Green Synthesis of Pure Superparamagnetic Fe3O4 Nanoparticles Using Shewanella sp. in a Non-Growth Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Source and Maintenance Conditions
2.2. Preparation and Characterization of Precursor Iron Oxyhydroxide
2.3. Preparation of Inoculum
2.4. Reduction of Iron Oxide
2.5. Estimation of Lactate and Soluble Fe2+ Ion Concentrations
2.6. Characterization of MNPs
3. Results
3.1. Characterization of FeOOH Precursor Particles
3.2. Culture Characteristics
3.3. Characterization of Magnetic Products
4. Discussion
- It eschews the temperature control requirements for psychrophilic/thermophilic species.
- Since S. putrefaciens CN-32 is a facultative anaerobe, greater quantities of pre-inoculum cells can be prepared quickly under aerobic conditions before the bioreduction process, which would increase the quantity of iron oxide/hydroxides that can be reduced in a batch and reduce overall production time.
- The reaction is carried out in plain saline. Thus, impurities (from growth media) that may affect the crystal structure of the reduction products can be avoided. Hence, pure magnetite crystals can be produced without undesired side products. Moreover, no toxic waste is generated.
- The MNPs obtained are superparamagnetic with saturation magnetization comparable to that of other biologically and chemically synthesized MNPs [40], making them ideal for biomedical applications.
- This system also permits easy experimentation to study the effects of dopants on the crystal structure of biomagnetites, which can be tailored for use in different fields such as catalysis and bioremediation.
- Furthermore, since Shewanella spp. are capable of using more than 10 different metals as terminal electron acceptors for growth [41], this system can be expanded to produce bimetallic and higher mixed metallic nanoparticles (including ferrites).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional Magnetic Iron Oxide Nanoparticles: Diverse Synthetic Approaches, Surface Modifications, Cytotoxicity towards Biomedical and Industrial Applications. BMC Mat. 2019, 1, 2. [Google Scholar] [CrossRef]
