A Study on the Differences in Vegetation Phenological Characteristics and Their Effects on Water–Carbon Coupling in the Huang-Huai-Hai and Yangtze River Basins, China
Abstract
:1. Introduction
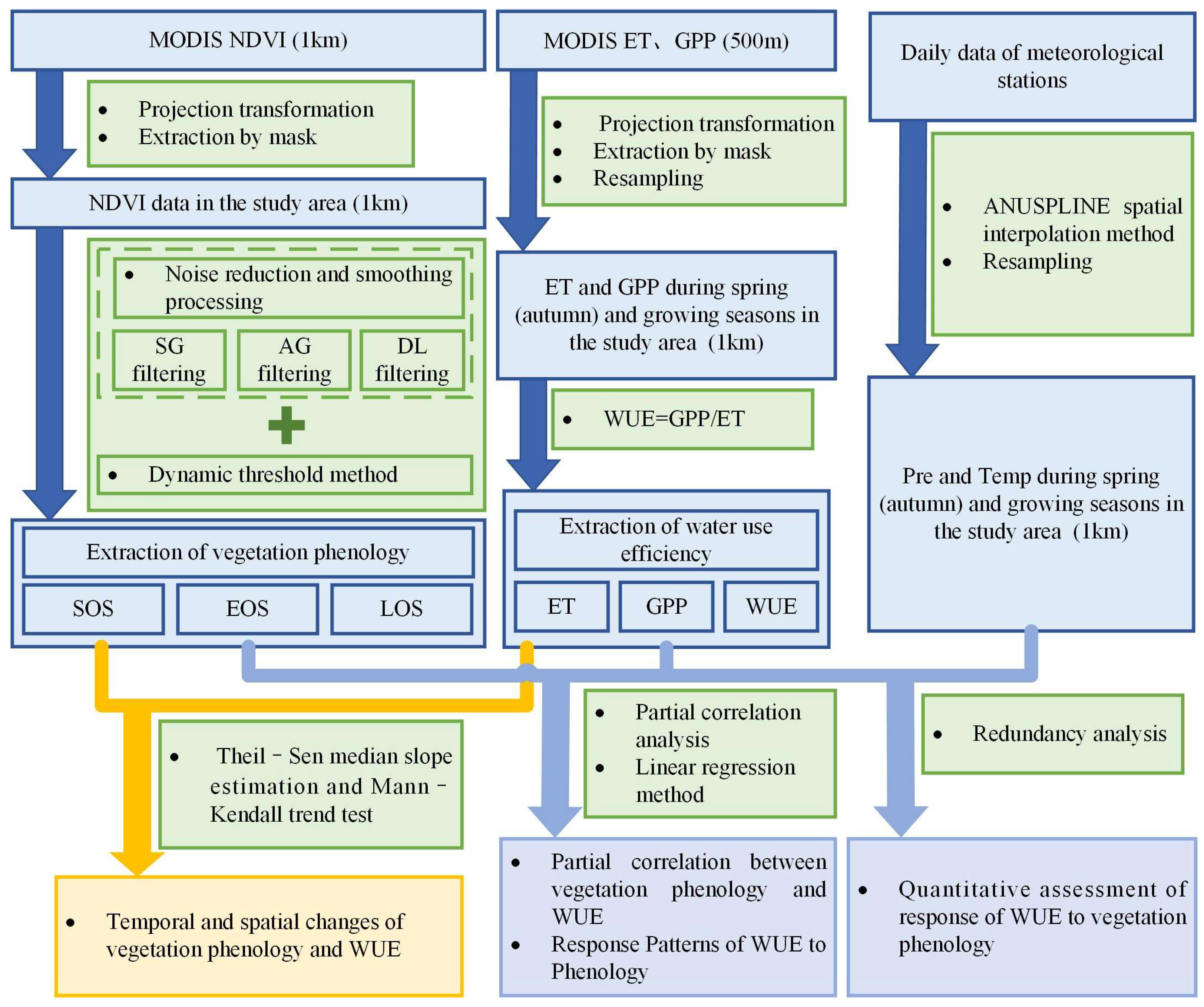
2. Materials and Methods
2.1. Study Area
2.2. Data
2.3. Research Method
2.3.1. Calculation of Water Use Efficiency
2.3.2. Determining the Phenology
- (1)
- Noise reduction and smoothing processing
- (a)
- Savitzky–Golay filtering method (SG)
- (b)
- Asymmetric Gaussian function fitting method (AG)
- (c)
- Double logistic function fitting method (DL)
- (2)
- Phenological extract
2.3.3. Analysis Methods
- (1)
- Trend analysis
- (2)
- Partial correlation analysis
- (3)
- Linear regression analysis
- (4)
- Redundancy analysis
3. Results
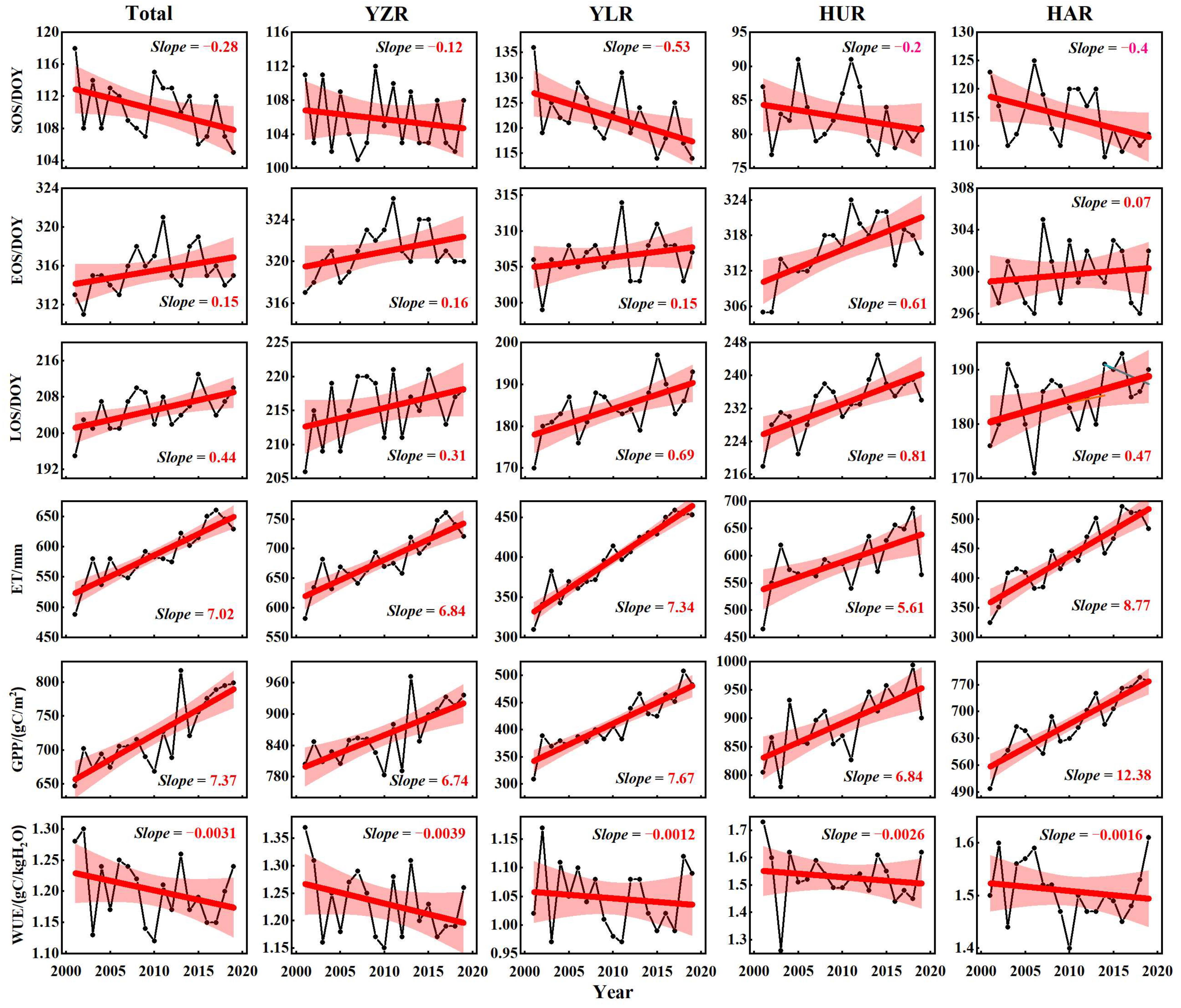
3.1. Temporal Variation of the Vegetation Phenology and WUE
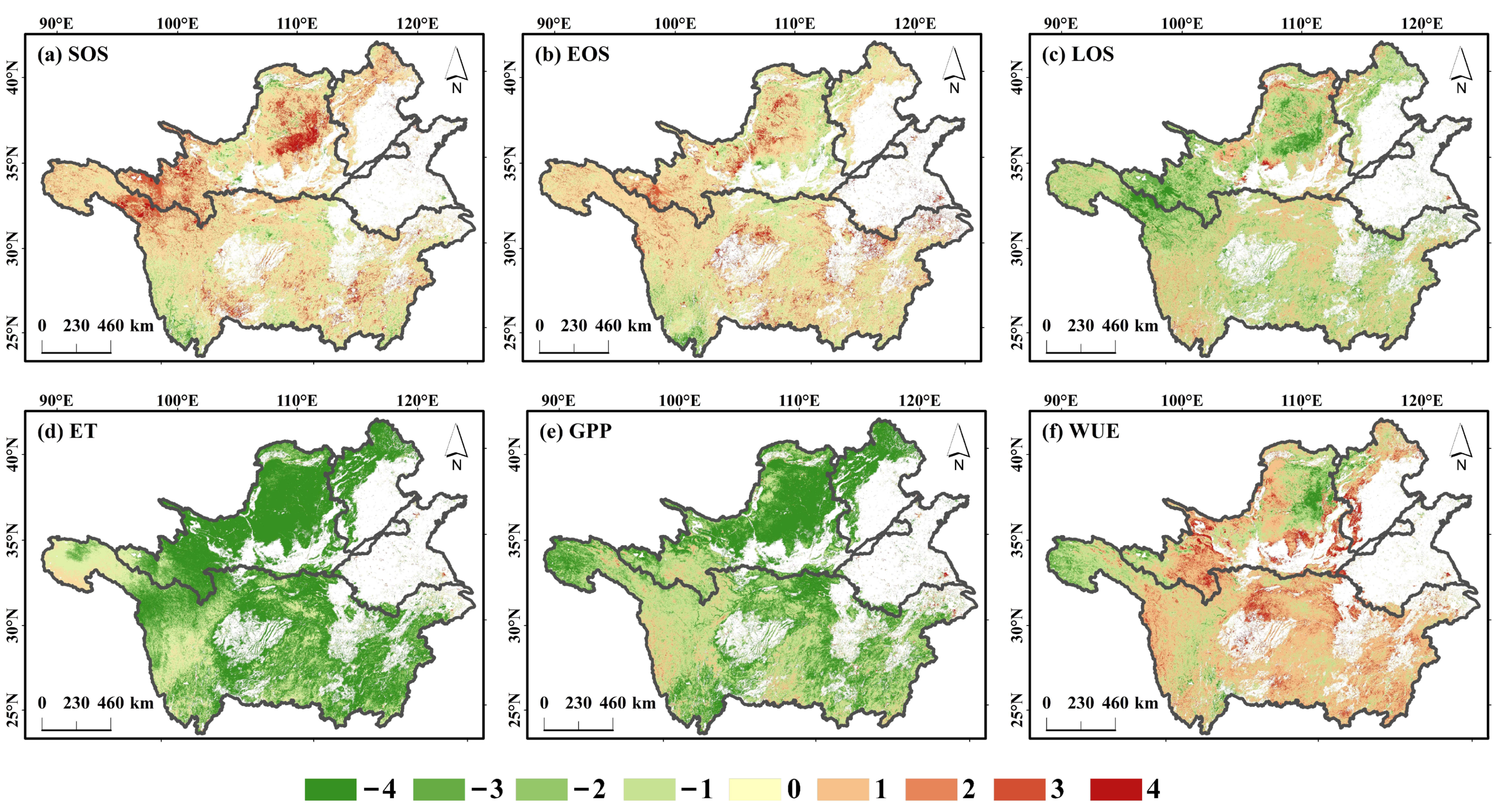
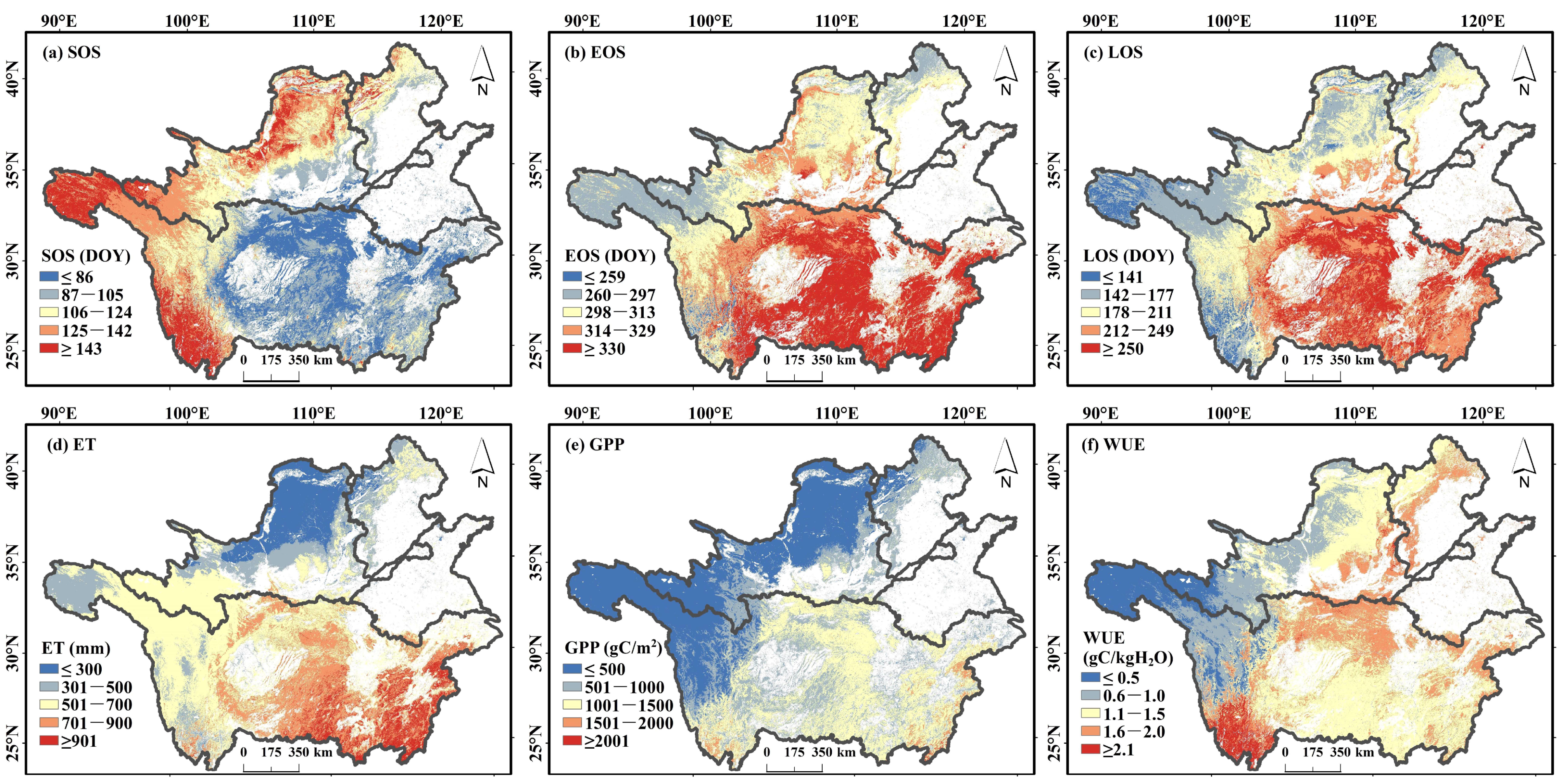
3.2. Spatial Distribution of the Vegetation Phenology and WUE
3.3. Relationship between Vegetation Phenology and WUE
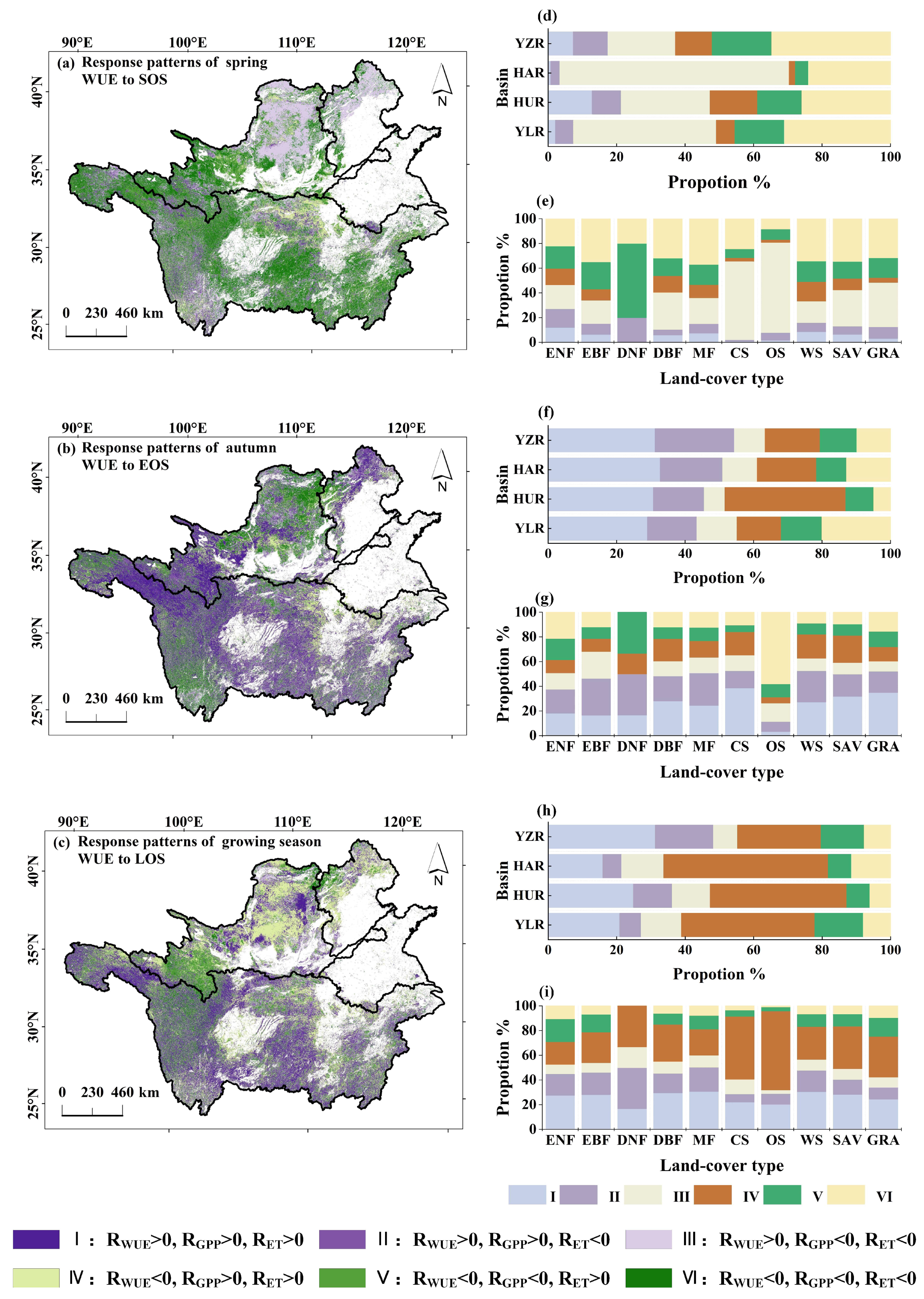
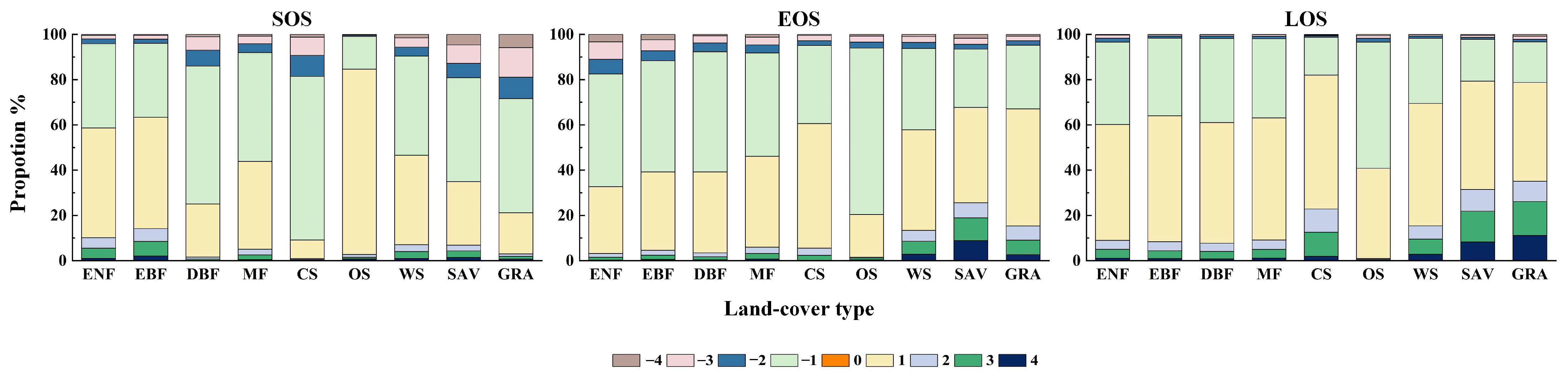
3.4. Response Patterns of WUE to Phenology
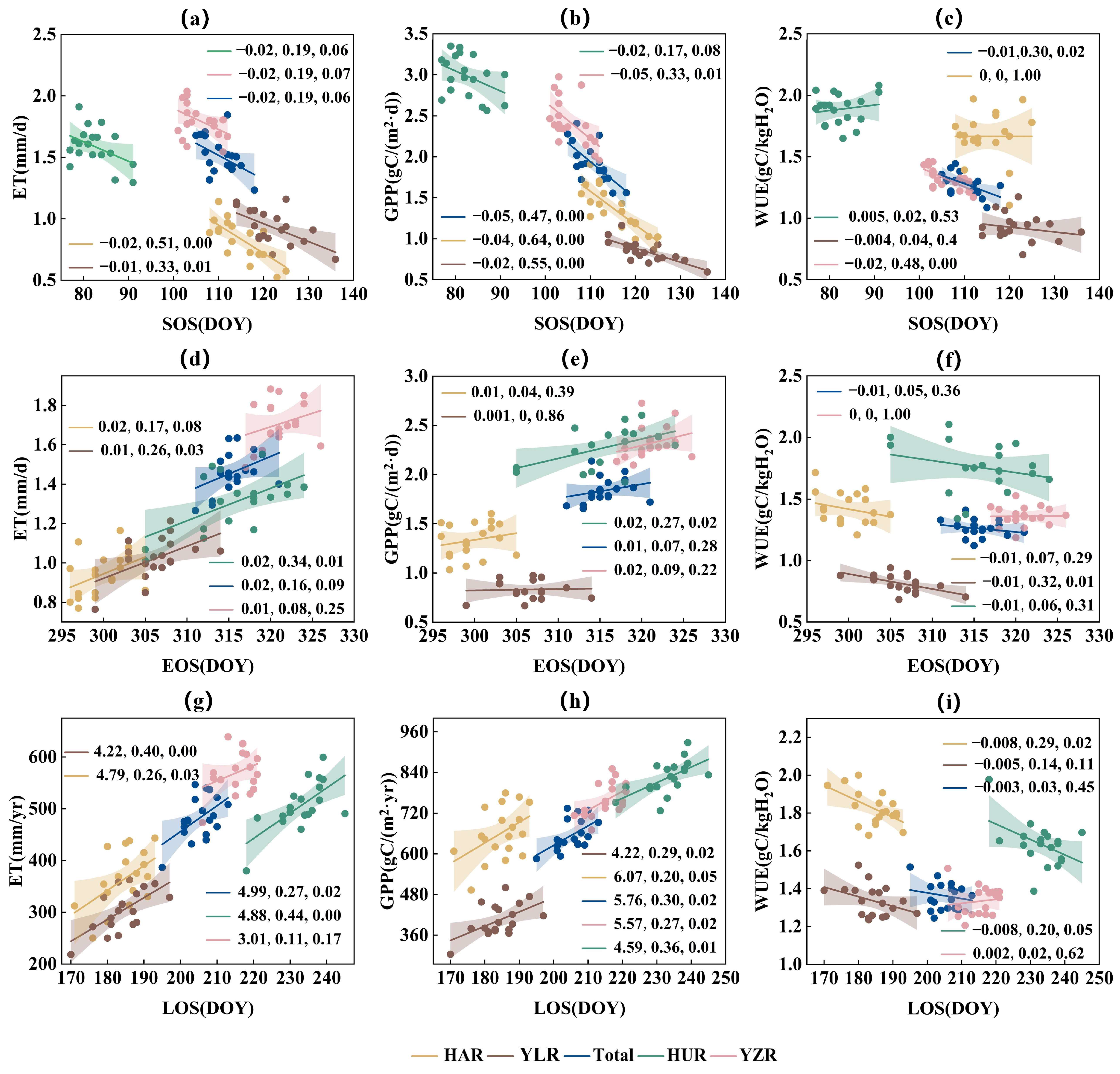
3.5. Quantitative Assessment of Response of WUE to Vegetation Phenology
4. Discussion
4.1. Phenological Changes and Spatial Heterogeneity
4.2. Response of WUE to Phenology, Temperature and Precipitation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study Area | Methods | Study Period | SOS (DOY) | EOS (DOY) | LOS (DOY) | Data Resources | Article |
|---|---|---|---|---|---|---|---|
| Loess Plateau (Mainly located in the YLR) | SG | 200–2018 | 96–144 | 288–304 | – | MODIS MOD13Q1 | Pei et al. [37] |
| YLR | SG | 2001–2020 | 90–160 | 270–310 | 110–190 | MODIS MOD13Q1 | Wang et al. [70] |
| YLR | DL | 2001–2020 | 80–160 | 270–300 | 150–200 | GOSIF dataset | Wang et al. [71] |
| Jing-jin-ji (Mainly located in the HAR) | SG | 2001–2020 | 99 (forest), 102 (grassland) | 287 (forest), 279 (grassland) | 188 (forest), 178 (grassland) | MODIS MOD13Q1 | Zhang [72] |
| Jing-jin-ji (Mainly located in the HAR) | – | 2000–2018 | 92–149 (forest), 90–132 (grassland) | 213–299 (forest), 236–300 (grassland) | – | Actual station phenological data | Zhang [72] |
| YZR | SG | 1982–2015 | 70–160 | 220–312 | 130–230 | GIMMS NDVI3g | Yuan et al. [73] |
| YLR, YZR, HAR, HUR | SG, DL, and AG | 200–2019 | 106 (YZR), 83 (HUR), 122 (YLR), 115 (HAR) | 321 (YZR), 316 (HUR), 306 (YLR), 300 (HAR) | 215 (YZR), 233 (HUR), 184 (YLR), 185 (HAR) | MODIS MOD13A2 | Our study |
| Study Area | Study Period | Annual Average WUE (gC/kgH2O) | Data Resources | Article |
|---|---|---|---|---|
| Yellow River Basin | 2001–2020 | 1.55 | MODIS MOD17A2(GPP) MOD16A2(ET) | Chen [74] |
| Yellow River Basin | 1982–2018 | 0.51–1.44 | GLASS | Liu et al. [75] |
| Haihe River basin | 2000–2014 | 1.52 | MODIS MOD17A2(GPP) MOD16A2(ET) | Zhao et al. [76] |
