Reducing Washout of Proteins from Defatted Soybean Flakes by Alkaline Extraction: Fractioning and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkaline Extraction Methods
2.3. Differential Protein Precipitation
2.4. Protein Resuspension and Drying
2.5. Separation of Fraction 2S in the Industrial Whey
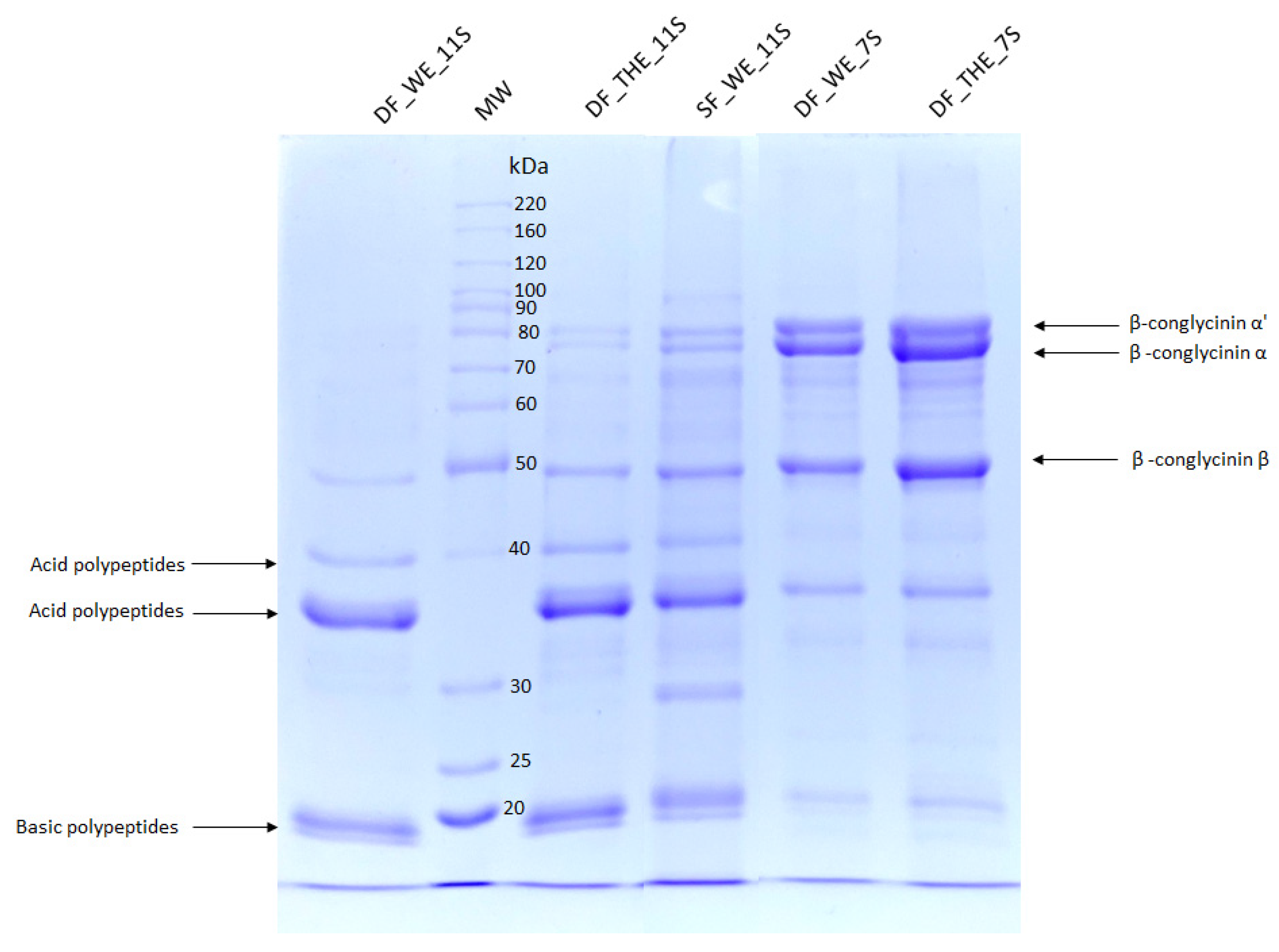
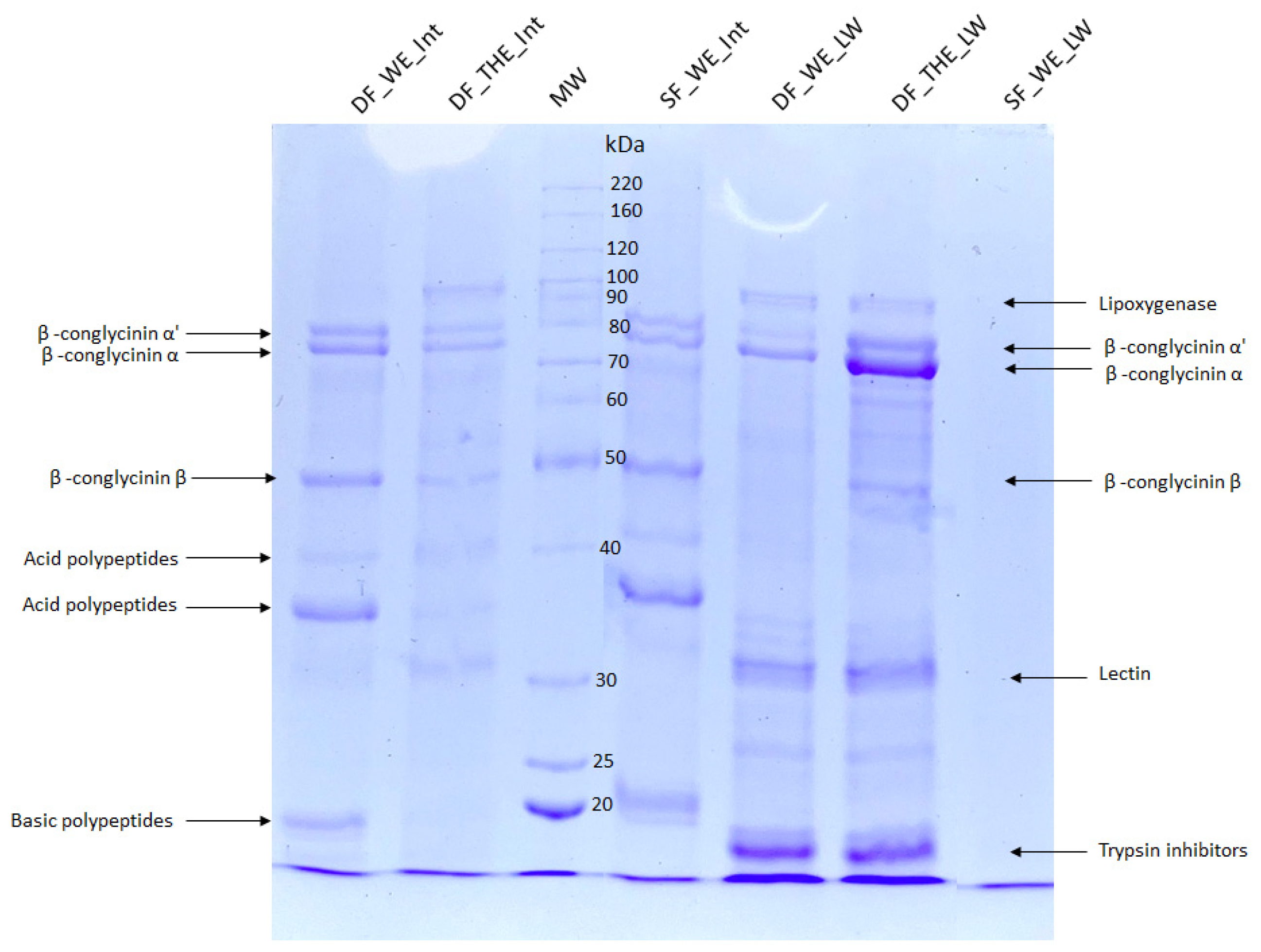
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
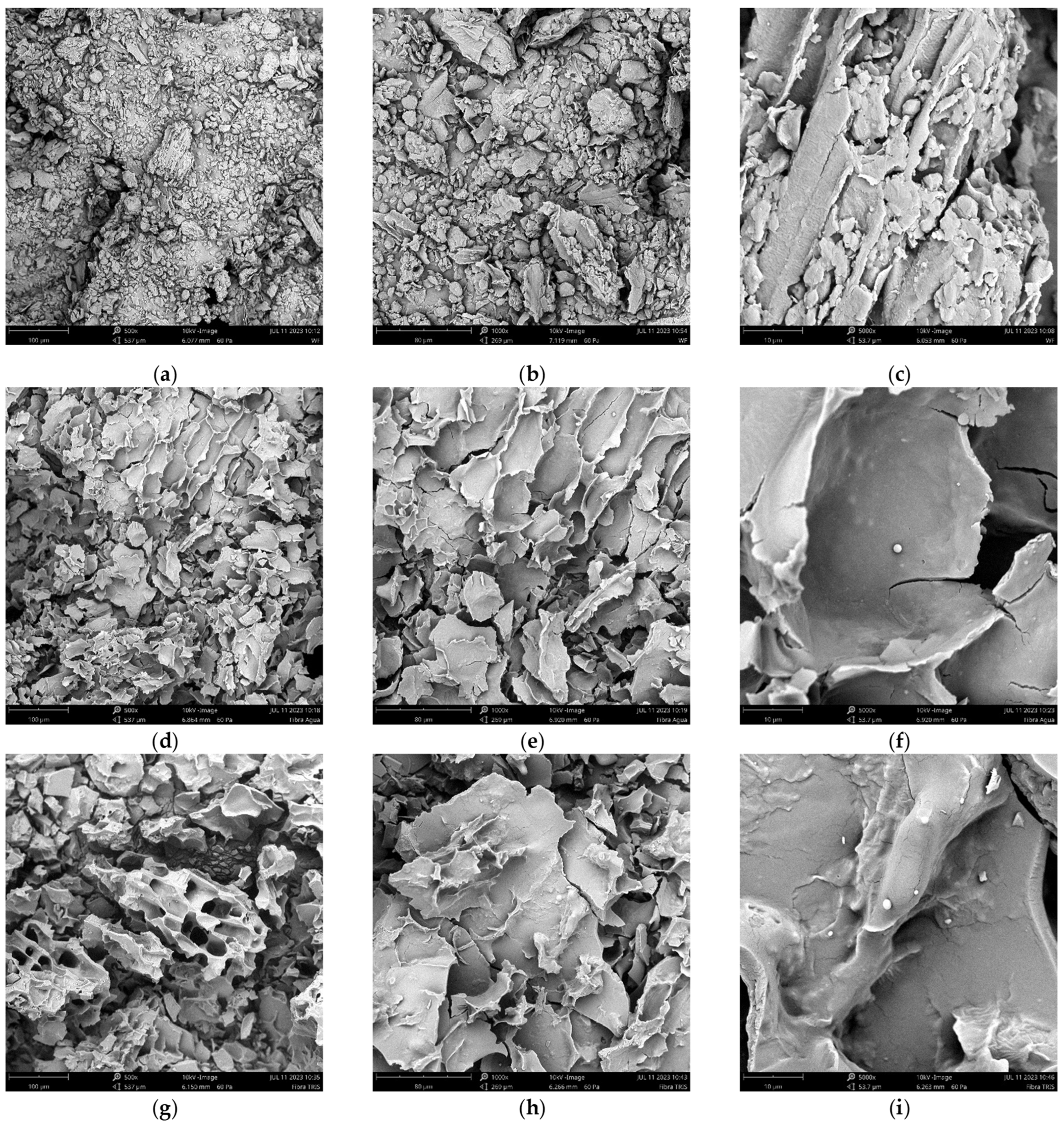
2.7. Scanning Electron Microscopy (SEM)
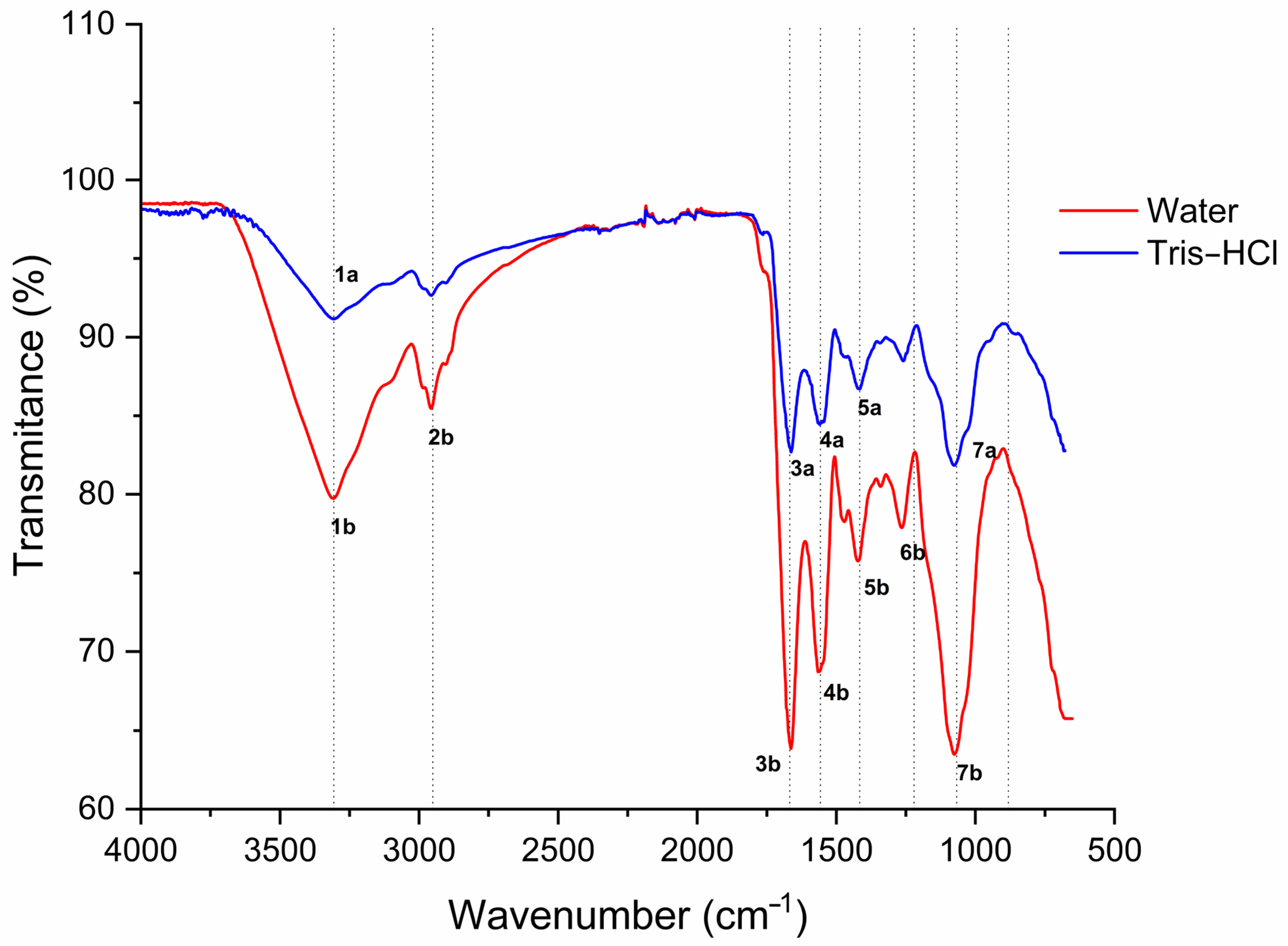
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Composition of Samples
3.2. Profile of Protein Fractions
3.3. Residual Protein in Spent Flakes
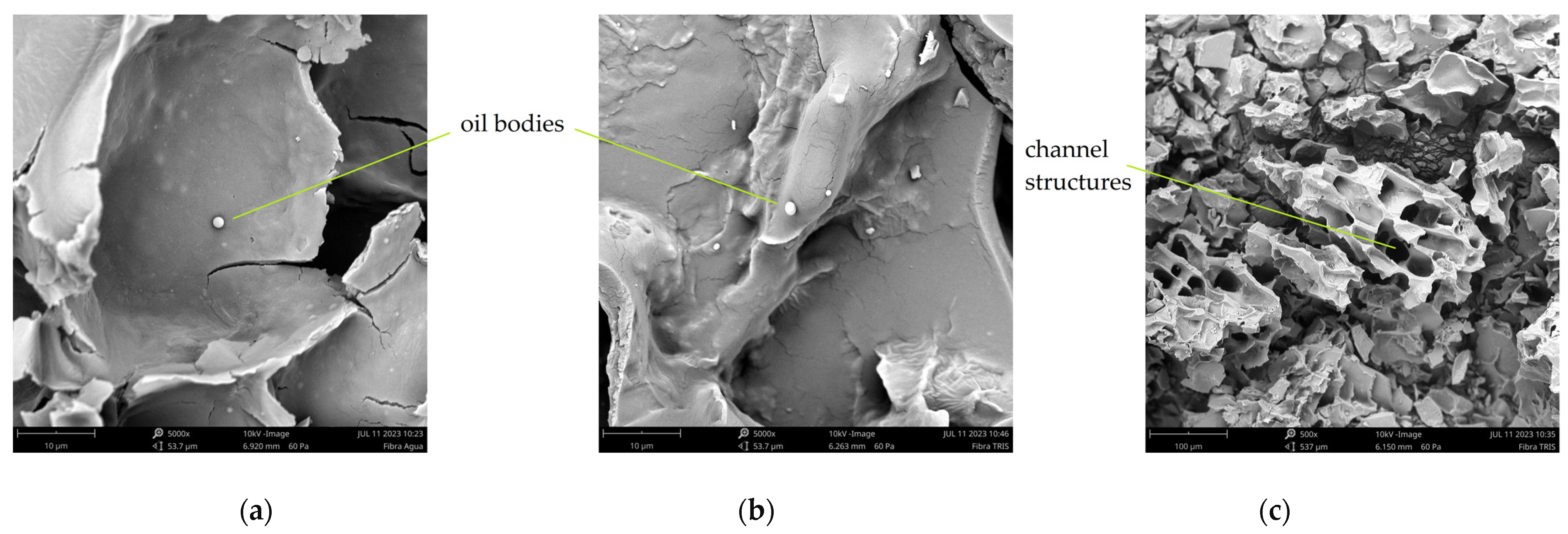
3.4. Microscopic Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messina, M. Perspective: Soybeans Can Help Address the Caloric and Protein Needs of a Growing Global Population. Front. Nutr. 2022, 9, 909464. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qu, Y.; Hua, X.; Wang, F.; Jia, X.; Yin, L. Recent Advances in Soybean Protein Processing Technologies: A Review of Preparation, Alterations in the Conformational and Functional Properties. Int. J. Biol. Macromol. 2023, 248, 125862. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Sun, X.S. Thermal and Mechanical Properties of Soy Proteins. In Bio-Based Polymers and Composites; Academic Press: Burlington, MA, USA, 2005; pp. 292–326. [Google Scholar]
