A Biomechanical Study of Potential Plants for Erosion Control and Slope Stabilization of Highland in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Plant Species and Sampling
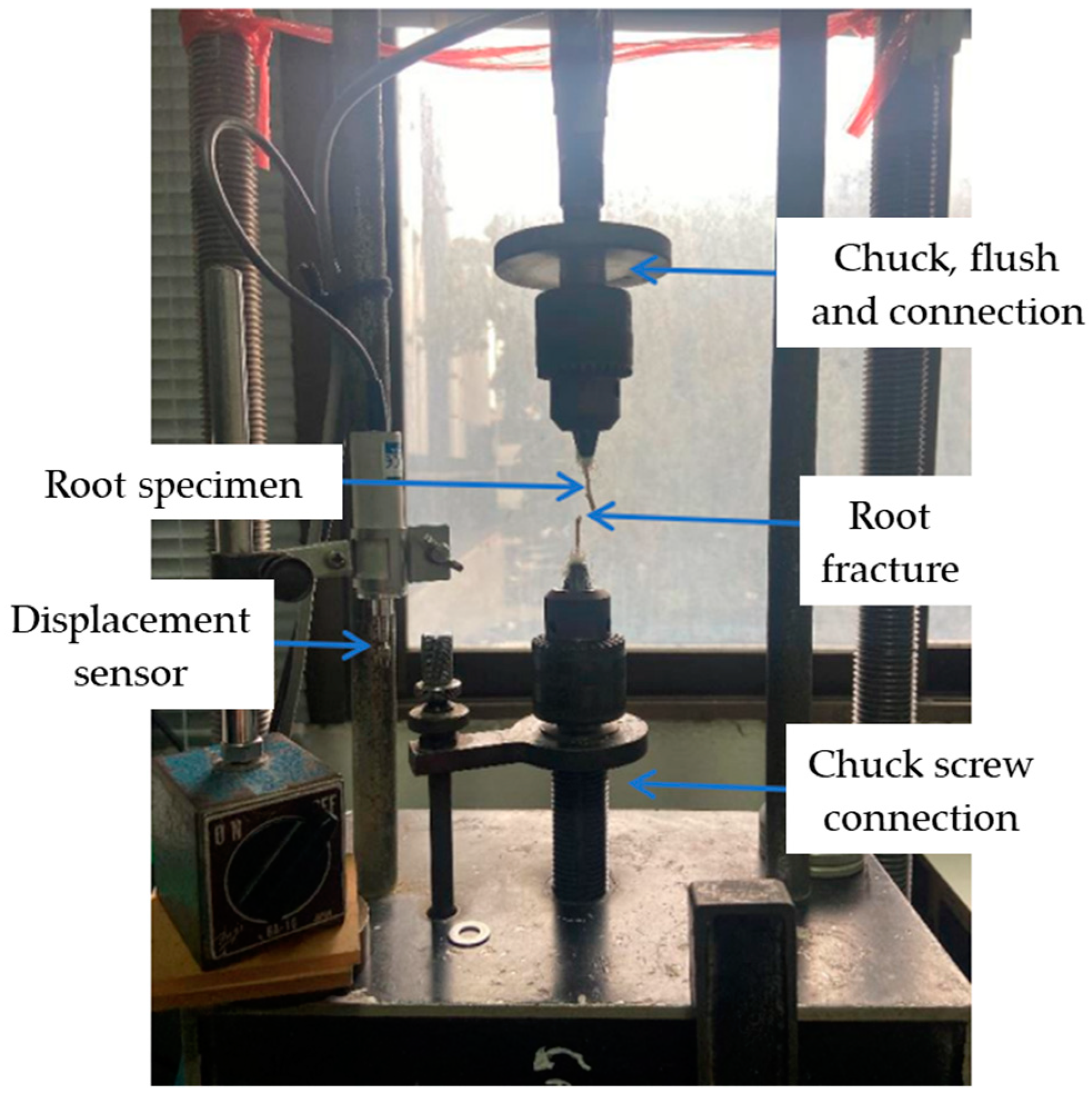
2.2. Measurements of Root Biomechanical Properties
3. Results and Discussions
3.1. Root Morphology and Architecture
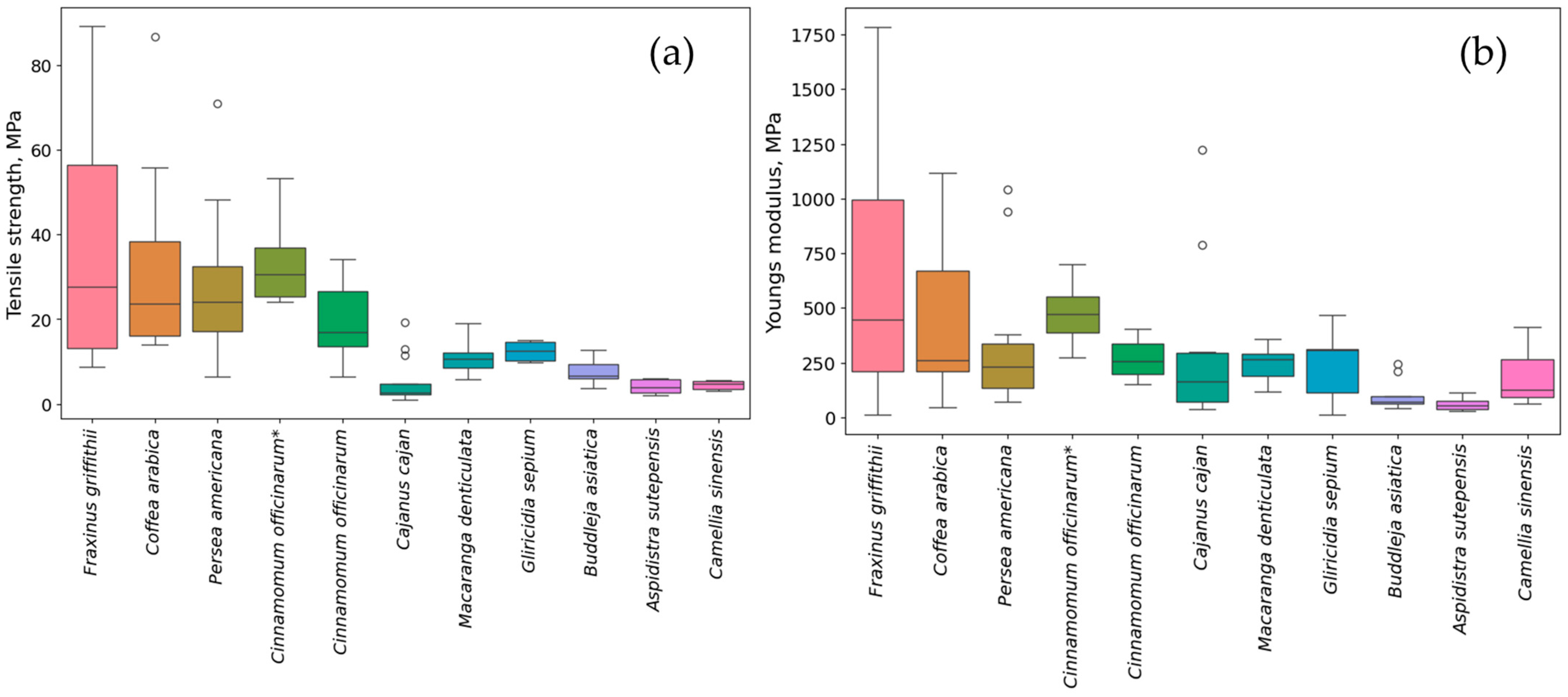
3.2. Root Tensile Strength
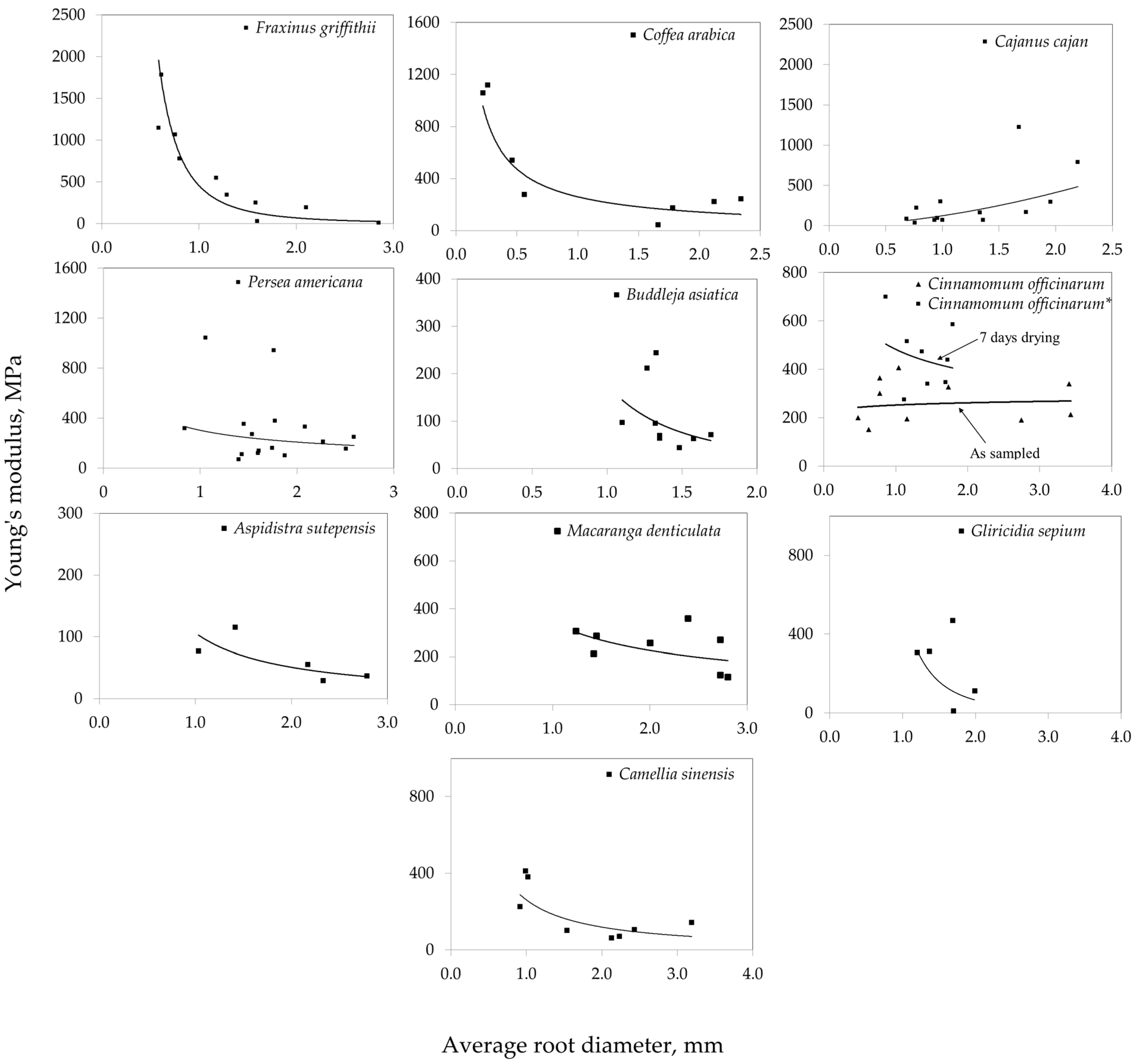
3.3. Young’s Modulus of Roots
3.4. Plant Response to Biotic and Abiotic Stresses
| Plant Species | Advantage | Disadvantage | References |
|---|---|---|---|
| Gliricidia sepium | Drought, frost and saline tolerant, enhancing soil fertility, resistant to rust and leaf spot diseases and stem borer insect. | Does not survive in waterlogged conditions | [54,55,56] |
| Persea americana | Improve soil physical properties, increase water holding capacity (WHC), mitigate nutrient loss. | Sensitive to water quality and few irrigations, drought, low soil fertility, susceptible to coffee berry disease. | [56,57,62] |
| Fraxinus griffithii | Drought and saline tolerant. | Requires ample space due to large canopy. | |
| Coffea arabica | Direct sunlight not necessary. | Susceptible to Xylotrechus quadripes, Xylosandrus compactus, and Hemileia vastatrix | [59] |
