Traceability of Phreatic Groundwater Contaminants and the Threat to Human Health: A Case Study in the Tabu River Basin, North China
Abstract
:1. Introduction
2. Materials and Methods
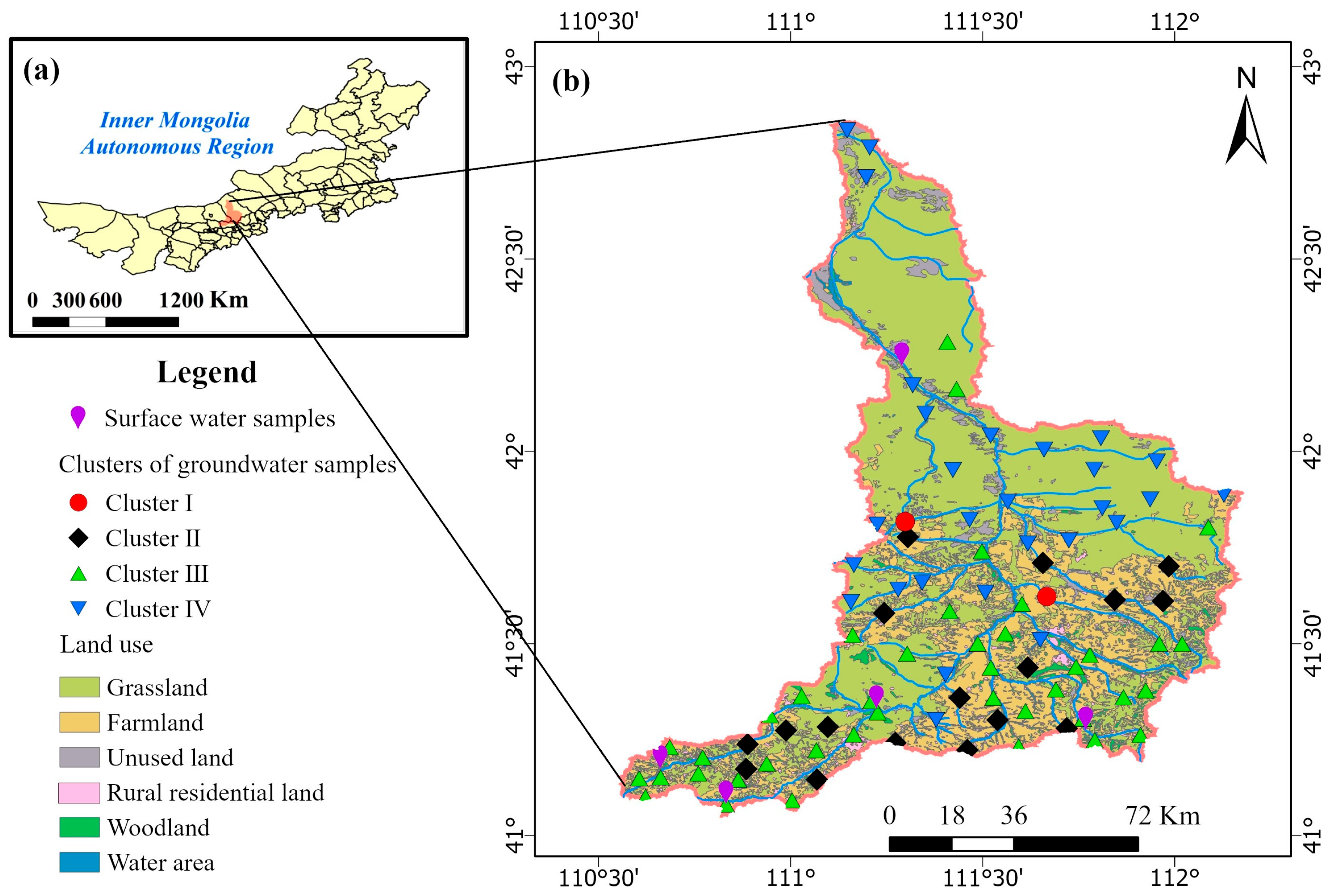
2.1. Study Area
2.2. Sampling and Analysis
2.3. Data Handling
2.4. Human Health Risk Assessment (HHRA)
3. Results and Discussion
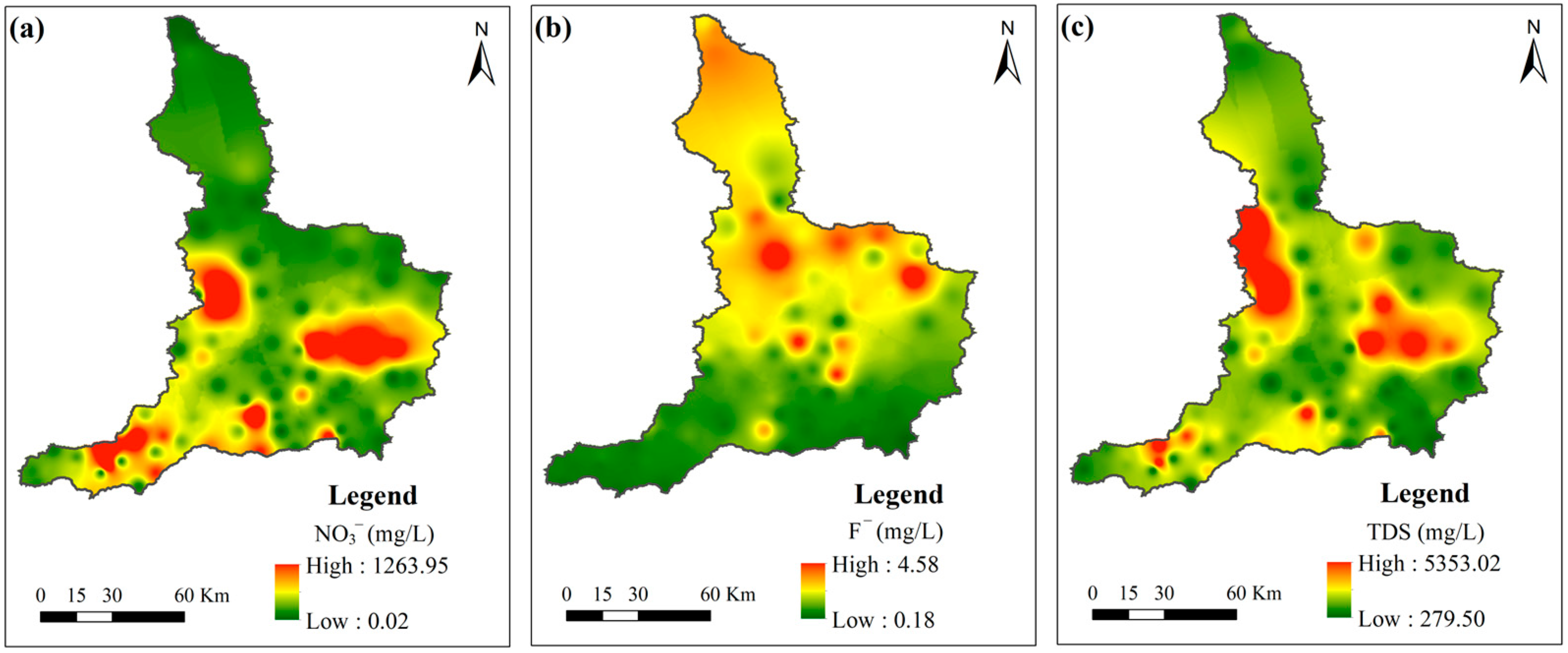
3.1. General Hydrogeochemical Parameters of Groundwater Samples and the Spatial Distribution of Groundwater Pollution
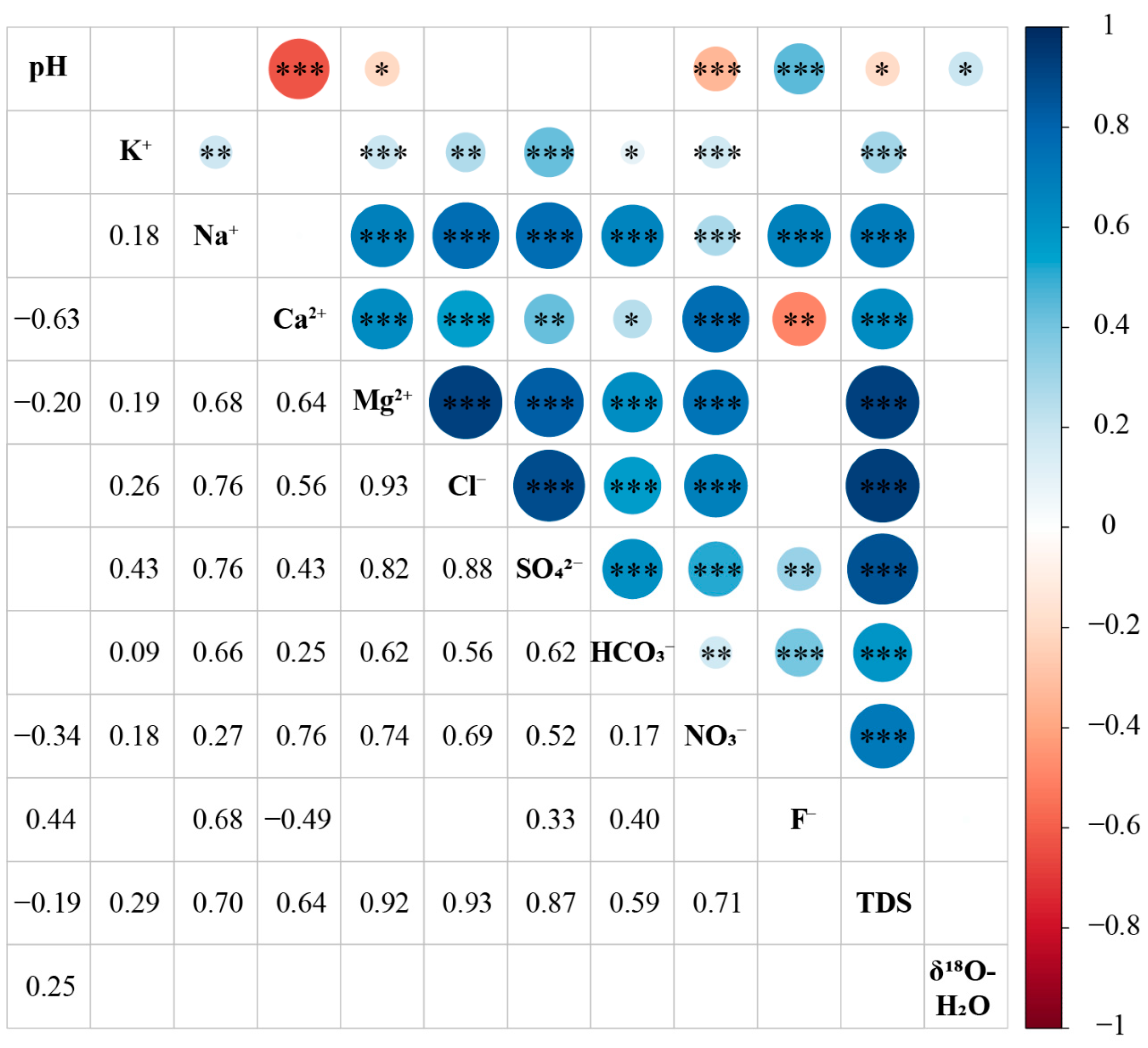
3.2. Influences of Anthropogenic Factors on Groundwater Quality
3.3. Influences of Geogenic Factors on Groundwater Quality
3.3.1. Evaporation
3.3.2. Interaction between Surface Water and Groundwater
3.3.3. Water–Rock Interactions
3.3.4. Alkaline Condition and Competitive Adsorption
3.4. HCA and PCA Based on Hydrogeochemical Parameters
3.5. Assessment of the Risk to Human Health
3.6. Development Trends and Countermeasures for Groundwater Contamination
4. Conclusions
- (1)
- Agricultural activity is the primary anthropogenic factor influencing the quality of phreatic groundwater. NO3− pollution in the groundwater primarily originates from manure, and the high level of TDS is highly associated with irrigation.
- (2)
- The enrichment of F− in the phreatic groundwater is dominated by geogenic factors, including alkaline conditions, competitive adsorption, the dissolution of fluorine-bearing minerals, and cation exchange.
- (3)
- Phreatic groundwater with high NO3− and F− contents poses significant threats to human health through the oral contact pathway, especially for children. Most of the phreatic groundwater in the Tabu River Basin can be utilized for domestic purposes, with the exception of drinking water.
