Effects of Gamma-Synthesized Chitosan on Morphological, Thermal, Mechanical, and Heavy-Metal Removal Properties in Natural Rubber Foam as Sustainable and Eco-Friendly Heavy Metal Sorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamma Irradiation of Chitin Powder
2.2. Preparation of Gamma-Synthesized Chitosan Powder
2.3. Preparation of Natural Rubber Foam Containing Gamma-Synthesized CS Powder
2.4. Characterization of CT and CS Powders
2.4.1. Morphology
2.4.2. Viscosity of CT and CS Powders in Acetic Acid
2.4.3. Functional Groups and Degree of Deacetylation
2.5. Characterization of NRF Samples
2.5.1. Morphology and Functional Groups
2.5.2. Density and Pore Characteristics
2.5.3. Thermal Stability
2.5.4. Compression Set and Hardness (Shore OO)
2.5.5. Heavy Metal Adsorption Measurement
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chitin and Chitosan Powders
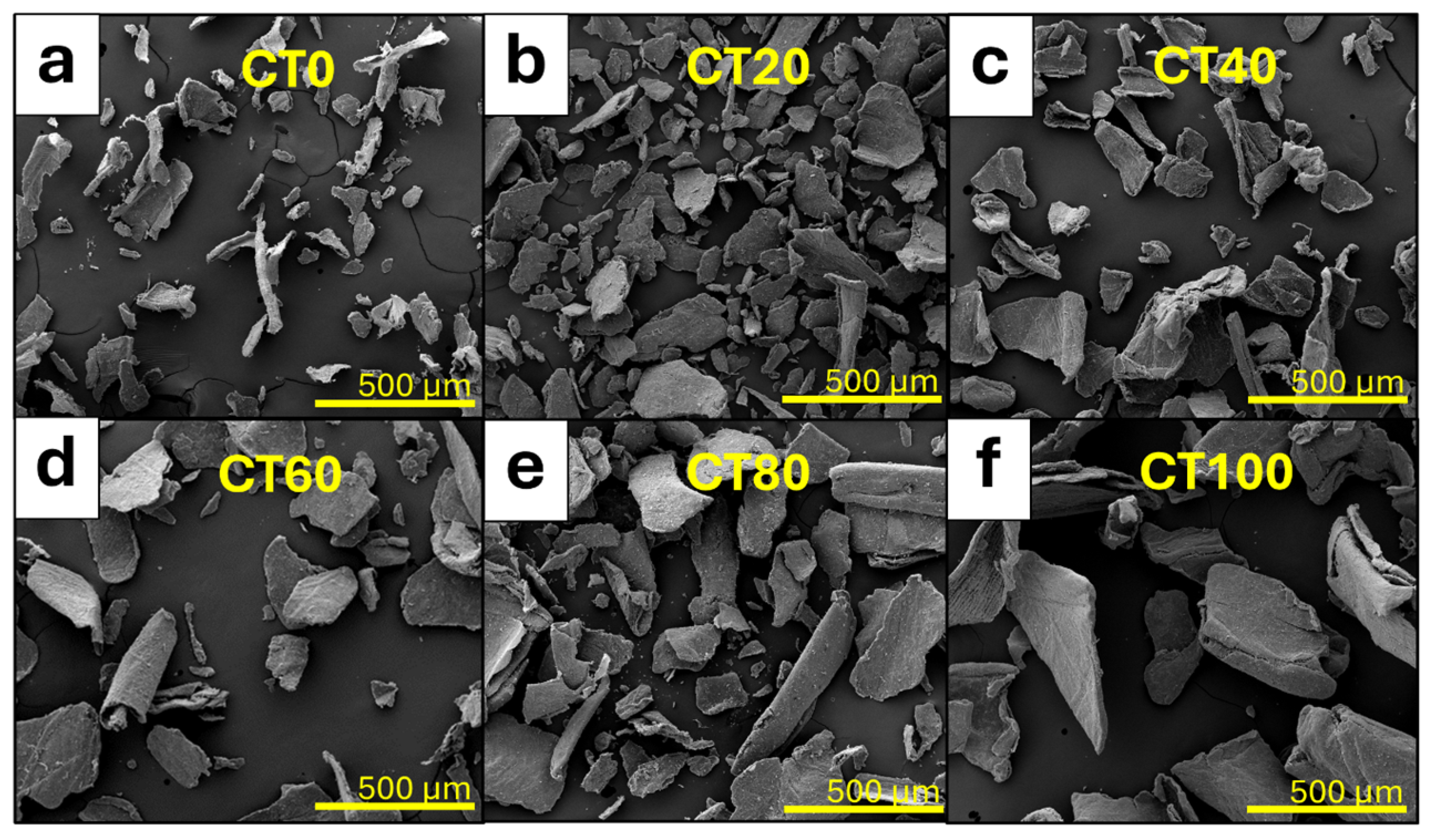
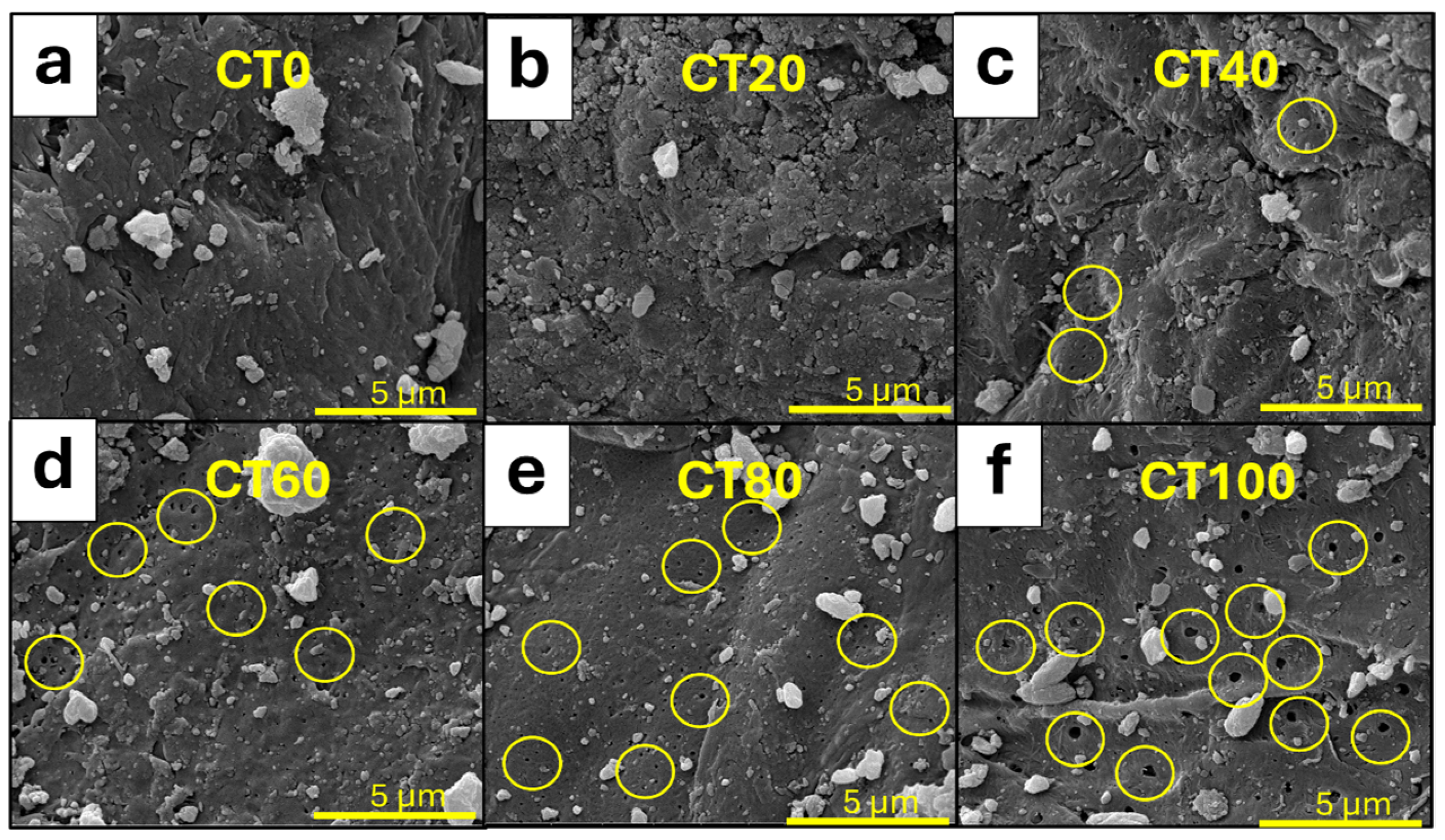
3.1.1. Morphological Properties of CT and CS Powders
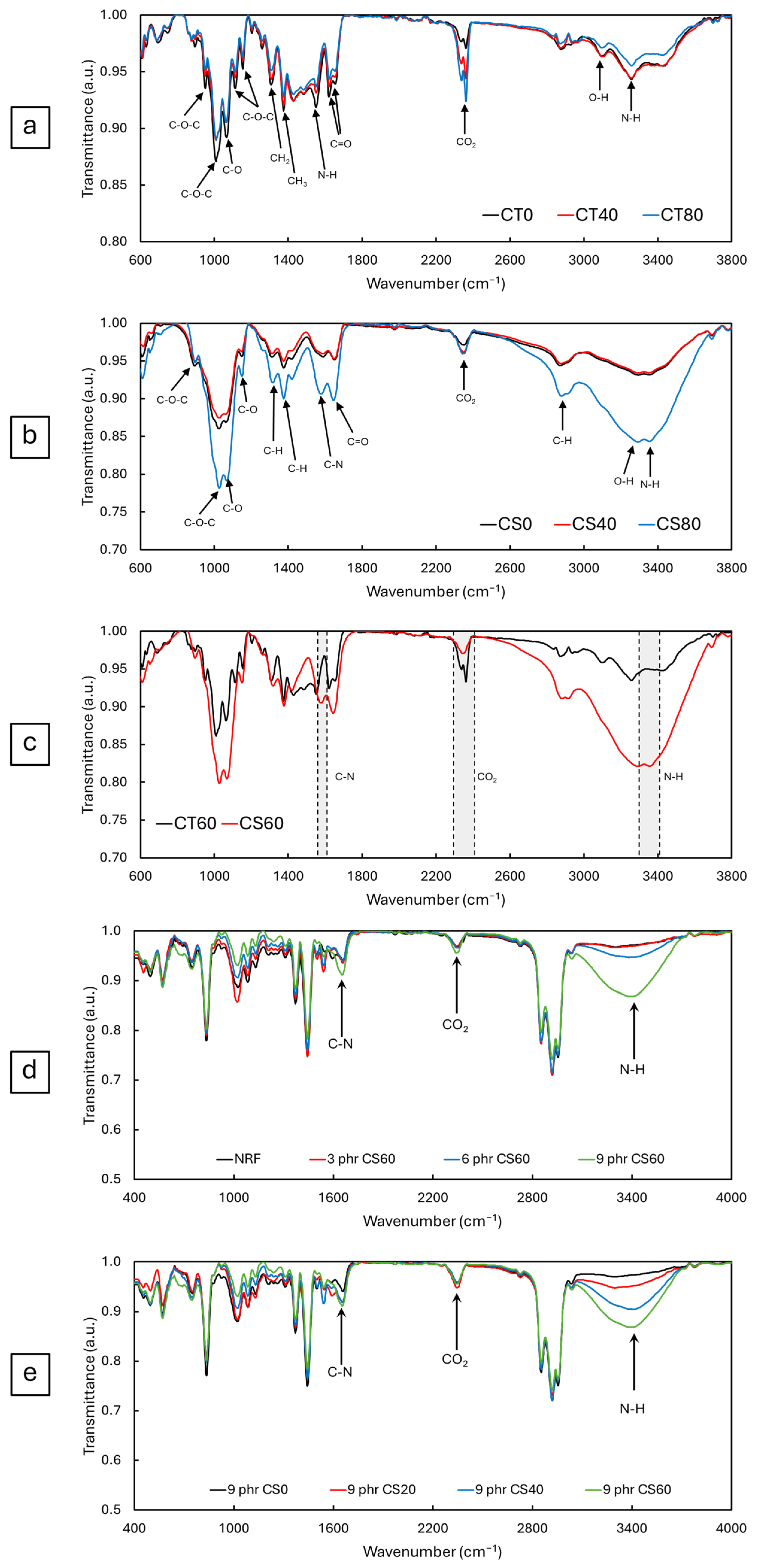
3.1.2. Functional Groups of CT and CS Powders and Viscosity of CS Powder in Acetic Acid
3.1.3. Degree of Deacetylation of CS Powder
3.2. Natural Rubber Foam Containing Chitosan Powder
3.2.1. Functional Groups of NRF Containing CS Powder
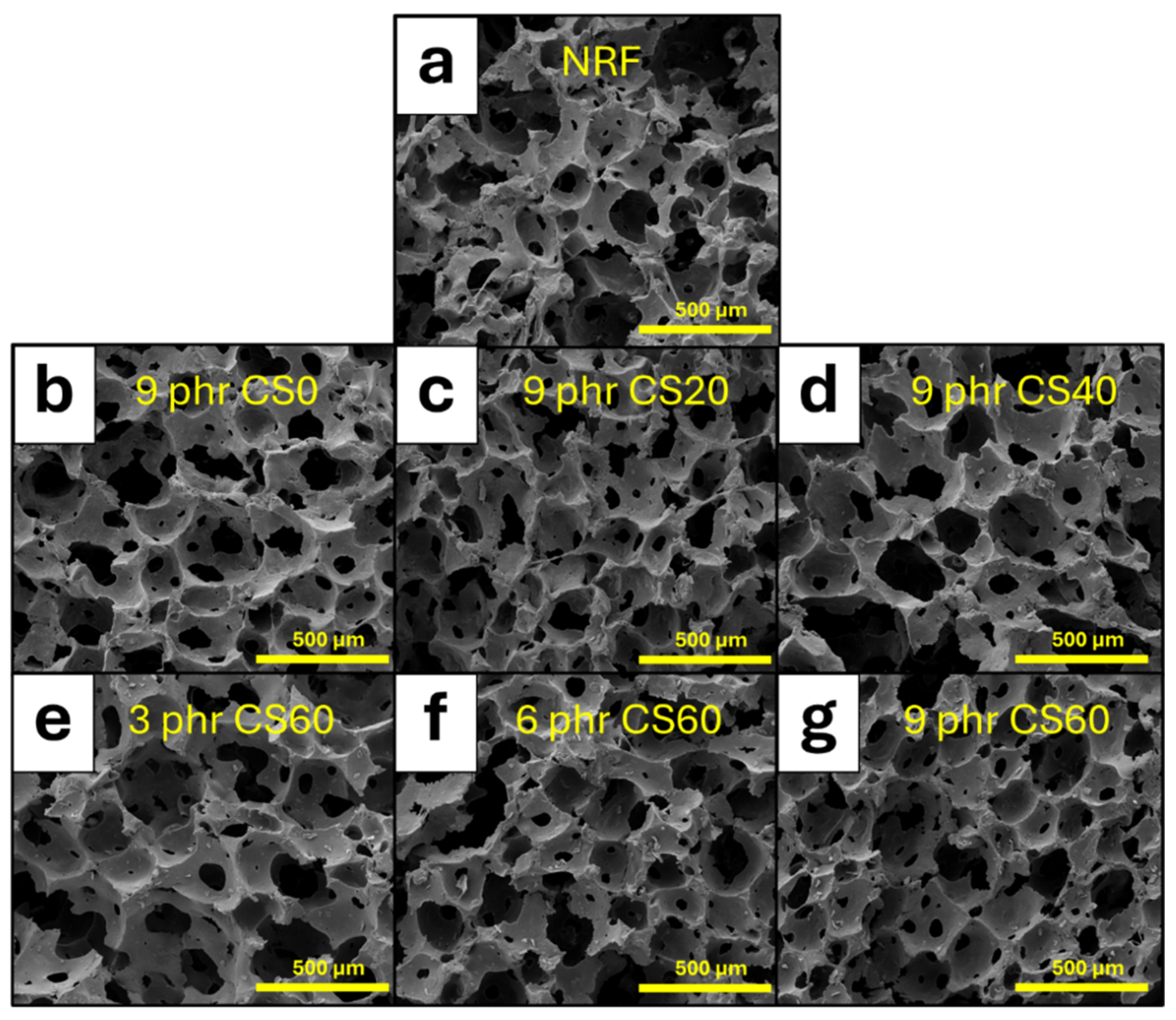
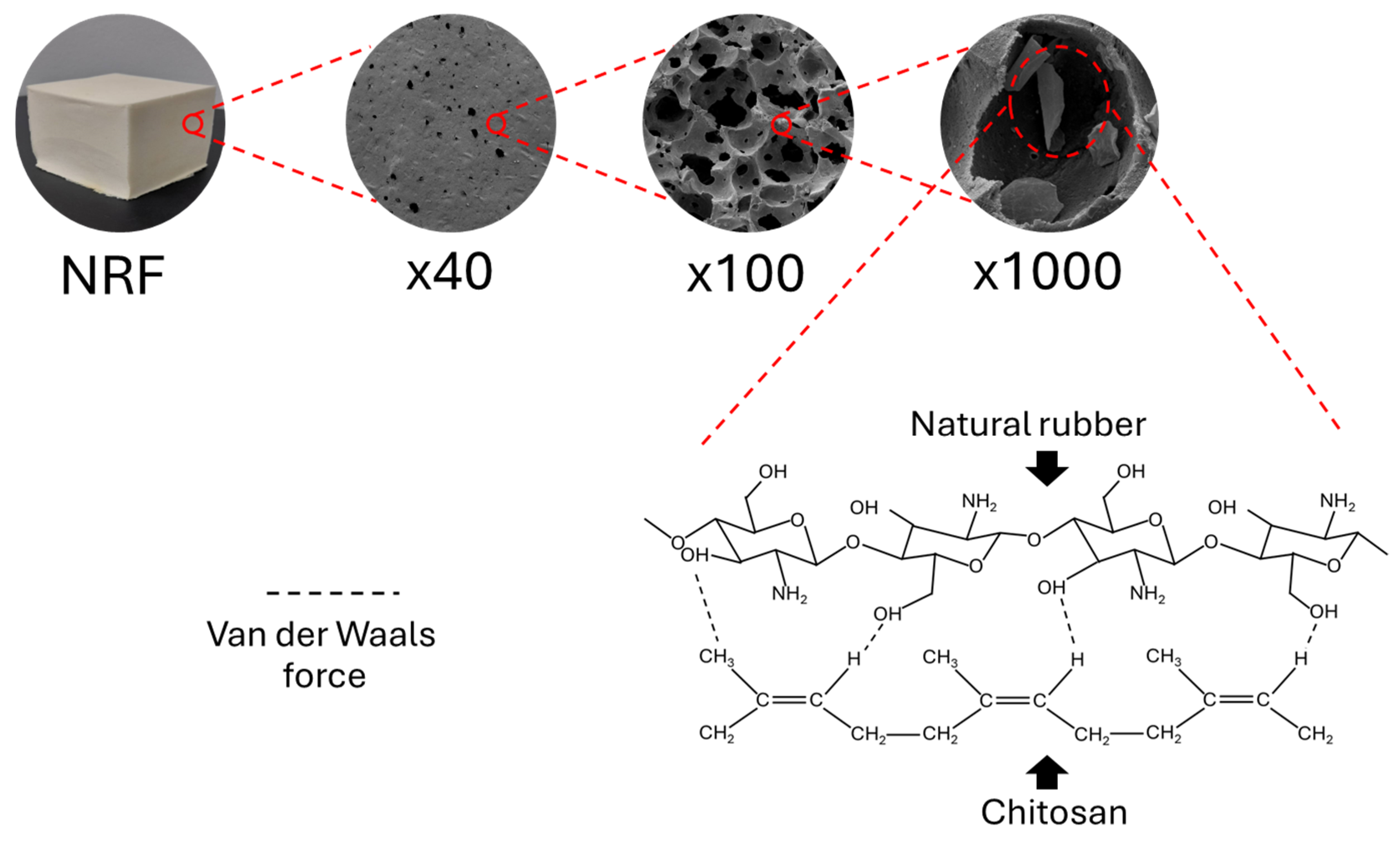
3.2.2. Densities, Pore Characteristics, and Morphological Properties of NRF Containing CS Powder
3.2.3. Thermal Stability of NRF Containing CS Powder
3.2.4. Compression Set and Hardness (Shore OO)
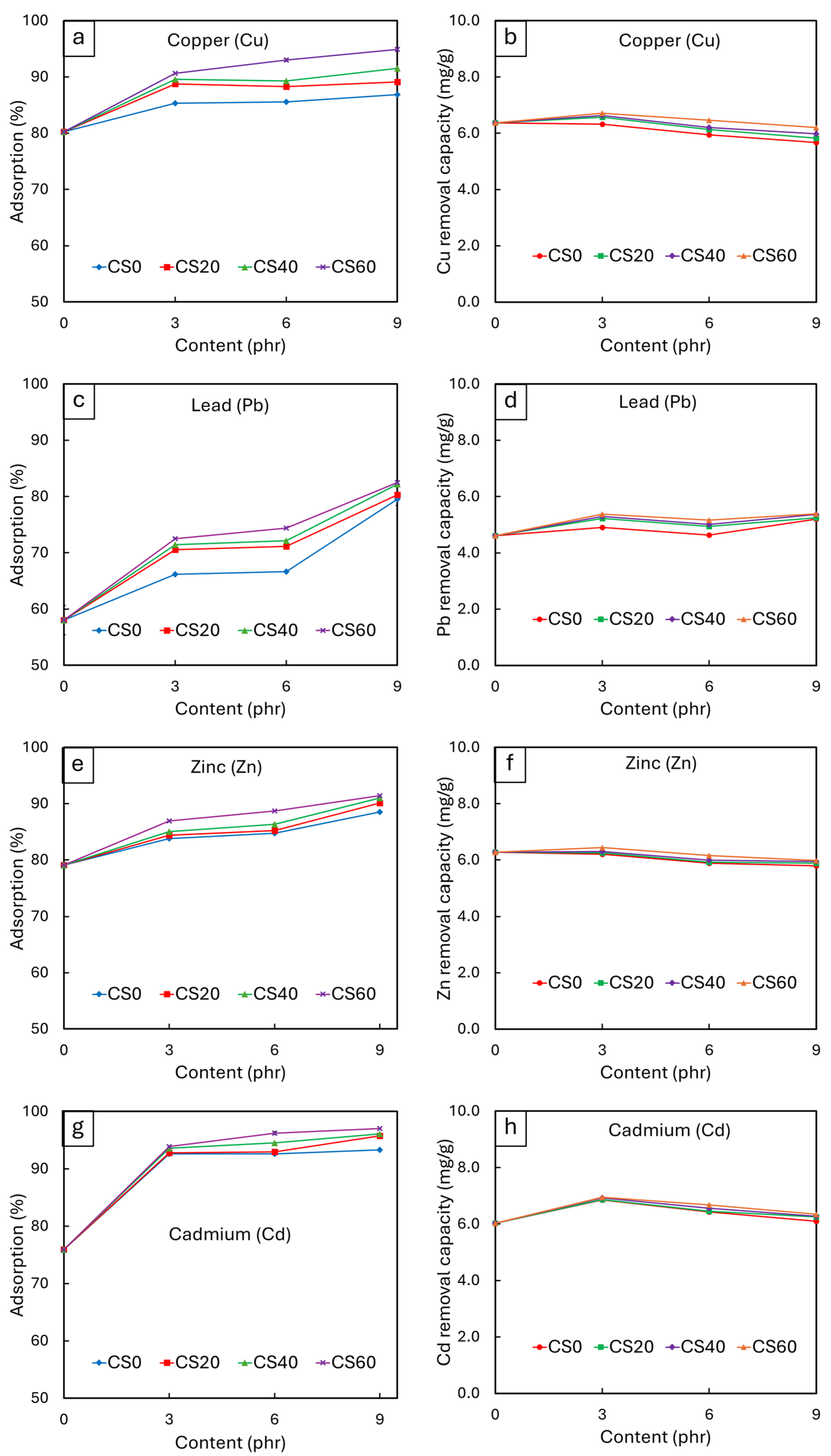
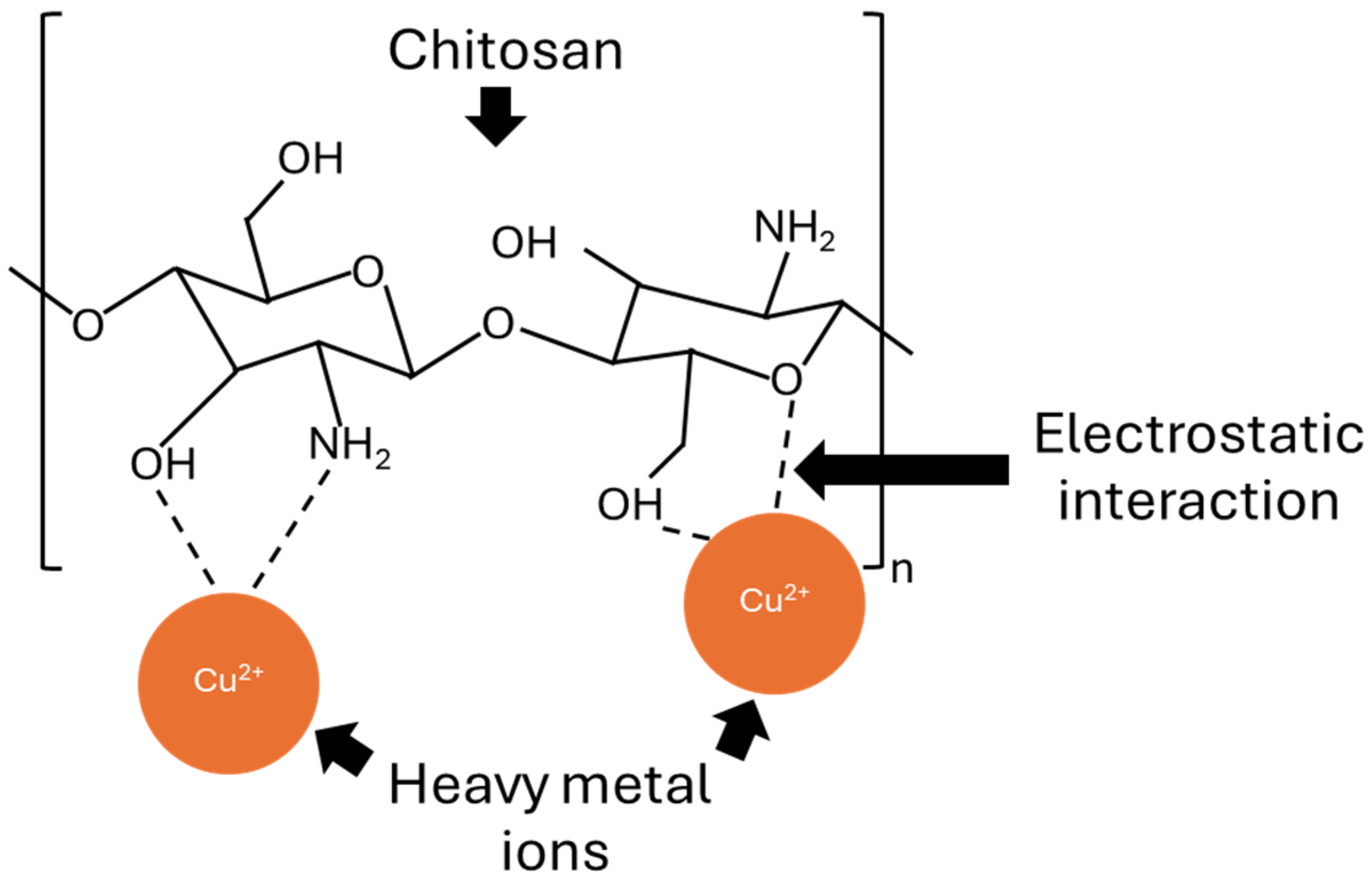
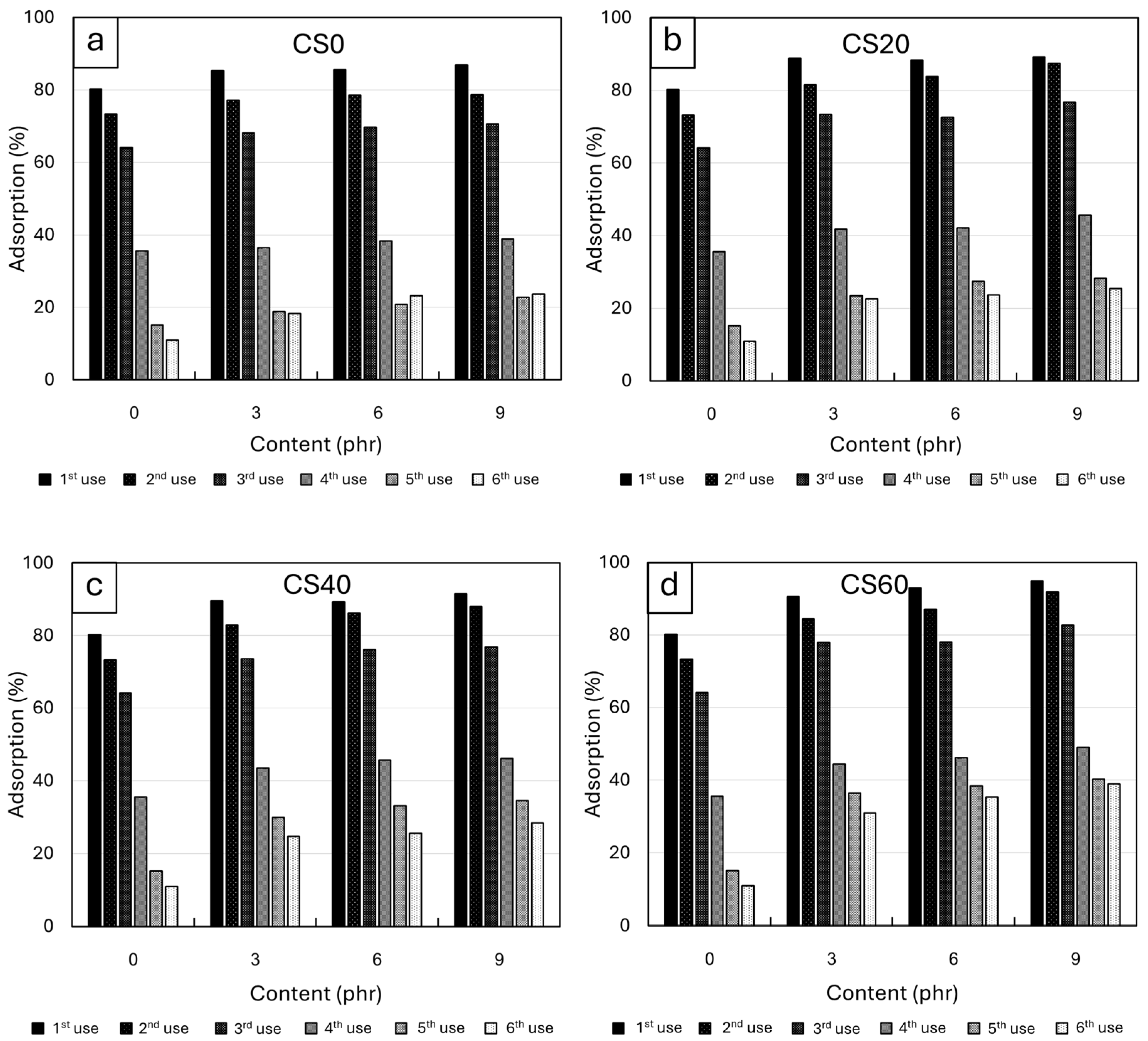