- Ogawa, T.; Tsuji, H.; Bando, N.; Kitamura, K.; Zhu, Y.L.; Zhu, Y.L.; Hirano, H.; Nishikawa, K. Identification of the Soybean Allergenic Protein, Gly m Bd 30K, with the Soybean Seed 34-KDa Oil-Body-Associated Protein. Biosci. Biotechnol. Biochem. 1993, 57, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Beilinson, V.; Chen, Z.; Shoemaker, R.C.; Fischer, R.L.; Goldberg, R.B.; Nielsen, N.C. Genomic Organization of Glycinin Genes in Soybean. Theor. Appl. Genet. 2002, 104, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Arellano, J.B. Vegetable Protein Isolates. In Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 383–419. ISBN 9781845695873. [Google Scholar]
- Lin, J.; Fido, R.; Shewry, P.; Archer, D.B.; Alcocer, M.J.C. The Expression and Processing of Two Recombinant 2S Albumins from Soybean (Glycine Max) in the Yeast Pichia Pastoris. Biochim. Biophys. Acta Proteins Proteom. 2004, 1698, 203–212. [Google Scholar] [CrossRef]
- Sok, T.L. Functional And Structural Properties Of Molecular Soy Protein Fractions. Ph.D. Thesis, National University of Singapore, Singapore, 2005. [Google Scholar]