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent Magnetic Nanoparticle Engineering: The Role of Structure-Property Relationship in Advanced Applications. Mater. Today Chem. 2022, 26, 101220. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest Disease Inhibition in Fruit by Synthesis and Characterization of Chitosan Iron Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Koo, K.N.; Ismail, A.F.; Othman, M.H.D.; Bidin, N.; Rahman, M.A. Preparation and Characterization of Superparamagnetic Magnetite (Fe3O4) Nanoparticles: A Short Review. Malaysian, J. Fundam. Appl. Sci. 2019, 15, 23–31. [Google Scholar] [CrossRef]
- Campaña, A.L.; Saragliadis, A.; Mikheenko, P.; Linke, D. Insights into the Bacterial Synthesis of Metal Nanoparticles. Front. Nanotechnol. 2023, 5, 1216921. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ullah, F.; Majeed, I.; Ayaz, A.; Hussain Munis, M.F. Organometallic Assembling of Chitosan-Iron Oxide Nanoparticles with Their Antifungal Evaluation Against Rhizopus oryzae. Appl. Organomet. Chem. 2019, 33, e5190. [Google Scholar] [CrossRef]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.D.; Ge, H.; Hu, Y. Top-Down Fabrication of Shape-Controlled, Monodisperse Nanoparticles for Biomedical Applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Carmona, M.; Poblete-Castro, I.; Rai, M.; Turner, R.J. Opportunities and Obstacles in Microbial Synthesis of Metal Nanoparticles. Microb. Biotechnol. 2023, 16, 871–876. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Liu, S.; Du, L.; Chen, Q.; Li, X.; Dong, J.; Zhang, Z.; Lu, S.; Gong, Y.; et al. Insights into the Synthesis, Types and Application of Iron Nanoparticles: The Overlooked Significance of Environmental Effects. Environ. Int. 2022, 158, 106980. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Silva, V.C.; Valladão, D.M.S.; Souto, R.S. Biosynthesis of Magnetic Iron Oxide Nanoparticles: A Review. Biotechnol. Lett. 2021, 43, 1–12. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological Synthesis of Metal Nanoparticles by Microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Atalah, J.; Espina, G.; Blamey, L.; Muñoz-Ibacache, S.A.; Blamey, J.M. Advantages of Using Extremophilic Bacteria for the Biosynthesis of Metallic Nanoparticles and its Potential for Rare Earth Element Recovery. Front. Microbiol. 2022, 13, 855077. [Google Scholar] [CrossRef]
