Americas. Heliyon 2024, 10, e31160. [Google Scholar] [CrossRef]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020, 738, 13982. [Google Scholar] [CrossRef]
- Mela, A.; Tousi, E.; Melas, E.; Varelidis, G. Spatial Distribution and Quality of Urban Public Spaces in the Attica Region (Greece) during the COVID-19 Pandemic: A Survey-Based Analysis. Urban Sci. 2024, 8, 2. [Google Scholar] [CrossRef]
- Schatke, M.; Meier, F.; Schröder, B.; Weber, S. Impact of the 2020 COVID-19 lockdown on NO2 and PM10 concentrations in Berlin, Germany. Atmos. Environ. 2022, 290, 119372. [Google Scholar] [CrossRef]
- Zhang, C.; Stevenson, D. Characteristic changes of ozone and its precursors in London during COVID-19 lockdown and the ozone surge reason analysis. Atmos. Environ. 2022, 273, 118980. [Google Scholar] [CrossRef]
- Núñez-Peiró, M.; Sánchez, C.S.-G.; González, F.J.N. Hourly evolution of intra-urban temperature variability across the local climate zones. The case of Madrid. Urban Clim. 2021, 39, 100921. [Google Scholar] [CrossRef]
- Ivanovic, S.; Wood, J.; Purves, R. Evaluation of bicycle sharing scheme data as a proxy for cycling mobility—How COVID-19 measures influenced cycling in Paris. Transp. Res. Interdiscip. Perspect. 2023, 22, 100937. [Google Scholar] [CrossRef]
- Thunis, P.; Degraeuwe, B.; Peduzzi, E.; Vignati, E.; Trombetti, M.; Degraeuwe, B.; Wilson, J.; Belis, C.A. Urban PM2.5 Atlas: Air Quality in European Cities; (online); Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Worldometer Info. 2023. Available online: https://www.worldometers.info/ (accessed on 20 January 2024).
- WHO. 2024. Available online: https://covid19.who.int/WHO-COVID-19-global-data.csv (accessed on 25 January 2024).
- Giovanni. 2024. Available online: https://giovanni.gsfc.nasa.gov/giovanni/ (accessed on 25 January 2024).
- MERRA. 2024. Available online: http://www.soda-pro.com/web-services/meteo-data/merra (accessed on 25 January 2024).
- Copernicus. 2024. Available online: https://cds.climate.copernicus.eu/ (accessed on 20 January 2024).
- AQICN. 2024. Available online: https://aqicn.org/city/ (accessed on 20 January 2024).
- Ireland, C. Multiple regression and non-linear regression analysis. In Experimental Statistics for Agriculture and Horticulture; CABI: Wallingford, UK, 2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Liu, J.; Petrosian, O. Long-Term Forecasting of Air Pollution Particulate Matter (PM2.5) and Analysis of Influencing Factors. Sustainability 2024, 16, 19. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Y.; Wang, F.; Huang, Z.; Qiu, W.; Mao, A. Case fatality rates of COVID-19 during epidemic periods of variants of concern: A meta-analysis by continents. Int. J. Infect. Dis. 2024, 141, 106950. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Han, C.; Fang, Q.; Cui, F.; Li, X. Effects of extreme temperature events on deaths and its interaction with air pollution. Sci. Total Environ. 2024, 915, 170212. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. The Spatiality of COVID-19 in Kermanshah Metropolis, Iran. Urban Sci. 2022, 6, 30. [Google Scholar] [CrossRef]
- Taczanowska, K.; Tansil, D.; Wilfer, J.; Jiricka-Pürrer, A. The impact of age on people’s use and perception of urban green spaces and their effect on personal health and wellbeing during the COVID-19 pandemic—A case study of the metropolitan area of Vienna, Austria. Cities 2024, 147, 104798. [Google Scholar] [CrossRef]
