Revealing the Nexus between Fertilizer Composition and the Performance of Common Bean (Phaseolus vulgaris L.) Genotypes in the Himalayan Heartland of India
Abstract
:1. Introduction
2. Materials and Methods
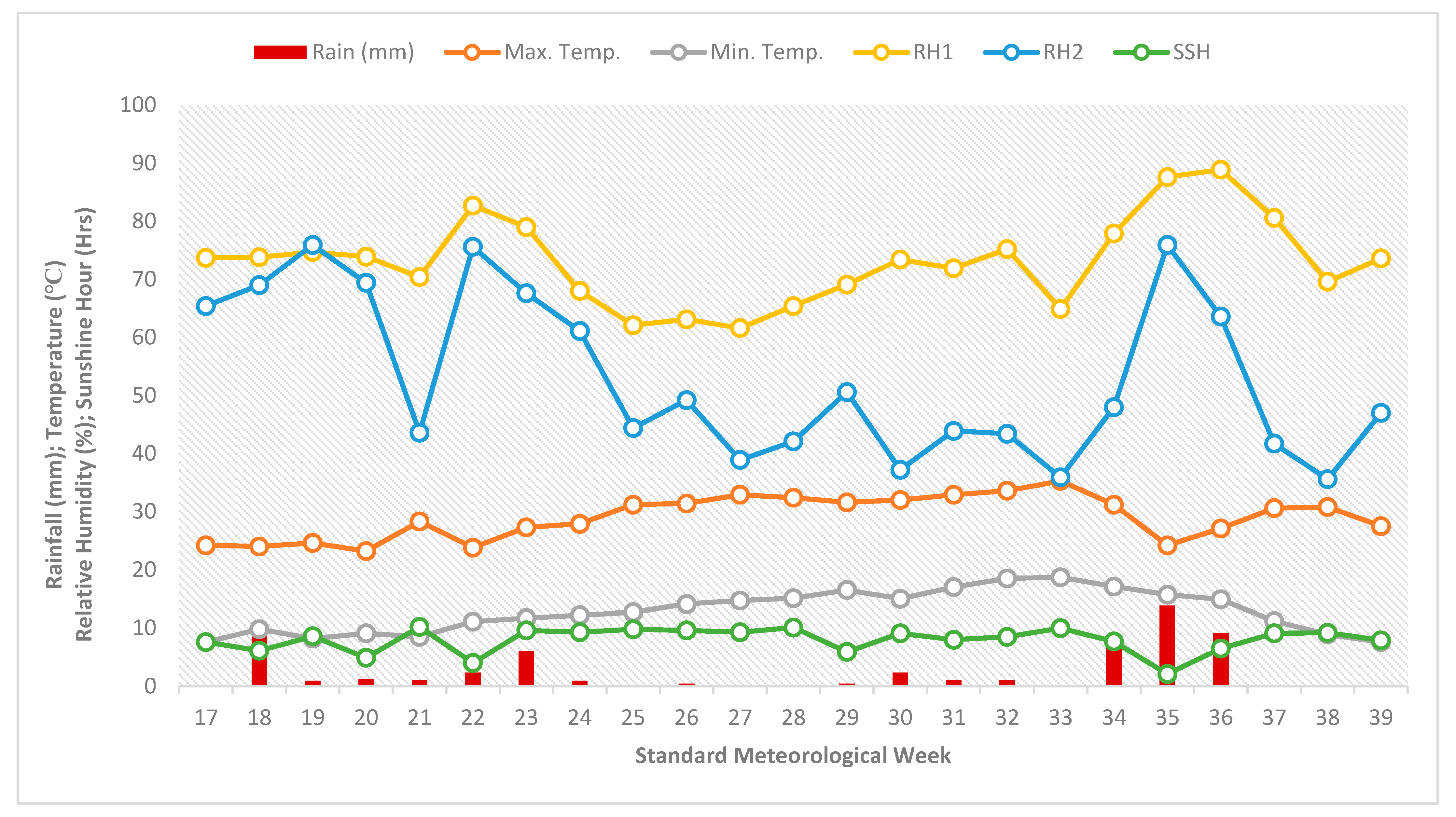
2.1. Experimental Site Description
2.2. Design of Experiment and Experimental Duration
2.3. Biometric Analysis
- -
- Plant height: 5 randomly selected plants per net plot were tagged, and their heights were recorded every 30 days, measured from the ground surface to the growing tip. Average height data in centimeters per plot were used to express plant height across the experimental area.
- -
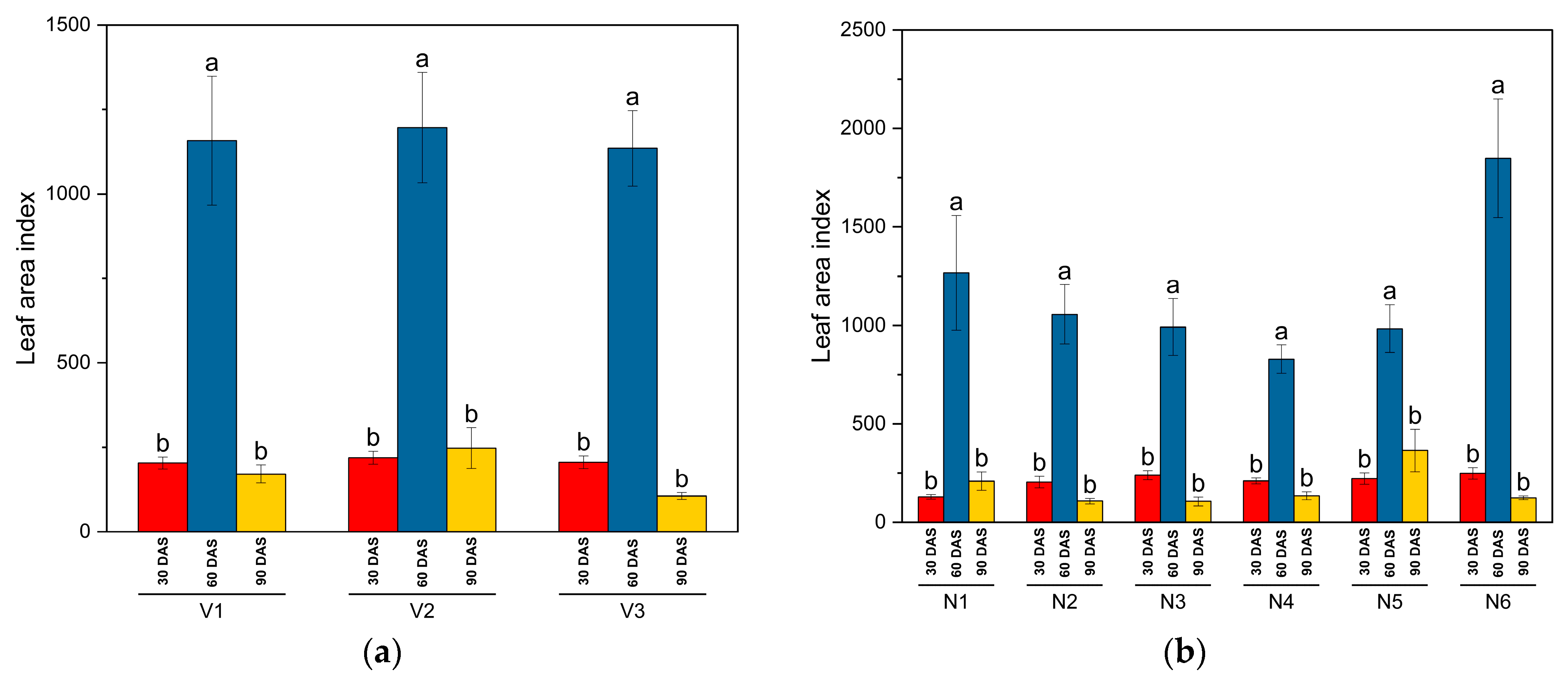
- Leaf area: Leaf area observations were also conducted at 30-day intervals from sowing. In a 0.5 m × 0.5 m quadrant outside each net plot, all plants were cut at ground level, and their leaves were separated. Total leaf area was measured using the Easy Leaf Area software from the University of California, USA, version 4.1 [43]. Leaf area was calculated as follows: Leaf area = (Green pixel count) × (Calibration area/Red pixel count). The leaf area index (LAI) was then estimated by dividing the total leaf area by the ground area of the sample quadrant.