- Brandon, D.L.; Friedman, M. Immunoassays of Soy Proteins. J. Agric. Food Chem. 2002, 50, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.G.; Murphy, P.A.; Johnson, L.A. SOY (SOYA) BEANS | Properties and Analysis. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Burlington, MA, USA, 2003; pp. 5389–5392. [CrossRef]
- Fridman, C.; Lis, H.; Sharon, N.; Katchalski, E. Isolation and Characterization of Soybean Cytochrome C. Arch. Biochem. Biophys. 1968, 126, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W. Chemistry and Technology of Soybeans. In Advances in Cereal Science and Technology; American Association of Cereal Chemists: St. Paul, MN, USA, 1976; pp. 325–377. [Google Scholar]
- Koshiyama, I.; Kikuchi, M.; Harada, K.; Fukushima, D. 2S Globulins of Soybean Seeds. 1. Isolation and Characterization of Protein Components. J. Agric. Food Chem. 1981, 29, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Rackis, J.J.; Wolf, W.J.; Baker, E.C. Protease Inhibitors in Plant Foods: Content and Inactivation. In Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1986; pp. 299–347. [Google Scholar]
- Shibasaki, M.; Suzuki, S.; Tajima, S.; Nemo, H.; Kuroume, T. Allergenicity of Major Component Proteins of Soybean. Int. Arch. Allergy Immunol. 1980, 61, 441–448. [Google Scholar] [CrossRef]