| Buddleja asiatica | Antifungal, antibacterial, and cytotoxic properties against rat, resistant against six bacteria and two fungi. | Sensitive to cold and dry/drought stress. | [59,60,63] |
| Macaranga denticulata | Antioxidant properties, survive in adverse soil and environment, have complex symbiotic mycorrhizal relationship that increase nutrient uptake. | Difficult management and hand weeding needed. | [64] |
| Cinnamomum officinarum | Antioxidant properties | No report found | |
| Aspidistra sutepensis | Grows under diverse environment | Does not survive in waterlogged soil. | [65] |
| Cajanus cajan | Drought, frost, saline tolerant, enhancing soil fertility, protest rust, leaf spot and stem borer. | Does not survive in waterlogged conditions | [65] |
| Camellia sinensis | Minimum runoff, high organic matter and water holding capacity, natural habitat. | Need a combination of cover crops, susceptible to Xylotrechus quadripes, Xylosandrus compactus, and Hemileia vastatrix | [59,66] |
4. Conclusions
- G. sepium, F. griffithii, P. americana, B. asiatica, and C. arabica possessed H-type roots and wide lateral spread. M. denticulata and C. officinarum demonstrated VH-type roots with deep taproots in addition to their lateral extent. A. sutepensis showed M-type roots with most root matrix in the top 0.3m, whereas C. cajan and C. sinensis had R-type roots with deep, oblique growth. This information is crucial for strategically arranging slope vegetation, enabling a more robust bioengineering system through diversified root reinforcement patterns and a variety of plant usages.
- The root tensile strength and Young’s modulus–diameter relationships of the ten species highlighted two different trends. For nine of the species tested, P. americana, F. griffithii, M. denticulata, G. sepium, A. sutepensis, C. arabica, C. sinensis, C. officinarum, and B. asiatica demonstrated a decrease in tensile strength with the diameter, the commonly observed negative power law relationship with the exponent values ranging from −0.187 to −1.983. Conversely, C. cajan exhibited an increase in tensile strength with the diameter, the positive power law with the exponent value of 1.5474 diverging from the typical trend.
- Plant species with relatively high values of 1-mm root tensile strength (exceeding 24 to 42 MPa) include P. americana, F. griffithii, C. officinarum, and C. arabica. Species exhibiting intermediate root tensile strength (ranging from 8 to 19 MPa) comprise M. denticulata, G. sepium, and B. asiatica. Conversely, A. sutepensis, C. cajan, and C. sinensis demonstrate the lowest root tensile strength, up to 7 MPa.