3.2.5. Heavy Metal Adsorption of NRF Containing CS Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nduka, J.K.; Kelle, H.I.; Amuka, J.O. Health risk assessment of cadmium, chromium and nickel from car paint dust from used automobiles at auto-panel workshops in Nigeria. Toxicol. Rep. 2019, 6, 449–456. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzeminska, E.; Wieszala, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Khlifi, R.; Hamza-Chaffai, A. Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: A review. Toxicol. Appl. Pharmacol. 2010, 248, 71–88. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y. Tackling heavy metal pollution: Evaluating governance models and frameworks. Sustainability 2023, 15, 15863. [Google Scholar] [CrossRef]
- Khan, M.U.; Muhammad, S.; Malik, R.N.; Khan, S.A.; Tariq, M. Heavy metals potential health risk assessment through consumption of wastewater irrigated wild plants: A case study. Hum. Ecol. Risk Assess. 2016, 22, 141–152. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef]
- Li, W.; Liu, S. Preparation and characterization of polyurethane foam/activated carbon composite adsorbents. J. Porous Mater. 2012, 19, 567–572. [Google Scholar] [CrossRef]
- Teodosiu, C.; Wenkert, R.; Tofan, L.; Paduraru, C. Advances in preconcentration/removal of environmentally relevant heavy metal ions from water and wastewater by sorbents based on polyurethane foam. Rev. Chem. Eng. 2014, 30, 403–420. [Google Scholar] [CrossRef]
- Hong, H.J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.P. Carboxymethlyated cellulose nanofibrils (CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Moonlek, B.; Saenboonruang, K. Mechanical and electrical properties of radiation-vulcanized natural rubber latex with waste eggshell powder as bio-fillers. Radiat. Eff. Defects Solids 2019, 174, 452–466. [Google Scholar] [CrossRef]