- Sobrino, N.; Arce-Ruiz, R.M. Effects of Mobility Restrictions on Air Pollution in the Madrid Region during the COVID-19 Pandemic and Post-Pandemic Periods. Sustainability 2023, 15, 12702. [Google Scholar] [CrossRef]
- Sohrabi, S.; Shu, F.; Gupta, A.; Sabbaghian, M.H.; Mehrara Molan, A.; Sajjadi, S. Health Impacts of COVID-19 through the Changes in Mobility. Sustainability 2023, 15, 4095. [Google Scholar] [CrossRef]
- Ju, T.; Geng, T.; Li, B.; An, B.; Huang, R.; Fan, J.; Liang, Z.; Duan, J. Impacts of Certain Meteorological Factors on Atmospheric NO2 Concentrations during COVID-19 Lockdown in 2020 in Wuhan, China. Sustainability 2022, 14, 16720. [Google Scholar] [CrossRef]
- Marquès, M.; Correig, E.; Domingo, J.L. Long-term exposure to PM10 above WHO guidelines exacerbates COVID-19 severity and mortality. Environ. Int. 2022, 158, 106930. [Google Scholar] [CrossRef]
- Lox, J.E.; Taylor, J.; Kaiser, J. Outdoor air pollution exposure and COVID-19 infection in the United States. Environ. Pollut. 2021, 292 Pt B, 118369. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Li, C.; Zhao, Y.; Wang, D.; Huang, Z.; Yang, K. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 2021, 195, 110874. [Google Scholar] [CrossRef]
- To, T.; Zhang, K.; Maguire, B.; Terebessy, E.; Fong, I.; Parikh, S.; Zhu, J.; Su, Y. UV, ozone, and COVID-19 transmission in Ontario, Canada using generalised linear models. Environ. Res. 2021, 194, 110645. [Google Scholar] [CrossRef]
- Feng, B.; Lian, J.; Yu, F.; Zhang, D.; Chen, W.; Wang, Q.; Shen, Y.; Xie, G.; Wang, R.; Teng, Y.; et al. Impact of short-term ambient air pollution exposure on the risk of severe COVID-19. J. Environ. Sci. 2024, 135, 610–618. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2022, 203, 111930. [Google Scholar] [CrossRef]
- Chen, B.; Liang, H.; Yuan, X.; Hu, Y.; Xu, M.; Zhao, Y.; Zhang, B.; Tian, F.; Zhu, X. Roles of meteorological conditions in COVID-19 transmission on a worldwide scale. MedRxiv 2020. [Google Scholar] [CrossRef]
- Jana, A.; Kundu, S.; Shaw, S.; Chakraborty, S.; Chattopadhyay, A. Spatial shifting of COVID-19 clusters and disease association with environmental parameters in India: A time series analysis. Environ. Res. 2023, 222, 115288. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, B.; Zhu, R.; Che, C.; Ma, D.; Wang, R.; Dai, H. Airborne microbial community structure and potential pathogen identification across the PM size fractions and seasons in the urban atmosphere. Sci. Total Environ. 2022, 831, 154665. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Yamamoto, N.; Hosaka, Y.; Wakita, M.; Hiki, M.; Matsushita, Y.; Mori, H.; Hori, S.; Misawa, S.; Miida, T.; et al. Molecular characterization of SARS-CoV-2 detected in Tokyo, Japan during five waves: Identification of the amino acid substitutions associated with transmissibility and severity. Front. Microbiol. 2022, 13, 912061. [Google Scholar] [CrossRef]
- Chen, H.; Du, R.; Zhang, Y. Evolution of PM2.5 bacterial community structure in Beijing’s suburban atmosphere. Sci. Total Environ. 2021, 799, 149387. [Google Scholar] [CrossRef]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Impacts of exposure to air pollution, radon and climate drivers on the COVID 19 pandemic in Bucharest, Romania: A time series study. Environ. Res. 2022, 212, 113437. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, K.; Yang, R.; Zhang, Y.; Xu, D.; Bai, K.; Guo, J. Impact of near-surface turbulence on PM2.5 concentration in Chengdu during the COVID-19 pandemic. Atmos. Environ. 2022, 268, 118848. [Google Scholar] [CrossRef]
