1. Introduction
The common bean (
Phaseolus vulgaris L.), a member of the Fabaceae family, is a popular grain legume representing a significant source of protein, fiber, minerals, and vitamins [
1].
Phaseolus vulgaris L., also known as the new world crop, is a plant that evolved 7000 years ago in two distinct regions of the North and South American continents [
2,
3]. This dry bean, often referred to as rajmash or rajma in India, is native to southern Mexico and Central America [
4,
5]. The common bean is an annual crop with minor differences in growth habit, crop duration, pod size, shape, and color of pods and seeds, grown in a mixture of agro-climatic regions [
6]. The crop is grown in humid, tropical, and subtropical climates up to 2000 m above sea level with 500 to 1500 mL of annual rainfall [
7,
8,
9]. It is widely consumed for its edible seeds and pods and is inexpensive in many countries. It has multiple health benefits, including cholesterol and coronary heart disease management, as well as reducing blood sugar levels in diabetic people [
10,
11]. The common bean is the primary staple food for more than 200 million people in sub-Saharan Africa, making it a grain that humankind depends upon [
12].
The common bean is grown worldwide over 29.39 million ha
−1 with a production of 26.83 million tons (t) [
13]. Myanmar, India, Brazil, the USA, Tanzania, China, and Mexico are the top dry bean producers [
14].
Phaseolus vulgaris L. is grown on 9.47 million ha
−1 in India, with a production of 3.90 million t and a productivity of 0.41 t ha
−1 [
13]. India’s leading agricultural producing regions are Jammu and Kashmir, Himachal Pradesh, Uttar Pradesh, Maharashtra, the Nilgiri and Palani Hills of Tamil Nadu, parts of the Western Ghats of Kerala, the Chickmagalur Hills of Karnataka, and the Darjeeling Hills of West Bengal. Pulses are often cultivated in the rainfed Kurewa regions of Jammu and Kashmir, either as a mono- or as an intercrop with maize under a low-input system, producing low yields. The districts of Baramulla, Bandipora, and Kupwara in the Kashmir Valley have the potential for rajmash cultivation [
15]. Common bean varieties vary in seed size, shape, and color. People mainly prefer local bean varieties such as Kashmiri, Bhaderwah, Harshil, Munsiyari, Auli, Barot, Chamba rajmash, and Kinnauree [
16]. Farmers typically prefer kidney-shaped plain red seed coats over cuboidal and cylindrical seed coat shapes and other seed coat colors in the Kashmir Valley.
The crop growth and yield depend on sufficient amounts of mineral nutrients, and the primary nutrients, nitrogen (N), phosphorus (P), and potassium (K), are essential for increased productivity. The application of these nutrients plays a vital role in shaping the quantitative and qualitative characteristics of the common bean.
Phaseolus vulgaris L. has reasonable prospects of N-fixation but has been reported less compared to other legumes [
17,
18]. Legume plants have a symbiotic relationship with rhizobia bacteria for the fixation of atmospheric N in the host plant. However, N-fixation is a high-energy-consuming process compared to artificial N supply to soil [
19].
N in the soil is affected by the high temperature of the soil surface, drought stress, soil reaction (pH), and deficiency of phosphorus and other nutrients. The limitations on crop growth are caused mainly by the low availability of nutrients in the soil, which is affected by abiotic stress and, particularly, soil moisture. N supply to the common bean affects the photosynthesis, leaf area, number and size of pods, nodules, water-use efficiency, and yield [
20,
21,
22]. N availability is crucial for optimizing the growth and yield of the common bean. It directly affects several key aspects of plant development and productivity. Firstly, adequate N levels enhance photosynthesis, thereby boosting the plant’s ability to produce energy and grow efficiently. Additionally, N supports the expansion of leaf area, which is essential for capturing more sunlight and maximizing photosynthetic activity. Moreover, N plays a pivotal role in the formation and development of pods and nodules. It contributes to the formation of larger and more numerous pods, thereby increasing the yield potential. In leguminous plants like common beans, N also facilitates the formation of nodules on roots, where symbiotic N-fixing bacteria reside. This symbiosis allows the plant to convert atmospheric N into a form that can be utilized for growth, further enhancing nutrient availability. Furthermore, N availability improves water-use efficiency in plants, helping them to better manage water resources and tolerate periods of water stress. Ultimately, the cumulative effect of an optimized nitrogen supply results in an increased overall yield of common bean crops, making it a critical factor in agronomic management and genotype selection [
20,
21,
22]. During genotype selection for common beans, increasing the protein levels in grains involves choosing plants that efficiently accumulate essential nutrients like N, P, and K. This process includes selecting genotypes with traits that enhance nutrient uptake and utilization. Agronomists apply balanced fertilization and optimize field management to ensure plants receive adequate nutrients throughout their growth. Post-harvest techniques then maintain grain quality. Through rigorous evaluation, breeders identify genotypes that consistently produce high-protein grains, aiming to enhance the nutritional quality in cultivated beans [
23].
