An Evaluation of the Physical Characteristics of Seeds of Selected Lilac Species for Seed Sorting Purposes and Sustainable Forest Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physical Properties
- Geometric mean diameter D:
- T/W, T/L, and W/L aspect ratios describing seed shape;
- Sphericity index Φ:
- m/T, m/W, m/L, and m/D aspect ratios (seed mass divided by seed dimensions) describing seed size.
2.3. Statistical Analysis
3. Results and Discussion
3.1. Experimental Material
- 0.2 m s−1 for terminal velocity;
- 0.3 mm for length;
- 0.2 mm for width;
- 0.05 mm for thickness;
- 1 mg for mass;
- 1° for the angle of external friction.
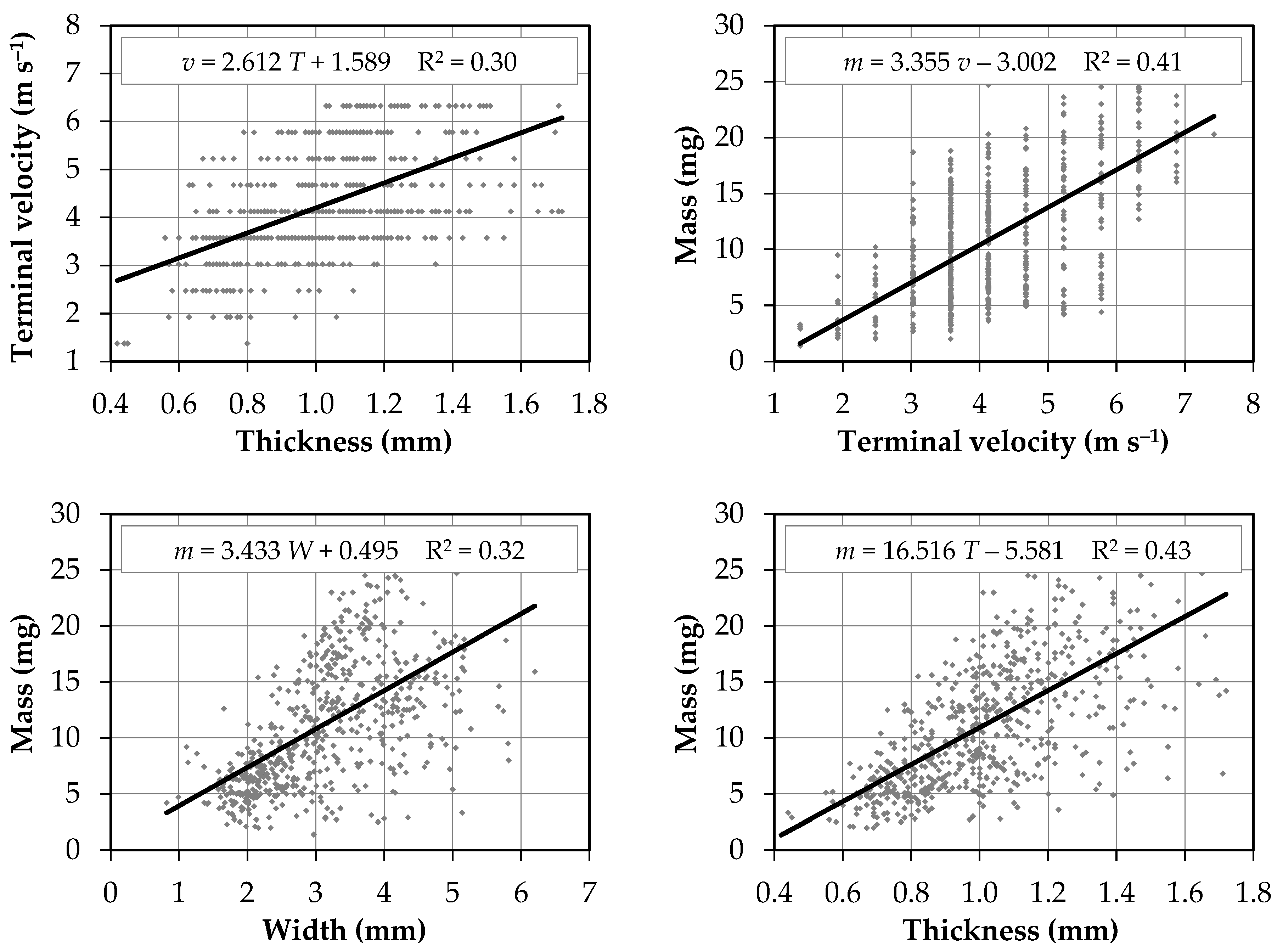
3.2. Correlations between the Physical Characteristics of Seeds
3.3. Suggestions for Seed Separation
- Fraction I (thinnest seeds) containing around 41% (Pekin lilac) to around 60% (Amur lilac) of class 1 seeds, around 15% (Japanese tree lilac) to around 21% (Hungarian lilac) of class 2 seeds, and around 3% (broadleaf lilac) to around 5% (Amur lilac, Hungarian lilac, and Japanese tree lilac) of class 3 seeds;
- Fraction II (medium-thick seeds) containing around 26% (Amur lilac and Japanese tree lilac) to around 50% (broadleaf lilac) of class 1 seeds, around 28% (Hungarian lilac) to around 56% (broadleaf lilac) of class 2 seeds, and around 14% (Japanese tree lilac) to around 50% (Hungarian lilac) of class 3 seeds;
- Fraction III (thickest seeds) containing around 8% (broadleaf lilac) to around 21% (Japanese tree lilac) of class 1 seeds, around 28% (broadleaf lilac) to around 51% (Hungarian lilac) of class 2 seeds, and around 45% (Hungarian lilac) to around 81% (Japanese tree lilac) of class 3 seeds.
- Fraction III (seeds with high terminal velocity) containing around 5% (Hungarian lilac) to around 43% (Pekin lilac) of all class 1 seeds, around 39% (Hungarian lilac) to around 61% (Pekin lilac) of all class 2 seeds, and around 55% (Hungarian lilac) to around 67% (Japanese tree lilac) of all class 3 seeds;