- -
- Dry matter accumulation: After leaf area observations, we used the same plant samples to estimate dry matter accumulation (DMA). These samples were oven-dried (60–65 °C) until reaching a constant weight, and then the total dry matter per quadrant was divided by the number of plants in that quadrant to calculate DMA, expressed as grams per plant (g plant−1).
- -
- Crop growth analysis: The traits of crop growth analysis—mean crop growth rate (mean CGR), mean relative growth rate (mean RGR), and mean net assimilation rate (mean NAR)—were estimated based on DMA and the leaf area produced per unit area.
- -
- Nodulation: Three carefully uprooted plants, preserving soil and minimizing nodule loss, had their roots washed in a sieve. Pink effective nodules were counted, air-dried, and weighed using a laboratory digital scale.
- -
- Days to different phenological stages: We recorded the days needed to reach key phenological stages, like 50% emergence, 50% flowering, and 50% maturity, for each plot. Each stage was marked when half of the plants in the plot reached it.
2.4. Post-Harvest Determination
- -
- Number of pods plant−1: At harvest, 5 plants were chosen randomly from the net plot area of each plot in the experimental region. The total number of pods was counted, and the average was calculated to ascertain the number of pods plant−1.
- -
- Number of seeds pod−1: All of the seeds were separated from the pods. The total number of seeds and the average number of seeds per pod−1 were calculated.
- -
- Seed index: Each net plot area had seed samples from bulk products, and the weight of 100 seeds was computed and expressed in grams (g).
- -
- Seed and stover yields: The biomass harvested from the net plot area of each plot of the experimental area was sun-dried and weighed (biological yield) before threshing. The seeds obtained from the net plot area of each plot were thoroughly cleaned, sun-dried, and weighed. The net plot seed yield was expressed in t ha−1. The rest of the parts after threshing the biomass from each plot constituted the stover yield. The net plot stover yield was expressed in t ha−1.
- -
- Harvest index: Total biological yields (t ha−1) recorded from each net plot area were used to compute the harvest index (HI). The HI was calculated by multiplying the seed yield (economic yield) by the overall biological yield (percentage yield) [45]:
2.5. Plant Chemical Analysis
- -
- N content and uptake: The N content was determined by the modified micro-Kjeldahl method [37]. The N uptakes by seeds and stovers were estimated by multiplying the N content (percent) by their respective yields.
- -
- P content and uptake: The vanado-molybdo-phosphoric yellow color method was used to detect P in seeds and stovers [39]. The P uptake by the crop was calculated by multiplying the P content in seeds and stovers by their corresponding yields.
- -
- K content and uptake: A flame photometer was used to determine the K content. The percent K concentration was multiplied by the relative yields to calculate the K uptake levels in seeds and straw.
2.6. Post-Harvest Soil Chemical Analysis and Nutrient-Use Efficiency (NUE)
2.7. Statistical Analysis
3. Results
3.1. Biometric and Crop Growth Traits
3.2. Phenological Development, Yield Characteristics, Harvest Index and Nutrient Content and Uptake
3.3. Soil Properties and Nutrient-Use Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the prevention of cardiovascular diseases—Cardioprotective potential of bioactive compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef]
- Camara, C.R.; Urrea, C.A.; Schlegel, V. Pinto beans (Phaseolus vulgaris L.) as a functional food: Implications on human health. Agriculture 2013, 3, 90–111. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Katoch, S.; Katoch, A.; Dhiman, S.; Sharma, P.; Rana, S.K.; Sharma, P.N. Recitation of R genes identified in common bean landrace KRC-5 and KRC-8 native to Himachal Pradesh against Colletotrichum lindemuthianum virulences. Himachal J. Agric. Res. 2019, 45, 51–56. [Google Scholar]
- Basavaraja, T.; Manjunatha, L.; Chandora, R.; Singh, M.; Rathod, S.; Dubey, V.; Singh, N.P. Assessment of phenotypic diversity and multi-locational screening against bean common mosaic virus (BCMV) disease resistance in dry bean (Phaseolus vulgaris L.) germplasm. Plant Genet. Resour. 2022, 20, 79–86. [Google Scholar] [CrossRef]
- Jan, S.; Rather, I.A.; Sofi, P.A.; Wani, M.A.; Sheikh, F.A.; Bhat, M.A.; Mir, R.R. Characterization of common bean (Phaseolus vulgaris L.) germplasm for morphological and seed nutrient traits from Western Himalayas. Legume Sci. 2021, 3, e86. [Google Scholar] [CrossRef]
- George, M.T.; Susan, N.M. Diversity of common bean (Phaseolus vulgaris L.) genotypes in iron and zinc contents under screenhouse conditions. Afr. J. Agric. Res. 2010, 5, 738–747. [Google Scholar]
- Souza, T.L.P.; Faleiro, F.G.; Dessaune, S.N.; Paula-Junior, T.J.D.; Moreira, M.A.; Barros, E.G.D. Breeding for common bean (Phaseolus vulgaris L.) rust resistance in Brazil. Trop. Plant Pathol. 2013, 38, 361–374. [Google Scholar] [CrossRef]
- Alemu, H. Review paper on breeding common bean (Phaseolus vulgaris L.) genotypes for acidic soil tolerance. Int. J. Adv. Res. Publ. 2017, 1, 39–46. [Google Scholar]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr. J. 2011, 10, 34. [Google Scholar] [CrossRef]
- Assefa, T.; Assibi Mahama, A.; Brown, A.V.; Cannon, E.K.; Rubyogo, J.C.; Rao, I.M.; Cannon, S.B. A review of breeding objectives, genomic resources, and marker-assisted methods in common bean (Phaseolus vulgaris L.). Mol. Breed. 2019, 39, 20. [Google Scholar] [CrossRef]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jackson, S.A. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2016. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 January 2023).