P is the second major nutrient that impacts common bean growth, nodulation, and root development. It is responsible for plant metabolic processes such as transpiration, photosynthesis, and amino acid synthesis [
24,
25,
26]. P also increases root growth, promotes adequate nodulation, and supports rhizobia in biological N-fixation [
27,
28,
29]. The energy-consuming nodulation and N-fixation processes depend on sugars transported downward from the host plant’s branches; in this regard, P is necessary for the synthesis of helpful energy as well as the production and translocation of sugars. P deficiency in the soil causes a reduction in leaf area, affects plant growth, and lowers dry matter accumulation [
30]. Also, P deficiency affects how well UV light is absorbed, which lowers photosynthesis and inhibits the production of buds and flowers [
31]. K plays a crucial role in most biochemical and physiological processes affecting plant growth. It contributes significantly to cell osmotic concentrations and the maintenance of stomata guard cell turgor, increases the photosynthesis rate and biomass production, and increases yields [
32,
33]. Additionally, it helps in enzyme activation and cell formation, reduces excessive intake of sodium (Na) from the soil, increases carbon (C) assimilation, translocates organic and inorganic nutrients from the soil, and effectively increases the yield quality [
34,
35,
36].
Recently, breeders have identified some promising genotypes of the common bean in the Kashmir Valley. Agronomic interference is required to evaluate the optimum utilization of primary nutrients—N, P, and K—to realize the yield potential of these genotypes. Common beans are a critical source of protein and essential nutrients, making them indispensable for food security and nutrition, especially in regions like the Himalayas. This study’s objective was to evaluate the effects of varying levels of primary nutrients (NPK) on the growth, yield, and nutrient-use efficiency of newly identified common bean genotypes in the temperate Himalayas. Emphasizing the identification of the most favorable agricultural conditions, this research aimed to enhance both the quality and yield of common beans. In doing so, we sought to promote sustainable development in the Himalayan region, ensuring that agricultural practices contribute to ensuring the long-term ecological balance and economic growth. This approach is vital for maximizing the potential of common bean genotypes, thereby supporting the region’s agricultural output and sustainability.
4. Discussion
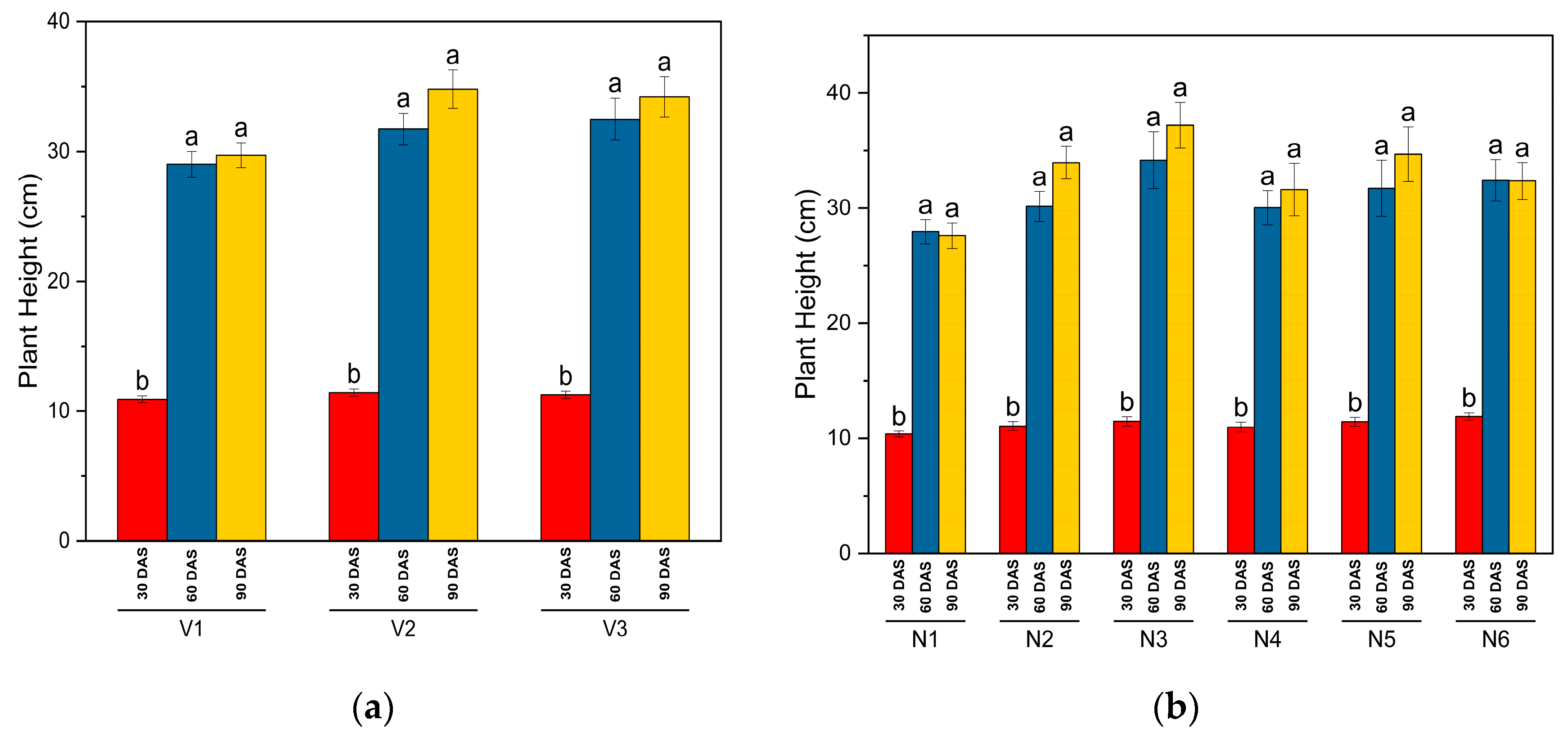
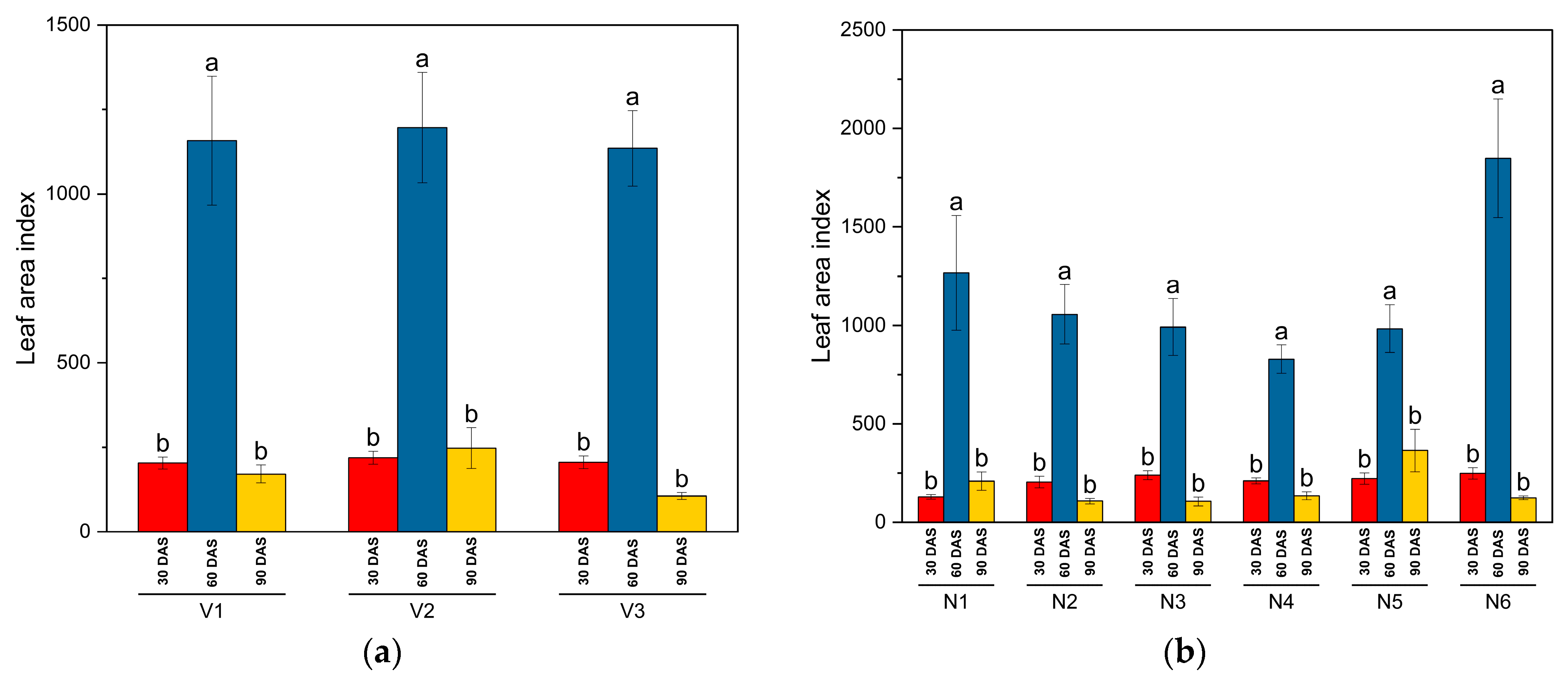
