Low Temperature Pyrolysis and Exfoliation of Waste Printed Circuit Boards: Recovery of High Purity Copper Foils
Abstract
1. Introduction
Aim of the Investigation
2. Background
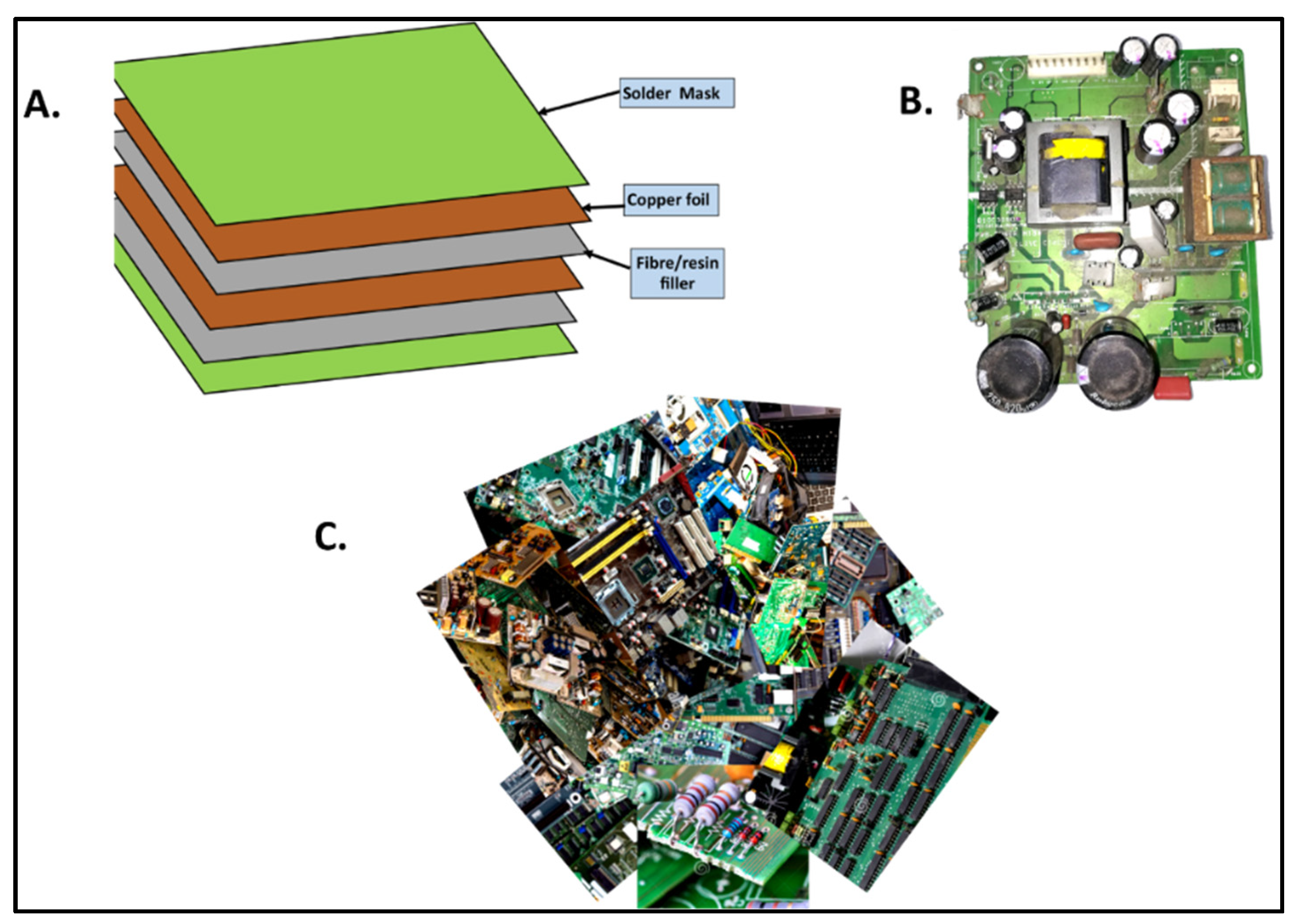
2.1. Structural Features of PCBs
2.2. Mechanical Pre-Treatments