- (4)
- The contamination of phreatic groundwater cannot be mitigated through natural attenuation under existing external pressures. Measures need to be taken to decrease contamination of phreatic groundwater and enhance groundwater sustainability in the Tabu River Basin.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, S.; Mohapatra, G.; Arora, R. Groundwater quality, human health risks and major driving factors in arid and semi-arid regions of Rajasthan, India. J. Clean. Prod. 2023, 427, 139149. [Google Scholar] [CrossRef]
- Wang, X.K.; Xiao, C.L.; Yang, W.F.; Liang, X.J.; Zhang, L.Z.; Zhang, J. Analysis of the quality, source identification and apportionment of the groundwater in a typical arid and semi-arid region. J. Hydrol. 2023, 625 Pt B, 130169. [Google Scholar] [CrossRef]
- Jolly, I.D.; Mcewan, K.L.; Holland, K.L. A review of groundwater-surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 2010, 1, 43–58. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Boving, T.B.; Kreamer, D.K.; Kebede, S.; Smedley, P.L. Groundwater quality: Global threats, opportunities and realising the potential of groundwater. Sci. Total Environ. 2022, 811, 152471. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hao, Q.C.; Zhang, Y.H.; Zhu, Y.C.; Yin, S.Y.; Qin, L.M.; Li, X.H. Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Sci. Total Environ. 2022, 802, 149909. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, H.; Chen, H.; Li, Z.; Zhang, S.Z. Source identification and potential health risks of fluoride and nitrate in groundwater of a typical alluvial plain. Sci. Total Environ. 2023, 904, 166920. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Zhou, H.L.; Qian, K.; Xie, X.J.; Xue, X.B.; Yang, Y.J.; Wang, Y.X. Fluoride and iodine enrichment in groundwater of North China Plain: Evidences from speciation analysis and geochemical modeling. Sci. Total Environ. 2017, 598, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.F.; Xi, B.D.; Jiang, Y.H.; Guo, H.M.; Yang, Y.; Lian, X.Y.; Han, S.B. Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: A review. Sci. Total Environ. 2018, 643, 967–993. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, J.X.; Ma, T.; Xie, X.J.; Deng, Y.M.; Gan, Y.Q. Genesis of geogenic contaminated groundwater: As, F and I. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2895–2933. [Google Scholar] [CrossRef]
- Ferraro, A.; de Sario, S.; Attanasio, A.; Di Capua, F.; Gorgoglione, A.; Fratino, U.; Mascolo, M.C.; Pirozzi, F.; Trancone, G.; Spasiano, D. Phosphorus recovery as struvite and hydroxyapatite from the liquid fraction of municipal sewage sludge with limited magnesium addition. J. Environ. Qual. 2022, 52, 584–595. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.Y.; Qian, H.; Ren, W.H.; Qu, W.G. Hydrogeochemical evidence for fluoride behavior in groundwater and the associated risk to human health for a large irrigation plain in the Yellow River Basin. Sci. Total Environ. 2021, 800, 149428. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.W.; Sun, Z.Y.; Zhou, A.G.; Bi, J.B.; Liu, Y.D. Source and enrichment mechanism of fluoride in groundwater of the Hotan Oasis within the Tarim Basin, Northwestern China. Environ. Pollut. 2022, 300, 118962. [Google Scholar] [CrossRef]
- Coyte, R.M.; Singh, A.; Furst, K.E.; Mitch, W.A.; Vengosh, A. Co-occurrence of geogenic and anthropogenic contaminants in groundwater from Rajasthan, India. Sci. Total Environ. 2019, 688, 1216–1227. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Krishan, G.; MacDonald, A.M.; Rao, M. Groundwater quality in the alluvial aquifer system of northwest India: New evidence of the extent of anthropogenic and geogenic contamination. Sci. Total Environ. 2017, 599–600, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Song, J.M.; Han, D.Y.; Liu, R.N.; Liu, C.Y.; Hou, Q.X. Assessing natural background levels of geogenic contaminants in groundwater of an urbanized delta through removal of groundwaters impacted by anthropogenic inputs: New insights into driving factors. Sci. Total Environ. 2023, 857 Pt 2, 159527. [Google Scholar] [CrossRef]