- Baron, Y.M. Are there medium to short-term multifaceted effects of the airborne pollutant PM2.5 determining the emergence of SARS-CoV-2 variants? Med. Hypotheses 2022, 158, 110718. [Google Scholar] [CrossRef]
- Beloconi, A.; Vounatsou, P. Long-term air pollution exposure and COVID-19 case-severity: An analysis of individual-level data from Switzerland. Environ. Res. 2023, 216, 114481. [Google Scholar] [CrossRef]
- Sanchez-Lorenzo, A.; Vaquero-Martínez, J.; Calbó, J.; Wild, M.; Santurtún, A.; Lopez-Bustins, J.A.; Vaquero, J.M.; Folini, D.; Antón, M. Anomalous atmospheric circulation favor the spread of COVID-19 in Europe? Environ. Res. 2021, 194, 110626. [Google Scholar] [CrossRef]
- Jerrett, M.; Nau, C.L.; Young, D.R.; Butler, R.K.; Batteate, C.M.; Su, J.; Burnett, R.T.; Kleeman, M.J. Air pollution and meteorology as risk factors for COVID-19 death in a cohort from Southern California. Environ. Int. 2023, 171, 107675. [Google Scholar] [CrossRef]
- Amrhein, S.; Hospers, G.-J.; Reiser, D. Transformative Effects of Overtourism and COVID-19-Caused Reduction of Tourism on Residents—An Investigation of the Anti-Overtourism Movement on the Island of Mallorca. Urban Sci. 2022, 6, 25. [Google Scholar] [CrossRef]
- Salvador, P.; Barreiro, M.; Gómez-Moreno, F.J.; Alonso-Blanco, E.; Artíñano, B. Synoptic classification of meteorological patterns and their impact on air pollution episodes and new particle formation processes in a south European air basin. Atmos. Environ. 2021, 245, 118016. [Google Scholar] [CrossRef]
- Rodríguez-Arias, R.; Rojo, J.; Fernández-González, F.; Pérez-Badia, R. Desert dust intrusions and their incidence on airborne biological content. Review and case study in the Iberian Peninsula. Environ. Pollut. 2023, 316, 120464. [Google Scholar] [CrossRef]
- Fu, D.; Shi, X.; Zuo, J.; Yabo, S.D.; Li, J.; Li, B.; Li, H.; Lu, L.; Tang, B.; Qi, H.; et al. Why did air quality experience little improvement during the COVID-19 lockdown in megacities, northeast China? Environ. Res. 2023, 221, 115282. [Google Scholar] [CrossRef]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N.; Baschir, L.A.; Tenciu, D.V. Assessing the impact of air pollution and climate seasonality on COVID-19 multiwaves in Madrid, Spain. Environ. Res. 2022, 203, 111849. [Google Scholar] [CrossRef]
- Renard, J.-B.; Surcin, J.; Annesi-Maesano, I.; Delaunay, G.; Poincelet, E.; Dixsaut, G. Relation between PM2.5 pollution and Covid-19 mortality in Western Europe for the 2020–2022 period. Sci. Total Environ. 2022, 848, 157579. [Google Scholar] [CrossRef]
- Cao, B.; Wang, X.; Ning, G.; Yuan, L.; Jiang, M.; Zhang, X.; Wang, S. Factors influencing the boundary layer height and their relationship with air quality in the Sichuan Basin, China. Sci. Total Environ. 2020, 727, 138584. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- European Commission. Urban Agenda for the EU—European Commission (europa.eu). 2024. Available online: https://futurium.ec.europa.eu/en/urban-agenda (accessed on 16 May 2024).
- European Commission. The European Green Deal—European Commission (europa.eu). 2024. Available online: https://ec.europa.eu/stories/european-green-deal/ (accessed on 16 May 2024).
- UN. Health—United Nations Sustainable Development; UN: New York, NY, USA, 2022. [Google Scholar]
- Han, Y.; Gu, X.; Lin, C.; He, M.; Wang, Y. Effects of COVID-19 on coastal and marine environments: Aggravated microplastic pollution, improved air quality, and future perspective. Chemosphere 2024, 355, 141900. [Google Scholar] [CrossRef]