| Huaihe River basin | 1995–2004 | 0.83–1.52 | Vegetation Integrative Simulator for Trace Gases | Ito and Inatomi [77] |
| Huaihe River basin | 2003–2012 | 1.54–1.72 | MODIS MOD17A3(GPP) MOD16A3(ET) | Qiu et al. [78] |
| China | 2001–2021 | 0.78–1.43 | MODIS MOD17A2(GPP) MOD16A2(ET) | Li et al. [79] |
| China | 2003–2010 | 0.37–3.1 | Flux data | Xiao et al. [80] |
| China | 2003–2020 | 1.3–2.7 | Flux data | Dou et al. [81] |
| YLR, YZR, HAR, HUR | 2001–2019 | 1.2 (YZR), 1.53 (HUR), 1.04 (YLR), 1.51 (HAR) | MODIS MOD17A2(GPP) MOD16A2(ET) | Our study |
References
- Wu, L.Z.; Ma, X.F.; Dou, X.; Zhu, J.T.; Zhao, C.Y. Impacts of climate change on vegetation phenology and net primary productivity in arid Central Asia. Sci. Total Environ. 2021, 796, 15. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.B.; Chapin, F.S.; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tommervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013, 3, 581–586. [Google Scholar] [CrossRef]
- Wu, C.Y.; Peng, D.L.; Soudani, K.; Siebicke, L.; Gough, C.M.; Arain, M.A.; Bohrer, G.; Lafleur, P.M.; Peichl, M.; Gonsamo, A.; et al. Land surface phenology derived from normalized difference vegetation index (NDVI) at global FLUXNET sites. Agric. For. Meteorol. 2017, 233, 171–182. [Google Scholar] [CrossRef]
- Piao, S.L.; Fang, J.Y.; Zhou, L.M.; Ciais, P.; Zhu, B. Variations in satellite-derived phenology in China’s temperate vegetation. Glob. Chang. Biol. 2006, 12, 672–685. [Google Scholar] [CrossRef]
- Zhang, X.K.; Du, X.D.; Lu, X.Y.; Wang, X.D. A Review on Alpine Grassland Phenology on the Tibetan Plateau. Remote Sens. Technol. Appl. 2019, 34, 337–344. [Google Scholar] [CrossRef]
- Osborne, C.P.; Chuine, I.; Viner, D.; Woodward, F.I. Olive phenology as a sensitive indicator of future climatic warming in the Mediterranean. Plant Cell Environ. 2000, 23, 701–710. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; SueWing, I.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Fu, Y.S.H.; Zhao, H.F.; Piao, S.L.; Peaucelle, M.; Peng, S.S.; Zhou, G.Y.; Ciais, P.; Huang, M.T.; Menzel, A.; Uelas, J.P.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104. [Google Scholar] [CrossRef]
- Piao, S.L.; Tan, J.G.; Chen, A.P.; Fu, Y.H.; Ciais, P.; Liu, Q.; Janssens, I.A.; Vicca, S.; Zeng, Z.Z.; Jeong, S.J.; et al. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 2015, 6, 8. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Luo, T.X. Rainy season onset mainly drives the spatiotemporal variability of spring vegetation green-up across alpine dry ecosystems on the Tibetan Plateau. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Jin, H.; Tong, S.Q.; Chen, J.Q.; Huang, X.J.; Bao, Y.H.; Shao, C.L.; Mandakh, U.; Chopping, M.; Du, L.T. Autumn Phenology and Its Covariation with Climate, Spring Phenology and Annual Peak Growth on the Mongolian Plateau. Agric. For. Meteorol. 2021, 298, 11. [Google Scholar] [CrossRef]