- Siddiq, M.; Uebersax, M.A.; Siddiq, F. Global production, trade, processing, and nutritional profile of dry beans and other pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Khan, M.N.; Sofi, P.A.; Dar, Z.A.; Sofi, N.R.; Bhat, J.A.; Bhat, M.A. Farmers’ Preference Ranking in Bush Type of Common Bean (Phaseolus vulgaris L.) in Kashmir—Participatory Varietal Selection. Int. J. Pure Appl. Biosci. 2017, 5, 712–719. [Google Scholar] [CrossRef]
- Rana, J.C.; Sharma, T.R.; Tyagi, R.K.; Chahota, R.K.; Gautam, N.K.; Singh, M.; Ojha, S.N. Characterisation of 4274 accessions of common bean (Phaseolus vulgaris L.) germplasm conserved in the Indian gene bank for phenological, morphological, and agricultural traits. Euphytica 2015, 205, 441–457. [Google Scholar] [CrossRef]
- Martinez-Romero, E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: Overview and perspectives. Plant Soil 2003, 252, 11–23. [Google Scholar] [CrossRef]
- Peoples, M.B.; Giller, K.E.; Jensen, E.S.; Herridge, D.F. Quantifying country-to-global scale nitrogen fixation for grain legumes: I. Reliance on nitrogen fixation of soybean, groundnut and pulses. Plant Soil 2021, 469, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.S.; Mahale, A.G.; Sultan, A.; Jadhav, S.C.; Ejaz, A.; Krishna, J.R.; Channa, T.K. Boosting Agricultural Production-Beneficial Biofertilizers. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 646–662. [Google Scholar] [CrossRef]
- Wilker, J.; Humphries, S.; Rosas-Sotomayor, J.C.; Gómez Cerna, M.; Torkamaneh, D.; Edwards, M.; Pauls, K.P. Genetic diversity, nitrogen fixation, and water use efficiency in a panel of Honduran common bean (Phaseolus vulgaris L.) landraces and modern genotypes. Plants 2020, 9, 1238. [Google Scholar] [CrossRef]
- Lavrenko, S.O.; Lavrenko, N.M.; Maksymov, D.O.; Maksymov, M.V.; Didenko, N.O.; Islam, K.R. Variable tillage depth and chemical fertilization impact on irrigated common beans and soil physical properties. Soil Tillage Res. 2021, 212, 105024. [Google Scholar] [CrossRef]
- dos Santos Sousa, W.; Soratto, R.P.; Peixoto, D.S.; Campos, T.S.; da Silva, M.B.; Souza, A.G.V.; Gitari, H.I. Effects of Rhizobium inoculum compared with mineral nitrogen fertilizer on nodulation and seed yield of common bean: A meta-analysis. Agron. Sustain. Dev. 2022, 42, 52. [Google Scholar] [CrossRef]
- Fageria, N.K.; Melo, L.C.; de Oliveira, J. Nitrogen use efficiency in dry bean genotypes. J. Plant Nutr. 2013, 36, 2179–2190. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Zeng, J.; Tu, Q.; Yu, X.; Qian, L.; Wang, C.; Shu, L.; He, Z. PCycDB: A comprehensive and accurate database for fast analysis of phosphorus cycling genes. Microbiome 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Amanuel, A.; Amisalu, N.; Merkeb, G. Growth and yield of common bean (Phaseolus vulgaris L.) cultivars as influenced by rates of phosphorus at Jimma, Southwest Ethiopia. J. Agric. Biotechnol. Sustain. Dev. 2018, 10, 104–115. [Google Scholar] [CrossRef]
- Chekanai, V.; Chikowo, R.; Vanlauwe, B. Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric. Ecosyst. Environ. 2018, 266, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of phosphorus fertilization on the growth, photosynthesis, nitrogen fixation, mineral accumulation, seed yield, and seed quality of a soybean low-phytate line. Plants 2019, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front. Plant Sci. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]
- Dawood, M.G.; Abdelhamid, M.T.; Schmidhalter, U. Potassium fertiliser enhances the salt-tolerance of common bean (Phaseolus vulgaris L.). J. Hortic. Sci. Biotechnol. 2014, 89, 185–192. [Google Scholar] [CrossRef]
- Dubey, R.S.; Srivastava, R.K.; Pessarakli, M. Physiological mechanisms of nitrogen absorption and assimilation in plants under stressful conditions. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 579–616. [Google Scholar]
- Piper, C.S. Annual International Science Publication; Asian Edition; University of Adelaide: Adelaide, Australia, 1966; p. 235. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In methods of soil structure and migration of colloidal materials soils. Soil Sci. Soc. Am. Proc. 1986, 26, 297–300. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Private Limited: New Delhi, India, 1967. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.; Asija, G.L. Alkaline permanganate method of available nitrogen determination. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular 939; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Watson, D.J. The physiological basis of variation in yield. Adv. Agron. 1952, 4, 101–145. [Google Scholar] [CrossRef]
- Donald, C.M. In search of yield. J. Aust. Inst. Agric. Sci. 1962, 28, 171–178. [Google Scholar]
- Sepat, S.; Bana, R.S.; Kumar, D.; Rana, K.S. A practical manual on principle and practices of managing soil and field crops. In New Delhi Publication; Division of Agronomy, IARI: New Delhi, India, 2013; p. 90. [Google Scholar]
- Cochran, G.C.; Cox, M.M. Experimental Designs; Asia Publishing House: Bombay, India, 1963; pp. 293–316. [Google Scholar]
- SPSS, Statistical Package for the Social Sciences, Version 26.0; IBM SPSS: Armonk, NY, USA, 2020.