This study has examined the plant height, LAI, DMA, and nodulation as growth metrics for different genotypes of common bean (SKUA-WB-5000/1446 (V1), SKUA-WB-5002/185 (V2), and SKUA-WB-5003/1492 (V3)) under various NPK treatments. The results revealed that NPK levels significantly influenced these growth metrics, particularly at 60 DAS. Genotypes V2 and V3 exhibited significantly higher plant heights compared to V1, with increases observed up to N10P20K10. For LAI, genotypes V1 and V3 showed higher values, with significant improvements up to N30P60K30. Genotype V1 had the highest DMA at all observation stages, followed by V3, with significant increases noted up to N50P100K50 at later growth stages. The interaction between genotypes and NPK levels showed that, at 60 DAS, plant height and LAI did not vary significantly across different NPK levels, except that LAI for V1 and V3 did not increase significantly beyond N30P60K30. For DMA, V1 and V3 did not show significant increases beyond N30P60K30 and N40P80K40, respectively. Genotype V2 did not exhibit a significant increase in LAI beyond N40P80K40 at 60 DAS, while DMA continued to increase significantly up to N50P100K50 at all growth stages.
The genotypes’ influences on the growth parameters were due to their genetic characteristics. Plant nutrients (NPK) play significant roles in controlling plant height, LAI, and DMA with varying intensity among the crop genotypes. The beneficial effects of N, P, and K could be attributed to the fact that the essential primary nutrients are directly engaged in plant metabolism actions such as photosynthesis, nutrient storage, and transportation, all of which contribute to plant growth and development [
49]. Leguminous crops have a symbiotic nodulation process; hence, they respond well to NPK application. However, the significant response to these growth parameters varied at different levels of NPK application, which might be due to inherent soil fertility status and genetic characteristic differences among common bean genotypes. Several studies have also reported on the growth parameters of the common bean as influenced by varying levels of NPK application. A considerably increased plant height of the bean crop was observed with fertilizer levels ranging at 25–120 kg N, 60–75 kg P, and 30–50 kg K ha
−1 in different studies [
50,
51,
52]. Ayub et al. [
53] reported that applying P and K fertilizers in the ratio of 70:70 kg ha
−1 increased the plant height. A significant increase in leaf area at 150 kg N and 75 kg P
2O
5 ha
−1 was reported by Kakon et al. [
54]. The maximum DMA in beans was reported with fertilizer levels at 80:75:30 kg NPK ha
−1 [
50] and 120:60:45 kg NPK ha
−1 [
50].