- Marchand, L.J.; Dox, I.; Gricar, J.; Prislan, P.; Leys, S.; Van den Bulcke, J.; Fonti, P.; Lange, H.; Matthysen, E.; Penuelas, J.; et al. Inter-individual variability in spring phenology of temperate deciduous trees depends on species, tree size and previous year autumn phenology. Agric. For. Meteorol. 2020, 290, 8. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Robinson, T.B.; Martin, N.; Loureiro, T.G.; Matikinca, P.; Robertson, M.P. Double trouble: The implications of climate change for biological invasions. NeoBiota 2020, 62, 463–487. [Google Scholar] [CrossRef]
- Yang, H.L.; Yang, X.; Heskel, M.; Sun, S.C.; Tang, J.W. Seasonal variations of leaf and canopy properties tracked by ground-based NDVI imagery in a temperate forest. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Guan, H.D.; Shen, M.G.; Liang, W.; Jiang, L. Changes in autumn vegetation dormancy onset date and the climate controls across temperate ecosystems in China from 1982 to 2010. Glob. Chang. Biol. 2015, 21, 652–665. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Q.; Wang, C.; Yu, M. Spatiotemporal patterns of vegetation phenology change and relationships with climate in the two transects of East China. Glob. Ecol. Conserv. 2017, 10, 206–219. [Google Scholar] [CrossRef]
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.F.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Glob. Biogeochem. Cycle 2009, 23, 13. [Google Scholar] [CrossRef]
- Huang, M.T.; Piao, S.L.; Sun, Y.; Ciais, P.; Cheng, L.; Mao, J.F.; Poulter, B.; Shi, X.Y.; Zeng, Z.Z.; Wang, Y.P. Change in terrestrial ecosystem water-use efficiency over the last three decades. Glob. Chang. Biol. 2015, 21, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.L.; Guo, Q.H.; Otto, A.; Xiao, J.F.; Tao, S.L.; Li, L. Global patterns, trends, and drivers of water use efficiency from 2000 to 2013. Ecosphere 2015, 6, 18. [Google Scholar] [CrossRef]
- Cai, X.; Li, L.; Fisher, J.B.; Zeng, Z.; Zhou, S.; Tan, X.; Liu, B.; Chen, X. The responses of ecosystem water use efficiency to CO2, nitrogen deposition, and climatic drivers across China. J. Hydrol. 2023, 622, 129696. [Google Scholar] [CrossRef]
- Liu, W.; Zhan, J.Y.; Zhao, F.; Wang, C.; Zhang, F.; Teng, Y.M.; Chu, X.; Kumi, M.A. Spatio-temporal variations of ecosystem services and their drivers in the Pearl River Delta, China. J. Clean Prod. 2022, 337, 11. [Google Scholar] [CrossRef]
- Liu, Y.B.; Xiao, J.F.; Ju, W.M.; Zhou, Y.L.; Wang, S.Q.; Wu, X.C. Water use efficiency of China’s terrestrial ecosystems and responses to drought. Sci. Rep. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Chen, X.Z.; Li, R.H.; Long, F.L.; Zhang, L.; Zhang, Q.M.; Li, J.Y. Water-use efficiency of an old-growth forest in lower subtropical China. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernandez-Clemente, R.; Zhao, Y.C.; Gaitan, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehman, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787. [Google Scholar] [CrossRef] [PubMed]