- It is recommended to design slope vegetation by strategically selecting species with diverse root morphologies and mechanical properties, as well as incorporating a variety of plant species that offer multiple benefits. This approach addresses both the engineering and socioeconomic requirements of the nature-based solution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadhoke, P. Walking the Middle Path of Food Sovereignty, Food Security, Nutrition, and Health in Chiang Mai Province, Thailand. In Transformations of Global Food Systems for Climate Change Resilience: Addressing Food Security, Nutrition, and Health; CRC PRESS: Boca Raton, FL, USA, 2023; pp. 263–289. [Google Scholar] [CrossRef]
- Jotisankasa, A.; Jamrueang, W.; Pramusandi, S.; Semmad, S.; Pilumwong, J. Field Observations of Soil Moisture, Suction and Movement of Cornfield in Tropical Highland with and without Vetiver System. In Proceedings of the 8th International Conference on Unsaturated Soils (UNSAT 2023), E3S Web of Conference, Milos, Greece, 2–5 May 2023; Volume 382, p. 24004. [Google Scholar] [CrossRef]
- Coppin, N.J.; Barker, D.L.; Richards, I. Use of Vegetation in Civil Engineering; Butterworths: London, UK, 1990. [Google Scholar]
- Gray, D.H.; Sotir, R.B. Biotechnical and Soil Bioengineering Slope Stabilization: A Practical Guide for Erosion Control; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Stokes, A.; Norris, J.E.; Van Beek, L.P.H.; Bogaard, T.; Cammeraat, E.; Mickovski, S.B.; Jenner, A.; Di Iorio, A.; Fourcaud, T. How Vegetation Reinforces Soil on Slopes. Slope Stab. Eros. Control Ecotechnol. Solut. 2008, 4, 65–118. [Google Scholar]
- Stokes, A.; Douglas, G.B.; Fourcaud, T.; Giadrossich, F.; Gillies, C.; Hubble, T.; Kim, J.; Loades, K.W.; Mao, Z.; Mclvor, I.R.; et al. Ecological mitigation of hillslope instability: Ten key issues facing researchers and practitioners. Plant Soil 2014, 377, 1–23. [Google Scholar] [CrossRef]
- Jotisankasa, A.; Sirirattanachat, T. Effects of Grass Roots on Soil-Water Retention Curve and Permeability Function. Can. Geotech. J. 2017, 54, 1612–1622. [Google Scholar] [CrossRef]
- Mahannopkul, K.; Jotisankasa, A. Influence of Root Suction on Tensile Strength of Chrysopogon zizanioides Roots and Its Implication on Bioslope Stabilization. J. Mt. Sci. 2019, 16, 275–284. [Google Scholar] [CrossRef]
- Inui, T.; Chau, C.; Soga, K.; Nicolson, D.; O’Riordan, N. Embodied Energy and Gas Emissions of Retaining Wall Structures. J. Geotech. Geoenviron. Eng. 2011, 137, 958–967. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, T.W.; Cai, C.F.; Li, Z.X.; Cheng, D.B. Effects of Vegetation on Runoff Generation, Sediment Yield and Soil Shear Strength on Road-Side Slopes under a Simulation Rainfall Test in the Three Gorges Reservoir Area, China. Sci. Total Environ. 2014, 485, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, F.; Zheng, F.; Zhang, Q. Effects and Mechanisms of Erosion Control Techniques on Stairstep Cut-Slopes. Sci. Total Environ. 2019, 656, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Fourcaud, T.; Jourdan, C.; Maeght, J.L.; Mao, Z.; Metayer, J.; Meylan, L.; Pierret, A.; Rapidel, B.; Roupsard, O.; et al. Vegetation as a Driver of Temporal Variations in Slope Stability: The Impact of Hydrological Processes. Geophys. Res. Lett. 2017, 44, 4897–4907. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Ai, Y.; Chen, J.; Luo, X.; Chen, J.; Zhong, S. Effects and Mechanisms of Revegetation Modes on Cadmium and Lead Pollution in Artificial Soil on Railway Rock-Cut Slopes. Sci. Total Environ. 2018, 644, 1602–1611. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Li, Y.; Yu, H. Vetiver Grass Hedgerows Significantly Reduce Nitrogen and Phosphorus Losses from Fertilized Sloping Lands. Sci. Total Environ. 2019, 661, 86–94. [Google Scholar] [CrossRef]
- Grima, N.; Edwards, D.; Edwards, F.; Petley, D.; Fisher, B. Landslides in the Andes: Forest can provide cost-effective landslide regulation services. Sci. Total Environ. 2020, 745, 141128. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Leung, A.K.; Boldrin, D.; Ganesan, S.P. Variability in root biomechanics of Chrysopogon zizanioides for soil eco-engineering solutions. Sci. Total Environ. 2021, 776, 145943. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Leung, A.K.; Ni, J.J. Plant-Soil Slope Interaction; Taylor & Francis: New York, NY, USA, 2019. [Google Scholar]
- Docker, B.B.; Hubble, T.C.T. Quantifying root-reinforcement of river bank soil by four Australian tree species. Geomorphology 2008, 100, 401–418. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Root biomechanical properties during establishment of woody perennials. Ecol. Eng. 2017, 109, 196–206. [Google Scholar] [CrossRef]
- Styczen, M.E.; Morgan, R.P.C. Engineering properties of vegetation. In Slope Stabilization and Erosion Control: A Bioengineering Approach; Morgain, R.P.C., Rickson, R.J., Eds.; E & F N Spon: London, UK, 1995. [Google Scholar]
- Yen, C. Tree Root Patterns and Erosion Control. 1987. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19930667066 (accessed on 13 June 2024).