- Van Holle, S.; Van Damme, E. Distribution and Evolution of the Lectin Family in Soybean (Glycine Max). Molecules 2015, 20, 2868–2891. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Teng, F.; Zhang, S.; Qi, B.; Wu, C.; Zhou, Y.; Li, L.; Wang, Z.; Li, Y. A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates. Foods 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy Protein: Impacts, Production, and Applications. In Sustainable Protein Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 23–45. ISBN 9780128027769. [Google Scholar]
- Fukushima, D. Soy Proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 210–232. [Google Scholar]
- Brazil. Ministério Da Agricultura, Pecuária e Abastecimento. Exportações Brasileiras: Soja em Grão. Brasília, Brazil. 2023. Available online: https://www.gov.br/agricultura/pt-br/assuntos/relacoes-internacionais/Sojaemgros.pdf (accessed on 3 June 2024).
- Din, J.U.; Sarwar, A.; Li, Y.; Aziz, T.; Hussain, F.; Shah, S.M.M.; Yi, G.; Liu, X. Separation of Storage Proteins (7S and 11S) from Soybean Seed, Meals and Protein Isolate Using an Optimized Method Via Comparison of Yield and Purity. Protein J. 2021, 40, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Cui, Z.; He, X.; Wang, X.; Zeng, X.; Ma, H. Optimization of Extraction and Isolation for 11S and 7S Globulins of Soybean Seed Storage Protein. Food Chem. 2007, 102, 1310–1316. [Google Scholar] [CrossRef]
- Laemmli, U.K. Clevage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kavran, J.M.; Leahy, D.J. Silver Staining of SDS-Polyacrylamide Gel. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 541, pp. 169–176. ISBN 9780124201194. [Google Scholar]