- Peichl, M.; Gazovic, M.; Vermeij, I.; de Goede, E.; Sonnentag, O.; Limpens, J.; Nilsson, M.B. Peatland vegetation composition and phenology drive the seasonal trajectory of maximum gross primary production. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.G.; Elmendorf, S.C.; Hollister, R.D.; Henry, G.H.R.; Clark, K.; Bjorkman, A.D.; Myers-Smith, I.H.; Prevey, J.S.; Ashton, I.W.; Assmann, J.J.; et al. Experimental warming differentially affects vegetative and reproductive phenology of tundra plants. Nat. Commun. 2021, 12, 12. [Google Scholar] [CrossRef]
- Piao, S.L.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycle 2007, 21, 11. [Google Scholar] [CrossRef]
- Guo, F.S.; Jin, J.X.; Yong, B.; Wang, Y.; Jiang, H. Responses of water use efficiency to phenology in typical subtropical forest ecosystems-A case study in Zhejiang Province. Sci. China-Earth Sci. 2020, 63, 145–156. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Zhang, J.; Xia, J.Y.; Guo, Y.H.; Fu, Y.S.H. Effects of Vegetation Phenology on Ecosystem Water Use Efficiency in a Semiarid Region of Northern China. Front. Plant Sci. 2022, 13, 10. [Google Scholar] [CrossRef]
- Cheng, M.; Jin, J.X.; Jiang, H. Strong impacts of autumn phenology on grassland ecosystem water use efficiency on the Tibetan Plateau. Ecol. Indic. 2021, 126, 9. [Google Scholar] [CrossRef]
- Fan, Y.; van den Dool, H. A global monthly land surface air temperature analysis for 1948-present. J. Geophys. Res.-Atmos. 2008, 113, 18. [Google Scholar] [CrossRef]
- Wang, J.B.; Wang, J.W.; Hui, Y.H.; Ya, L.Y.; He, H.L. An interpolated temperature and precipitation dataset at 1-km grid resolution in China (2000–2012). China Sci. Data 2017, 2, 73–80. [Google Scholar] [CrossRef]
- Bao, G.; Chen, J.Q.; Chopping, M.; Bao, Y.H.; Bayarsaikhan, S.; Dorjsuren, A.; Tuya, A.; Jirigala, B.; Qin, Z.H. Dynamics of net primary productivity on the Mongolian Plateau: Joint regulations of phenology and drought. Int. J. Appl. Earth Obs. Geoinf. 2019, 81, 85–97. [Google Scholar] [CrossRef]
- Pei, T.T.; Ji, Z.X.; Chen, Y.; Wu, H.W.; Hou, Q.Q.; Qin, G.X.; Xie, B.P. The Sensitivity of Vegetation Phenology to Extreme Climate Indices in the Loess Plateau, China. Sustainability 2021, 13, 7623. [Google Scholar] [CrossRef]
- Chen, J.; Jonsson, P.; Tamura, M.; Gu, Z.H.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky-Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. TIMESAT—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. Seasonality extraction by function fitting to time-series of satellite sensor data. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1824–1832. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Hogda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Wu, C.Y.; Gough, C.M.; Chen, J.M.; Gonsamo, A. Evidence of autumn phenology control on annual net ecosystem productivity in two temperate deciduous forests. Ecol. Eng. 2013, 60, 88–95. [Google Scholar] [CrossRef]