- Fraction II (seeds with average terminal velocity) containing around 11% (Amur lilac) to around 73% (Hungarian lilac) of all class 1 seeds, around 25% (Japanese tree lilac) to around 49% (Hungarian lilac) of all class 2 seeds, and around 28% (Japanese tree lilac) to around 45% (Hungarian lilac) of all class 3 seeds;
- Fraction I (seeds with low terminal velocity) containing around 19% (broadleaf lilac) to around 82% (Amur lilac) of all class 1 seeds, 0% (broadleaf lilac) to around 22% (Japanese tree lilac) of all class 2 seeds, and 0% (broadleaf lilac and Hungarian lilac) to around 5% (Japanese tree lilac) of all class 3 seeds.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyce, R.L. Invasive shrubs and forest tree regeneration. J. Sustain. For. 2009, 28, 152–217. [Google Scholar] [CrossRef]
- Jaworski, A. Hodowla Lasu. Tom III. In Charakterystyka Hodowlana Drzew i Krzewów Leśnych; Silviculture. Volume 3. Breeding Characteristics of Forest Trees and Shrubs); PWRiL: Warszawa, Poland, 2011; pp. 481–511. (In Polish) [Google Scholar]
- Götmark, F.; Götmark, E.; Jensen, A.M. Why be a shrub? A basic model and hypotheses for the adaptive values of a common growth form. Front. Plant Sci. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Chen, L.; Liu, T.; Hu, H.; Zhao, X.; Zhou, L.; Zhang, P.; Fang, J. Effects of shrub encroachment on soil organic carbon in global grasslands. Sci. Rep. 2016, 6, 28974. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, P.V.; Elliott, K.J.; Miniat, C.F. Forests, shrubs, and terrain: Top-down and bottom-up controls on forest structure. Ecosphere 2018, 9, e02185. [Google Scholar] [CrossRef]
- Philips, R.; Rix, M. The Botanical Garden. Vol. 1. Trees and Shrubs; Macmillan: London, UK, 2002. [Google Scholar]
- Rudolf, P.O.; Slabaugh, P.E.; Shaw, N.L. Syringa L.: Lilac. In The Woody Plant Seed Manual. Agric. Handbook No. 727; Bonner, F.T., Karrfalt, R.P., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2008; pp. 1083–1086. [Google Scholar]
- Jedrzejuk, A.; Szlachetka, W. Development of flower organs in common lilac [Syringa vulgaris L.] cv. Mme Florent Stepman. Acta Biol. Crac. Ser. Bot. 2005, 74, 41–52. [Google Scholar]