- Rahman, M.M.; Dutta, S.; Lamsal, B.P. High-Power Sonication-Assisted Extraction of Soy Protein from Defatted Soy Meals: Influence of Important Process Parameters. J. Food Process Eng. 2021, 44, e13720. [Google Scholar] [CrossRef]
- Perkins, E.G. Composition of Soybeans and Soybean Products. In Practical Handbook of Soybean Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 1995; pp. 9–28. [Google Scholar]
- Azam, M.; Zhang, S.; Qi, J.; Abdelghany, A.M.; Shaibu, A.S.; Ghosh, S.; Feng, Y.; Huai, Y.; Gebregziabher, B.S.; Li, J.; et al. Profiling and Associations of Seed Nutritional Characteristics in Chinese and USA Soybean Cultivars. J. Food Compos. Anal. 2021, 98, 103803. [Google Scholar] [CrossRef]
- Zarkadas, C.G.; Gagnon, C.; Poysa, V.; Khanizadeh, S.; Cober, E.R.; Chang, V.; Gleddie, S. Protein Quality and Identification of the Storage Protein Subunits of Tofu and Null Soybean Genotypes, Using Amino Acid Analysis, One- and Two-Dimensional Gel Electrophoresis, and Tandem Mass Spectrometry. Food Res. Int. 2007, 40, 111–128. [Google Scholar] [CrossRef]
- Pierce, B.C.; Wichmann, J.; Tran, T.H.; Cheetamun, R.; Bacic, A.; Meyer, A.S. Formation of Water-Soluble Soybean Polysaccharides from Spent Flakes by Hydrogen Peroxide Treatment. Carbohydr. Polym. 2016, 144, 504–513. [Google Scholar] [CrossRef]