- Che, M.L.; Chen, B.Z.; Innes, J.L.; Wang, G.Y.; Dou, X.M.; Zhou, T.M.; Zhang, H.F.; Yan, J.W.; Xu, G.; Zhao, H.W. Spatial and temporal variations in the end date of the vegetation growing season throughout the Qinghai-Tibetan Plateau from 1982 to 2011. Agric. For. Meteorol. 2014, 189, 81–90. [Google Scholar] [CrossRef]
- Ivits, E.; Cherlet, M.; Toth, G.; Sommer, S.; Mehl, W.; Vogt, J.; Micale, F. Combining satellite derived phenology with climate data for climate change impact assessment. Glob. Planet. Chang. 2012, 88–89, 85–97. [Google Scholar] [CrossRef]
- Sen Kumar, P. Estimates of the Regression Coefficient Based on Kendall’s Tau. Publ. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Bai, Y.; Li, S.; Zhou, J.; Liu, M.; Guo, Q. Revisiting vegetation activity of Mongolian Plateau using multiple remote sensing datasets. Agric. For. Meteorol. 2023, 341, 109649. [Google Scholar] [CrossRef]
- Dang, C.; Shao, Z.; Huang, X.; Zhuang, Q.; Cheng, G.; Qian, J. Climate warming-induced phenology changes dominate vegetation productivity in Northern Hemisphere ecosystems. Ecol. Indic. 2023, 151, 110326. [Google Scholar] [CrossRef]
- Huang, M.T.; Piao, S.L.; Janssens, I.A.; Zhu, Z.C.; Wang, T.; Wu, D.H.; Ciais, P.; Myneni, R.B.; Peaucelle, M.; Peng, S.S.; et al. Velocity of change in vegetation productivity over northern high latitudes. Nat. Ecol. Evol. 2017, 1, 1649–1654. [Google Scholar] [CrossRef]
- Cong, N.; Shen, M.G.; Piao, S.L. Spatial variations in responses of vegetation autumn phenology to climate change on the Tibetan Plateau. J. Plant Ecol. 2017, 10, 744–752. [Google Scholar] [CrossRef]
- Cleland, E.E.; Allen, J.M.; Crimmins, T.M.; Dunne, J.A.; Pau, S.; Travers, S.E.; Zavaleta, E.S.; Wolkovich, E.M. Phenological tracking enables positive species responses to climate change. Ecology 2012, 93, 1765–1771. [Google Scholar] [CrossRef]
- Arfin Khan, M.A.S.; Beierkuhnlein, C.; Kreyling, J.; Backhaus, S.; Varga, S.; Jentsch, A. Phenological Sensitivity of Early and Late Flowering Species Under Seasonal Warming and Altered Precipitation in a Seminatural Temperate Grassland Ecosystem. Ecosystems 2018, 21, 1306–1320. [Google Scholar] [CrossRef]
- Song, Z.Q.; Fu, Y.S.H.; Du, Y.J.; Li, L.; Ouyang, X.J.; Ye, W.H.; Huang, Z.L. Flowering phenology of a widespread perennial herb shows contrasting responses to global warming between humid and non-humid regions. Funct. Ecol. 2020, 34, 1870–1881. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Y.; Zeng, Z.; Huang, M.; Li, X.; Piao, S. Temperature, precipitation, and insolation effects on autumn vegetation phenology in temperate China. Glob. Chang. Biol. 2015, 22, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Prevey, J.S.; Seastedt, T.R. Seasonality of precipitation interacts with exotic species to alter composition and phenology of a semi-arid grassland. J. Ecol. 2014, 102, 1549–1561. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Yue, X.J.; Li, H.; Zhang, J.J.; Liang, J.Q.; Yuan, X.T.; Ru, J.Y.; Song, J.; Li, Y.; Zheng, M.M.; et al. Climate warming enhances precipitation sensitivity of flowering phenology in temperate steppes on the Mongolian Plateau. Agric. For. Meteorol. 2022, 324, 13. [Google Scholar] [CrossRef]