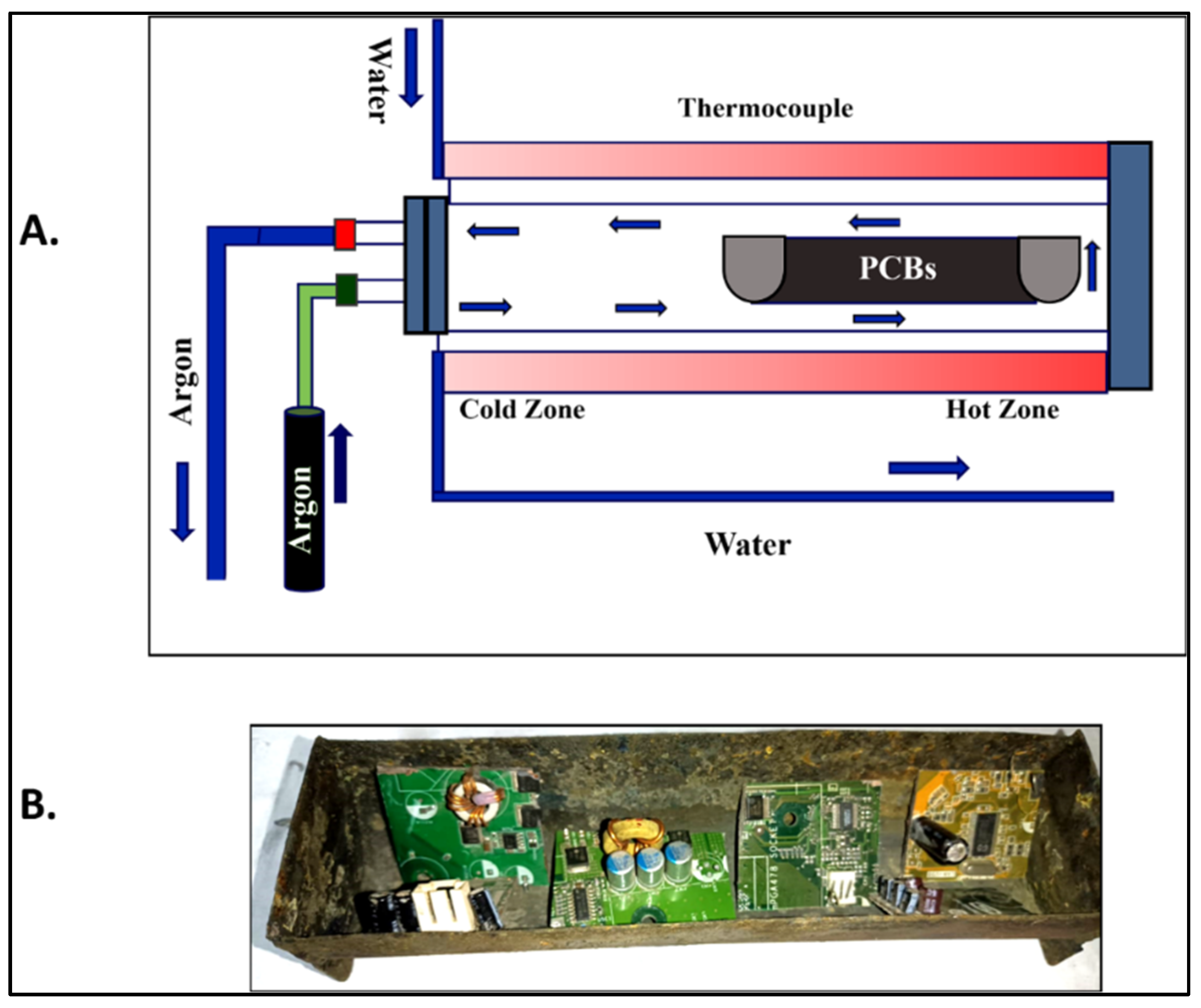
3. Experimental Section
4. Results
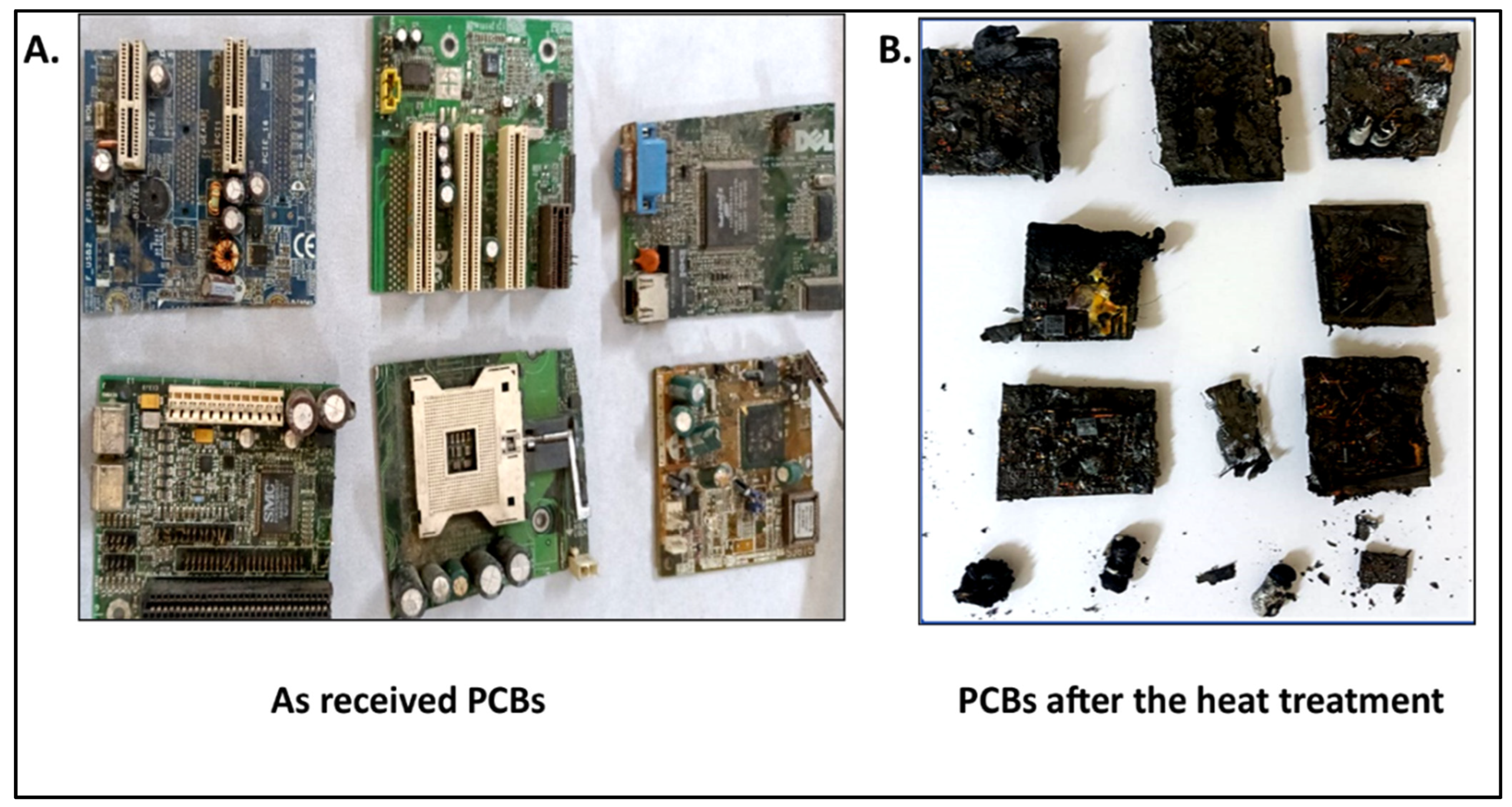
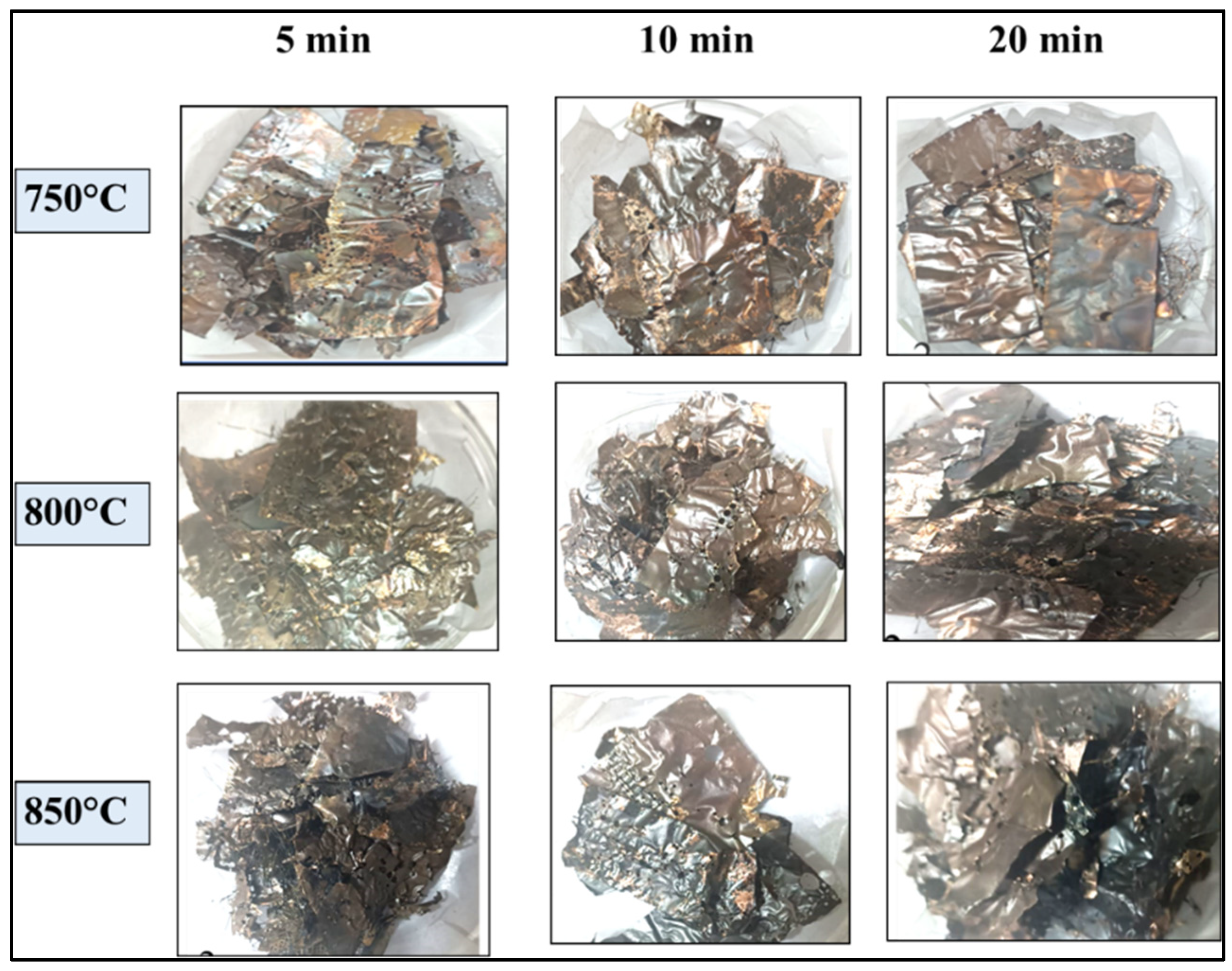
4.1. Morphology of PCBs after Pyrolysis
4.2. Net Weight Loss after Pyrolysis
4.3. Extraction of Copper Foils
4.4. Characterization of Copper Foils