- Houweling, L.; der Zee, A.-H.M.-V.; Holtjer, J.C.; Bazdar, S.; Vermeulen, R.C.; Downward, G.S.; Bloemsma, L.D. The effect of the urban exposome on COVID-19 health outcomes: A systematic review and meta-analysis. Environ. Res. 2024, 240 Pt 2, 117351. [Google Scholar] [CrossRef]





| Metropolis | Berlin | Paris | Madrid | London |
|---|---|---|---|---|
| Population size (million inhabitants) | 5.35 | 12.92 | 6.98 | 14.4 |
| Population density (inhabitants/km2) | 3910.82 | 4542.90 | 5113.55 | 8285.39 |
| Total COVID-19 cases (DNC) during March 2020–March 2022 | 905,272 | 1,155,528 | 2,246,443 | 3,278,230 |
| Total COVID-19 deaths (DND) during March 2020–March 2022 | 4381 | 25,312 | 30,284 | 30,321 |
| Mean Daily Average Variable | London | Paris | Madrid | Berlin |
|---|---|---|---|---|
| PM2.5 (µg/m3) (Particulate matter 2.5 µm) | (18.55 ± 9.61) In the range (2–68) | (24.80 ± 11.61) In the range (4–75) | (14.55 ± 9.61) In the range (2–68) | (24.80 ± 11.61) In the range (4–75) |
| PM10 (Particulate matter 10 µm) (µg/m3) | (45.02 ± 20.44) In the range (11–154) | (50.37 ± 21.19) In the range (9–145) | (45.01 ± 20.45) In the range (11–154) | (50.37 ± 21.19) In the range (9–145) |
| O3 (Ozone) (µg/m3) | (22.34 ± 11.06) In the range (1–73) | (26.01 ± 11.6) In the range (1–71) | (22.35 ± 11.06) In the range (0–73) | (26.0 ± 11.05) In the range (0–71) |
| NO2 (Nitrogen dioxide) (µg/m3) | (16.60 ± 8.78) In the range (0–52) | (19.88 ± 11.34) In the range (2–70) | (16.60 ± 8.78) In the range (1–52) | (19.88 ± 11.35) In the range (2–70) |
| AOD | (0.247 ± 0.161) In the range (0.096–0.664) | (0.213 ± 0.145) In the range (0.090–0.699) | (0.124 ± 0.102) In the range (0.038–0.547) | (0.184 ± 0.089) In the range (0.054–0.44) |
| Daily Average Variable | Berlin | London | Madrid | Paris | ||||
|---|---|---|---|---|---|---|---|---|
| DNC | DND | DNC | DND | DNC | DND | DNC | DND | |
| PM2.5 (µg/m3) (Particulate matter 2.5 µm) | 0.20 * | 0.19 * | 0.22 * | 0.21 * | 0.18 * | 0.16 * | 0.22 * | 0.27 * |
| PM10 (Particulate matter 10 µm) (µg/m3) | 0.27 * | 0.26 * | 0.11 ** | 0.03 ** | 0.14 * | 0.19 * | 0.12 ** | 0.14 * |
| O3 (Ozone) (µg/m3) | −0.57 * | −0.44 * | −0.33 * | −0.41 * | −0.27 * | −0.49 * | −0.51 * | −0.31 * |
| NO2 (Nitrogen dioxide) (µg/m3) | 0.47 * | 0.40 * | 0.12 * | 0.06 ** | 0.29 * | 0.19 * | 0.05 ** | 0.10 ** |
| T (air Temperature at 2 m height) (°C) | −0.60 * | −0.67 * | −0.48 * | −0.58 * | −0.25 * | −0.61 * | −0.51 * | −0.63 * |
| RH (Relative Humidity) (%) | 0.47 * | 0.35 * | 0.55 * | 0.32 * | 0.28 * | 0.69 * | 0.41 * | 0.35 * |
| w (wind intensity) (m/s) | −0.06 ** | −0.08 ** | 0.18 * | −0.20 * | 0.14 * | −0.19 * | 0.04 ** | −0.04 ** |
| SI (surface solar irradiance) (W/m2) | −0.73 * | −0.57 * | −0.73 * | −0.48 * | −0.36 * | −0.30 * | −0.66 * | −0.58 * |
| PBL (Planetary Boundary Layer height) (m) | −0.59 * | −0.45 * | −0.46 * | −0.28 * | −0.26 * | −0.52 * | −0.44 * | −0.42 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoran, M.; Radvan, R.; Savastru, D.; Tautan, M. Urban Air Pollution Exposure Impact on COVID-19 Transmission in a Few Metropolitan Regions. Sustainability 2024, 16, 6119. https://doi.org/10.3390/su16146119
Zoran M, Radvan R, Savastru D, Tautan M. Urban Air Pollution Exposure Impact on COVID-19 Transmission in a Few Metropolitan Regions. Sustainability. 2024; 16(14):6119. https://doi.org/10.3390/su16146119
Chicago/Turabian StyleZoran, Maria, Roxana Radvan, Dan Savastru, and Marina Tautan. 2024. "Urban Air Pollution Exposure Impact on COVID-19 Transmission in a Few Metropolitan Regions" Sustainability 16, no. 14: 6119. https://doi.org/10.3390/su16146119