- Fu, Y.S.H.; Zhou, X.C.; Li, X.X.; Zhang, Y.R.; Geng, X.J.; Hao, F.H.; Zhang, X.; Hanninen, H.; Guo, Y.H.; De Boeck, H.J. Decreasing control of precipitation on grassland spring phenology in temperate China. Glob. Ecol. Biogeogr. 2021, 30, 490–499. [Google Scholar] [CrossRef]
- Geng, X.J.; Zhou, X.C.; Yin, G.D.; Hao, F.H.; Zhang, X.; Hao, Z.C.; Singh, V.P.; Fu, Y.S.H. Extended growing season reduced river runoff in Luanhe River basin. J. Hydrol. 2020, 582, 9. [Google Scholar] [CrossRef]
- Li, X.X.; Fu, Y.S.H.; Chen, S.Z.; Xiao, J.F.; Yin, G.D.; Li, X.; Zhang, X.; Geng, X.J.; Wu, Z.F.; Zhou, X.C.; et al. Increasing importance of precipitation in spring phenology with decreasing latitudes in subtropical forest area in China. Agric. For. Meteorol. 2021, 304, 9. [Google Scholar] [CrossRef]
- Jin, J.X.; Wang, Y.; Zhang, Z.; Magliulo, V.; Jiang, H.; Cheng, M. Phenology Plays an Important Role in the Regulation of Terrestrial Ecosystem Water-Use Efficiency in the Northern Hemisphere. Remote Sens. 2017, 9, 664. [Google Scholar] [CrossRef]
- Richardson, A.D.; Anderson, R.S.; Arain, M.A.; Barr, A.G.; Bohrer, G.; Chen, G.; Chen, J.M.; Ciais, P.; Davis, K.J.; Desai, A.R. Terrestrial biosphere models need better representation of vegetation phenology: Results from the North American Carbon Program Site Synthesis. Glob. Chang. Biol. 2012, 18, 566–584. [Google Scholar] [CrossRef]
- Luyssaert, S.; Janssens, I.A.; Sulkava, M.; Papale, D.; Dolman, A.J.; Reichstein, M.; Hollmen, J.; Martin, J.G.; Suni, T.; Vesala, T.; et al. Photosynthesis drives anomalies in net carbon-exchange of pine forests at different latitudes. Glob. Chang. Biol. 2007, 13, 2110–2127. [Google Scholar] [CrossRef]
- Silva, A.C.; Mendes, K.R.; Silva, C.; Rodrigues, D.T.; Costa, G.B.; da Silva, D.T.C.; Mutti, P.R.; Ferreira, R.R.; Bezerra, B.G. Energy Balance, CO2 Balance, and Meteorological Aspects of Desertification Hotspots in Northeast Brazil. Water 2021, 13, 2962. [Google Scholar] [CrossRef]
- Costa, G.B.; Mendes, K.R.; Viana, L.B.; Almeida, G.V.; Mutti, P.R.; Silva, C.; Bezerra, B.G.; Marques, T.V.; Ferreira, R.R.; Oliveira, C.P.; et al. Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data. Remote Sens. 2022, 14, 3955. [Google Scholar] [CrossRef]
- Cleverly, J.; Eamus, D.; Van Gorsel, E.; Chen, C.; Rumman, R.; Luo, Q.Y.; Coupe, N.R.; Li, L.H.; Kljun, N.; Faux, R.; et al. Productivity and evapotranspiration of two contrasting semiarid ecosystems following the 2011 global carbon land sink anomaly. Agric. For. Meteorol. 2016, 220, 151–159. [Google Scholar] [CrossRef]