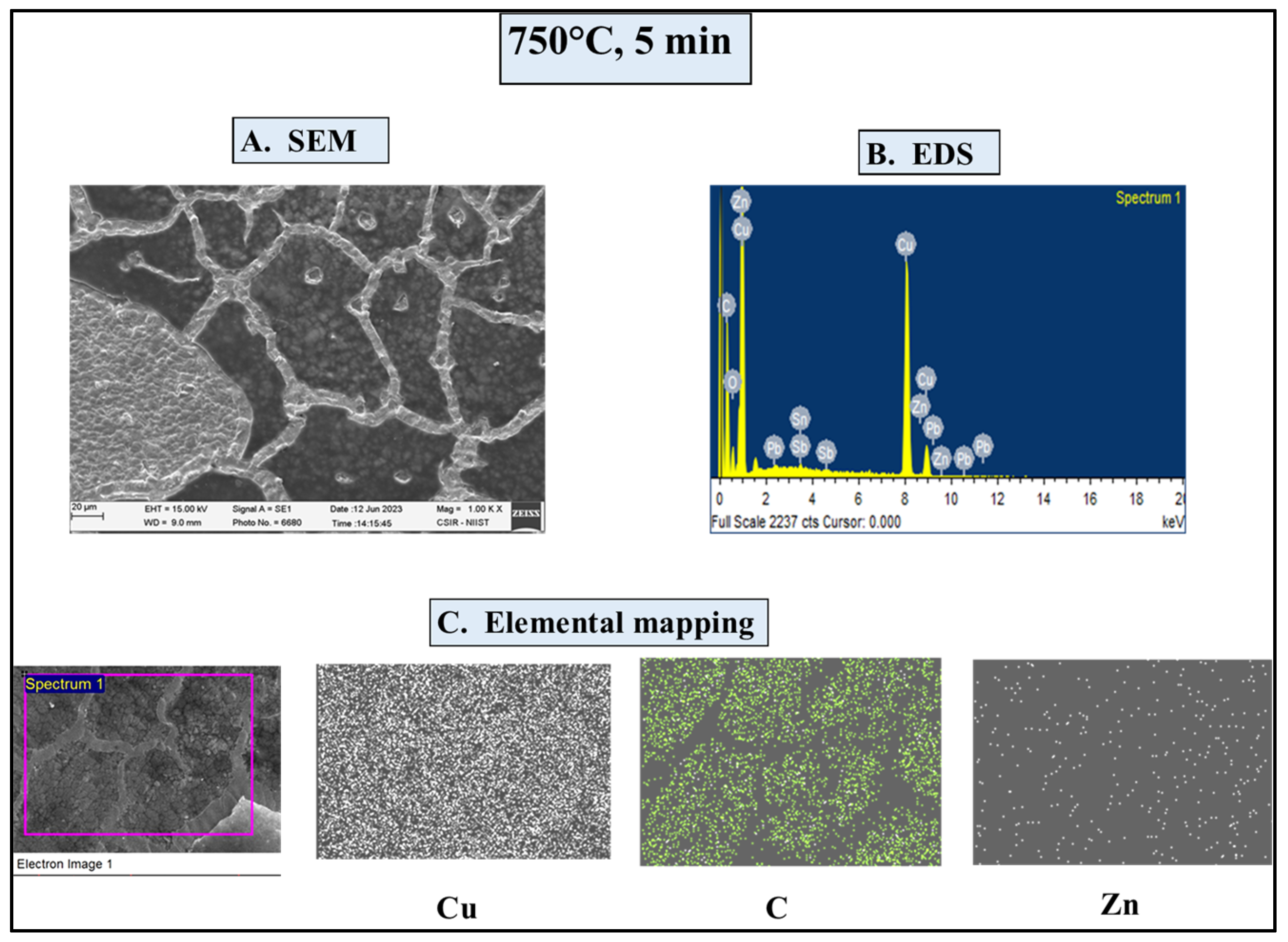
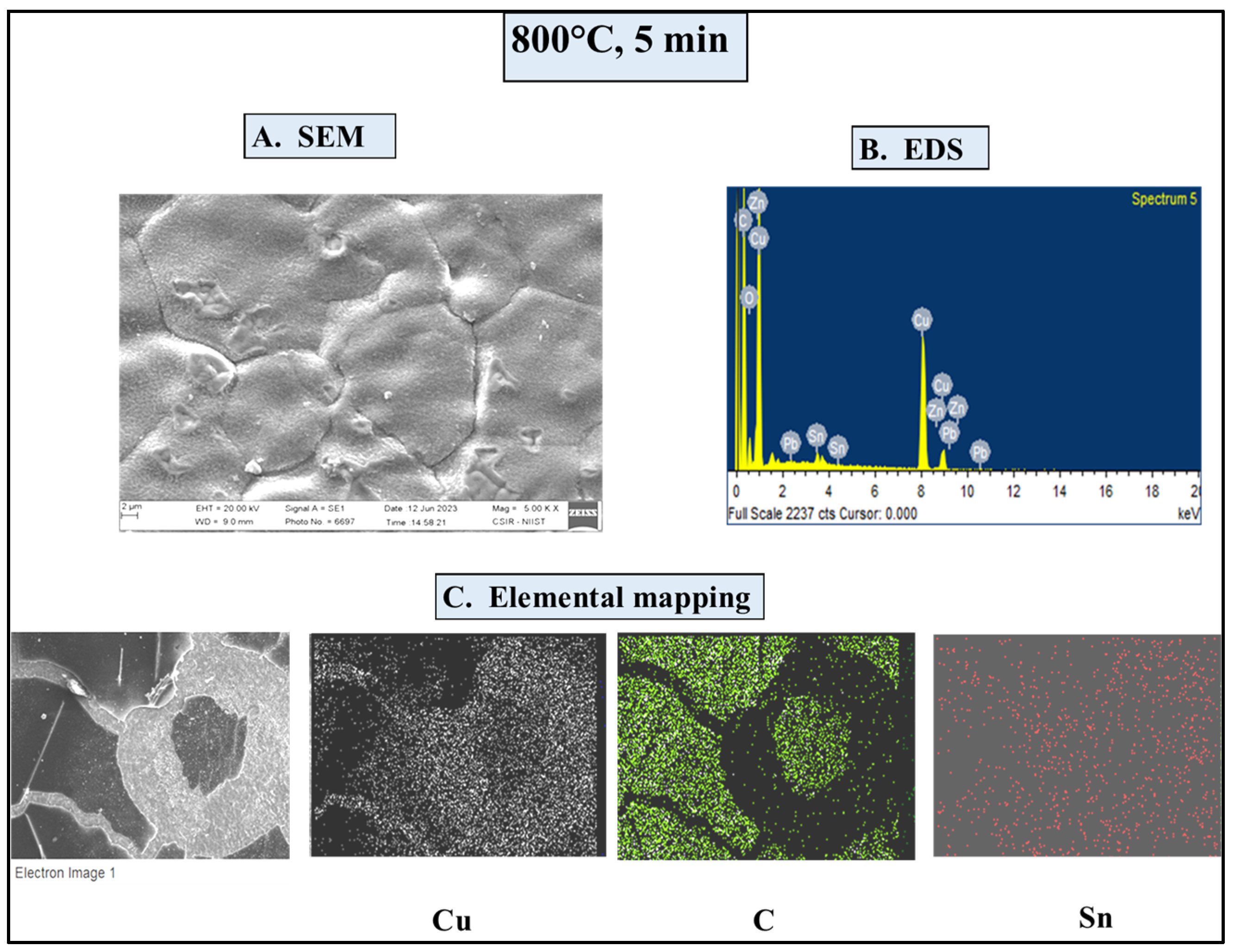
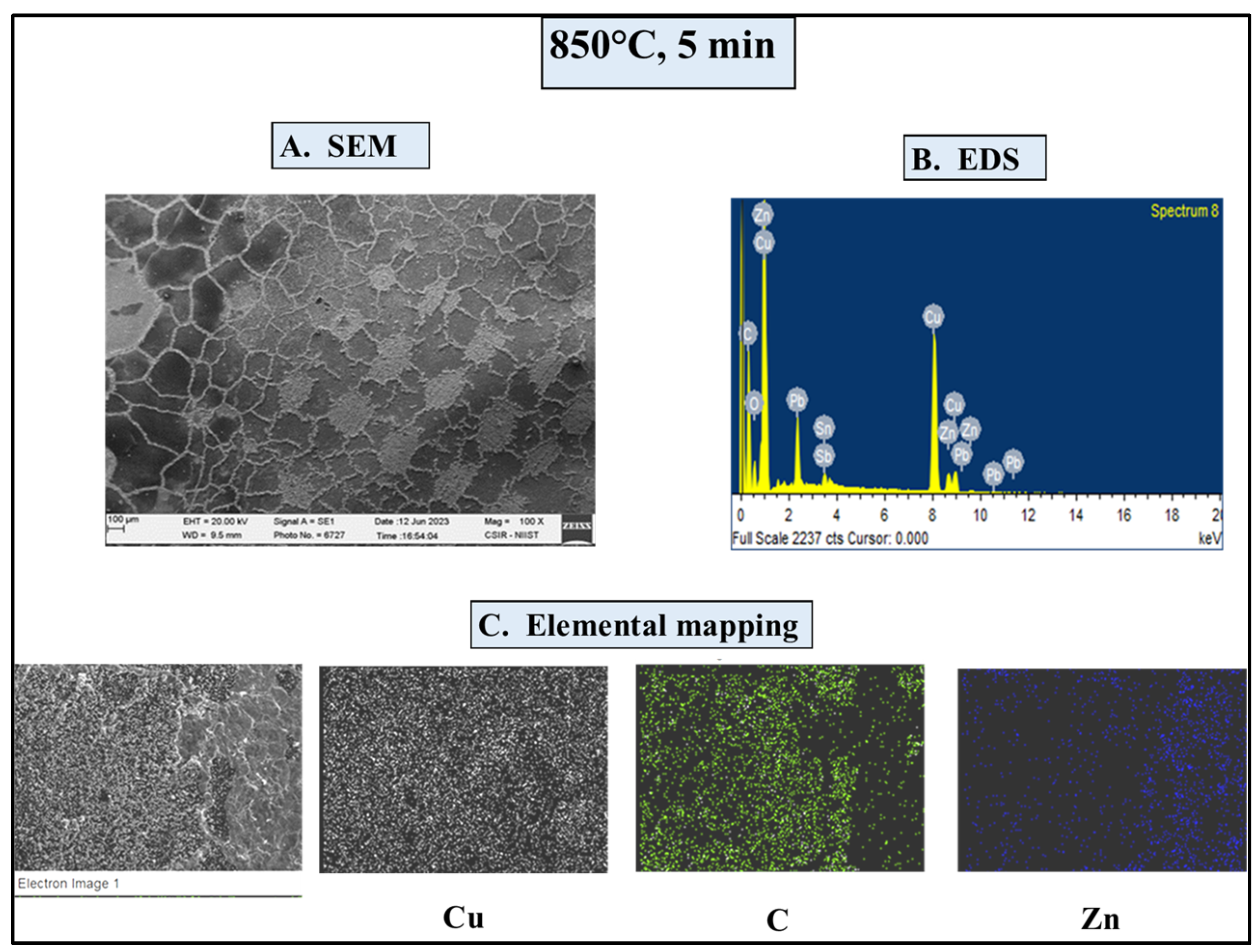
4.4.1. SEM/EDS Investigations
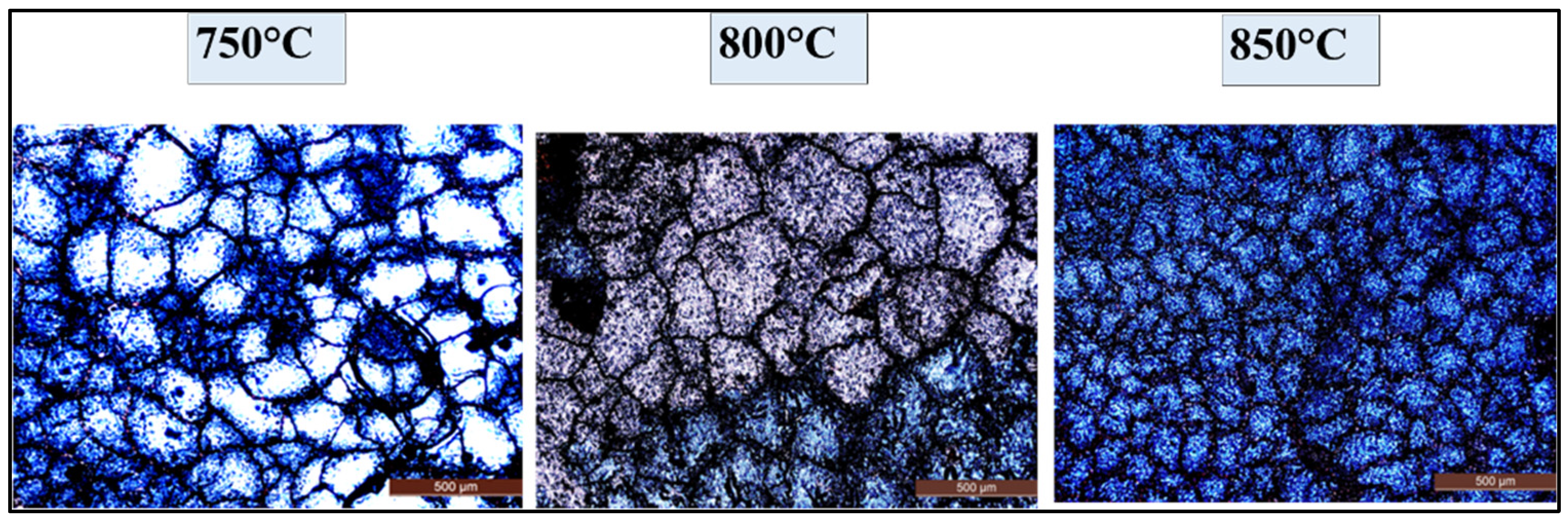
4.4.2. Optical Microscopic Investigations
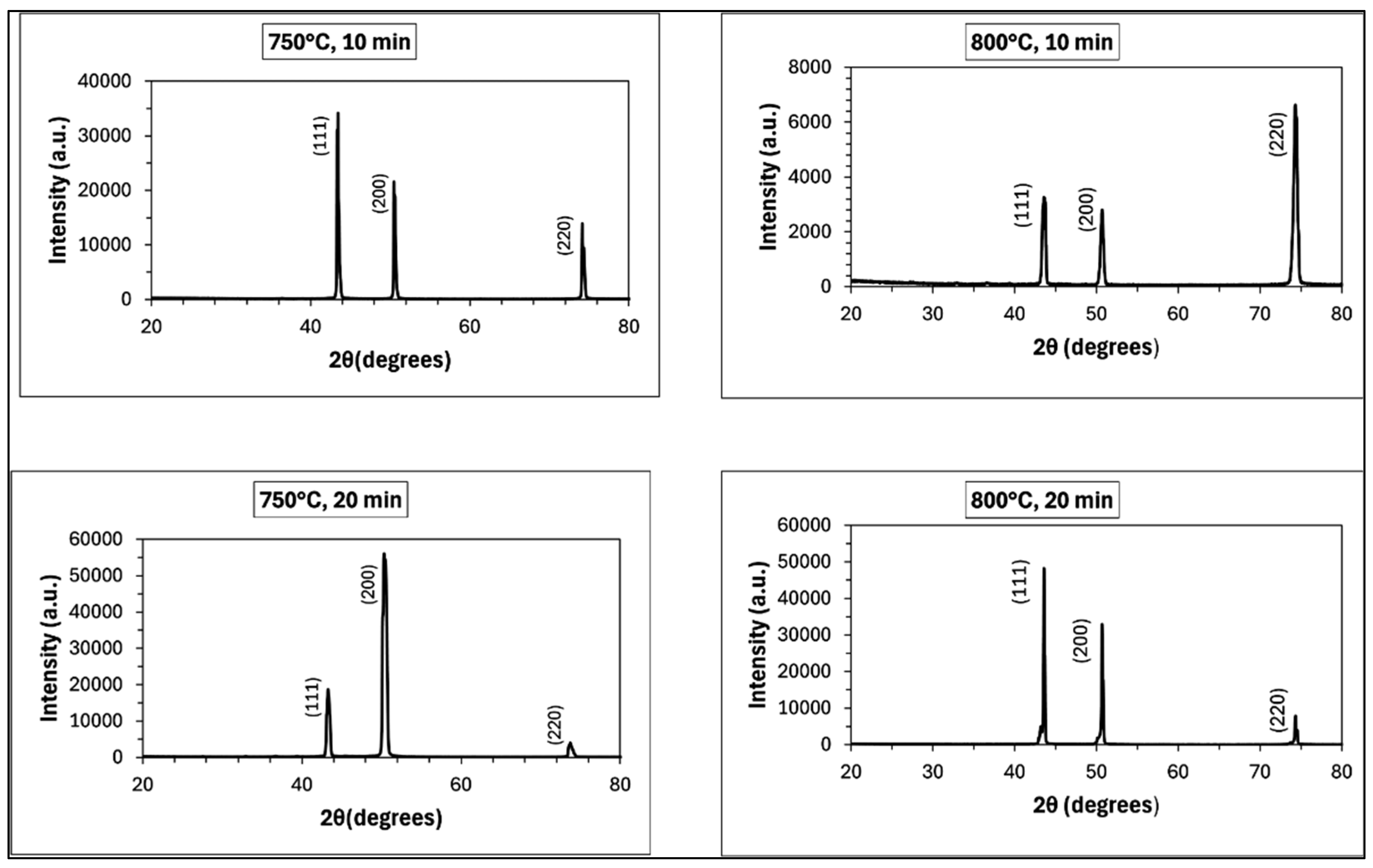