- Sun, J.-B.; Zhao, F.; Tang, T.; Jiang, W.; Tian, J.; Li, Y.; Li, J.L. High-Yield Growth and Magnetosome Formation by Magnetospirillum gryphiswaldense MSR-1 in an Oxygen-Controlled Fermentor Supplied Solely with Air. Appl. Microbiol. Biotechnol. 2008, 79, 389. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Esther, J.; Sukla, L.B.; Pradhan, N.; Panda, S. Fe (III) Reduction Strategies of Dissimilatory Iron Reducing Bacteria. Korean J. Chem. Eng. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Abhilash; Revati, K.; Pandey, B.D. Microbial Synthesis of Iron-Based Nanomaterials—A Review. Bull. Mater. Sci. 2011, 34, 191–198. [Google Scholar] [CrossRef]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and Characterization of Metal-Reducing Thermoanaerobacter Strains from Deep Subsurface Environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S. Biogenic Iron Mineralization Accompanying the Dissimilatory Reduction of Hydrous Ferric Oxide by a Groundwater Bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Z.; Yi, X.; Tang, J.; Feng, C.; Dang, Z. Biogenic Iron Mineralization of Polyferric Sulfate by Dissimilatory Iron Reducing Bacteria: Effects of Medium Composition and Electric Field Stimulation. Sci. Total Environ. 2019, 684, 466–475. [Google Scholar] [CrossRef]
- Yang, J.; Ju, P.; Dong, X.; Duan, J.; Xiao, H.; Tang, X.; Zhai, X.; Hou, B. Green Synthesis of Functional Metallic Nanoparticles by Dissimilatory Metal-Reducing Bacteria “Shewanella”: A Comprehensive Review. J. Mater. Sci. Technol. 2023, 158, 63–76. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 49, pp. 219–286. [Google Scholar]
- Lovley, D.R.; Phillips, E.J.P. Organic Matter Mineralization with Reduction of Ferric Iron in Anaerobic Sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. A Serum Bottle Modification of the Hungate Technique for Cultivating Obligate Anaerobes. Appl. Microbiol. 1974, 27, 985–987. [Google Scholar] [CrossRef]