- Pandey, R. Mineral nutrition of plants. Plant Biol. Biotechnol. 2015, 499–538. [Google Scholar] [CrossRef]
- Kalmani, B.R.; Sunad, L.K.; Gopur, S.T. Studies on the effect of mother plant nutrition on crop growth, yield and quality of French bean. Leg. Res. 2002, 19, 34–41. [Google Scholar]
- Kumar, M.; Sinha, K.K.; Sharma, R.R. Effect of organic manure, NPK and boron application on the productivity of French bean in sandy loam soil of north Bihar. Indian J. Pulses Res. 2004, 17, 42–44. [Google Scholar]
- Kumar, E.S.; Channaveerswami, A.S.; Merwade, M.N.; Naik, V.R.; Krishna, A. Influence of Nipping and Hormonal Sprays on Growth and Seed Yield in Field Bean [Lablab purpureus (L.) Sweet] Genotypes. Int. J. Econ. Plants 2018, 5, 008–014. [Google Scholar] [CrossRef]
- Ayub, M.; Nadeem, M.A.; Naeem, M.; Tahir, M.; Tariq, M.; Ahmad, W. Effect of different levels of P and K on growth, forage yield and quality of cluster bean (Cyamopsis tetragonolobus L.). J. Anim. Plants Sci. 2012, 22, 479–483. [Google Scholar]
- Kakon, S.S.; Bhuiya, M.S.U.; Hossain, S.M.A.; Naher, Q.; Bhuiyan, M.D. Effect of nitrogen and phosphorus on growth and seed yield of French bean. Bangladesh J. Agric. Res. 2016, 41, 759–772. [Google Scholar] [CrossRef]
- Amare, G.; Assaye, D.; Tuma, A. The response of haricot bean varieties to different rates of phosphorus at Arba Minch, Southern Ethiopia. J. Agric. Biol. Sci. 2014, 9, 344–350. [Google Scholar]
- Soratto, R.P.; Perez, A.A.; Fernandes, A.M. Age of No-Till system and nitrogen management on common bean nutrition and yield. Agron. J. 2014, 106, 809–820. [Google Scholar] [CrossRef]
- Dwivedi, Y.C.; Sharma, R.S.; Sengupata, S.K. Effect of phosphorus and potassium fertilization on grain yield of French bean (Phaseolus vulgaris L.). Veg. Sci. 1995, 22, 36–38. [Google Scholar]
- Davis, J.G.; Brick, M.A. Colorado State University Extension. Fertilizing Dry Beans. Crop Series. Fact Sheet No. 0.539. Available online: https://extension.colostate.edu/docs/pubs/crops/00539.pdf (accessed on 1 January 2023).
- Alemayehu, D.; Shumi, D. Response of faba bean (Vicia faba L.) to phosphorus nutrient application in Bore Highlands, Guji Zone, Southern Ethiopia. Agric. Res. Technol. 2018, 17, 107–114. [Google Scholar]
- Talwar, H.S.; Soni, M.L.; Beniwal, R.K. Evaluation of crop physiological traits in clusterbean (Cyamopsis tetragonoloba L. Taub) and mothbean (Vigna aconitifolia Jacq. Marechal). Arid Legumes Sustain. Agric. Trade 2019, 2, 274. [Google Scholar]
- Rawat, G.S.; Rajput, R.L.; Rawat, U. Response of Varying Levels of Organic Manure and PSB on the Productivity of Clusterbean Cyamopsis tetragonoloba Taub. Bhartiya Krishi Anusandhan Patrika 2010, 24, 71–73. [Google Scholar]
- Bhattacharya, A. Mungbean seed yield IV. Effect of various physiological parameters during pre and post flowering periods. Legume Res.-Int. J. 2006, 29, 169–174. [Google Scholar]
- De Ron, A.M.; Rodiño, A.P.; Santalla, M.; González, A.M.; Lema, M.J.; Martín, I.; Kigel, J. Seedling emergence and phenotypic response of common bean germplasm to different temperatures under controlled conditions and in open field. Front. Plant Sci. 2016, 7, 1087. [Google Scholar] [CrossRef] [PubMed]
- Beruktawit, M.; Tamada, T.; Nigussie, D. Response of Common Bean (Phaseolus vulgaris L.) Cultivars with Different Growth Habits to Plant Density at Haramaya; Haramaya University: Haramaya, Ethiopia, 2012. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Tewari, J.K.; Singh, S.S. Effect of nitrogen and phosphorus on growth and seed yield of French bean (Phaseolus vulgaris L.). Veg. Sci. 2000, 27, 172–175. [Google Scholar]