4.4.3. X-ray Diffraction Investigations
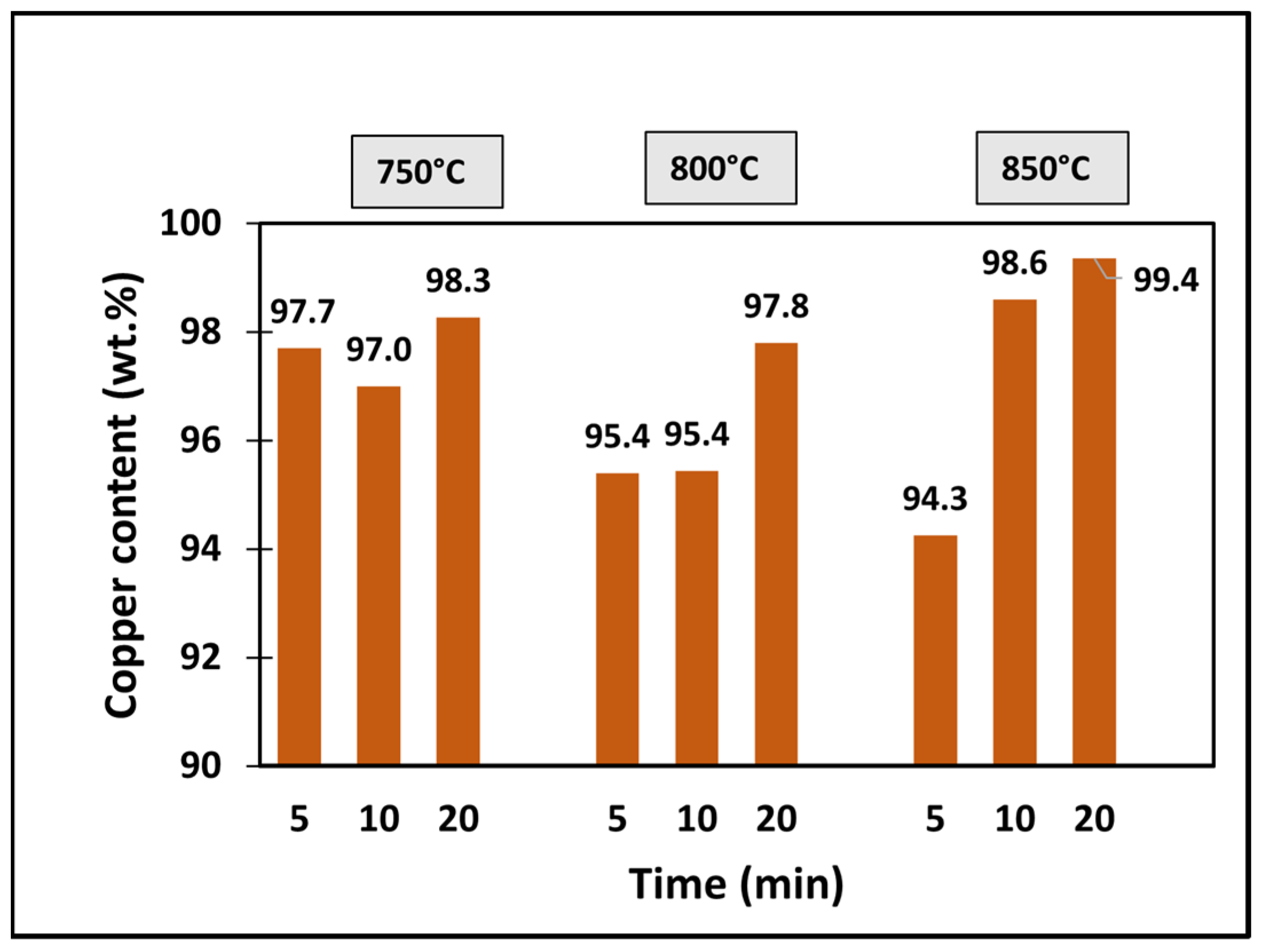
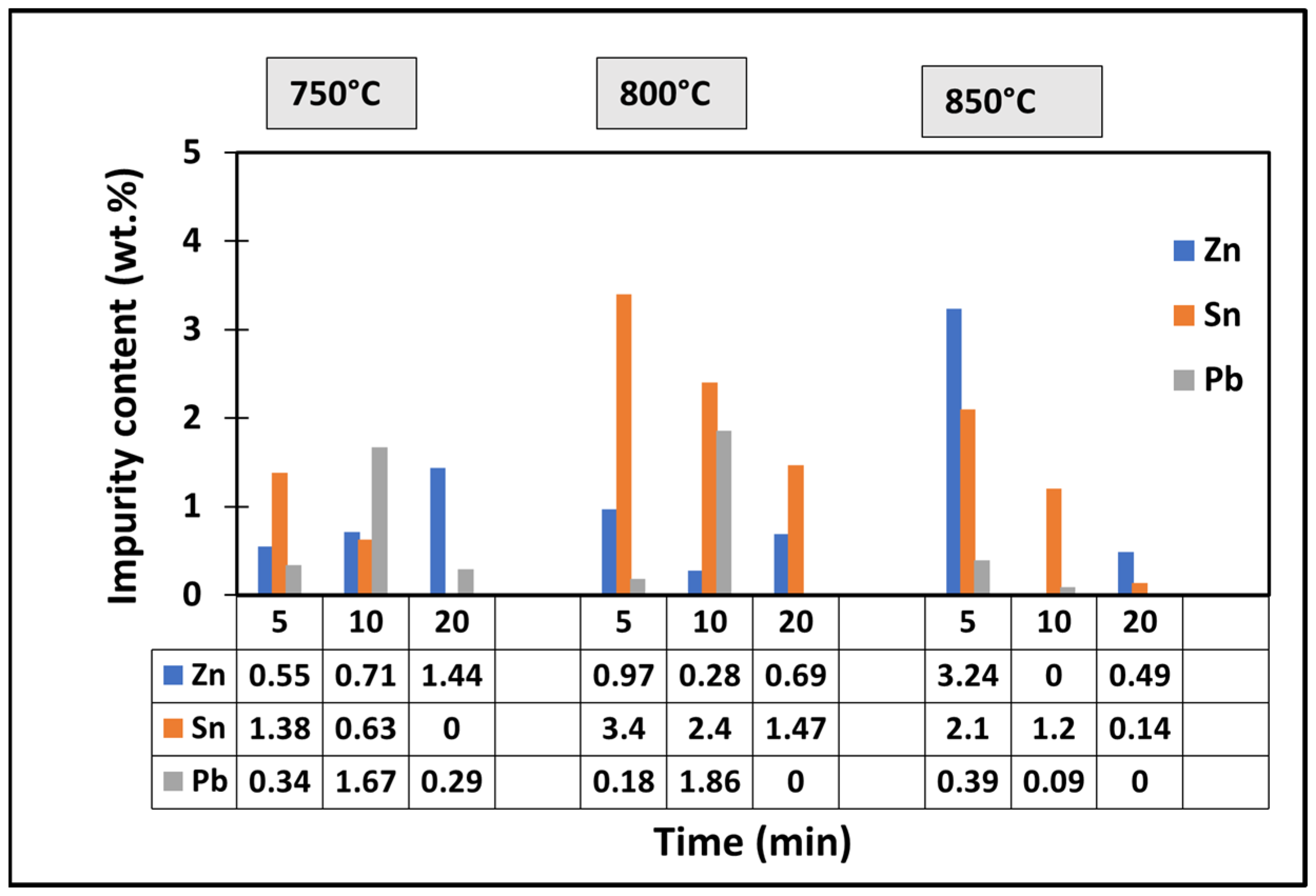
4.4.4. Chemical Composition of Copper Foils
5. Discussion
5.1. Pre-Treatment Steps
5.2. Exfoliation Technique
5.3. Choice of Operational Parameters