- Ninyong, K.; Wimolmala, E.; Sombatsompop, N.; Saenboonruang, K. Properties of natural rubber (NR) and wood/NR composites as gamma shielding materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 012038. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [PubMed]
- Kaczorowska, M.A.; Bozejewicz, D. The Application of chitosan-based adsorbents for the removal of hazardous pollutants from aqueous solutions—A review. Sustainability 2024, 16, 2615. [Google Scholar] [CrossRef]
- Thumwong, A.; Poltabtim, W.; Kerdsang, P.; Saenboonruang, K. Roles of Chitosan as bio-fillers in radiation-vulcanized natural rubber latex and hybrid radiation and peroxide-vulcanized natural rubber latex: Physical/mechanical properties under thermal aging and biodegradability. Polymers 2021, 13, 3940. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.; Ray, S.K. Chitosan based nano composite adsorbent—Synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr. Polym. 2018, 182, 159–171. [Google Scholar] [CrossRef]
- Das, N.C.; Rahman, M.M.; Kabir, S.F. Preparation of novel clay/chitosan/ZnO bio-composite as an efficient adsorbent for tannery wastewater treatment. Int. J. Biol. Macromol. 2023, 249, 126136. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, E.S.; Oliveira, C.P.; Bellato, C.R. An approach to understanding the deacetylation degree of chitosan. Carbohydr. Polym. 2010, 80, 1155–1160. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Zakaria, Z.; Izzah, Z.; Jawaid, M.; Hassan, A. Effect of degree of deacetylation of chitosan on thermal stability and compatibility of chitosan-polyamide blend. Bioresources 2012, 7, 5568–5580. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Tahriri, M.; Rasoulianboroujeni, M.; Koturi, H.; Tayebi, L. Preparation of natural chitosan from shrimp shell with different deacetylation degree. Mater. Res. Innov. 2018, 22, 177–181. [Google Scholar] [CrossRef]
- Rashid, T.U.; Rahman, M.M.; Kabir, S.; Shamsuddin, S.M.; Khan, M.A. A new approach for the preparation of chitosan from γ-irradiation of prawn shell: Effects of radiation on the characteristics of chitosan. Polym. Int. 2012, 61, 1302–1308. [Google Scholar] [CrossRef]
- Darwis, S.; Pangerteni, D.S.; Abbas, B.; Puspitasari, T.; Nuryanthi, N.; Nurrochmad, A.; Airin, C.M. Molecular weight, degree of deacetylation, and acute oral toxicity evaluation of irradiated and nonirradiated chitosan using mice. Macromol. Symp. 2020, 391, 1900149. [Google Scholar] [CrossRef]
- ISO 124: 2014; Latex, Rubber—Determination of Total Solids Content. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 126:2005; Natural Rubber Latex Concentrate—Determination of Dry Rubber Content. International Organization for Standardization: Geneva, Switzerland, 2005.
- Thumwong, A.; Chinnawet, M.; Intarasena, P.; Rattanapongs, C.; Tokonami, S.; Ishikawa, T.; Saenboonruang, K. A comparative study on X-ray shielding and mechanical properties of natural rubber latex nanocomposites containing Bi2O3 or BaSO4: Experimental and numerical determination. Polymers 2022, 14, 3654. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Adikary, S.U. Adsorption characteristics of cadmium and lead heavy metals into locally synthesized chitosan biopolymer. Trop. Agri. Res. 2015, 26, 395–401. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Inamdar, M.S. Aqueous behavior of chitosan. Int. J. Polym. Sci. 2010, 2010, 939536. [Google Scholar] [CrossRef]