- Nie, C.; Huang, Y.F.; Zhang, S.; Yang, Y.T.; Zhou, S.; Lin, C.J.; Wang, G.Q. Effects of soil water content on forest ecosystem water use efficiency through changes in transpiration/evapotranspiration ratio. Agric. For. Meteorol. 2021, 308, 10. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, M.; Tao, M.; Tan, Y.; Wang, Q. Large Divergence of Satellite Monitoring of Diffuse Radiation Effect on Ecosystem Water-Use Efficiency. Geophys. Res. Lett. (USA) 2023, 50, e2023GL106086. [Google Scholar] [CrossRef]
- Lawal, S.; Sitch, S.; Lombardozzi, D.; Nabel, J.; Wey, H.W.; Friedlingstein, P.; Tian, H.Q.; Hewitson, B. Investigating the response of leaf area index to droughts in southern African vegetation using observations and model simulations. Hydrol. Earth Syst. Sci. 2022, 26, 2045–2071. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, J.F.; Li, J.; Wang, K.; Lei, L.P.; Guo, H.D. The 2010 spring drought reduced primary productivity in southwestern China. Environ. Res. Lett. 2012, 7, 10. [Google Scholar] [CrossRef]
- Mumtaz, F.; Li, J.; Liu, Q.; Arshad, A.; Dong, Y.; Liu, C.; Zhao, J.; Bashir, B.; Gu, C.; Wang, X.; et al. Spatio-temporal dynamics of land use transitions associated with human activities over Eurasian Steppe: Evidence from improved residual analysis. Sci. Total Environ. 2023, 905, 166940. [Google Scholar] [CrossRef]
- Wang, Y.C.; He, J.; He, L.; Zhang, Y.J.; Zhang, X.P. Vegetation phenology and its response to climate change in the Yellow River Basin from 2001 to 2020. Acta Ecol. Sin. 2024, 44, 844–857. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, Q. Temporal and Spatial Changes of Vegetation Phenology and Their Response to Climate in the Yellow River Basin. IEEE Access (USA) 2023, 11, 141776–141788. [Google Scholar] [CrossRef]
- Zhang, C.C.; Meng, D.; Li, X.J. Spatial and temporal changes of vegetation phenology and its response to urbanization in the Beijing-Tianjin-Hebei region. Acta Ecol. Sin. 2023, 43, 249–262. [Google Scholar]
- Yuan, M.X.; Wang, L.C.; Lin, A.W.; Liu, Z.J.; Qu, S. Variations in land surface phenology and their response to climate change in Yangtze River basin during 1982–2015. Theor. Appl. Climatol. 2019, 137, 1659–1674. [Google Scholar] [CrossRef]
- Chen, L.W. Spatio-temporal variation of water use efficiency and its responses to environmental fators in Yellow River basin during 2001–2020. Bull. Soil Water Conserv. 2022, 42, 222–230. [Google Scholar] [CrossRef]
- Liu, S.; Xue, L.; Xiao, Y.; Yang, M.; Liu, Y.; Han, Q.; Ma, J. Dynamic process of ecosystem water use efficiency and response to drought in the Yellow River Basin, China. Sci. Total Environ. 2024, 934, 173339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.Z.; Zhang, A.B.; Feng, L.L.; Wang, D.L.; Cheng, D.Y. Spatio-temporal characteristics of water-use efficiency and its relationship with climatic factors in the Haihe River basin. Acta Ecol. Sin. 2019, 39, 1452–1462. [Google Scholar]