Differences in the effective nodule count and total nodule weight were not significant among the genotypes. However, NPK levels greatly influenced the effective nodule count and total nodule weight. The effective nodule count was significantly higher at N
50P
100K
50, while the total nodule weight was not increased dramatically beyond N
30P
60K
30. Many reports suggest that the common bean is a poor N-fixer compared to other legumes. Therefore, the crop is more responsive to N than other legumes [
55,
56]. N is recommended in legumes with a low soil N status (less than 34 kg N ha
−1) to avoid manifest deficiency symptoms [
57]. However, the common bean also exhibited nodulation in hilly conditions as the soil contained suitable
Rhizobium strains. In these conditions, the nodulation was affected by the application of more N irrespective of the genotype as nitrogenous activity declined with applied N, decreasing the sink strength and reducing the quantity of photo-assimilate partitioned to nodules [
58]. Common bean genotypes had a substantial impact on whether the nodule number and nodule weight responded well to NPK application from 30:60:30 to 50:100:20 kg ha
−1 of N:P:K [
27,
59].
Based on the DMA and leaf area throughout a 30-day growth interval, crop growth analysis traits such as the mean CGR, RGR, and NAR were determined. Irrespective of NPK levels, genotype V
1 showed the marked maximum mean CGR, followed by genotype V
3, during the growth intervals of 0–30 and 30–60 DAS. The maximum mean RGR was logged with genotype V
1 during 0–30 DAS, while for genotype V
2, we logged a higher mean RGR during 30–60 and 60–90 DAS. A significantly higher mean NAR was computed with genotype V
1 during 0–30 DAS and 30–60 DAS and with genotypes V
2 and V
3 during the growth interval of 60–90 DAS. Irrespective of the common bean genotype, the mean CGR was significantly increased with the application of all NPK levels during all the growth intervals. However, it was not increased considerably above the level of N
40P
80K
40. The mean RGR was not significantly increased above N
40P
80K
40 and N
10P
20K
10 during the early growth interval (0–30 DAS) and later growth intervals (30–60 DAS and 60–90 DAS), respectively. The mean NAR was not increased significantly above N
40P
80K
40 during 0–30 DAS and 30–60 DAS. At 60–90 DAS, the mean NAR was not raised above N
30P
60K
30. Variability in how genetic features and NPK impacted the leaf area and dry matter production per unit area per unit time were blamed for the disparities in growth analysis traits. Earlier researchers also reported variation in crop growth traits due to plant nutrients in crops like the cluster bean, moth bean [
60,
61], and green gram [
62].