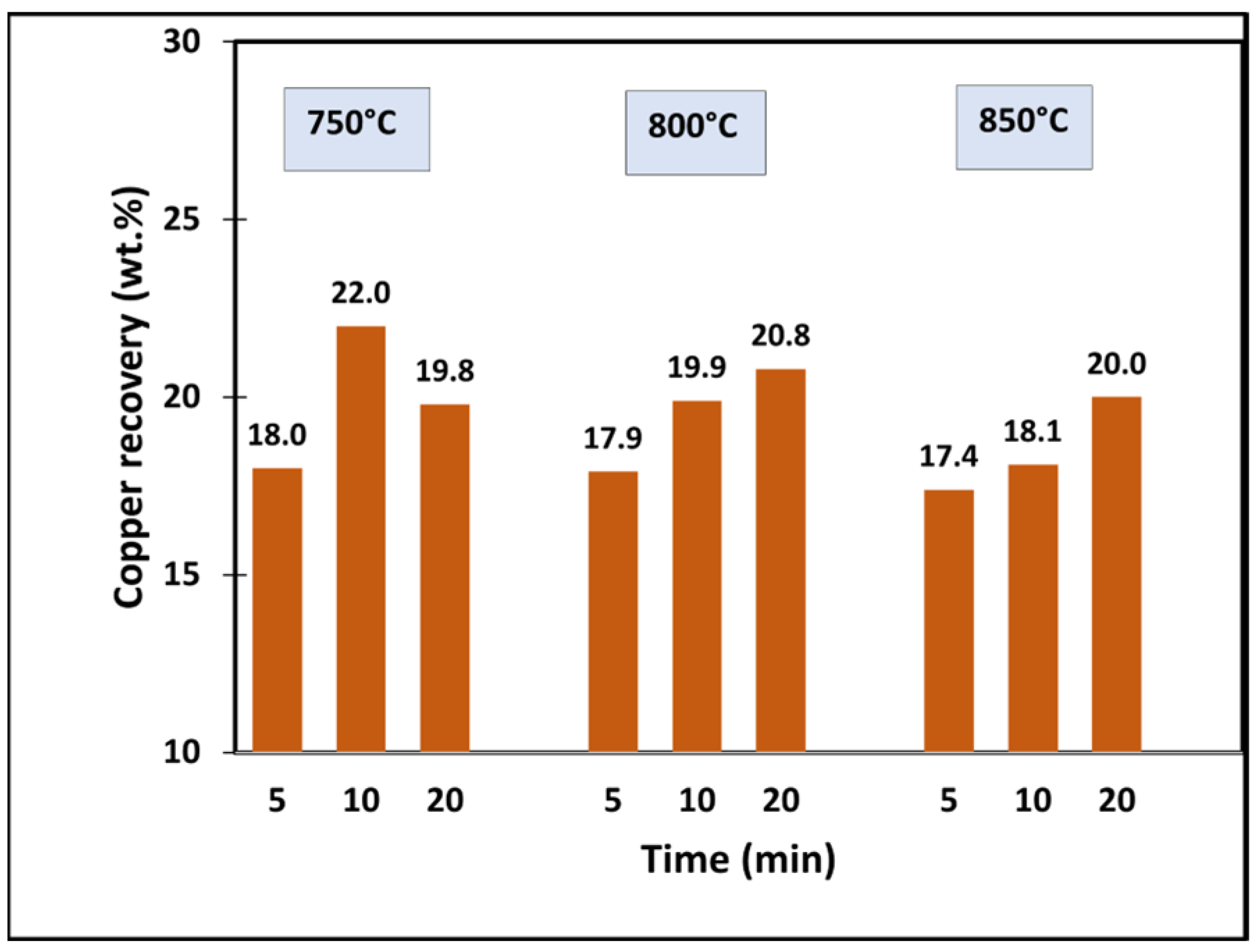
5.4. Copper Recovery
5.5. Economic Impact and Future Directions
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forecast Number of Mobile Users Worldwide from 2020 to 2025. 2021. Available online: https://www.statista.com/statistics/218984/number-of-global-mobile-users-since-2010/ (accessed on 6 June 2024).
- Forecast Number of Mobile Devices Worldwide from 2020 to 2025 (in Billions). 2021. Available online: https://www.statista.com/statistics/245501/multiple-mobile-device-ownership-worldwide/ (accessed on 7 June 2024).