- Qu, S.; Duan, L.; Shi, Z.M.; Mao, H.R.; Wang, G.C.; Liu, T.X.; Yu, R.H.; Peng, X.H. Identifying the spatial pattern, driving factors and potential human health risks of nitrate and fluoride enriched groundwater of Ordos Basin, Northwest China. J. Clean. Prod. 2022, 376, 134289. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Carrey, R.; Ballesté, E.; Blanch, A.R.; Lucena, F.; Pons, P.; López, J.M.; Rull, M.; Solà, J.; Micola, N.; Fraile, J.; et al. Combining multi-isotopic and molecular source tracking methods to identify nitrate pollution sources in surface and groundwater. Water Res. 2021, 188, 116537. [Google Scholar] [CrossRef]
- Varol, M. Use of water quality index and multivariate statistical methods for the evaluation of water quality of a stream affected by multiple stressors: A case study. Environ. Pollut. 2020, 266, 115417. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, Y.D.; Zhou, A.G.; Zhang, L. Identification of groundwater pollution from livestock farming using fluorescence spectroscopy coupled with multivariate statistical methods. Water Res. 2021, 206, 117754. [Google Scholar] [CrossRef]
- Yang, J.; Ye, M.; Tang, Z.; Jiao, T.; Song, X.; Pei, Y.; Liu, H.H. Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J. Hydrol. 2020, 583, 124594. [Google Scholar] [CrossRef]
- Paca, J.M.; Santos, F.M.; Pires, J.C.M.; Leitão, A.A.; Boaventura, R.A.R. Quality assessment of water intended for human consumption from Kwanza, Dande and Bengo rivers (Angola). Environ. Pollut. 2019, 254, 113037. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Qian, H. Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo. Health 2016, 8, 349–359. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Heltshe, S.L.; Nuckols, J.R.; Sabra, M.M.; Shuldiner, A.R.; Mitchell, B.D.; Airola, M.; Holford, T.R.; Zhang, Y.W.; Ward, M.H. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the old order amish in Pennsylvania. Environ. Health 2012, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S.J.S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.A.; Kurella, S. Fluoride in drinking water: An in-depth analysis of its prevalence, health effects, advances in detection and treatment. Mater. Today Proc. 2023; in press. [Google Scholar]
- Zhai, Y.Z.; Lei, Y.; Wu, J.; Teng, Y.G.; Wang, J.S.; Zhao, X.B.; Pan, X.D. Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ. Sci. Pollut. Res. 2017, 24, 3640–3653. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A), Interim Final; EPA/540/1-89/002; Office of Emergency and Remedial Response U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- Yang, Y.; Liu, H.; Tao, W.H.; Shan, Y.Y. Spatiotemporal variation characteristics and driving force analysis of precipitation use efficiency at the north foot of Yinshan Mountain. Water 2024, 16, 99. [Google Scholar] [CrossRef]
- Jin, J.; Liu, T.; Wang, M.; Liao, Z.; Zhang, J. Hydrochemical and isotopic explanations of the interaction between surfacewater and groundwater in a typical-desertified steppe of northern China. Sustainability 2023, 15, 11034. [Google Scholar] [CrossRef]
- Muscetta, M.; Bianco, F.; Trancone, G.; Race, M.; Siciliano, A.; D’Agostino, F.; Sprovieri, M.; Clarizia, L. Washing bottom sediment for the removal of arsenic from contaminated italian coast. Processes 2023, 11, 902. [Google Scholar] [CrossRef]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A combined system for asbestos-cement waste degradation by dark fermentation and resulting supernatant valorization in anaerobic digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Shen, L.J.; Kang, H.Q. Size distributions of aerosol and water-soluble ions in Nanjing during a crop residual burning event. J. Environ. Sci. 2012, 24, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- El Housse, M.; Hadfi, A.; Karmal, I.; Tadoumant, S.; Ben-aazza, S.; Errami, M.; Belattar, M.; Mohareb, S.; Tounsi, A.; Driouiche, A. Study of scaling problem in the region of tata (Morocco): Analysis of the elemental composition, crystalline phases, and morphologies of scale deposition in water installations. Appl. Radiat. Isot. 2022, 188, 110388. [Google Scholar] [CrossRef] [PubMed]
- Weigand, M.A.; Foriel, J.; Barnett, B.; Oleynik, S.; Sigman, D.M. Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Commun. Mass. Spectrom. 2016, 30, 1365–1383. [Google Scholar] [CrossRef] [PubMed]
- Balacco, G.; Alfio, M.R.; Fidelibus, M.D. Groundwater Drought Analysis under Data Scarcity: The Case of the Salento Aquifer (Italy). Sustainability 2022, 14, 707. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 1 March 2024).