- Dejene, T.; Tana, T.; Urage, E. Response of common bean (Phaseolus vulgaris L.) to application of lime and phosphorus on acidic soil of Areka, Southern Ethiopia. J. Nat. Sci. Res. 2016, 6, 90–100. [Google Scholar]
- Leal, F.T.; Filla, V.A.; Bettiol, J.V.T.; Sandrini, F.D.O.T.; Mingotte, F.L.C.; Lemos, L.B. Use efficiency and responsivity to nitrogen of common bean cultivars. Ciência E Agrotecnologia 2019, 43, e004919. [Google Scholar] [CrossRef]
- Kwizera, C.; Ong’or, B.T.I.; Kaboneka, S.; Nkeshimana, F.; Ahiboneye, N. Effects of Potassium Fertilizer on Bean growth and Yield parameters. Int. J. Adv. Sci. Res. Eng. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Zebire, D.A.; Gelgelo, S. Effect of phosphorus fertilizer levels on growth and yield of haricot bean (Phaseolus vulgaris. L.) in South Ommo Zone, Ethiopia. Agric. Sci. Dig.-A Res. J. 2019, 39, 55–58. [Google Scholar] [CrossRef]
- Sofi, P.A.; Rehman, K.; Gull, M.; Kumari, J.; Djanaguiraman, M.; Prasad, P.V.V. Integrating root architecture and physiological approaches for improving drought tolerance in common bean (Phaseolus vulgaris L.). Plant Physiol. Rep. 2021, 26, 4–22. [Google Scholar] [CrossRef]
- Emam, S.M.; Semida, W.M. Foliar-applied Amcoton® and potassium thiosulphate enhances the growth and productivity of three faba beans varieties by improving photosynthetic efficiency. Arch. Agric. Environ. Sci. 2020, 5, 89–96. [Google Scholar] [CrossRef]
- Yin, Z.; Guo, W.; Xiao, H.; Liang, J.; Hao, X.; Dong, N.; Yin, F. Nitrogen, phosphorus, and potassium fertilization to achieve expected yield and improve yield components of mung bean. PLoS ONE 2018, 13, e0206285. [Google Scholar] [CrossRef]
- Sofi, P.; Zargar, M.Y.; Debouck, D.G.; Graner, A. Evaluation of common bean (Phaseolus vulgaris L) germplasm under temperate conditions of Kashmir Valley. J. Phytol. 2011, 3, 47–52. [Google Scholar]
- Singh, A.K.; Singh, S.S. Effect of planting dates, nitrogen and phosphorus levels on yield contributing factors in french bean. Legume Res.-Int. J. 2000, 23, 33–36. [Google Scholar]
- Veeresh, N.K. Response of French bean (Phaseolus vulgaris L.) to fertilizer levels in Northern Transitional Zone of Karnataka. Ph.D. Thesis, University of Agricultural Sciences Bangalore, Dharwad, Indian, 2004; pp. 37–79. [Google Scholar]
- Shubhashree, K.S.; Alagundagi, S.C.; Hiremath, S.M.; Chittapur, B.M.; Hebsur, N.S.; Patil, B.C. Effect of nitrogen, phosphorus and potassium levels on growth, yield and economics of rajmash (Phaseolus vulgaris). Karnataka J. Agric. Sci. 2011, 24, 283–285. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates Inc. Publisher: Sunderland, MA, USA, 2006. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Root strategies for phosphorus acquisition. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Dordrecht, The Netherlands, 2008; pp. 83–116. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Vadez, V. Physiological traits for crop yield improvement in low N and P environments. Plant Soil 2002, 245, 1–15. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Savvas, D. Agronomic practices to increase the yield and quality of common bean (Phaseolus vulgaris L.): A systematic review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Islam, M.R.; Sarker, U.; Azam, M.G.; Hossain, J.; Alam, M.A.; Ullah, R.; Islam, M.S. Potassium augments growth, yield, nutrient content, and drought tolerance in mung bean (Vigna radiata L. Wilczek.). Sci. Rep. 2024, 14, 9378. [Google Scholar] [CrossRef] [PubMed]