The influence of the NPK level on phenological development phases such as the days to 50% emergence, days to 50% flowering, days to 50% pod development, and days to 50% maturity was investigated among genotypes. Significant earliness in the days to 50% emergence was noted with genotype V3. Meanwhile, significantly earliness of the days to 50% flowering and 50% pod development was seen in the genotype V1. No significant differences were observed among the genotypes in the days to 50% maturity. The growth and development of common bean genotypes differed with fertility levels. Earliness of phenological development phases occurred at lower levels of NPK application, and with each successive increase in NPK level, these phases were delayed. Irrespective of the genotype, 50% emergence was observed in about 12 days below N30P60K30 and increased significantly beyond that level. The days to 50% flowering, 50% pod development, and 50% maturity were increased considerably from N10P20K10.
Varietal differences in early germination and seedling emergence occur in response to low temperature and soil moisture tolerance. De Ron et al. [
63] reported that the rate of emergence in bean genotypes was greatly reduced by a cool temperature. Beruktawit et al. [
64] reported that differences in the days to flowering among common bean cultivars suggested a high percentage of seed germination when there was a balanced supply of N, P, and K. However, a lack of P and K with a high N supply resulted in limited germination ability. A delay in flowering might occur because an excessive collection of N promotes luxuriant and succulent vegetative growth, dominating the reproductive phase. Marschner [
65] suggested that the nutrients taken up were used for increased cell division and synthesis of carbohydrates, which will predominantly be partitioned to the vegetative sink of the plants, resulting in plants with a luxurious foliage growth and delayed in maturity. Tewari and Singh [
66] reported that increased N levels resulted in more plant height but delayed blooming in beans. Dejene et al. [
67] hypothesized that greater P application increased cytokinin production, resulting in early flowering.
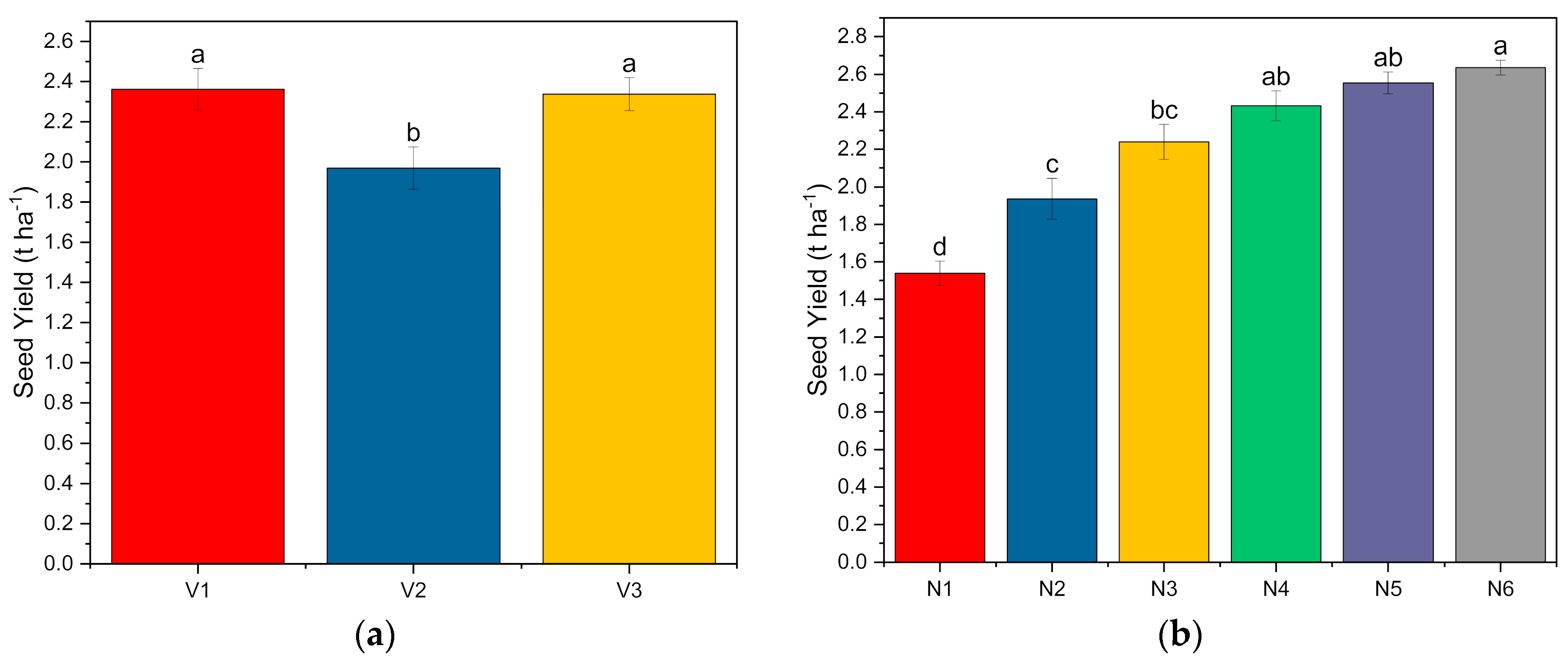
Except for the number of seeds pod
−1, the yield characteristics—the number of pods plant
−1 and seed index—were significantly influenced by the common bean genotypes. Both these yield characteristics were substantially higher with genotype V
1. An increased level of NPK resulted in a higher number of pods plant
−1, number of seeds pod
−1, and seed index. However, beyond the level of N
30P
60K
30, neither the number of pods plant
−1 nor the number of seeds pod