- Barros, I.; Guzman, L.; Taron, A. Extraction and quantitative comparison of chitin obtained from the shell of Callinectes sapidus and Penaus vannamei. Rev. UDCA Actual. Divulg. Cient. 2015, 18, 227–234. [Google Scholar]
- Thumwong, A.; Darachai, J.; Thamrongsiripak, N.; Tokonami, S.; Ishikawa, T.; Saenboonruang, K. Fruit peel powder as natural antioxidant and reinforcing bio-filler in natural rubber latex gloves: Cases of mangosteen, pomelo and durian. Antioxidants 2023, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- ISO 1856:2018; Flexible Cellular Polymeric Materials—Determination of Compression Set. International Organization for Standardization: Geneva, Switzerland, 2018.
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- Garcia, M.A.; Paz, N.; Castro, C.; Rodriguez, J.L.; Rapado, M.; Zuluaga, R.; Casariego, A. Effect of molecular weight reduction by gamma irradiation on the antioxidant capacity of chitosan from lobster shells. J. Radiat. Res. Appl. Sci. 2015, 8, 190–200. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, W.H.; Ihm, D.; Hudson, S.M. Effect of the degree of deacetylation on the thermal decomposition of chitin and chitosan nanofibers. Carbohydr. Polym. 2010, 80, 291–295. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Nayan, M.B.; Jagadish, K.; Abhilash, M.R.; Namratha, K.; Srikantaswamy, S. Comparative study on the effects of surface area, conduction band and valence band positions on the photocatalytic activity of ZnO-MxOy heterostructures. J. Water Resour. Prot. 2019, 11, 357–370. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Tan, H.; Wang, Y.; Gao, C. Chitosan modified poly(L-lactide) microspheres as cell microcarriers for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2008, 66, 218–225. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel synthesis method combining a foaming agent with freeze-drying to obtain hybrid highly macroporous bone scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kraynik, A.M.; Kyriakides, S. On the microstructure of open-cell foams and its effect on elastic properties. Int. J. Solids Struct. 2008, 45, 1845–1875. [Google Scholar] [CrossRef]
- Boonrasri, S.; Sae-Oui, P.; Rachtanapun, P. Chitosan and natural rubber latex biocomposite prepared by incorporating negatively charged chitosan dispersion. Molecules 2020, 25, 2777. [Google Scholar] [CrossRef] [PubMed]
- Kudori, S.N.I.; Ismail, H. The effects of filler contents and particle sizes on properties of green kenaf-filled natural rubber latex foam. Cell. Polym. 2020, 39, 57–68. [Google Scholar] [CrossRef]
- Kudori, S.N.I.; Ismail, H.; Shuib, R. Kenaf core and bast loading vs. properties of natural rubber latex foam (NRLF). BioResources 2019, 14, 1765–1780. [Google Scholar] [CrossRef]
- He, J.; Xue, H.X.; Lu, C.W.; Fan, Q.Y.; Liang, Y.; Sun, Y.; Shen, L.L.; Bai, S. The impacts of common ions on the adsorption of heavy metal. Environ. Geol. 2009, 58, 1499–1508. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Jorge, M.; Gomes, P. Interaction of chitosan and chitin with Ni, Cu and Zn ions: A computational study. J. Chem. Thermodym. 2014, 73, 121–129. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Monroy-Zepeda, R.; Leyva-Ramos, E.; Diaz-Flores, P.E.; Shirai, K. Chitosan selectivity for removing cadmium (II), copper (II), and lead (II) from aqueous phase: pH and organic matter effect. J. Hazard. Mater. 2009, 162, 503–511. [Google Scholar] [CrossRef]





| Chemical | Dry Content (phr) | Role |
|---|---|---|
| 60% Natural rubber latex (NRL) | 100 | Main matrix |
| 10% Potassium oleate (C18H33KO2) | 1.5 | Foaming agent |
| 50% Sulfur | 2 | Crosslinker |
| 50% Zinc diethyldithiocarbamate (ZDEC) | 1 | Accelerator |
| 50% Zinc-2-mercaptobenzthiazole (ZMBT) | 1 | Accelerator |
| 50% WingStay-L | 1 | Antioxidant |
| 10% Chitosan (CS) | 0, 3, 6, and 9 | Heavy metal adsorber |
| 50% Zinc oxide (ZnO) | 5 | Activator |
| 33% 1,3 Diphenylguanidine (DPG) | 0.9 | Secondary gelling agent |
| 12.5% Sodium silicofluoride (SSF) | 1 | Primary gelling agent |
| Sample | Viscosity (cP) | %DD (%) |
|---|---|---|
| CS0 | 1004.7 ± 0.6 | 54.7 ± 3.5 |
| CS20 | 295.7 ± 0.6 | 67.9 ± 3.6 |
| CS40 | 35.7 ± 0.6 | 75.6 ± 3.9 |
| CS60 | 15.7 ± 0.6 | 81.3 ± 2.0 |
| CS80 | 15.3 ± 0.6 | 79.6 ± 1.2 |
| CS100 | 15.7 ± 0.6 | 73.3 ± 0.4 |
| Filler | Content (phr) | Density (g/cm3) |
|---|---|---|
| None | 0 | 0.14 ± 0.01 |
| CS0 | 3 | 0.15 ± 0.01 |
| 6 | 0.16 ± 0.01 | |
| 9 | 0.17 ± 0.01 | |
| CS20 | 3 | 0.15 ± 0.01 |
| 6 | 0.16 ± 0.02 | |
| 9 | 0.17 ± 0.01 | |
| CS40 | 3 | 0.15 ± 0.01 |
| 6 | 0.16 ± 0.01 | |
| 9 | 0.17 ± 0.01 | |
| CS60 | 3 | 0.15 ± 0.01 |
| 6 | 0.16 ± 0.01 | |
| 9 | 0.17 ± 0.01 |
| Filler | Content (phr) | Surface Area (m2/g) | Pore Volume (mm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| None | 0 | 1.64 ± 0.02 | 1.86 ± 0.39 | 5.03 ± 2.38 |