- Dias, K.; Myers, D.J.; Bian, Y.; Lihono, M.A.; Wu, S.; Murphy, P.A. Functional Properties of the Acidic and Basic Subunits of the Glycinin (11S) Soy Protein Fraction. J. Am. Oil Chem. Soc. 2003, 80, 551–555. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A.I. Protein Enrichment of Defatted Soybean Flour by Fine Milling and Electrostatic Separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, W.; Li, D.; Cui, C.; Jiang, L.; Hou, J. Differences in the Structure and Properties of Seed Oil Bodies from Diverse Oilseed Crops. Food Sci. 2018, 39, 133–139. [Google Scholar]
- Idogawa, S.; Abe, N.; Abe, K.; Fujii, T. Effect of Oleosins on the Stability of Oil Bodies in Soymilk. Food Sci. Technol. Res. 2018, 24, 677–685. [Google Scholar] [CrossRef]



| Sample | Moisture (%) | Solids (%) | Protein (%) | Oil (%) | Fiber (%) |
|---|---|---|---|---|---|
| Defatted flakes | 5.72 ± 0.09 | - | 52.80 ± 0.16 | 0.52 ± 0.03 | 3.28 ± 0.18 |
| Spent Flakes | - | 13.10 ± 0.85 | 3.77 ± 0.97 | - | - |
| Peak # | X (cm−1) | Transmittance (%) | Extracting Solution | Band Name |
|---|---|---|---|---|
| 1a | 3279.67 | 91.16 | Tris-HCl | Amide A |
| 1b | 3280.25 | 79.74 | Water | |
| 2b | 2929.06 | 85.46 | Water | Amide B |
| 3a | 1634.84 | 82.70 | Tris-HCl | Amide I |
| 3b | 1635.29 | 63.85 | Water | |