−1 grew appreciably. Meanwhile, the seed index obtained with N
40P
80K
40 was similar to that with N
50P
100K
50. The yield parameters—seed yield, stover yield, biological yield, and harvest index of the common bean—were significantly higher with genotypes V
1 and V
3. Irrespective of the genotype, the seed yield and biological yield were not significantly above the level of N
40P
80K
40. In comparison, the stover yield and harvest index were not significantly above the level of N
30P
60K
30. The harvest index in genotypes V
1 and V
2 was not significantly above the level of N
30P
60K
30. In the case of genotype V
3, the harvest index was not improved above the level of N
20P
40K
20. The yield characteristics are the crucial parameters that are supposed to play a role directly in achieving potential yield recovery in legumes. The number of pods plant
−1 can positively correlate with nodulation and the crop yield [
19]. Common bean cultivars vary in yield characteristics [
27]. The application of 25:75:50 kg ha
−1 of NPK in dolichos bean resulted in a significantly greater number of pods plant
−1 [
52]. Chekanai et al. [
28] also reported that the application of N in the primary season increased the number of pods in the subsequent season. Similarly, Leal et al. [
68] also suggested that the application of 120 kg N ha
−1 increased the number of pods plant
−1. Elsewere, the number of pods plant
−1 increased with N and K application [
69].
A considerable increase in the number of seeds pod
−1 with N, P, and K applications was reported by Zebire and Gelgelo [
70]. Sofi et al. [
71] observed that number of seeds pod
−1 was significantly increased up to 60 kg P
2O
5 ha
−1. The genotypes also created significant variation in the yield characteristics. Emam and Semida [
72] reported that the seed index increased with K supply. Yin et al. [
73] reported that the amounts of NPK for a higher seed index were 41.9 kg N ha
−1, 20.7 kg P
2O
5 ha
−1, and 66.50 kg K
2O ha
−1. The yield of a crop can be defined as the outcome and highly dependent on the vegetative growth, and numerically, it is the product of plant population and yield characteristics. In pulses, a higher vegetative growth may render a greater final yield. According to Sofi et al. [
74], an increase in yield-attributing features among the diverse genotypes of the common bean resulted in increased seed production. The application of nutrients highly influences the yield parameters. A significant response in the seed yield of bean was observed with N application varied from 120 to 240 kg ha
−1 and P application from 60 to 100 kg P
2O
5 ha
−1 [
66,
75,
76]. Shubhashree et al. [
77] reported that the yield of rajmash was significantly higher at 80:75:30 kg N:P
2O
5:K
2O ha
−1.