- Nourani, V.; Ghaneei, P.; Kantoush, S.A. Robust clustering for assessing the spatiotemporal variability of groundwater quantity and quality. J. Hydrol. 2022, 604, 127272. [Google Scholar] [CrossRef]
- NGWA. Brackish Groundwater. National Groundwater Association. Information Brief. 25 September 2017. Available online: https://www.ngwa.org/docs/default-source/default-document-library/publications/information-briefs/brackish-groundwater.pdf (accessed on 1 February 2024).
- WHO. Guidelines for Drinking Water Quality. Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, S.Q.; Zheng, W.B.; Currell, M.; Yang, Y.H.; Zhao, H.; Lv, M.Y. Relationship between land-use and sources and fate of nitrate in groundwater in a typical recharge area of the North China plain. Sci. Total Environ. 2017, 609, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.X.; Tang, C.Y.; Cao, Y.J. Mechanisms of nitrate accumulation in highly urbanized rivers: Evidence from multi-isotopes in the Pearl River Delta, China. J. Hydrol. 2020, 587, 124924. [Google Scholar] [CrossRef]
- Jalali, M. Nitrate pollution of groundwater in Toyserkan, western Iran. Environ. Earth Sci. 2011, 62, 907–913. [Google Scholar] [CrossRef]
- Fabro, A.Y.R.; Ávila, J.G.P.; Alberich, M.V.E.; Sansores, S.A.C.; Camargovalero, M.A. Spatial distribution of nitrate health risk associated with groundwater use as drinking water in Merida, Mexico. Appl. Geogr. 2015, 65, 49–57. [Google Scholar] [CrossRef]
- Xue, D.M.; Botte, J.; Baets, B.D.; Accoe, F.; Nestler, A.; Taylor, P.; Cleemput, O.V.; Berglund, M.; Boeckx, P. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. Stable Isot. Ecol. Environ. Sci. 2007, 2, 375–449. [Google Scholar]
- Han, D.; Song, X.; Currell, M.J.; Cao, G.; Zhang, Y.; Kang, Y. A survey of groundwater levels and hydrogeochemistry in irrigated fields in the Karamay Agricultural Development Area, northwest China: Implications for soil and groundwater salinity resulting from surface water transfer for irrigation. J. Hydrol. 2011, 405, 217–234. [Google Scholar] [CrossRef]
- Pauloo, R.A.; Fogg, G.E.; Guo, Z.L.; Harter, T. Anthropogenic basin closure and groundwater salinization (ABCSAL). J. Hydrol. 2021, 593, 125787. [Google Scholar] [CrossRef]
- Li, C.C.; Gao, X.B.; Liu, Y.S.; Wang, Y.X. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Su, H.; Wang, J.D.; Liu, J.T. Geochemical factors controlling the occurrence of highfluoride groundwater in the western region of the Ordos basin, northwestern China. Environ. Pollut. 2019, 252, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Das, N.; Goswami, R.; Sarma, K.P.; Bhattacharya, P.; Ramanathan, A. Coupling fractionation and batch desorption to understand arsenic and fluoride co-contamination in the aquifer system. Chemosphere 2016, 164, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Karroum, M.; Elgettafi, M.; Elmandour, A.; Wilske, C.; Himi, M.; Casas, A. Geochemical processes controlling groundwater quality under semi arid environment: A case study in central Morocco. Sci. Total Environ. 2017, 609, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jia, Y.F.; Yang, Y.; Huan, H.; Lian, X.Y.; Xu, X.J.; Xia, F.; Han, X.; Jiang, Y.H. Hydrogeochemical and statistical analysis of high fluoride groundwater in northern China. Environ. Sci. Pollut. Res. 2020, 27, 34840–34861. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Li, Z.; Su, H.; Xiao, J.; Han, F.P.; Li, Z. Spatial and seasonal variability, control factors and health risk of fluoride in natural water in the Loess Plateau of China. J. Hazard. Mater. 2022, 434, 128897. [Google Scholar] [CrossRef]