- Pooler, M.R. ‘Betsy Ross’, ‘Old Glory’, and ‘Declaration’ lilacs. HortScience 2008, 43, 544–545. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Li, J.; Li, H.; You, Y.; Zang, S.; Zhang, Y.; Ye, J.; Lv, Z.; Zhang, Z.; et al. A chromosome-level genome of Syringa oblata provides new insights into chromosome formation in Oleaceae and evolutionary history of lilacs. Plant J. 2022, 111, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ştefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical profile, cytotoxic activity and oxidative stress reduction of different Syringa vulgaris L. extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Sun, Y.; Yang, B.; Wang, Q.; Kuang, H. Traditional uses, phytochemistry and pharmacology of genus Syringa: A comprehensive review. J. Ethnopharmacol. 2021, 266, 113465. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Michalak, B.; Wyszomierska, J.; Dudek, M.K.; Kiss, A.K. Effects of phytochemically characterized extracts from Syringa vulgaris and isolated secoiridoids on mediators of inflammation in a human neutrophil model. Front. Pharmacol. 2018, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Wyszomierska, J.; Michalak, B.; Kiss, A.K. Syringa vulgaris bark as a source of compounds affecting the release of inflammatory mediators from human neutrophils and monocytes/macrophages. Phytochem. Lett. 2019, 30, 309–313. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Liu, H.; Zhou, Y. Enrichment and isolation of syringin and oleuropein from Syringa reticulata subsp. amurensis branch bark ionic liquid extract via macroporous resin adsorption and desorption. Ind. Crop. Prod. 2022, 189, 115813. [Google Scholar] [CrossRef]
- Tai, B.; Bai, L.; Ji, R.; Yu, M.; NAla; Huang, L.; Zheng, H. Phytochemical and pharmacological progress on Syringa oblata, a traditional Mongolian medicine. Chin. Herb. Med. 2022, 14, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, W.; Sasaki, T.; Li, Q.; Mitsuhata, N.; Asada, Y.; Zhang, Q.; Koike, K. Secoiridoid glucosides and related compounds from Syringa reticulata and their antioxidant activities. Bioorg. Med. Chem. Lett. 2011, 21, 6426–6429. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Cao, Y.; Li, C.; Yu, X.; Gao, X.; Tu, P.; Chai, X. Phytochemical and pharmacological progress on the genus Syringa. Chem. Cent. J. 2015, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; DeMarais, S.L.; Lee, C.W. Germination of nonstratified Japanese tree lilac seeds as influenced by seed capsule maturity and moisture content. HortTechnology 2014, 24, 177–180. [Google Scholar] [CrossRef]
- van Nocker, S.; Gardiner, S.E. Breeding better cultivars, faster: Applications of new technologies for the rapid deployment of superior horticultural tree crops. Hort. Res. 2014, 1, 14022. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, T.C.; Lattier, J.D.; Contreras, R.N. Sowing green seed without stratification does not shorten juvenility or increase plant size in common lilac (Syringa vulgaris). HortScience 2020, 55, 1781–1787. [Google Scholar] [CrossRef]
- Refouvelet, E.; Le Nours, S.; Tallon, C.; Daguin, F. A new method for in vitro propagation of lilac (Syringa vulgaris L.): Regrowth and storage conditions for axillary buds encapsulated in alginate beads, development of a pre-acclimatisation stage. Sci. Hortic. 1998, 74, 233–241. [Google Scholar] [CrossRef]
- Nesterowicz, S.; Kulpa, D.; Moder, K.; Kurek, J. Micropropagation of an old specimen of common lilac [Syringa vulgaris L.] from the Dendrological Garden at Przelewice. Acta Sci. Pol. Hortorum Cultus 2006, 5, 27–35. [Google Scholar]
- Liu, C.-P.; Liu, H.-Y.; Yang, B.-W.; Yang, L.; Zhang, P.; Shen, H.-L. Shoot multiplication of Syringa reticulata var. mandshurica from in vitro cultured seedlings. J. For. Res. 2017, 28, 41–46. [Google Scholar] [CrossRef]
- Chaisurisri, K.; Edwards, D.G.W.; El-Kassaby, Y.A. Effects of seed size on seedling attributes in Sitka spruce. New For. 1994, 8, 81–87. [Google Scholar] [CrossRef]
- Oliver, C.D. Sustainable forestry: What is it? How do we achieve it? J. For. 2003, 101, 8–14. [Google Scholar] [CrossRef]
- Kruk, H.; Kornatowska, B. Sustainable forest management in Poland—Theory and practice. Folia For. Pol. Ser. A For. 2014, 56, 45–55. [Google Scholar] [CrossRef]