The influence of NPK on the productivity of common beans is multifaceted, impacting several physiological and biochemical processes essential for plant growth and the yield. N is a crucial component of amino acids, proteins, and chlorophyll, all of which are vital for plant growth and development. An adequate N supply enhances photosynthesis by increasing the chlorophyll content, thereby improving the plant’s ability to capture light energy and convert it into biomass [
78]. Furthermore, N promotes leaf area expansion, providing a larger surface area for photosynthesis, which is critical for biomass accumulation and the yield [
79]. P is essential for energy transfer within the plant, as it is a key component of ATP (adenosine triphosphate), which fuels various metabolic processes [
80]. It plays a significant role in root development, enhancing the plant’s ability to absorb water and nutrients from the soil [
81]. Additionally, P is critical for the formation and development of flowers, seeds, and pods, directly affecting reproductive success and the yield [
82]. P regulates the water-use efficiency and stomatal function, which are crucial for maintaining cellular turgor and controlling water loss, particularly under drought conditions [
65]. It activates enzymes involved in photosynthesis, protein synthesis, and carbohydrate metabolism, thereby facilitating overall plant growth and productivity [
32]. P also enhances the plant’s ability to withstand biotic and abiotic stresses, contributing to more stable and higher yields [
83]. The balanced application of NPK fertilizers ensures that common bean plants receive all essential macronutrients in appropriate proportions, promoting optimal growth and development. The synergistic effects of combined NPK application can lead to enhanced root biomass, improved nutrient uptake, and better overall plant health [
84]. For instance, N and P together can enhance root development more effectively than either nutrient alone, resulting in improved nutrient acquisition and growth [
85]. Adequate NPK nutrition leads to increased biomass production, higher pod and seed counts, and improved seed quality, including a higher protein content [
86]. N primarily influences vegetative growth and protein synthesis, P impacts reproductive development and energy transfer, and K improves stress tolerance and water-use efficiency. Collectively, these effects contribute to higher yields and better productivity of common beans. The influence of NPK on the productivity of common beans involves enhancing photosynthesis, energy transfer, nutrient uptake, water-use efficiency, and stress resistance, leading to improved growth, development, and yield [
87,
88,
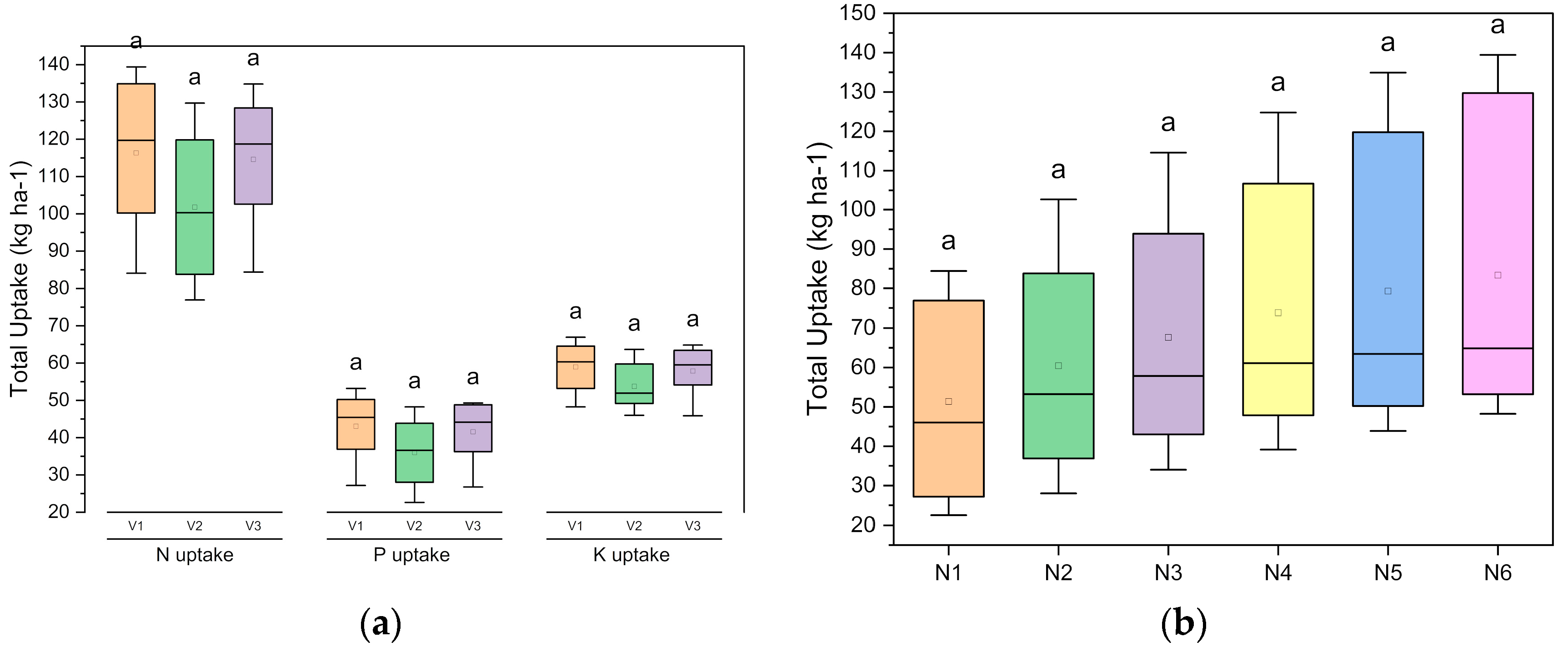
89]. Our findings revealed that the nutrient contents (N, P, and K) in genotypes V
1, V
2, and V
3 were on a par. However, the N, P, and K uptake levels in seeds and whole plants (seeds and stovers) were significantly higher with genotypes V
1 and V
3. The higher seed yield and total biomass obtained with genotypes V
1 and V
3 might have increased the uptake of these nutrients. Irrespective of the genotype, the contents of these nutrients in seeds and stovers increased significantly with the increasing levels of NPK applied. However, the N and P contents in the seeds were not increased significantly above the level of N
30P
60K
30, while the K content was not increased significantly above the level of N
40P
80K
40. Consequently, the uptake of these nutrients by crop was not significantly above the level of N
40P
80K
40. Increasing the application of nutrients leads to more available nutrients in the soil; consequently, the nutrient content and uptake increase [
90]. Thus, when the nutrient application is increased, the nutrient uptake by the plants is also higher because of increased biomass production [
91,
92,
93].