- Ito, A.; Inatomi, M. Water-Use Efficiency of the Terrestrial Biosphere: A Model Analysis Focusing on Interactions between the Global Carbon and Water Cycles. J. Hydrometeorol. 2012, 13, 681–694. [Google Scholar] [CrossRef]
- Qiu, K.B.; Cheng, J.F.; Jia, B.Q. Spatio-temporal distribution of cropland water use efficiency and influential factors in middle and east of China. Trans. Chin. Soc. Agric. Eng. 2015, 31, 103–109. [Google Scholar]
- Li, X.; Zou, L.; Xia, J.; Wang, F.; Li, H. Identifying the Responses of Vegetation Gross Primary Productivity and Water Use Efficiency to Climate Change under Different Aridity Gradients across China. Remote Sens. 2023, 15, 1563. [Google Scholar] [CrossRef]
- Xiao, J.F.; Sun, G.; Chen, J.Q.; Chen, H.; Chen, S.P.; Dong, G.; Gao, S.H.; Guo, H.Q.; Guo, J.X.; Han, S.J.; et al. Carbon fluxes, evapotranspiration, and water use efficiency of terrestrial ecosystems in China. Agric. For. Meteorol. 2013, 182, 76–90. [Google Scholar] [CrossRef]
- Dou, X.; Yu, G.; Chen, Z.; Yang, M.; Hao, T.; Han, L.; Liu, Z.; Ma, L.; Lin, Y.; Zhu, X.; et al. High spatial variability in water use efficiency of terrestrial ecosystems throughout China is predominated by biological factors. Agric. For. Meteorol. 2024, 345, 109834. [Google Scholar] [CrossRef]




| Basin | Range | Area/ 104 km2 | Ta/°C | Pre/mm | Proportion of Vegetation Types/% | Climate Zoning | ||
|---|---|---|---|---|---|---|---|---|
| Forest | Grassland | Croplands | ||||||
| YZR | 24°27′–35°44, 90°32′–121°54′ | 180 | 13.3 | 1100 | 60.09 | 18.44 | 16.70 | Humid areas |
| HAR | 35°03′–42°43′, 111°57′–119°44′ | 32.06 | 8.8 | 530 | 14.07 | 29.17 | 48.34 | Semi-humid and semi-arid areas |
| HUR | 30°57′–37°49′, 111°56′–122°40′ | 27 | 14.1 | 920 | 7.79 | 2.37 | 79.77 | Humid areas |
| YLR | 32°09′–41°49′, 95°54′–119°12′ | 79.5 | 9.4 | 480 | 9.57 | 65.54 | 20.57 | Arid and semi-arid areas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhai, J.; Ma, M.; Zhao, Y.; Li, X.; Li, L.; Li, H. A Study on the Differences in Vegetation Phenological Characteristics and Their Effects on Water–Carbon Coupling in the Huang-Huai-Hai and Yangtze River Basins, China. Sustainability 2024, 16, 6245. https://doi.org/10.3390/su16146245
Han S, Zhai J, Ma M, Zhao Y, Li X, Li L, Li H. A Study on the Differences in Vegetation Phenological Characteristics and Their Effects on Water–Carbon Coupling in the Huang-Huai-Hai and Yangtze River Basins, China. Sustainability. 2024; 16(14):6245. https://doi.org/10.3390/su16146245
Chicago/Turabian StyleHan, Shuying, Jiaqi Zhai, Mengyang Ma, Yong Zhao, Xing Li, Linghui Li, and Haihong Li. 2024. "A Study on the Differences in Vegetation Phenological Characteristics and Their Effects on Water–Carbon Coupling in the Huang-Huai-Hai and Yangtze River Basins, China" Sustainability 16, no. 14: 6245. https://doi.org/10.3390/su16146245