| 4a | 1532.15 | 84.44 | Tris-HCl | Amide II |
| 4b | 1537.94 | 68.72 | Water | |
| 5a | 1387.87 | 86.70 | Tris-HCl | Amide III |
| 5b | 1394.81 | 75.76 | Water | |
| 6b | 1236.71 | 77.88 | Water | Amide III |
| 7a | 1048.03 | 81.84 | Tris-HCl | |
| 7b | 1047.48 | 63.48 | Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittmann, G.; Duarte, L.S.; Ayub, M.A.Z.; Rossi, D.M. Reducing Washout of Proteins from Defatted Soybean Flakes by Alkaline Extraction: Fractioning and Characterization. Sustainability 2024, 16, 6238. https://doi.org/10.3390/su16146238
Wittmann G, Duarte LS, Ayub MAZ, Rossi DM. Reducing Washout of Proteins from Defatted Soybean Flakes by Alkaline Extraction: Fractioning and Characterization. Sustainability. 2024; 16(14):6238. https://doi.org/10.3390/su16146238
Chicago/Turabian StyleWittmann, Giovana, Lovaine Silva Duarte, Marco Antônio Záchia Ayub, and Daniele Misturini Rossi. 2024. "Reducing Washout of Proteins from Defatted Soybean Flakes by Alkaline Extraction: Fractioning and Characterization" Sustainability 16, no. 14: 6238. https://doi.org/10.3390/su16146238
APA StyleWittmann, G., Duarte, L. S., Ayub, M. A. Z., & Rossi, D. M. (2024). Reducing Washout of Proteins from Defatted Soybean Flakes by Alkaline Extraction: Fractioning and Characterization. Sustainability, 16(14), 6238. https://doi.org/10.3390/su16146238