Irrespective of the nutrients applied, the soil reaction, EC, SOC, and available soil nutrients (N, P, and K) were not significantly affected among the common bean genotypes. These soil properties were found to be very stable and may not be varied considerably by varied genotypes in a short period of one crop cycle. The various levels of NPK had significant effects on soil properties. The level of N
50P
100K
50 had significantly higher values of soil reaction and EC. The SOC was not markedly increased beyond the level of N
40P
80K
40. The available NPK in soil was also not increased significantly above the level of N
30P
60K
30. SOC is an essential soil quality index, playing a significant role in affecting crop productivity [
94]. The findings by Sharma and Verma [
95] indicated that a build-up of SOC and available N, P, and K (over the control) occurred due to the inoculation of rajmash with
Rhizobium and the application of organic manure and chemical fertilizers. The SOC and available NPK in the soil can be maintained at an optimum level or improved through fertility management by using inorganic fertilizers and organic manures, tillage methods, crop rotation, and other cropping system components. However, a report indicated that increasing the P levels did not impact the pH, SOC, or soil-available N [
96]. Phosphorous fertilization in the common bean was expected to improve the cropping system performance, with well-documented P residual benefits to crop rotations [
28]. Sofi et al. [
71] noted no changes in pH, EC, SOC, or available NPK in the soil when growing various genotypes of the common bean and varying the phosphorus levels. The SOC levels were also measured at various P levels, but the percentage increase over a single season was near-negligible.
Irrespective of the NPK level applied, PFP, AE, and ARE were found to be higher with genotypes V
1 and V
3. PE was found to be at its maximum with genotype V
2. The nutrient-use efficiency in terms of PFP, AE, ARE, and PE was higher at lower levels of NPK applied. AER in genotype V
2 increased with the NPK level, reaching a maximum at N
40P
80K
40. At N
30P
60K
30 and N
40P
80K
40, the genotype V
1 showed a relatively higher nutrient-use efficiency, followed by V
3 and V
2. The mineral sustenance law states that there will be a greater expansion in yield with the first addition of applied supplement and the yield increment will be lower with higher supplement rates [
97]. In this study, the higher growth rates and yields of different genotypes suggested that the optimum application rates of NPK fertilizers were at N
30P
60K
30 and N
40P
80K
40. Akter et al. [
98] reported that increasing the nutrient-use efficiency helps reduce fertilizers’ costs and control pollution. When lowering the application of N fertilizer, rhizobia will be free to fix atmospheric N. K fertilizer doses should be regulated to optimize crop growth and development. P fertilizer application should be stabilized to improve its utilization by plants. With precise, optimized fertilization, the utilization of these nutrients by crops can be increased, improving the crop fertilization efficacy, environmental protection, crop quality, and crop yield. Most of the N and P fertilizers available in the market have a use efficiency of
<30% because of rapid volatilization into greenhouse gases or fixation in soil [
73]. Therefore, it was previously suggested that fertilizer application in green gram should reduce N, increase K, and stabilize P. Dejene et al. [
67] commented that common beans’ P-use efficiency was associated with their variation in P uptake ability. In contrast, Ortiz-Monasterio et al. [
99] reported that this mainly depended on root morphological characteristics. Leal et al. [
68] reported that the agronomic, physiological, and recovery-use efficiencies of N application in some common bean cultivars stood out, which may have been due to better nodulation at lower rates of N application.