- Tansel, B. From Electronic Consumer Products to E-Wastes: Global Outlook, Waste Quantities, Recycling Challenges. Environ. Int. 2017, 98, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; United Nations University: Bonn, Germany, 2020. [Google Scholar]
- Baldé, C.P.; van den, B.S.; Forti, V.; van der, S.A.; Hopstaken, F. The Dutch WEEE Flows 2020: What Happened between 2010 and 2018; United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR): Tokyo, Japan, 2020. [Google Scholar]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- Duran, J.A.G.; Arroyo, Z.G.; Gómez Castro, F.I.; Owen, P.Q.; Cadena, L.E.S.; Gómez, M.V.A. Evaluation of the effect of physical and chemical factors in the recovery of Cu, Pb and Fe from waste PCB through acid leaching. Heliyon 2023, 9, e21348. [Google Scholar] [CrossRef] [PubMed]
- Segura-Bail’, B.; Lapidus, G.T. Selective recovery of copper contained in waste PCBs from cell phones with impurity inhibition in the citrate-phosphate system. Hydrometallurgy 2021, 203, 105699. [Google Scholar] [CrossRef]
- Nagarajan, N.; Panchatcharam, P. Cost-Effective and Eco-Friendly Copper Recovery from Waste Printed Circuit Boards Using Organic Chemical Leaching. Heliyon 2023, 9, e13806. [Google Scholar] [CrossRef] [PubMed]
- Esyra, K.; Ishak, H.K.; Ismail, S.; Irfan, M.; Razak, B.A. Recovery of copper and valuable metals from E-waste via hydrometallurgical method. J. Mater. Today 2022, 66, 3077–3081. [Google Scholar]
- Faraji, F.; Golmohammadzadeh, R.; Pickles, C.A. Potential and current practices of recycling waste printed circuit boards: A review of the recent progress in pyrometallurgy. J. Environ. Manag. 2022, 316, 115242. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, S.; Lee, J.Y.; Park, Y.K. Pyrolysis Reaction Pathways of Waste Epoxy-Printed Circuit Board. Environ. Eng. Sci. 2013, 30, 706–712. [Google Scholar] [CrossRef]
- Chen, W.; Shu, Y.; Li, Y.; Chen, Y.; Wei, J. Co-Pyrolysis of Waste Printed Circuit Boards with Iron Compounds for Br-Fixing and Material Recovery. Environ. Sci. Pollut. Res. 2021, 28, 64642–64651. [Google Scholar] [CrossRef]
- Guo, J.; Guo, J.; Xu, Z. Recycling of Non-Metallic Fractions from Waste Printed Circuit Boards: A Review. J. Hazard. Mater. 2009, 168, 567–590. [Google Scholar] [CrossRef]
- Rajagopal, R.R.; Aravinda, L.S.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Activated Carbon Derived from Non-Metallic Printed Circuit Board Waste for Supercapacitor Application. Electrochim. Acta 2016, 211, 488–498. [Google Scholar] [CrossRef]
- Manikandan, S.; Inbakandan, D.; Valli Nachiyar, C.; Karthick Raja Namasivayam, S. Towards Sustainable Metal Recovery from E-Waste: A Mini Review. Sustain. Chem. Environ. 2023, 2, 100001. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in Sustainable Approaches to Recover Metals from E-Waste-A Review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Williams, P.T. Valorization of Printed Circuit Boards from Waste Electrical and Electronic Equipment by Pyrolysis. Waste Biomass Valoriz. 2010, 1, 107–120. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chou, S.L.; Lo, S.L. Gold Recovery from Waste Printed Circuit Boards of Mobile Phones by Using Microwave Pyrolysis and Hydrometallurgical Methods. Sustain. Environ. Res. 2022, 32, 6. [Google Scholar] [CrossRef]
- Cayumil, R.; Khanna, R.; Ikram-Ul-Haq, M.; Rajarao, R.; Hill, A.; Sahajwalla, V. Generation of Copper Rich Metallic Phases from Waste Printed Circuit Boards. Waste Manag. 2014, 34, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, A.; Engström, F.; Samuelsson, C.; Björkman, B.; Pettersson, J. Large-Scale WEEE Recycling Integrated in an Ore-Based Cu-Extraction System. J. Sustain. Metall. 2018, 4, 222–232. [Google Scholar] [CrossRef]
- Khanna, R. Global Perspectives on Regulatory Measures and Collection Procedures for Electronic Waste: A Critical Assessment. Open Access J. Waste Manag. Xenobiot. 2018, 1, 000101. [Google Scholar] [CrossRef]
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic Waste Management Approaches: An Overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef]
- Sthiannopkao, S.; Wong, M.H. Handling E-Waste in Developed and Developing Countries: Initiatives, Practices, and Consequences. Sci. Total Environ. 2013, 463–464, 1147–1153. [Google Scholar] [CrossRef]
- Choubey, P.K.; Panda, R.; Jha, M.K.; Lee, J.C.; Pathak, D.D. Recovery of Copper and Recycling of Acid from the Leach Liquor of Discarded Printed Circuit Boards (PCBs). Sep. Purif. Technol. 2015, 156, 269–275. [Google Scholar] [CrossRef]
- Cayumil, R. Recycling of Electronic Waste for the Recovery of Value-Added Materials. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2016. [Google Scholar]
- Patel, A.; Kumar, S.; Awasthi, A. Hydrometallurgy: Urban mining of E-waste and its future implications. In Global E-Waste Management Strategies and Future Implications; Arya, S., Kumar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 6, pp. 105–123. ISBN 9780323999199. [Google Scholar] [CrossRef]
- Xua, Y.; Lia, J.; Liu, L. Current status and future perspective of recycling copper by hydrometallurgy from waste printed circuit boards. Procedia Environ. Sci. 2016, 31, 162–170. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Xu Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Adhapure, N.N.; Dhakephalkar, P.K.; Dhakephalkar, A.P.; Tembhurkar, V.R.; Rajgure, A.V.; Deshmukh, A.M. Use of large pieces of printed circuit boards for bioleaching to avoid ‘precipitate contamination problem’ and to simplify overall metal recovery. MethodX 2014, 1, e181–e186. [Google Scholar] [CrossRef] [PubMed]
- Jujun, R.; Jie, Z.; Jian, H.; Zhang, J. A Novel Designed Bioreactor for Recovering Precious Metals from Waste Printed Circuit Boards. Sci. Rep. 2015, 5, 13481. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Sun, S.; Zhong, S.; Dai, W.; Liu, J.; Song, W. Using vacuum pyrolysis and mechanical processing for recycling waste printed circuit boards. J. Hazard. Mater. 2019, 177, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Luo, Z.; Zhai, X. Experimental study on metal recycling from waste PCB. In Proceedings of the International Conference on Sustainable Solid Waste Management 2007, Chennai, India, 5–7 September 2007; pp. 155–162. [Google Scholar]
- Zhan, L.; Xu, Z. Separating and Recycling Metals from Mixed Metallic Particles of Crushed Electronic Wastes by Vacuum Metallurgy. Environ. Sci. Technol. 2009, 43, 7074–7078. [Google Scholar] [CrossRef] [PubMed]
- Cayumil, R.; Ikram-Ul-Haq, M.; Khanna, R.; Saini, R.; Mukherjee, P.S.; Mishra, B.K.; Sahajwalla, V. High Temperature Investigations on Optimising the Recovery of Copper from Waste Printed Circuit Boards. Waste Manag. 2018, 73, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.R.; Zhang, F.S. Recovery of Copper and Lead from Waste Printed Circuit Boards by Supercritical Water Oxidation Combined with Electrokinetic Process. J. Hazard. Mater. 2009, 165, 1002–1007. [Google Scholar] [CrossRef]
- Silvas, F.P.C.; Jiménez Correa, M.M.; Caldas, M.P.K.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Printed Circuit Board Recycling: Physical Processing and Copper Extraction by Selective Leaching. Waste Manag. 2015, 46, 503–510. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Imre-Lucaci, Á.; Ilea, P. Copper Recovery and Gold Enrichment from Waste Printed Circuit Boards by Mediated Electrochemical Oxidation. J. Hazard. Mater. 2014, 273, 215–221. [Google Scholar] [CrossRef]
- Cayumil, R.; Khanna, R.; Rajarao, R.; Ikram-ul-Haq, M.; Mukherjee, P.S.; Sahajwalla, V. Environmental Impact of Processing Electronic Waste—Key Issues and Challenges. In E-Waste in Transition—From Pollution to Resource; InTech: London, UK, 2016. [Google Scholar]