- Kim, G.T.; Hyun, M.S.; Chang, I.S.; Kim, H.J.; Park, H.S.; Kim, B.H.; Kim, S.D.; Wimpenny, J.W.T.; Weightman, A.J. Dissimilatory Fe(III) Reduction by an Electrochemically Active Lactic Acid Bacterium Phylogenetically Related to Enterococcus gallinarum Isolated from Submerged Soil. J. Appl. Microbiol. 2005, 99, 978–987. [Google Scholar] [CrossRef]
- Salas, E.C.; Berelson, W.M.; Hammond, D.E.; Kampf, A.R.; Nealson, K.H. The Impact of Bacterial Strain on the Products of Dissimilatory Iron Reduction. Geochim. Cosmochim. Acta 2010, 74, 574–583. [Google Scholar] [CrossRef]
- Moon, J.-W.; Rawn, C.J.; Rondinone, A.J.; Love, L.J.; Roh, Y.; Everett, S.M.; Lauf, R.J.; Phelps, T.J. Large-Scale Production of Magnetic Nanoparticles Using Bacterial Fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef]
- Byrne, J.M.; Muhamadali, H.; Coker, V.S.; Cooper, J.; Lloyd, J.R. Scale-up of the Production of Highly Reactive Biogenic Magnetite Nanoparticles Using Geobacter sulfurreducens. J. R. Soc. Interface 2015, 12, 20150240. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Phelps, T.J.; Cole, D.R.; Horita, J.; Fortier, S.M.; Elless, M.; Valley, J.W. Physiochemical, Mineralogical, and Isotopic Characterization of Magnetite-Rich Iron Oxides Formed by Thermophilic Iron-Reducing Bacteria. Geochim. Cosmochim. Acta 1997, 61, 4621–4632. [Google Scholar] [CrossRef]
- Roh, Y.; Zhang, C.-L.; Vali, H.; Lauf, R.J.; Zhou, J.; Phelps, T.J. Biogeochemical and Environmental Factors in Fe Biomineralization: Magnetite and Siderite Formation. Clays Clay Miner. 2003, 51, 83–95. [Google Scholar] [CrossRef]
- Shimizu, M.; Zhou, J.; Schröder, C.; Obst, M.; Kappler, A.; Borch, T. Dissimilatory Reduction and Transformation of Ferrihydrite-Humic Acid Coprecipitates. Environ. Sci. Technol. 2013, 47, 13375–13384. [Google Scholar] [CrossRef]
- Bingjie, O.; Xiancai, L.; Huan, L.; Juan, L.; Tingting, Z.; Xiangyu, Z.; Jianjun, L.; Rucheng, W. Reduction of Jarosite by Shewanella oneidensis MR-1 and Secondary Mineralization. Geochim. Cosmochim. Acta 2014, 124, 54–71. [Google Scholar] [CrossRef]