- Molinari, R.; Avci, A.H.; Argurio, P.; Curcio, E.; Meca, S.; Plà-Castellana, M.; Cortina, J.L. Selective precipitation of calcium ion from seawater desalination reverse osmosis brine. J. Clean. Prod. 2021, 328, 129645. [Google Scholar] [CrossRef]
- Schoeller, H. Geochemistry of groundwater. In Groundwater Studies—An International Guide for Research and Practice; UNESCO: Paris, France, 1977; pp. 1–18. [Google Scholar]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.T.; Li, Y.R.; Mei, J.; Ding, Y.; Wang, X.B. Preparation of poly(divinylbenzene-co-methyl acrylate) adsorbent with tunable surface hydrophilicity for atrazine removal. Surf. Interfaces 2024, 49, 104371. [Google Scholar] [CrossRef]
- Xi, Z.M.; Diao, S.J.; Xu, R.Z.; Wei, G.Y.; Wen, J.F.; Hu, G.H.; Tang, T.; Jiang, L.; Li, X.Y.; Li, M.; et al. Construction of carboxylated-GO and MOFs composites for efficient removal of heavy metal ions. Appl. Surf. Sci. 2023, 636, 157827. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhou, H.; He, S.; Zhang, Y.X. Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ. Earth Sci. 2019, 78, 446. [Google Scholar] [CrossRef]
- Gibson, J.J.; Edwards, T.W.D.; Bursey, G.G.; Prowse, T.D. Estimating evaporation using stable isotopes: Quantitative results and sensitivity analysis for two catchments in Northern Canada: Paper presented at the 9th Northern Res. Basin Symposium/Workshop (Whitehorse/Dawson/Inuvik, Canada-August 1992). Hydrol. Res. 1993, 24, 79–94. [Google Scholar] [CrossRef]








| Parameter | Min | Max | Median | SD |
|---|---|---|---|---|
| K+ (mg/L) | 0.8 | 272.6 | 2.6 | 29.2 |
| Na+ (mg/L) | 8.9 | 1130.0 | 76.0 | 166.1 |
| Ca2+ (mg/L) | 23.5 | 565.9 | 85.3 | 108.8 |
| Mg2+ (mg/L) | 11.5 | 292.4 | 39.2 | 51.6 |
| Cl− (mg/L) | 17.7 | 1156.0 | 98.4 | 190.9 |
| SO42− (mg/L) | 17.9 | 974.7 | 100.5 | 155.5 |
| HCO3− (mg/L) | 174.0 | 716.0 | 293.1 | 128.9 |
| F− (mg/L) | 0.2 | 4.6 | 0.8 | 1.0 |
| NO3− (mg/L) | 0.0 | 1274.0 | 81.3 | 308.0 |
| pH | 7.37 | 8.66 | 8.10 | 0.29 |
| TDS (mg/L) | 278.0 | 5398.5 | 712.0 | 882.4 |
| Burial depth (m) | 0.75 | 22.15 | 5.82 | 3.91 |
| δ2H-H2O (‰) | −111.6 | −32.6 | −75.4 | 10.1 |
| δ18O-H2O (‰) | −15.1 | −4.0 | −10.2 | 1.5 |
| δ15N-NO3− (‰) | −4.7 | 31.2 | 14.0 | 4.7 |
| δ18O-NO3− (‰) | −7.6 | 10.8 | −1.1 | 2.9 |
| SIcalcite | 0.26 | 1.6 | 0.91 | 0.25 |
| SIdolomite | 0.01 | 3.12 | 1.71 | 0.62 |
| SIfluorite | −2.39 | 0.05 | −1.11 | 0.5 |
| SIgypsum | −2.41 | −0.74 | −1.66 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liao, Z.; Jin, J.; Ni, Y.; Xu, J.; Wang, M.; Wang, Z.; Zhao, Y.; Zhang, Y. Traceability of Phreatic Groundwater Contaminants and the Threat to Human Health: A Case Study in the Tabu River Basin, North China. Sustainability 2024, 16, 6328. https://doi.org/10.3390/su16156328
Zhang J, Liao Z, Jin J, Ni Y, Xu J, Wang M, Wang Z, Zhao Y, Zhang Y. Traceability of Phreatic Groundwater Contaminants and the Threat to Human Health: A Case Study in the Tabu River Basin, North China. Sustainability. 2024; 16(15):6328. https://doi.org/10.3390/su16156328
Chicago/Turabian StyleZhang, Jing, Zilong Liao, Jing Jin, Yanyan Ni, Jian Xu, Mingxin Wang, Zihe Wang, Yiping Zhao, and Yuanzheng Zhang. 2024. "Traceability of Phreatic Groundwater Contaminants and the Threat to Human Health: A Case Study in the Tabu River Basin, North China" Sustainability 16, no. 15: 6328. https://doi.org/10.3390/su16156328