| CS0 | 9 | 1.55 ± 0.02 | 2.06 ± 0.41 | 5.04 ± 2.37 |
| CS20 | 9 | 2.19 ± 0.05 | 2.46 ± 0.73 | 5.44 ± 2.37 |
| CS40 | 9 | 2.22 ± 0.03 | 2.57 ± 0.47 | 5.62 ± 2.37 |
| CS60 | 9 | 2.07 ± 0.04 | 2.66 ± 0.46 | 5.61 ± 2.37 |
| CS60 | 6 | 1.78 ± 0.02 | 2.09 ± 0.38 | 5.59 ± 2.38 |
| CS60 | 3 | 1.29 ± 0.05 | 1.55 ± 0.30 | 5.18 ± 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intha, T.; Wimolmala, E.; Lertsarawut, P.; Saenboonruang, K. Effects of Gamma-Synthesized Chitosan on Morphological, Thermal, Mechanical, and Heavy-Metal Removal Properties in Natural Rubber Foam as Sustainable and Eco-Friendly Heavy Metal Sorbents. Sustainability 2024, 16, 6282. https://doi.org/10.3390/su16156282
Intha T, Wimolmala E, Lertsarawut P, Saenboonruang K. Effects of Gamma-Synthesized Chitosan on Morphological, Thermal, Mechanical, and Heavy-Metal Removal Properties in Natural Rubber Foam as Sustainable and Eco-Friendly Heavy Metal Sorbents. Sustainability. 2024; 16(15):6282. https://doi.org/10.3390/su16156282
Chicago/Turabian StyleIntha, Thitiwan, Ekachai Wimolmala, Pattra Lertsarawut, and Kiadtisak Saenboonruang. 2024. "Effects of Gamma-Synthesized Chitosan on Morphological, Thermal, Mechanical, and Heavy-Metal Removal Properties in Natural Rubber Foam as Sustainable and Eco-Friendly Heavy Metal Sorbents" Sustainability 16, no. 15: 6282. https://doi.org/10.3390/su16156282
APA StyleIntha, T., Wimolmala, E., Lertsarawut, P., & Saenboonruang, K. (2024). Effects of Gamma-Synthesized Chitosan on Morphological, Thermal, Mechanical, and Heavy-Metal Removal Properties in Natural Rubber Foam as Sustainable and Eco-Friendly Heavy Metal Sorbents. Sustainability, 16(15), 6282. https://doi.org/10.3390/su16156282