- Fan, C.-C.; Chen, Y.-W. The effect of root architecture on the shearing resistance of root-permeated soils. Ecol. Eng. 2010, 36, 813–826. [Google Scholar] [CrossRef]
- Capilleri, P.P.; Motta, E.; Raciti, E. Experimental Study on Native Plant Root Tensile Strength for Slope Stabilization. Procedia Eng. 2016, 158, 116–121. [Google Scholar] [CrossRef]
- Persichillo, M.G.; Bordoni, M.; Meisina, C. The role of land use changes in the distribution of shallow landslides. Sci. Total Environ. 2017, 574, 924–937. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Norris, J.E.; Di Iorio, A.; Stokes, A.; Nicoll, B.C.; Achim, A. Species selection for soil reinforcement and protection. In Slope Stability and Erosion Control: Ecotechnological Solutions; Norris, J.E., Stokes, A., Mickovski, S.B., Cammeraat, E., van Beek, R., Nicoll, B.C., Achim, A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 167–210. [Google Scholar]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.-F.; van Beek, R. The Influence of Cellulose Content on Tensile Strength in Tree Roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, Y.; McCormack, M.L.; Rowe, N.; Deng, X.; Yang, X.; Xia, S.; Nespoulous, J.; Sidle, R.C.; Guo, D.; et al. Mechanical traits of fine roots as a function of topology and anatomy. Ann. Bot. 2018, 122, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Loades, K.W.; Bengough, A.G.; Bransby, M.F.; Hallett, P.D. Biomechanics of nodal, seminal and lateral roots of barley: Effects of diameter, waterlogging and mechanical impedance. Plant Soil 2013, 370, 407–418. [Google Scholar] [CrossRef]
- Ghestem, M.; Cao, K.; Ma, W.; Rowe, N.; Leclerc, R.; Gadenne, C.; Stokes, A. A framework for identifying plant species to be used as ‘Ecological Engineers’ for fixing soil on unstable slopes. PLoS ONE 2014, 9, e95876. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Effects of root de-hydration on bio-mechanical properties of woody roots of U. europaeus. Plant Soil 2018, 431, 347–369. [Google Scholar] [CrossRef]
- Hales, T.C.; Miniat, C.F. Soil moisture causes dynamic adjustments to root reinforcement that reduce slope stability. Earth Surf. Process. Landf. 2016, 42, 803–813. [Google Scholar] [CrossRef]
- Zhang, C.B.; Zhou, X.; Jiang, J.; Wei, Y.; Ma, J.; Hallett, P.D. Root moisture content influence on root tensile tests of herbaceous plants. Catena 2019, 172, 140–147. [Google Scholar] [CrossRef]
- Loades, K.W.; Bengough, A.G.; Bransby, M.F.; Hallett, P.D. Effect of root age on the biomechanics of seminal and nodal roots of barley (Hordeum vulgare L.) in contrasting soil environments. Plant Soil 2015, 395, 253–261. [Google Scholar] [CrossRef]
- Cofie, P.; Koolen, A.J. Test speed and other factors affecting the measurements of tree root properties used in soil reinforcement models. Soil Tillage Res. 2001, 63, 51–56. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Jiang, J.; Zhou, S. Effects of gauge length and strain rate on the tensile strength of tree roots. Trees 2012, 26, 1577–1584. [Google Scholar] [CrossRef]
- Truong, P.; Van, T.T.; Pinners, E. Vetiver System Applications: Technical Reference Manual. Vetiver Netw. Int. 2008, 89. [Google Scholar]
- Cuong, D.C.; Truong, P.; Van Minh, V.; Minh, P.T.; Truong, P.N. Confusion between Chrysopogon nemoralis and Chrysopogon zizanioides at Bo Bo Mountain in Quang Nam Province, Vietnam. Available online: https://d1wqtxts1xzle7.cloudfront.net/88699934/2_20Doan_20Chi_20Cuong_20Paper-libre.pdf?1658101759=&response-content-disposition=inline%3B+filename%3D1_CONFUSION_BETWEEN_Chrysopogon_nemorali.pdf&Expires=1721788768&Signature=FKeOhqDSU6j4XPbuFlujtkUoam81QeLjFizuQgDIks9j1HmEJnpqANThkoaW4~-ykMIABiLi~rljJuRgc05uIhTOZbx3xvl0eOdvNFiCHqX4PDlXlIlk9JKaC0S-XKTzPRUu3I994MPab0qY2yUGkKDdQL5kR4fJWL1DhrwDfjvXfYKzAGQKyOYOMcE3DTucDhdXrZAT0P-coegf2JyVbOSd05XdZG2vJ--5Kv5NGMPZ3IsKM8kQq9Zpjf7JFAAgj8hWtp5JGQOi16rOfc32NaRDm2GYl9DcPMBySvx4Ybljc56Eh1XgC3pyyUcH~ZbTwXcRI8KuzBJJgVvmgRmviA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 13 June 2024).
- Lewis, L. Soil Bioengineering: An Alternative for Roadside Management; US Department of Agriculture, Forest Service, Technology & Development Program: Washington, DC, USA, 2000.
- Zhang, Z.; Fan, B.; Song, C.; Zhang, X.; Zhao, Q.; Ye, B. Advances in Root System Architecture: Functionality, Plasticity, and Research Methods. J. Resour. Ecol. 2023, 14, 15–24. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhang, X.J.; Wu, C. Advances in experimental methods for root system architecture and root development. J. For. Res. 2015, 26, 23–32. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 9 May 2024).