- Poorter, L.; Rose, S.A. Light-dependent changes in the relationship between seed mass and seedling traits: A meta-analysis for rain forest tree species. Oecologia 2005, 142, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Norden, N.; Daws, M.I.; Antoine, C.; Gonzalez, M.A.; Garwood, N.C.; Chave, J. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Func. Ecol. 2009, 23, 203–210. [Google Scholar] [CrossRef]
- Karki, H.; Bargali, K.; Bargali, S.S. Effect of sowing time on germination and early seedling growth of Quercus floribunda Lindl. J. For. Environ. Sci. 2018, 34, 199–208. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Żuk, Z.; Kusińska, E. Physical properties of seeds of eleven spruce species. Forests 2018, 9, 617. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Markowski, P.; Anders, A.; Jadwisieńczak, K.; Żuk, Z.; Krzysiak, Z. Physical properties of seeds of eleven fir species. Forests 2019, 10, 142. [Google Scholar] [CrossRef]
- Załęski, A. Nasiennictwo Leśnych Drzew i Krzewów Iglastych (Management of Coniferous Forest Trees and Shrubs for Seed Production); Oficyna Edytorska, Wydawnictwo Świat: Warszawa, Poland, 1995; pp. 117–121. (In Polish) [Google Scholar]
- Kaliniewicz, Z.; Choszcz, D.J. Analysis of the physical properties of seeds of selected Viburnum species for the needs of seed sorting operations. Processes 2021, 9, 711. [Google Scholar] [CrossRef]
- Ludwikowska, A.; Kowalkowski, W.; Tarasiuk, S. The growth of small-leaved lime (Tilia cordata Mill.) clones in a seed orchard in the Susz Forest Discrict. For. Res. Pap. 2011, 72, 121–130. [Google Scholar]
- Kim, K.H.; Shin, S.H.; Park, S.; Park, J.C.; Kang, C.S.; Park, C.S. Relationship between pre-harvest sprouting and functional markers associated with grain weight, TaSUS2-2B, TaGW2-6A, and TaCWI-A1, in Korean wheat cultivars. SABRAO J. Breed. Genet. 2014, 46, 319–328. [Google Scholar]
- Kasraei, M.; Nejadi, J.; Shafiei, S. Relationship between grain physicochemical and mechanical properties of some Iranian wheat cultivars. J. Agric. Sci. Technol. 2015, 17, 635–647. [Google Scholar]