- Shankarlingappa, B.C.; Shivaraj, B.; Viswanatha, K.P. Interaction effect of phosphorus and sulphur on uptake of nitrogen, phosphorus, potassium and sulphur by cowpea. Karnataka J. Agric. Sci. 2000, 13, 295–298. [Google Scholar]
- Kanwar, K.; Paliyal, S.S. Influence of phosphorus management and organic manuring on uptake and yield of chickpea (Cicer arietinum). Ann. Agric. Res. New Ser. 2002, 23, 642–645. [Google Scholar]
- Jain, A.K.; Kumar, S.; Panwar, J.D.S. Response of mungbean (Vigna radiata) to phosphorus and micronutrients on N and P uptake and seed quality. Legume Res. 2007, 30, 201–204. [Google Scholar]
- Fatima, Z.; Zia, M.; Chaudhary, M.F. Interactive effect of Rhizobium strains and P on soybean yield, nitrogen fixation and soil fertility. Pak. J. Bot. 2007, 39, 255. [Google Scholar]
- Kumar, S.S.; Mir, S.A.; Wani, O.A.; Babu, S.; Yeasin, M.; Bhat, M.A.; Dar, S.R. Land-use systems regulate carbon geochemistry in the temperate Himalayas, India. J. Environ. Manag. 2022, 320, 115811. [Google Scholar] [CrossRef]
- Sharma, R.; Verma, M.L. Effect of Rhizobium, farm yard manure and chemical fertilizers on sustainable production and profitability of rajmash (Phaseolus vulgaris L.) and soil fertility in dry temperate region of North-Western Himalayas. Legume Res. 2011, 34, 251–258. [Google Scholar]
- Gidago, G.; Beyene, S.; Worku, W.; Sodo, E. The response of haricot bean (Phaseolus vulgaris L.) to phosphorus application on Ultisols at Areka, Southern Ethiopia. J. Biol. Agric. Healthc. 2011, 1, 38–49. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Akter, Z.; Lupwayi, N.Z.; Balasubramanian, P.M. Nitrogen use efficiency of irrigated dry bean (Phaseolus vulgaris L.) genotypes in southern Alberta. Can. J. Plant Sci. 2017, 97, 610–619. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, I.; Manske, G.G.B.; Ginkel, M.V. Nitrogen and phosphorus use efficiency. In Application of Physiology in Wheat Breeding (No. CIS-3162. CIMMYT.); International Maize and Wheat Improvement Center (CIMMYT): Mexico City, Mexico, 2001. [Google Scholar]






| Particular | Value | Rating | Method |
|---|---|---|---|
| Physical characteristics | |||
| Soil texture | Sand 19.5% | Silty–clayey loam | [37] |
| Silt 50.0% | |||
| Clay 28.5% | |||
| Bulk density | 1.33 Mg m−3 | [38] | |
| Physico-chemical characteristics | |||
| pH | 6.8 | Normal | [39] |
| Electrical conductivity | 0.07 dS m−1 | Normal | [39] |
| Organic C | 0.74% | Medium | [40] |
| Available N | 275.5 kg ha−1 | Medium | [41] |
| Available P | 17.5 kg ha−1 | Medium | [42] |
| Available K | 174.2 kg ha−1 | Medium | [39] |
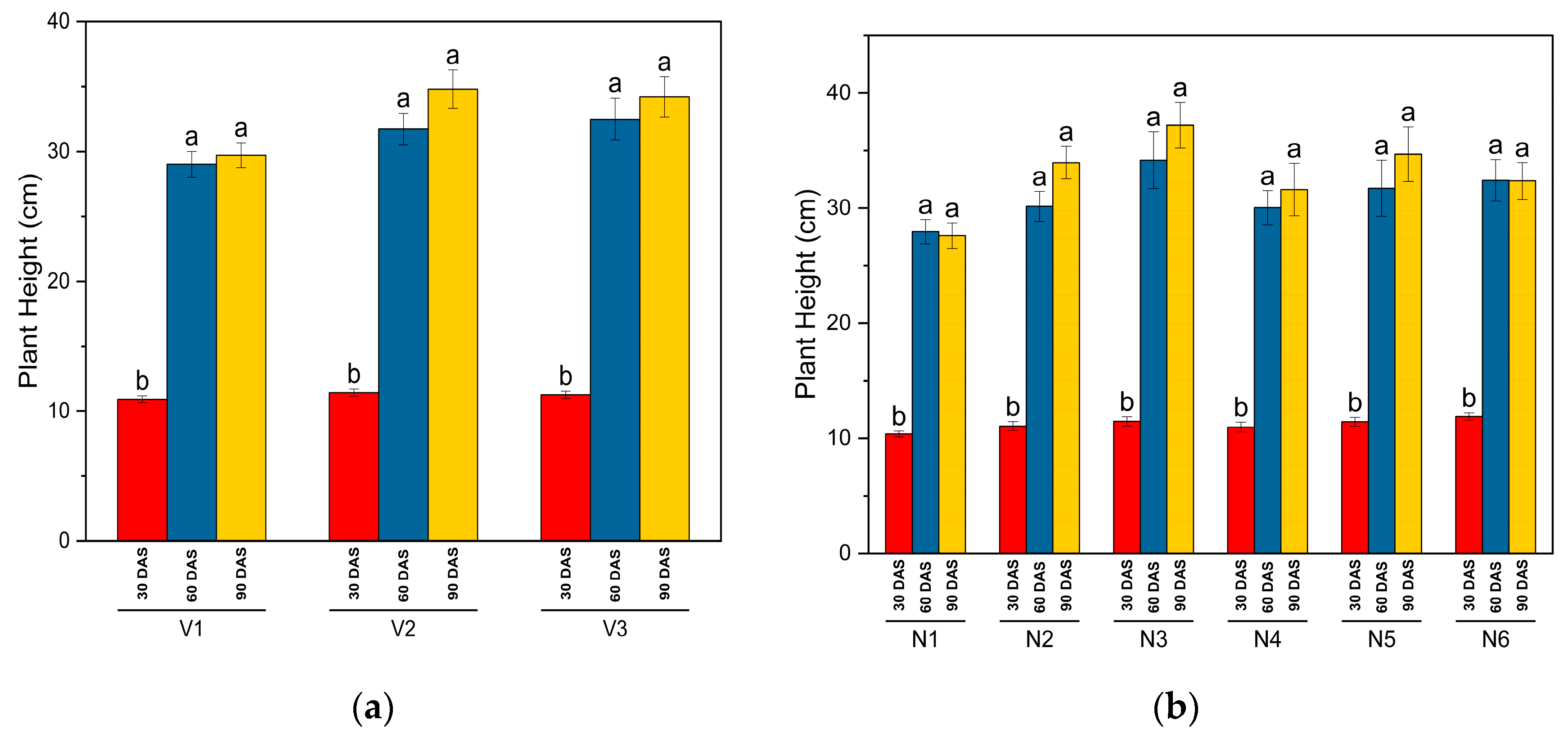
| Treatment | Plant Height (cm) | Leaf Area Index (LAI) | ||||
|---|---|---|---|---|---|---|
| 30 DAS | 60 DAS | 90 DAS | 30 DAS | 60 DAS | 90 DAS | |
| Genotype | ||||||
| SKUA-WB-5000/1446 (V1) | 10.9 | 29 | 30.2 | 0.73 | 1.65 | 0.196 |
| SKUA-WB-5002/185 (V2) | 11.4 | 30.8 | 31.8 | 0.72 | 1.52 | 0.197 |
| SKUA-WB-5003/1492 (V3) | 11.3 | 31 | 31.7 | 0.72 | 1.64 | 0.195 |
| SEM (±) | 0.24 | 0.59 | 0.68 | 0.01 | 0.03 | 0.003 |
| LSD (p ≤ 0.05) | NS | 1.7 | NS | NS | 0.07 | NS |
| NPK level (N:P2O5:K2O kg ha−1) | ||||||
| N0P0K0 | 10.9 | 29 | 29.7 | 0.67 | 1.43 | 0.181 |
| N10P20K10 | 11.1 | 30 | 31 | 0.73 | 1.55 | 0.185 |
| N20P40K20 | 11.4 | 30.8 | 32.2 | 0.75 | 1.68 | 0.207 |
| N30P60K30 | 11.6 | 31.6 | 32.9 | 0.77 | 1.81 | 0.215 |
| N40P80K40 | 11.9 | 32.3 | 33.7 | 0.77 | 1.87 | 0.219 |
| N50P100K50 | 10.9 | 29 | 29.7 | 0.67 | 1.43 | 0.181 |
| SEM (±) | 0.34 | 0.83 | 0.96 | 0.01 | 0.04 | 0.005 |