- Mckay, G. Dioxin Characterisation, Formation and Minimisation during Municipal Solid Waste (MSW) Incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Yu, X.; Zhang, H.; Han, M. Multilayer flexible electronics: Manufacturing approaches and applications. Mater. Today Physics. 2022, 23, 100647. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Lenny Koh, S.C.; Rosa, P. Recycling of WEEEs: An Economic Assessment of Present and Future E-Waste Streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Daware, S.; Chandel, S.; Rai, B. A Machine Learning Framework for Urban Mining: A Case Study on Recovery of Copper from Printed Circuit Boards. Miner. Eng. 2022, 180, 107479. [Google Scholar] [CrossRef]
- Mesquita, R.A.; Silva, R.A.F.; Majuste, D. Chemical Mapping and Analysis of Electronic Components from Waste PCB with Focus on Metal Recovery. Process Saf. Environ. Prot. 2018, 120, 107–117. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of Metals and Nonmetals from Electronic Waste by Physical and Chemical Recycling Processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Mukherjee, P.S.; Park, M. A Critical Assessment on Resource Recovery from Electronic Waste: Impact of Mechanical Pre-Treatment. J. Clean. Prod. 2020, 268, 122319. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z. Disposing and Recycling Waste Printed Circuit Boards: Disconnecting, Resource Recovery, and Pollution Control. Environ. Sci. Technol. 2015, 49, 721–733. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of Waste Printed Circuit Boards through Pyrometallurgical Processing: A Review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Xu, Z. An Environmentally Friendly Technology of Disassembling Electronic Components from Waste Printed Circuit Boards. Waste Manag. 2016, 53, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Maurice, A.A.; Dinh, K.N.; Charpentier, N.M.; Brambilla, A.; Gabriel, J.C.P. Dismantling of Printed Circuit Boards Enabling Electronic Components Sorting and Their Subsequent Treatment Open Improved Elemental Sustainability Opportunities. Sustainability 2021, 13, 10357. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, L.; Li, Y.T.; Wu, Y.; Ma, Y.X.; Yang, B. Copper Leaching from Waste Printed Circuit Boards Using Typical Acidic Ionic Liquids Recovery of E-Wastes’ Surplus Value. Waste Manag. 2018, 78, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Iseli, C.; Gaydardzhiev, S.; Streicher-Porte, M.; Weh, A. Electro Dynamic Fragmentation of Printed Wiring Boards as a Preparation Tool for Their Recycling. Miner. Eng. 2017, 107, 20–26. [Google Scholar] [CrossRef]
- Veit, H.M.; Pereira, C.C.; Hörlle, L.F.; Bernardes, A.M. Recycling of metals from PCB by dense medium separation process. In Recycling and Waste Treatment in Mineral and Metal Processing: Technical and Economic Aspects; June: Luleå, Sweden, 2002; pp. 16–20. [Google Scholar]
- Zhang, S.; Forssberg, E. Mechanical Separation-Oriented Characterization of Electronic Scrap. Resour. Conserv. Recycl. 1997, 21, 247–269. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of Metals from Electronic Waste by Physical and Chemical Recycling Processes. Int. Sch. Sci. Res. Innov. 2016, 10, 259–270. [Google Scholar] [CrossRef]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-26592-2. [Google Scholar] [CrossRef]
- Sander, S.; Schubert, G.; Jäckel, H.G. The Fundamentals of the Comminution of Metals in Shredders of the Swing-Hammer Type. Int. J. Miner. Process. 2004, 74, S385–S393. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kishore, S.; Vasireddi, R.; Mahapatra, D.R.; Ajayan, P.M.; Chattopadhyay, K. Electronic Waste Recycling via Cryo-Milling and Nanoparticle Beneficiation. Mater. Today 2017, 20, 67–73. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Ye, B.; Qian, W. Recycling of Printed Circuit Boards by Abrasive Waterjet Cutting. Process Saf. Environ. Prot. 2021, 148, 805–812. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Ruan, J.; Xu, Z. Assessment of Noise and Heavy Metals (Cr, Cu, Cd, Pb) in the Ambience of the Production Line for Recycling Waste Printed Circuit Boards. Environ. Sci. Technol. 2012, 46, 494–499. [Google Scholar] [CrossRef]
- Saini, R.; Khanna, R.; Dutta, R.K.; Cayumil, R.; Ikram-Ul-Haq, M.; Agarwala, V.; Ellamparuthy, G.; Jayasankar, K.; Mukherjee, P.S.; Sahajwalla, V. A Novel Approach for Reducing Toxic Emissions during High Temperature Processing of Electronic Waste. Waste Manag. 2017, 64, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Saini, R. Novel Approaches for Processing Waste Printed Circuit Boards. Ph.D. Thesis, Indian Institute of Technology, Roorkee, India, 2018. [Google Scholar]
- Liu, M.; Huang, J.; Meng, H.; Liu, C.; Chen, Z.; Yang, H.; Feng, Z.; Li, X.; Luo, R.; Huang, Z.; et al. A novel approach to prepare graphite nanoplatelets exfoliated by three-roll milling in phenolic resin for low-carbon MgO-C refractories. J. Eur. Ceram. Soc. 2023, 43, 4198–4208. [Google Scholar] [CrossRef]
- Alzate, A.; López, M.E.; Serna, C. Recovery of gold from waste electrical and electronic equipment (WEEE) using ammonium persulfate. Waste Manag. 2016, 57, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.D.; Ilankoon, I.M.S.K.; Chong, M.N.; Wu, T.Y. Exfoliation of coarse printed circuit boards using dimethylacetamide: Production of copper concentrates. Miner. Eng. 2023, 191, 107963. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, H.; Sippel, B. Mining discarded electronics. UNEP Ind. Environ. 1994, 17, 7–11. [Google Scholar]
- Zhan, Z.; Qiu, K. Pyrolysis kinetics and tg-ftir analysis of waste epoxy printed circuit boards. J. Cent. S. Univ. 2011, 18, 331–336. [Google Scholar] [CrossRef]
- Mairizal, A.Q.; Sembada, A.Y.; Tse, K.M.; Haque, N.; Rhamdhani, M.A. Techno-economic analysis of waste PCB recycling in Australia. Resour. Conserv. Recycl. 2023, 190, 106784. [Google Scholar] [CrossRef]
- Copper Price (USD/Kilogram) Chart for the Last 10 Years. Available online: https://www.dailymetalprice.com/metalpricecharts.php?c=cu&u=kg&d=24 (accessed on 12 July 2024).


| Temperature, Time | Sn | Zn | Sb | Cu | Pb |
|---|---|---|---|---|---|
| 750 °C, 5 min | 14.63 | 0.03 | 0.04 | 85.30 | <0.01 |
| 800 °C, 5 min | 11.50 | 0.19 | 0.27 | 88.04 | <0.01 |
| 850 °C, 5 min | 7.05 | 0.68 | 0.02 | 92.24 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjana, E.I.; Jayasankar, K.; Khanna, R.; Venkatesan, J.; Konyukhov, Y.V.; Mukherjee, P.S. Low Temperature Pyrolysis and Exfoliation of Waste Printed Circuit Boards: Recovery of High Purity Copper Foils. Sustainability 2024, 16, 6269. https://doi.org/10.3390/su16156269
Anjana EI, Jayasankar K, Khanna R, Venkatesan J, Konyukhov YV, Mukherjee PS. Low Temperature Pyrolysis and Exfoliation of Waste Printed Circuit Boards: Recovery of High Purity Copper Foils. Sustainability. 2024; 16(15):6269. https://doi.org/10.3390/su16156269
Chicago/Turabian StyleAnjana, Elanjikkal Indran, Kalidoss Jayasankar, Rita Khanna, Jayapalan Venkatesan, Yury V. Konyukhov, and Partha Sarathy Mukherjee. 2024. "Low Temperature Pyrolysis and Exfoliation of Waste Printed Circuit Boards: Recovery of High Purity Copper Foils" Sustainability 16, no. 15: 6269. https://doi.org/10.3390/su16156269
APA StyleAnjana, E. I., Jayasankar, K., Khanna, R., Venkatesan, J., Konyukhov, Y. V., & Mukherjee, P. S. (2024). Low Temperature Pyrolysis and Exfoliation of Waste Printed Circuit Boards: Recovery of High Purity Copper Foils. Sustainability, 16(15), 6269. https://doi.org/10.3390/su16156269