- Sivacioglu, A.; Ayan, S. Variation in cone and seed characteristic in a clonal seed orchard of Anatolian black pine [Pinus nigra Arnold subsp. paliasiana (Lamb.) Holmboe]. J. Environ. Biol. 2010, 31, 119–123. [Google Scholar] [PubMed]
- Nazarova, N.M. Seeds of Syringa vulgaris L. as a possible object for phytoindication studies of urban environment in Orenburg. RUDN J. Agron. Anim. Ind. 2023, 18, 350–360. [Google Scholar] [CrossRef]
- Gleiser, G.; Picher, M.C.; Veintimilla, P.; Martinez, J.; Verdú, M. Seed dormancy in relation to seed storage behavior in Acer. Bot. J. Linn. Soc. 2004, 145, 203–208. [Google Scholar] [CrossRef]
- Aguinagalde, I.; Hampe, A.; Mohanty, A.; Martin, J.P.; Duminil, J.; Petit, R.J. Effects of life-history traits and species distribution on genetic structure at maternally inherited markers in European trees and shrubs. J. Biogeogr. 2005, 32, 329–339. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Anders, A.; Markowski, P.; Tylek, P.; Owoc, D. Analysis of the physical properties of spindle seeds for seed sorting operations. Sci. Rep. 2021, 11, 13625. [Google Scholar] [CrossRef] [PubMed]
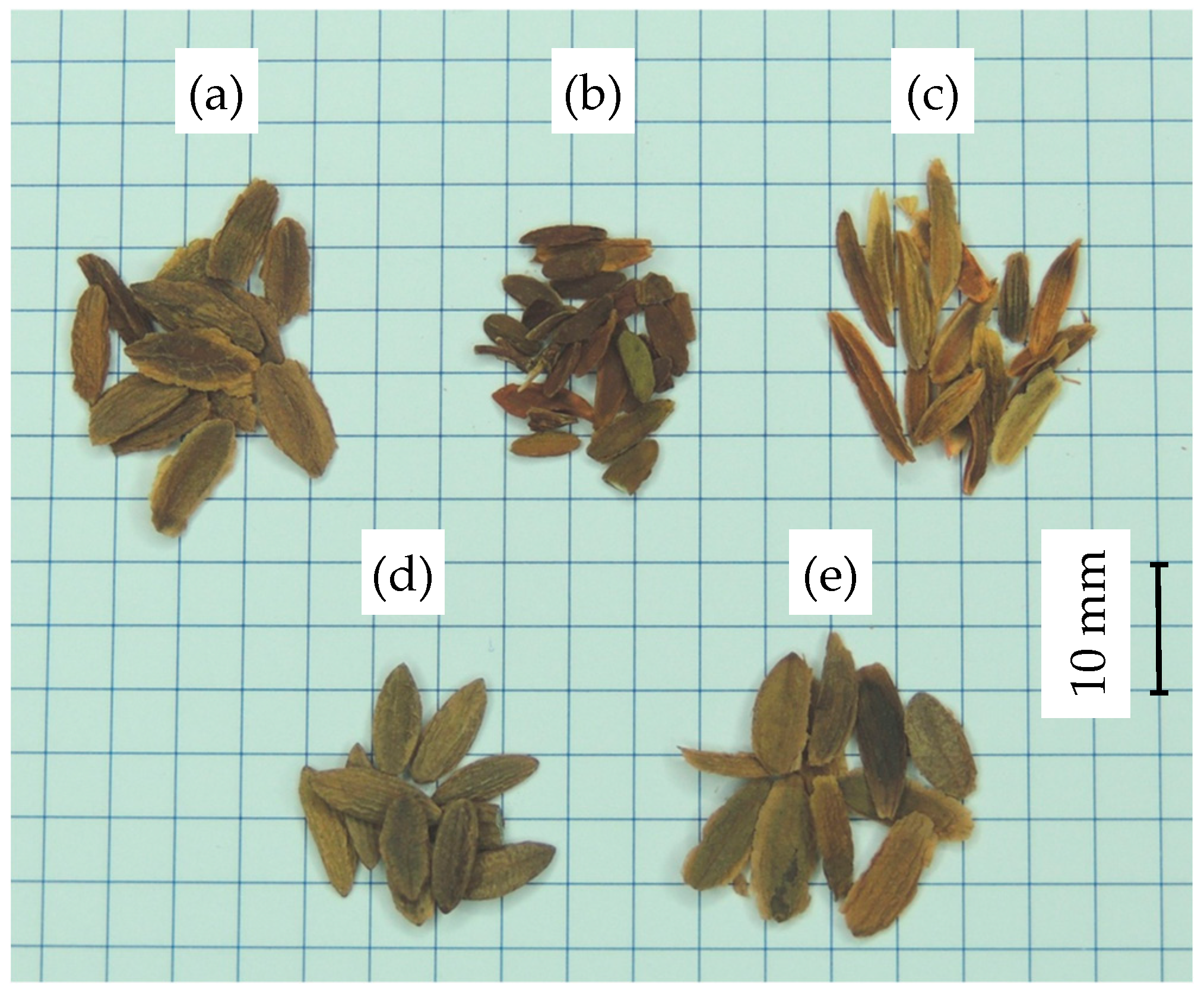
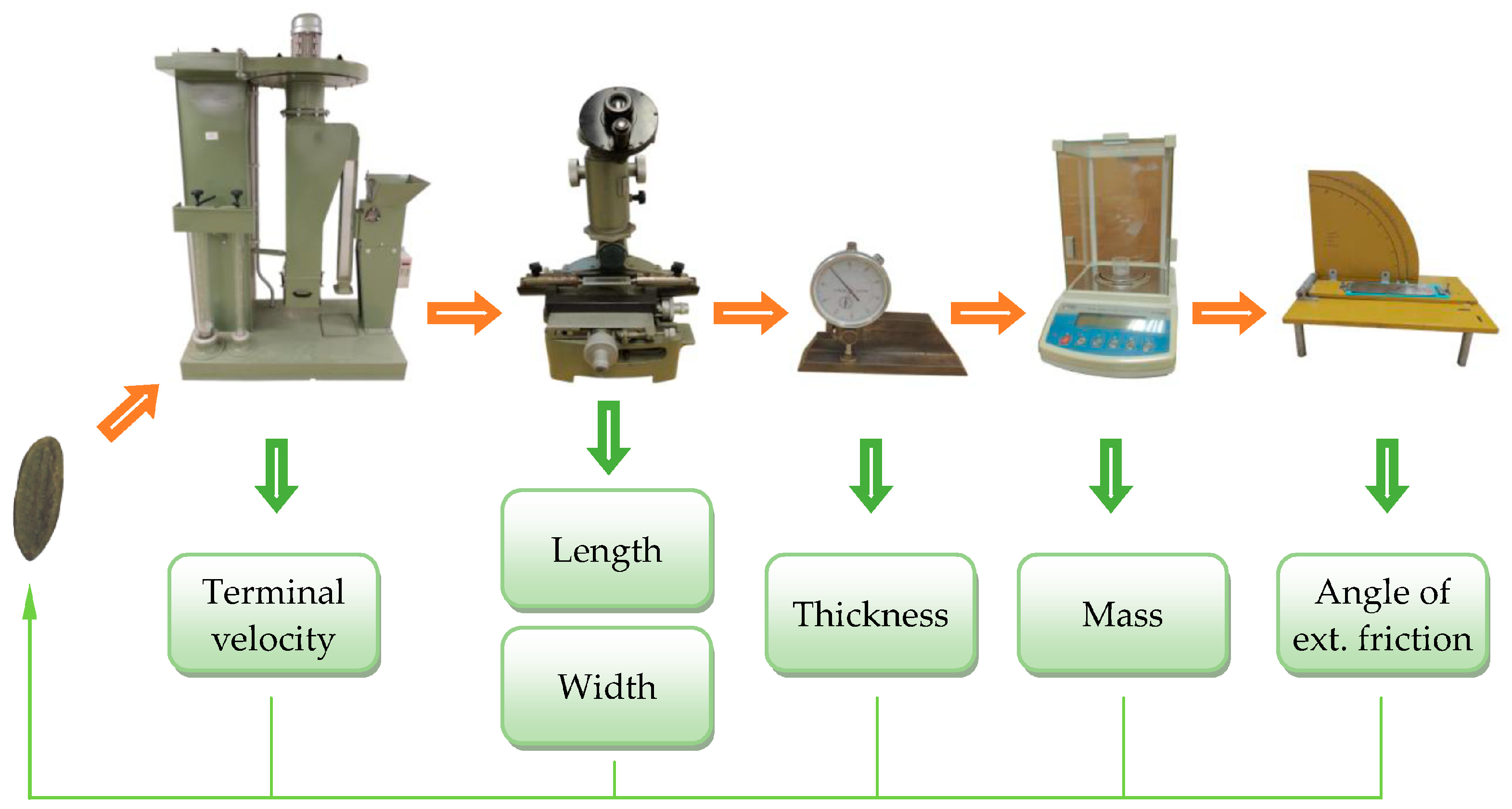



| Indicator | Lilac Species | ||||
|---|---|---|---|---|---|
| Amur | Broadleaf | Hungarian | Japanese Tree | Pekin | |
| Geom. mean diameter (mm) | 3.29 ± 0.38 c | 2.28 ± 0.29 a | 2.67 ± 0.31 b | 3.36 ± 0.30 c | 3.38 ± 0.39 c |
| Aspect ratio T/W (-) | 0.26 ± 0.09 a | 0.38 ± 0.11 b | 0.47 ± 0.16 c | 0.36 ± 0.07 b | 0.30 ± 0.12 a |
| Aspect ratio T/L (-) | 0.11 ± 0.03 a | 0.14 ± 0.03 b | 0.10 ± 0.03 a | 0.13 ± 0.03 b | 0.11 ± 0.03 a |
| Aspect ratio W/L (-) | 0.43 ± 0.10 d | 0.38 ± 0.09 bc | 0.23 ± 0.06 a | 0.37 ± 0.06 b | 0.41 ± 0.09 cd |
| Sphericity index (-) | 0.36 ± 0.04 b | 0.37 ± 0.05 c | 0.29 ± 0.04 a | 0.36 ± 0.04 bc | 0.36 ± 0.04 b |
| Aspect ratio m/T (g m−1) | 11.39 ± 3.45 b | 6.96 ± 1.76 a | 7.25 ± 2.30 a | 15.86 ± 3.35 c | 12.34 ± 3.27 b |
| Aspect ratio m/W (g m−1) | 3.03 ± 1.22 b | 2.61 ± 0.76 a | 3.24 ± 1.18 bc | 5.62 ± 0.99 d | 3.46 ± 1.07 c |