- Miot, J.; Li, J.; Benzerara, K.; Sougrati, M.T.; Ona-Nguema, G.; Bernard, S.; Jumas, J.-C.; Guyot, F. Formation of Single Domain Magnetite by Green Rust Oxidation Promoted by Microbial Anaerobic Nitrate-Dependent Iron Oxidation. Geochim. Cosmochim. Acta 2014, 139, 327–343. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on Magnetic Nanoparticle-Mediated Hyperthermia for Cancer Therapy. J. Nanoparticle Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic Fluid Hyperthermia: Focus on Superparamagnetic Iron Oxide Nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Altaf, S.; Zafar, R.; Zaman, W.Q.; Ahmad, S.; Yaqoob, K.; Syed, A.; Khan, A.J.; Bilal, M.; Arshad, M. Removal of Levofloxacin from Aqueous Solution by Green Synthesized Magnetite (Fe3O4) Nanoparticles Using Moringa olifera: Kinetics and Reaction Mechanism Analysis. Ecotoxicol. Environ. Saf. 2021, 226, 112826. [Google Scholar] [CrossRef]
- Yeary, L.W.; Moon, J.-W.; Love, L.J.; Thompson, J.R.; Rawn, C.J.; Phelps, T.J. Magnetic Properties of Biosynthesized Magnetite Nanoparticles. IEEE Trans. Magn. 2005, 41, 4384–4389. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kimber, R.L.; Singh, V.K.; Shende, S.; Behal, A.; Sushkova, S.; Mandzhieva, S.; Lloyd, J.R. Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella. Appl. Environ. Microbiol. 2021, 87, e01390-21. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Joshi, N.; Shemfe, M.; Lloyd, J.R. Life Cycle Assessment of Sustainable Raw Material Acquisition for Functional Magnetite Bionanoparticle Production. J. Environ. Manag. 2017, 199, 116–125. [Google Scholar] [CrossRef] [PubMed]
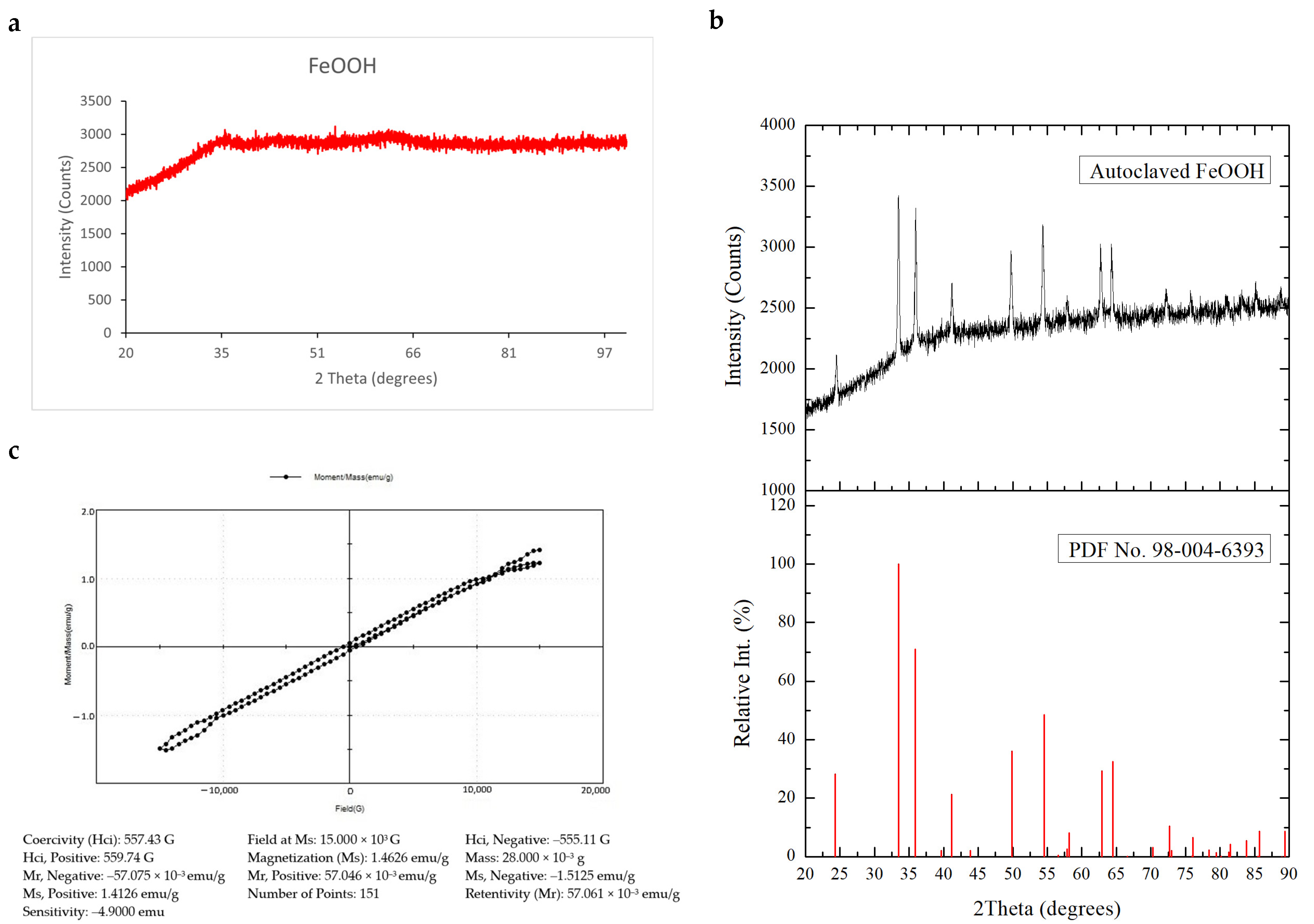
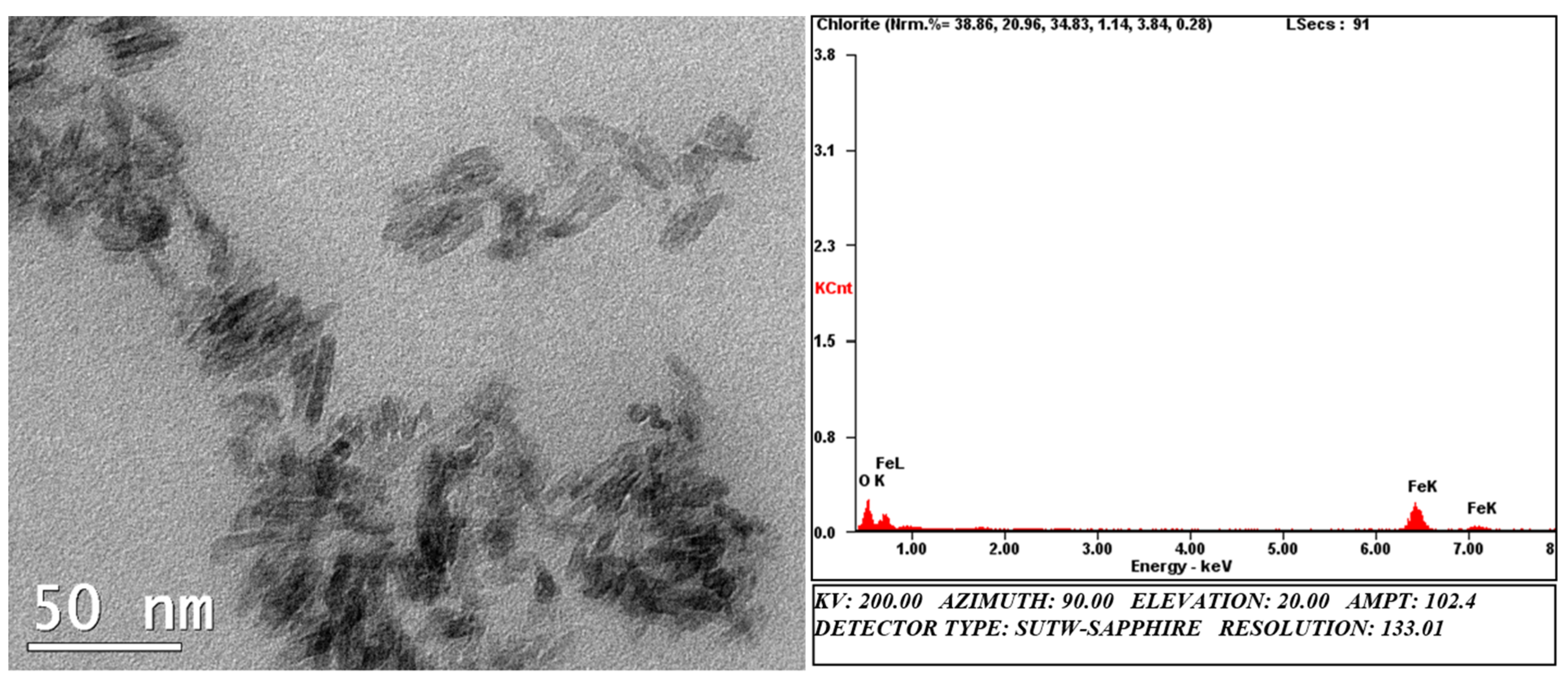
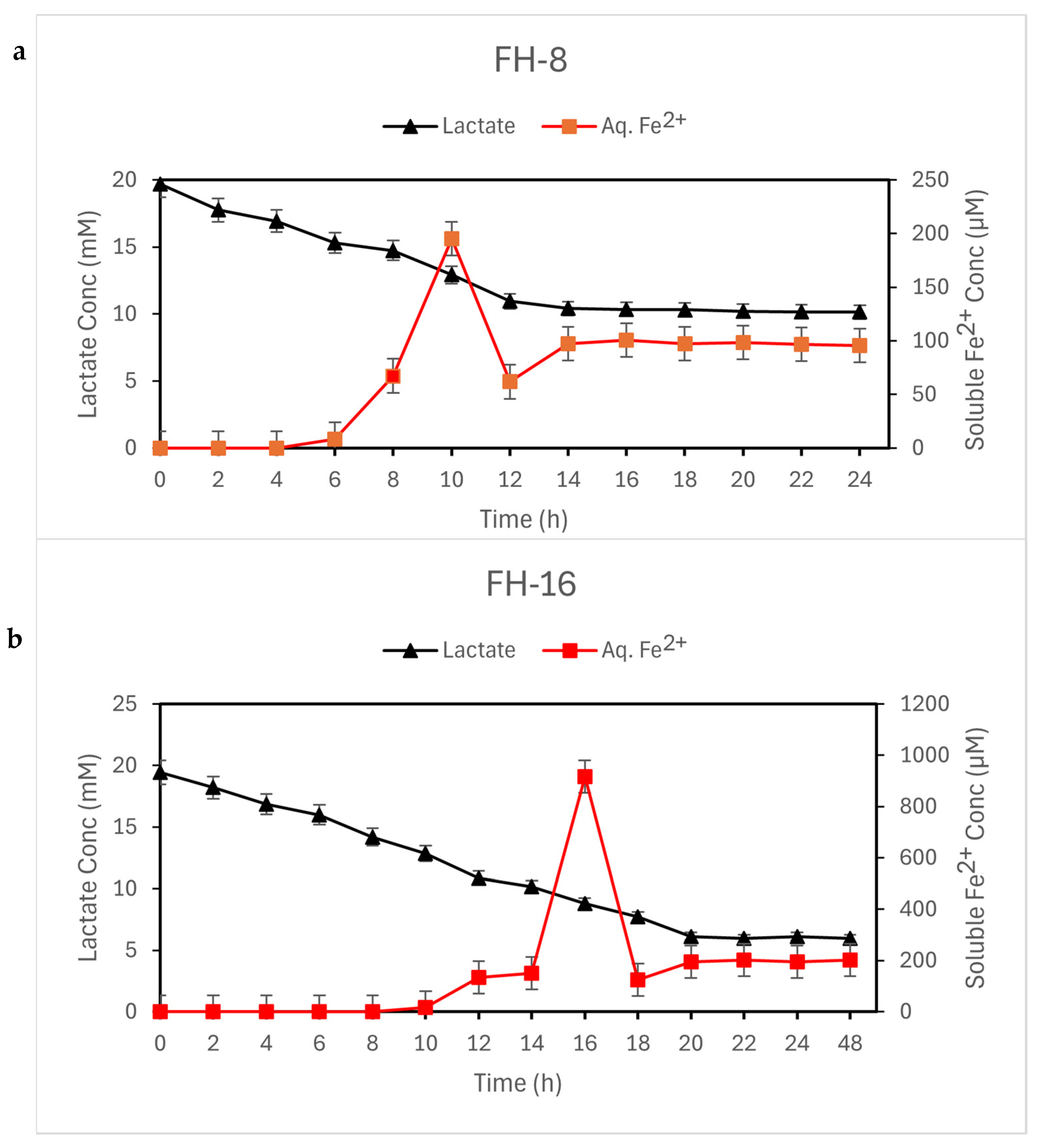



| Vial | Precursor Concentration (mM) | Inoculum Concentration (cells/mL) | Product Characteristic | Time (h) |
|---|---|---|---|---|
| FH-8 | 8 | 1 × 107 | Magnetic | 12 |
| FH-16 | 16 | 1 × 107 | Magnetic | 18 |
| FH-24 | 24 | 1 × 107 | Weakly magnetic | >1800 |
| FH-24 | 24 | 2 × 107 | Weakly magnetic | >1800 |
| Strain | Type | Product Characteristics | Total Production Time | Reference |
|---|---|---|---|---|
| Enrichment cultures | TAs a | Mixture | >2 d | [31] |
| Thermoanaerobacter ethanolicus TOR-39 | TA a | SD e magnetite | >2 d | [32] |
| Shewanella alga BrY | MFA b | Magnetite | NA | |
| Shewanella alga NV-1 | PFA c | Magnetite | >5 d | |
| Shewanella pealeana W3-7-1 | PFA c | SP f magnetite | NA | |