| LSD (p ≤ 0.05) | 1 | 2.4 | 2.8 | 0.03 | 0.1 | 0.013 |
| Treatment | DMA (g Plant−1) | Nodulation | |||
|---|---|---|---|---|---|
| 30 DAS | 60 DAS | 90 DAS | Effective Nodule Count (No. Plant−1) | Nodule Weight (mg Plant−1) | |
| Genotype | |||||
| SKUA-WB-5000/1446 (V1) | 2.13 | 11.95 | 16.55 | 26.6 | 1213 |
| SKUA-WB-5002/185 (V2) | 1.66 | 10.14 | 15.18 | 25.5 | 1145 |
| SKUA-WB-5003/1492 (V3) | 1.95 | 10.83 | 15.78 | 25.9 | 1209 |
| SEM (±) | 0.03 | 0.15 | 0.19 | 0.4 | 23 |
| LSD (p ≤ 0.05) | 0.08 | 0.43 | 0.54 | NS | NS |
| NPK level (N:P2O5:K2O kg ha−1) | |||||
| N0P0K0 | 1.38 | 7.03 | 9.7 | 20.7 | 1114 |
| N10P20K10 | 1.7 | 9.63 | 13.74 | 23.7 | 1164 |
| N20P40K20 | 1.87 | 10.76 | 15.47 | 25.2 | 1176 |
| N30P60K30 | 2.02 | 11.75 | 17.12 | 27.2 | 1187 |
| N40P80K40 | 2.22 | 13.02 | 19.02 | 28.9 | 1224 |
| N50P100K50 | 2.31 | 13.64 | 19.96 | 30.4 | 1269 |
| SEM (±) | 0.04 | 0.21 | 0.26 | 0.6 | 32 |
| LSD (p ≤ 0.05) | 0.12 | 0.6 | 0.76 | 1.8 | 92 |
| Treatment | Mean CGR (g m−2 Day−1) | Mean RGR (mg g−1 Day−1) | Mean NAR (g m−2 Day−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–30 DAS | 30–60 DAS | 60–90 DAS | 0–30 DAS | 30–60 DAS | 60–90 DAS | 0–30 DAS | 30–60 DAS | 60–90 DAS | |
| Genotype | |||||||||
| SKUA-WB-5000/1446 (V1) | 2.37 | 10.91 | 5.11 | 24.53 | 57.33 | 10.75 | 17.39 | 9.61 | 7.42 |
| SKUA-WB-5002/185 (V2) | 1.85 | 9.42 | 5.6 | 16.56 | 59.74 | 13.41 | 13.79 | 8.73 | 8.57 |
| SKUA-WB-5003/1492 (V3) | 2.17 | 9.86 | 5.5 | 21.73 | 56.93 | 12.31 | 16.09 | 8.77 | 7.98 |
| SEM (±) | 0.03 | 0.16 | 0.15 | 0.49 | 0.68 | 0.33 | 0.25 | 0.18 | 0.23 |
| LSD (p ≤ 0.05) | 0.09 | 0.47 | NS | 1.4 | 1.95 | 0.95 | 0.72 | 0.51 | 0.66 |
| NPK levels (N:P2O5:K2O kg ha−1) | |||||||||
| N0P0K0 | 1.53 | 6.28 | 2.97 | 10.67 | 54.23 | 10.79 | 12.55 | 6.92 | 5.65 |
| N10P20K10 | 1.89 | 8.81 | 4.57 | 17.37 | 57.98 | 11.89 | 14.97 | 8.79 | 7.54 |
| N20P40K20 | 2.08 | 9.88 | 5.23 | 20.47 | 58.48 | 12.22 | 15.37 | 9.08 | 8.18 |
| N30P60K30 | 2.24 | 10.82 | 5.96 | 22.97 | 58.91 | 12.66 | 16.18 | 9.39 | 8.53 |
| N40P80K40 | 2.47 | 12 | 6.66 | 26.35 | 59.12 | 12.68 | 17.43 | 9.89 | 8.91 |
| N50P100K50 | 2.56 | 12.59 | 7.02 | 27.8 | 59.28 | 12.71 | 18.05 | 10.15 | 9.13 |
| SEM (±) | 0.05 | 0.23 | 0.21 | 0.69 | 0.96 | 0.47 | 0.36 | 0.25 | 0.33 |
| LSD (p ≤ 0.05) | 0.13 | 0.67 | 0.61 | 1.98 | 2.75 | 1.35 | 1.02 | 0.72 | 0.94 |
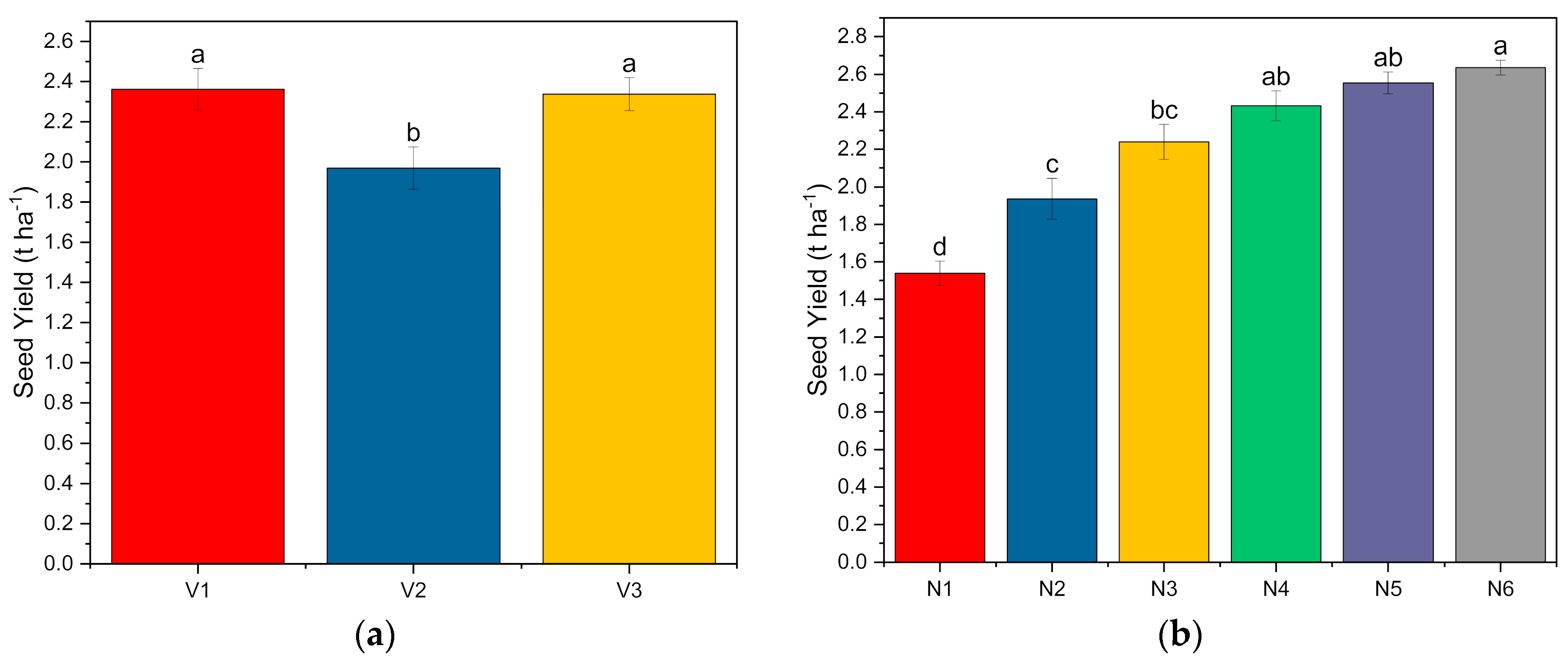
| Treatment | No. of Pods Plant−1 | No. of Seeds Pod−1 | Seed Index (g) | Seed Yield (t ha−1) | Stover Yield (t ha−1) | Biological Yield (t ha−1) | Harvest Index (%) |
|---|---|---|---|---|---|---|---|
| Genotype | |||||||
| SKUA-WB-5000/1446 (V1) | 8.02 | 3.84 | 39.47 | 2.36 | 3.66 | 6.03 | 38.9 |
| SKUA-WB-5002/185 (V2) | 7.53 | 3.71 | 36.82 | 1.97 | 3.49 | 5.46 | 35.7 |
| SKUA-WB-5003/1492 (V3) | 7.82 | 3.86 | 37.23 | 2.34 | 3.62 | 5.95 | 39.1 |
| SEM (±) | 0.11 | 0.06 | 0.26 | 0.03 | 0.04 | 0.06 | 0.3 |
| LSD (p ≤ 0.05) | 0.31 | NS | 0.75 | 0.08 | 0.13 | 0.18 | 0.9 |
| NPK level (N:P2O5:K2O kg ha−1) | |||||||
| N0P0K0 | 6.62 | 3.32 | 35 | 1.54 | 3.33 | 4.87 | 31.5 |
| N10P20K10 | 7.58 | 3.34 | 36.63 | 1.94 | 3.48 | 5.41 | 35.6 |
| N20P40K20 | 7.9 | 3.87 | 37.3 | 2.24 | 3.54 | 5.78 | 38.6 |
| N30P60K30 | 8.08 | 4 | 38.44 | 2.43 | 3.64 | 6.07 | 40 |
| N40P80K40 | 8.13 | 4.11 | 39.66 | 2.55 | 3.74 | 6.3 | 40.5 |
| N50P100K50 | 8.44 | 4.16 | 40.02 | 2.64 | 3.81 | 6.45 | 40.9 |
| SEM (±) | 0.15 | 0.09 | 0.37 | 0.04 | 0.06 | 0.09 | 0.42 |
| LSD (p ≤ 0.05) | 0.44 | 0.25 | 1.05 | 0.11 | 0.18 | 0.26 | 1.2 |
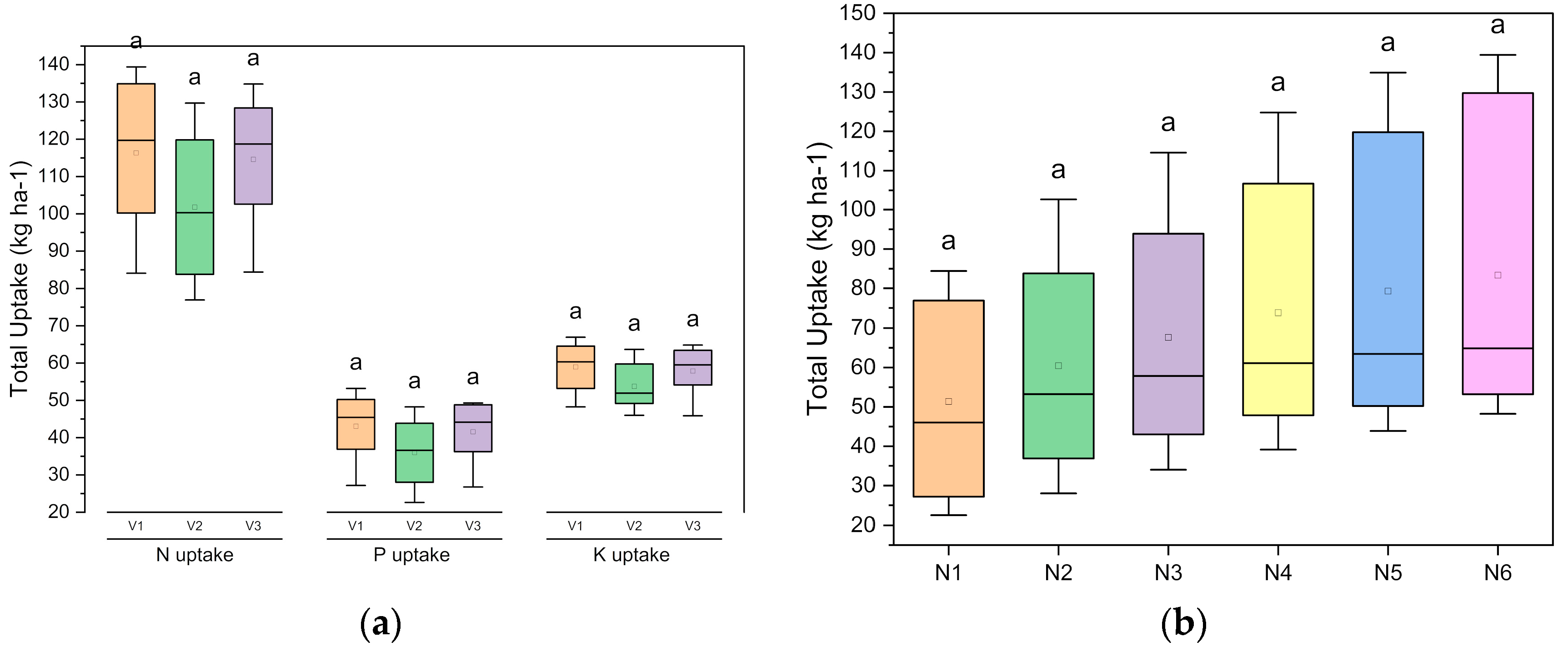