| Aspect ratio m/L (g m−1) | 1.25 ± 0.43 c | 0.97 ± 0.27 b | 0.72 ± 0.21 a | 2.04 ± 0.41 d | 1.38 ± 0.41 c |
| Aspect ratio m/D (g m−1) | 3.47 ± 1.11 b | 2.57 ± 0.61 a | 2.54 ± 0.67 a | 5.64 ± 0.95 d | 3.84 ± 0.95 c |
| Lilac Species | Coefficients Denoting the Strength of Correlations between Seed Mass m and | ||||
|---|---|---|---|---|---|
| Terminal Velocity | Length | Width | Thickness | Angle of External Friction | |
| Amur lilac | 0.768 | 0.480 | 0.135 | 0.635 | −0.038 |
| Broadleaf lilac | 0.444 | 0.336 | 0.427 | 0.589 | −0.128 |
| Hungarian lilac | 0.553 | 0.550 | 0.340 | 0.348 | −0.008 |
| Japanese tree lilac | 0.395 | 0.607 | 0.730 | 0.513 | 0.030 |
| Pekin lilac | 0.592 | 0.279 | 0.368 | 0.506 | −0.139 |
| Property | Terminal Velocity | Length | Width | Thickness | Mass |
|---|---|---|---|---|---|
| Length | −0.073 | 1 | |||
| Width | −0.059 | 0.397 | 1 | ||
| Thickness | 0.545 | 0.316 | 0.184 | 1 | |
| Mass | 0.637 | 0.498 | 0.562 | 0.654 | 1 |
| Angle of ext. friction | −0.543 | −0.072 | −0.250 | −0.404 | −0.042 |
| Lilac Species | Seed Class | Percentage Share in the Total Number of Seeds (%) | Percentage Share in Total Seed Mass (%) |
|---|---|---|---|
| Amur lilac | 1 (m < 10 mg) | 30.8 | 17.2 |
| 2 (m = 10–14 mg) | 40.0 | 41.3 | |
| 3 (m > 14 mg) | 29.2 | 41.5 | |
| Broadleaf lilac | 1 (m < 5 mg) | 32.0 | 21.7 |
| 2 (m = 5–7 mg) | 42.6 | 42.1 | |
| 3 (m > 7 mg) | 25.4 | 36.2 | |
| Hungarian lilac | 1 (m < 6 mg) | 34.2 | 22.6 |
| 2 (m = 6–8 mg) | 35.0 | 35.3 | |
| 3 (m > 8 mg) | 30.8 | 42.1 | |
| Japanese tree lilac | 1 (m < 17 mg) | 33.9 | 25.6 |
| 2 (m = 17–21 mg) | 35.6 | 34.9 | |
| 3 (m > 21 mg) | 30.5 | 39.5 | |
| Pekin lilac | 1 (m < 12 mg) | 34.4 | 23.1 |
| 2 (m = 12–15 mg) | 30.3 | 30.4 | |
| 3 (m > 15 mg) | 35.3 | 46.5 |
| Lilac Species | Seed Fraction | Percentage Share in Total Seed Mass (%) | Coefficient of Variation (%) of Seed Mass | |
|---|---|---|---|---|
| Fraction | Total | |||
| Amur lilac | I (v < 3.30 m s−1) | 22.0 | 44.4 | 37.4 |
| II (v = 3.30–3.85 m s−1) | 31.5 | 18.1 | ||