| Shewanella putrefaciens strain CN32 | MFA b | Mixture | >2 d | [33] |
| Shewanella oneidensis MR-1 | MFA b | Mixture | >2 d | [34] |
| Acidovorax sp. strain BoFeN1 | Anaerobe | Mixture with SD e magnetite | >2 d | [35] |
| Thermoanaerobacter sp. TOR-39 | TA a | SD e magnetite | >2 d | [29] |
| Geobacter sulfurreducens | MA d | Mixture | >2 d | [30] |
| Shewanella putrefaciens strain CN32 | MFA b | SP f magnetite | 1–2 d | This study |
| Material/Aspect | Unit Cost (USD) | Chemically Synthesized (Ref. [39]) | Green Synthesized (Ref. [39]) | This Study | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amount Used (g) | Cost (USD) | NP Yield (g) | Amount Used (g) | Cost (USD) | NP Yield (g) | Amount Used (g) | Cost (USD) | NP Yield (g) | ||
| FeCl3.6H2O | 5.76 | 19.8 | 114.04 | 0.80 | 5.3 | 30.52 | 4.55 | 5.3 | 30.52 | 4.26 |
| FeCl2.4H2O | 0.726 | 27.03 | 19.62 | 2.65 | 1.92 | - | - | |||
| NaOH | 0.15 | 3.99 | 0.59 | 3.99 | 0.59 | 2.35 | 0.35 | |||
| Nitrogen gas | 0.010/L | 500 (mL) | 0.005 | - | - | 500 (mL) | 0.005 | |||
| Lactate | 0.063 | - | - | - | - | 2.207 | 0.14 | |||
| Heating requirement | Yes | Yes | No | |||||||
| Waste generated | Spent liquor | Spent liquor | Re-usable cells and saline | |||||||
| Total Chemical Cost (USD) | 134.25 | 33.03 | 31.01 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parthasarathy, S.; Chandra, T.S. Green Synthesis of Pure Superparamagnetic Fe3O4 Nanoparticles Using Shewanella sp. in a Non-Growth Medium. Sustainability 2024, 16, 6278. https://doi.org/10.3390/su16156278
Parthasarathy S, Chandra TS. Green Synthesis of Pure Superparamagnetic Fe3O4 Nanoparticles Using Shewanella sp. in a Non-Growth Medium. Sustainability. 2024; 16(15):6278. https://doi.org/10.3390/su16156278
Chicago/Turabian StyleParthasarathy, Saranath, and T. S. Chandra. 2024. "Green Synthesis of Pure Superparamagnetic Fe3O4 Nanoparticles Using Shewanella sp. in a Non-Growth Medium" Sustainability 16, no. 15: 6278. https://doi.org/10.3390/su16156278
APA StyleParthasarathy, S., & Chandra, T. S. (2024). Green Synthesis of Pure Superparamagnetic Fe3O4 Nanoparticles Using Shewanella sp. in a Non-Growth Medium. Sustainability, 16(15), 6278. https://doi.org/10.3390/su16156278