| Treatments | N Uptake (kg N ha−1) | P Uptake (kg P2O5 ha−1) | K Uptake (kg K2O ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed | Stover | Total | Seed | Stover | Total | Seed | Stover | Total | |
| Genotype | |||||||||
| SKUA-WB-5000/1446 (V1) | 67.3 | 49.1 | 116.4 | 34.85 | 8.21 | 43.06 | 15.7 | 43.2 | 58.93 |
| SKUA-WB-5002/185 (V2) | 55.4 | 46.39 | 101.8 | 28.47 | 7.52 | 35.99 | 13 | 40.79 | 53.75 |
| SKUA-WB-5003/1492 (V3) | 66.5 | 48.1 | 114.6 | 33.7 | 7.88 | 41.58 | 15.6 | 42.29 | 57.87 |
| SEM (±) | 1.06 | 0.9 | 1.29 | 0.59 | 0.15 | 0.68 | 0.31 | 0.74 | 0.81 |
| LSD (p ≤ 0.05) | 3.06 | NS | 3.7 | 1.69 | 0.42 | 1.96 | 0.89 | NS | 2.32 |
| NPK level (N:P2O5:K2O kg ha−1) | |||||||||
| N0P0K0 | 41.3 | 40.51 | 81.8 | 20.17 | 5.37 | 25.54 | 9.4 | 37.32 | 46.72 |
| N10P20K10 | 52.4 | 43.12 | 95.6 | 27.24 | 6.48 | 33.72 | 12.3 | 39.91 | 52.18 |
| N20P40K20 | 62.7 | 45.02 | 107.7 | 32.34 | 7.55 | 39.89 | 14.5 | 40.87 | 55.36 |
| N30P60K30 | 69.5 | 48.55 | 118.1 | 36.1 | 8.12 | 44.22 | 16.2 | 42.99 | 59.14 |
| N40P80K40 | 74.1 | 53.55 | 127.7 | 38.33 | 9.32 | 47.64 | 17.6 | 45.02 | 62.57 |
| N50P100K50 | 78.2 | 56.43 | 134.7 | 39.87 | 10.38 | 50.25 | 18.7 | 46.45 | 65.13 |
| SEM (±) | 1.5 | 1.27 | 1.82 | 0.83 | 0.21 | 0.96 | 0.44 | 1.05 | 1.14 |
| LSD (p ≤ 0.05) | 4.32 | 3.66 | 5.2 | 2.39 | 0.59 | 2.77 | 1.26 | 3.03 | 3.28 |
| Treatment | PFP | AE | ARE | PE |
|---|---|---|---|---|
| (kg Seed kg−1 NPK Applied) | ||||
| Genotype | ||||
| SKUA-WB-5000/1446 (V1) | 26.9 | 8.7 | 0.63 | 13.45 |
| SKUA-WB-5002/185 (V2) | 21.5 | 6.3 | 0.44 | 14.37 |
| SKUA-WB-5003/1492 (V3) | 27.1 | 7.8 | 0.64 | 11.85 |
| SEM (±) | - | - | - | - |
| LSD (p ≤ 0.05) | - | - | - | - |
| NPK level (N:P2O5:K2O kg ha−1) | ||||
| N0P0K0 | - | - | - | - |
| N10P20K10 | 48.4 | 9.9 | 0.68 | 14.28 |
| N20P40K20 | 28.0 | 8.8 | 0.61 | 14.89 |
| N30P60K30 | 20.3 | 7.4 | 0.56 | 13.40 |
| N40P80K40 | 16.0 | 6.3 | 0.52 | 12.13 |
| N50P100K50 | 13.2 | 5.5 | 0.48 | 11.41 |
| SEM (±) | - | - | - | - |
| LSD (p ≤ 0.05) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vemulakonda, A.L.; Saad, A.A.; Kumar, S.S.; Wani, O.A.; Singh, L.; Babu, S.; Khan, I.M.; Wani, F.J.; Jan, S.K.; Elhindi, K.M.; et al. Revealing the Nexus between Fertilizer Composition and the Performance of Common Bean (Phaseolus vulgaris L.) Genotypes in the Himalayan Heartland of India. Sustainability 2024, 16, 6234. https://doi.org/10.3390/su16146234
Vemulakonda AL, Saad AA, Kumar SS, Wani OA, Singh L, Babu S, Khan IM, Wani FJ, Jan SK, Elhindi KM, et al. Revealing the Nexus between Fertilizer Composition and the Performance of Common Bean (Phaseolus vulgaris L.) Genotypes in the Himalayan Heartland of India. Sustainability. 2024; 16(14):6234. https://doi.org/10.3390/su16146234
Chicago/Turabian StyleVemulakonda, Amani Lakshmi, Ahmad Abdullah Saad, Shamal Shasang Kumar, Owais Ali Wani, Lal Singh, Subhash Babu, Inayat Mustafa Khan, Fahim Jeelani Wani, Shaheen Kauser Jan, Khalid M. Elhindi, and et al. 2024. "Revealing the Nexus between Fertilizer Composition and the Performance of Common Bean (Phaseolus vulgaris L.) Genotypes in the Himalayan Heartland of India" Sustainability 16, no. 14: 6234. https://doi.org/10.3390/su16146234
APA StyleVemulakonda, A. L., Saad, A. A., Kumar, S. S., Wani, O. A., Singh, L., Babu, S., Khan, I. M., Wani, F. J., Jan, S. K., Elhindi, K. M., & Mattar, M. A. (2024). Revealing the Nexus between Fertilizer Composition and the Performance of Common Bean (Phaseolus vulgaris L.) Genotypes in the Himalayan Heartland of India. Sustainability, 16(14), 6234. https://doi.org/10.3390/su16146234