- Chang, M.C.; Qiu, L.Q.; Green, P.S. Oleaceae. In Flora of China; Wu, C.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1996; Volume 15, pp. 272–319. [Google Scholar]
- Li, X.W.; Li, J.; Huang, P.H.; Wei, F.N.; Cui, H.B.; van der Werff, H. Lauraceae. In Flora of China; Wu, C.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 7, pp. 102–254. [Google Scholar]
- Keng, H. Theaceae. In Flora of Thailand; Smitinand, T., Larsen, K., Eds.; The Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 1972; Volume 2, Part 2, pp. 142–158. [Google Scholar]
- Bartholomew, B. Theaceae. In Flora of China; Wu, C.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 12, pp. 366–478. [Google Scholar]
- Ding, H.B.; Xiong, K.C.; Yang, B.; Yin, J.T.; Bouamanivong, S.; Tani, Y.H. New species and taxonomic notes of Aspidistra (Asparagaceae) for the flora of China and Laos. Taiwania 2021, 66, 439–449. [Google Scholar] [CrossRef]
- van Welzen, P.C.; Chayamarit, K. Euphorbiaceae (Genera G–Z). In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; The Forest Herbarium, National Park, Wildlife and Plant Conservation Department: Bangkok, Thailand, 2007; Volume 8, Part 2, pp. 305–592. [Google Scholar]
- Yang, Y.; Chen, L.; Li, N.; Zhang, Q. Effect of root moisture content and diameter on root tensile properties. PLoS ONE 2016, 11, e0151791. [Google Scholar] [CrossRef]
- Nilaweera, N.S. Effects of Tree Roots on Slope Stability—The Case of Khao Luang Mountain Area, Southern Thailand. Ph.D. Thesis, Asian Institute of Technology, Pathum Thani, Thailand, 1994; p. 452. [Google Scholar]
- Nilaweera, N.S.; Nutalaya, P. Role of tree roots in slope stabilisation. Bull. Eng. Geol. Environ. 1999, 57, 337–342. [Google Scholar] [CrossRef]
- Gargi, B.; Semwal, P.; Jameel Pasha, S.B.; Singh, P.; Painuli, S.; Thapliyal, A.; Cruz-Martins, N. Revisiting the Nutritional, Chemical and Biological Potential of Cajanus cajan (L.) Millsp. Molecules 2022, 27, 6877. [Google Scholar] [CrossRef]
- Fagbola, O.; Osonubi, O.; Mulongoy, K.; Odunfa, S. Effects of drought stress and arbuscular mycorrhiza on the growth of Gliricidia sepium (Jacq). Walp, and Leucaena leucocephala (Lam.) de Wit. in simulated eroded soil conditions. Mycorrhiza 2001, 11, 215–223. [Google Scholar] [CrossRef]
- Wartenberg, A.C.; Blaser, W.J.; Roshetko, J.M.; Van Noordwijk, M.; Six, J. Soil fertility and Theobroma cacao growth and productivity under commonly intercropped shade-tree species in Sulawesi, Indonesia. Plant Soil 2020, 453, 87–104. [Google Scholar] [CrossRef]
- Elevitch, C.R.; Francis, J.K. Gliricidia sepium (gliricidia). Species Profiles Pac. Isl. Agrofor. 2006, 2, 1–8. [Google Scholar]
- Juma, I. Production, local trade and diversity of avocado (Persea americana Mill.) in the southern highlands of Tanzania. Acta Univ. Agric. Sueciae 2020, 2020, 26. [Google Scholar]
- Sina, B.; Demissie, H.; Rezene, Y. Assessment of the Constraints and Challenges in Avocado (Persea americana Mill.) Production and Marketing in Southern Ethiopia. Int. J. Fruit Sci. 2024, 24, 60–72. [Google Scholar] [CrossRef]
- Liao, Y.H.; Houghton, P.J.; Hoult, J.R. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J. Nat. Prod. 1999, 62, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Mishra, D.; Khetwal, K.S.; Bisht, G. Antibacterial and Antifungal Properties of Crude Extracts of Buddleja asiatica L. Aerial Parts. J. Pharm. Res. 2011, 4, 2282. [Google Scholar]
- Schaefer, J.W.; Dhar, A.; Chanasyk, D.S.; Naeth, M.A. Reclamation of an eroded lakeshore slope using small vegetation islands and terraces. Ecol. Eng. 2024, 206, 107327. [Google Scholar] [CrossRef]
- Atucha, A.; Merwin, I.A.; Brown, M.G.; Gardiazabal, F.; Mena, F.; Adriazola, C.; Lehmann, J. Soil erosion, runoff and nutrient losses in an avocado (Persea americana Mill) hillside orchard under different groundcover management systems. Plant Soil 2013, 368, 393–406. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Watt, M.S.; Potter, K.J.; Manning, L.K.; Alexander, N.S.; Tallent-Halsell, N. Managing invasive weeds under climate change: Considering the current and potential future distribution of Buddleja davidii. Weed Res. 2011, 51, 85–96. [Google Scholar] [CrossRef]