| III (v > 3.85 m s−1) | 46.5 | 23.7 | ||
| I (T ≤ 0.90 mm) | 20.4 | 48.5 | ||
| II (T = 0.91–1.10 mm) | 43.5 | 26.0 | ||
| III (T > 1.10 mm) | 36.1 | 25.1 | ||
| Broadleaf lilac | I (v < 3.30 m s−1) | 4.5 | 37.5 | 30.9 |
| II (v = 3.30–4.40 m s−1) | 46.4 | 31.9 | ||
| III (v > 4.40 m s−1) | 49.1 | 23.9 | ||
| I (T ≤ 0.70 mm) | 17.1 | 35.2 | ||
| II (T = 0.71–0.90 mm) | 43.3 | 23.3 | ||
| III (T > 0.90 mm) | 39.6 | 23.1 | ||
| Hungarian lilac | I (v < 3.30 m s−1) | 9.3 | 42.3 | 31.7 |
| II (v = 3.30–3.85 m s−1) | 52.9 | 29.6 | ||
| III (v > 3.85 m s−1) | 37.8 | 21.1 | ||
| I (T ≤ 0.80 mm) | 20.3 | 33.7 | ||
| II (T = 0.81–1.00 mm) | 38.9 | 30.9 | ||
| III (T > 1.00 mm) | 40.8 | 22.9 | ||
| Japanese tree lilac | I (v < 5.50 m s−1) | 18.4 | 23.1 | 25.2 |
| II (v = 5.50–6.05 m s−1) | 29.8 | 22.6 | ||
| III (v > 6.05 m s−1) | 51.8 | 24.0 | ||
| I (T ≤ 1.10 mm) | 20.9 | 21.3 | ||
| II (T = 1.11–1.20 mm) | 26.4 | 19.1 | ||
| III (T > 1.20 mm) | 52.7 | 23.2 | ||
| Pekin lilac | I (v < 3.30 m s−1) | 11.6 | 48.7 | 30.5 |
| II (v = 3.30–3.85 m s−1) | 30.4 | 20.8 | ||
| III (v > 3.85 m s−1) | 58.0 | 23.9 | ||
| I (T ≤ 0.90 mm) | 16.4 | 36.5 | ||
| II (T = 0.91–1.10 mm) | 42.0 | 26.4 | ||
| III (T > 1.10 mm) | 41.6 | 24.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliniewicz, Z.; Konopka, S.; Krzysiak, Z.; Tylek, P. An Evaluation of the Physical Characteristics of Seeds of Selected Lilac Species for Seed Sorting Purposes and Sustainable Forest Management. Sustainability 2024, 16, 6340. https://doi.org/10.3390/su16156340
Kaliniewicz Z, Konopka S, Krzysiak Z, Tylek P. An Evaluation of the Physical Characteristics of Seeds of Selected Lilac Species for Seed Sorting Purposes and Sustainable Forest Management. Sustainability. 2024; 16(15):6340. https://doi.org/10.3390/su16156340
Chicago/Turabian StyleKaliniewicz, Zdzisław, Stanisław Konopka, Zbigniew Krzysiak, and Paweł Tylek. 2024. "An Evaluation of the Physical Characteristics of Seeds of Selected Lilac Species for Seed Sorting Purposes and Sustainable Forest Management" Sustainability 16, no. 15: 6340. https://doi.org/10.3390/su16156340