- Changkija, S.; Thakuria, D.; Cynthia, A. Traditional Use of Macaranga Trees for Soil Fertility: By Naga shifting cultivators in northeast India. In Farmer Innovations and Best Practices by Shifting Cultivators in Asia-Pacific; CABI: New York, NY, USA, 2023; pp. 557–570. [Google Scholar]
- Dwivedi, A.K.; Tripathi, B.D. Pollution tolerance and distribution pattern of plants in surrounding area of coal-fired industries. J. Environ. Biol. 2007, 28, 257–263. [Google Scholar]
- Sahoo, D.C.; Madhu, M.G.; Bosu, S.S.; Khola, O.P. Farming methods impact on soil and water conservation efficiency under tea [Camellia sinensis (L.)] plantation in Nilgiris of South India. Int. Soil Water Conserv. Res. 2016, 4, 195–198. [Google Scholar] [CrossRef]





| Plant Species | Family | Site Cordinates | Age, Year | Height, m | Tree Perimeter, cm | Longest Measurable Root Length, m | Type of Root * |
|---|---|---|---|---|---|---|---|
| Persea americana | Lauraceae | 17.507069°,98.216669° | 10 | 1.35 | 31 | 3.37 | H |
| Fraxinus griffithii | Oleaceae | 17.503434°,98.216081° | 2 | 2.2 | 5.87 | 2.3 | H |
| Cajanus cajan | Fabaceae | 17.519346°,98.098063° | 0.75 | 3 | 19.64 | 1.3 | R |
| Cinnamomum officinarum | Lauraceae | 17.503434°,98.216081° | 2 | 2.35 | 16 | 4.5 | VH |
| Camellia sinensis | Theaceae | 20.152026°,99.616434° | 35 | 0.72 | 29.42 | 0.9 | R |
| Aspidistra sutepensis | Asparagaceae | 20.148751°,99.614794° | 2 | 0.82 | NA | 0.3 | M |
| Coffea arabica | Rubiaceae | 20.148751°,99.614794° | 2 | 2.26 | 5.76 | 1.07 | H |
| Gliricidia sepium | Fabaceae | 17.860048°,97.842293° | 2 | 3.65 | 13.72 | 1.5 | H |
| Macaranga denticulata | Euphorbiaceae | 17.861017°,97.841098° | Unknown | 1.8 | 5.59 | 2.1 | VH |
| Buddleja asiatica | Scrophulariaceae | 17.861017°,97.841098° | Unknown | 2.55 | 5.2 | 1.4 | H |
| Scientific Names | Distributions | Native/Exotic Species | Invasive/Non-Invasive Species | References |
|---|---|---|---|---|
| Persea americana Mill. | Central Mexico to Costa Rica. | It is a non-invasive exotic species and is cultivated as a fruit tree, especially in the highland areas of northem Thailand. | Non-invasive species | [43] |
| Fraxims griffinh C. B. Clarke | Bangladesh to Nansei-shoto, Java to Lesser Sunda Islands (Bali). | It is a non-invasive exotic species and is cultivated for its wood in the highland habitats of northem Thailand | Non-invasive species | [43,44] |
| Cajanus cajan (L.) Huth | Indian Subcontinent. | It is a non-invasive exotic species and is used as animal food. | Non-invasive species | [43] |
| Cirnamonum officinarian (L.) J. Presl | Korea (Jeju-do), W. Central & S. Japan to E. & S. Taiwan. | It is a non-invasive exotic species. | Non-invasive species | [43,45] |
| Camellia sinensis (L.) Kuntze | E. Himalaya to S. China and N. Indo-China, Hainan. | Camellia sinensis (L.) Kuntze var. assamica (Royle ex Hook.) Steenis is a native variety. Camellia sinensis (L.) Kuntze var. sinensis (Royle ex Hook) Steenis is a non-invasive exotic variety. The native range of this variety is S. China. | Unknown | [43,46,47] |
| Aspidistra sutepensis K. Larsen | China (Yunnan) to N. Thailand. | It is anative species. | Unknown | [43,48] |
| Coffee arabica L. | E. South Sudan, SW. Ethiopia, N. Kenya (Mt. Marsibit). | It is a non-invasive exotic species and is cultivated for its seeds. | Non-invasive species | [43] |
| Gliricidia sepium (Jacq.) Kunth | Mexico to Colombia. | It is a non-invasive exotic species. | Non-invasive species | [43] |
| Macaranga denticulata (Blume) Müll. Arg. | S. China to Tropical Asia. | It is a native species. | Unknown | [43,49] |
| Buddleja asiatica Lour. | Central & S. China to Tropical Asia and Marianas. | It is a native species. | Invasive species | [43] |
| Chrysopogon zizanioides (L.) Roberty Poaceae | NE. India to Indo-China. | It is a native species. | Unknown | [43] |
| Plant Species | Diameter Range, (mm) | No of Samples, n | Average Tensile Strength, (MPa) | Average Young’s Modulus, (MPa) | Fitting Equation, R2 and p-Values | |
|---|---|---|---|---|---|---|
| Tensile Strength | Young’s Modulus | |||||
| Persea americana | 0.84–2.59 | 16 | 26.99 | 309.74 | Tr = 39.838d−1.109 (R2 = 0.277, p = 0.036) | Mr = 302.81d−0.538 (R2 = 0.044, p = 0.437) |
| Fraxinus griffithii | 0.58–2.85 | 10 | 36.82 | 615.63 | Tr = 34.442d−1.414 (R2 = 0.815, p = 0.0003) | Mr = 455.65d−2.71 (R2 = 0.768, p = 0.0009) |
| Cajanus cajan | 0.68–2.20 | 13 | 5.53 | 276.97 | Tr = 2.9691d1.5474 (R2 = 0.472, p = 0.0095) | Mr = 124.24d1.732 (R2 = 0.436, p = 0.014) |
| Cinnamomum officinarum* | 0.86–1.79 | 8 | 33.37 | 476.03 | Tr = 42.002d−0.884 (R2 = 0.617, p = 0.021) | Mr = 482.87d−0.297 (R2 = 0.061, p = 0.743) |
| Cinnamomum officinarum | 0.48–3.43 | 10 | 19.11 | 269.28 | Tr = 17.89d−0.187 (R2 = 0.066, p = 0.472) | Mr = 252.63d0.0523 (R2 = 0.012, p = 0.760) |
| Camellia sinensis | 0.92–3.19 | 8 | 4.48 | 187.94 | Tr = 5.2934d−0.395 (R2 = 0.514, p = 0.045) | Mr = 260.97d−1.135 (R2 = 0.555, p = 0.034) |
| Aspidistra sutepensis | 1.03–2.78 | 5 | 4.06 | 62.90 | Tr = 6.3501d−0.882 (R2 = 0.569, p = 0.141) | Mr = 106.23d−1.064 (R2 = 0.618, p = 0.115) |
| Coffea arabica | 0.22–2.34 | 8 | 33.36 | 460.47 | Tr = 24.654d−0.483 (R2 = 0.506, p = 0.048) | Mr = 259.21d−0.865 (R2 = 0.646, p = 0.016) |
| Gliricidia sepium | 1.20–1.99 | 5 | 12.44 | 242.35 | Tr = 15.79d−0.566 (R2 = 0.331, p = 0.310) | Mr = 558.7d−3.087 (R2 = 0.1612, p = 0.503) |
| Macaranga denticulata | 1.24–2.80 | 8 | 10.97 | 242.07 | Tr = 16.995d−0.712 (R2 = 0.458, p = 0.065) | Mr = 343.67d−0.606 (R2 = 0.235, p = 0.224) |
| Buddleja asiatica | 1.10–1.69 | 9 | 7.40 | 106.82 | Tr =13.107d−1.983 (R2 = 0.4491, p = 0.048) | Mr = 176.91d−2.084 (R2 = 0.213, p = 0.211) |
| Plant Species | Soil Reingforcement | Benefits | ||||
|---|---|---|---|---|---|---|
| Tensile Strength | Root Density | General Use | Economic Use | Edible Fruits | Soil and Water Conservation | |
| Persea americana | High | Medium | Medium | High | High | Medim-High |
| Fraxinus griffithii | High | High | High | Unknown | Unknown | High |
| Cajanus cajan | Low | High | Medium | Unknown | High | Medium |
| Cinnamomum officinarum | Medium | High | High | Unknown | Medium | Medim-High |
| Camellia sinensis | Low | High | Low | High | High | Medium |
| Aspidistra sutepensis | Low | Medium | No | Unknown | High | Medium |
| Coffea arabica | High | High | Low | High | High | High |
| Gliricidia sepium | Medium | Medium | Medium | Unknown | Medium | Medium |
| Macaranga denticulata | Medium | Medium | Low | Unknown | Unknown | Medium |
| Buddleja asiatica | Medium | Medium | High | Unknown | Unknown | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mairaing, W.; Jotisankasa, A.; Leksungnoen, N.; Hossain, M.; Ngernsaengsaruay, C.; Rangsiwanichpong, P.; Pilumwong, J.; Pramusandi, S.; Semmad, S.; Ahmmed, A.N.F. A Biomechanical Study of Potential Plants for Erosion Control and Slope Stabilization of Highland in Thailand. Sustainability 2024, 16, 6374. https://doi.org/10.3390/su16156374
Mairaing W, Jotisankasa A, Leksungnoen N, Hossain M, Ngernsaengsaruay C, Rangsiwanichpong P, Pilumwong J, Pramusandi S, Semmad S, Ahmmed ANF. A Biomechanical Study of Potential Plants for Erosion Control and Slope Stabilization of Highland in Thailand. Sustainability. 2024; 16(15):6374. https://doi.org/10.3390/su16156374
Chicago/Turabian StyleMairaing, Warakorn, Apiniti Jotisankasa, Nisa Leksungnoen, Monir Hossain, Chatchai Ngernsaengsaruay, Prem Rangsiwanichpong, Jarunee Pilumwong, Sony Pramusandi, Surat Semmad, and Abu Noman Faruq Ahmmed. 2024. "A Biomechanical Study of Potential Plants for Erosion Control and Slope Stabilization of Highland in Thailand" Sustainability 16, no. 15: 6374. https://doi.org/10.3390/su16156374








