Performance of a Diesel Engine Fueled by Blends of Diesel Fuel and Synthetic Fuel Derived from Waste Car Tires
Abstract
:1. Introduction
2. Materials and Methods
- Density determination at 40 °C—DMA 4500 M; Anton Paar GmbH; Graz, Austria;
- Flash point determination—HFP 339; Petroleum Analyzer Company; Houston, TX, USA;
- Cold filter plugging point determination—FPP 5 Gs; Petroleum Analyzer Company; Houston, TX, USA;
- Determination of kinematic viscosity at 40 °C—HVU 482; Petroleum Analyzer Company; Houston, TX, USA;
- Heating value designation—IKA C5000; IKA-Werke GmbH & Co. KG; Staufen, Germany;
- Determination of derived cetane number—Cetane ID510; Petroleum Analyzer Company; Houston, TX, USA;
- Lubricity—HFRR PCS Instruments; PCS Instruments Ltd; London, UK;
- Determination of fractional composition (including determination of distillation curve)—OpiDist; Petroleum Analyzer Company; Houston, TX, USA;
- Sulfur content determination—ANTEK MultiTek; Petroleum Analyzer Company; Houston, TX, USA;
- Elemental analysis using the VARIO EL III (CHNS method).; Elementar Analysensysteme GmbH; Langenselbold, Germany
- GEXH—mass flow rate of exhaust gas in the exhaust system [kg/h];
- q—degree of dilution.
- GTOT—diluted exhaust gas flow [dm3/min];
- GDIL—dilution air flow [dm3/min].
- MPM—mass of particulate matter accumulated on the measurement filter [mg];
- MSAM—mass of diluted exhaust gas that flowed through the filter in the test [kg].
- Ne—brake power of the engine at the selected measurement point [kW].
- mgas—emission mass flow rates [g/h] of individual pollutants (NOx, HC);
- ugas—the ratio between the density of the exhaust gas component and the density of the exhaust gas [–] (for NOx ugas = 0.001587; for HC ugas = 0.00479);
- cgas—the concentration of a pollutant in the exhaust gas [ppm].
3. Results and Discussion
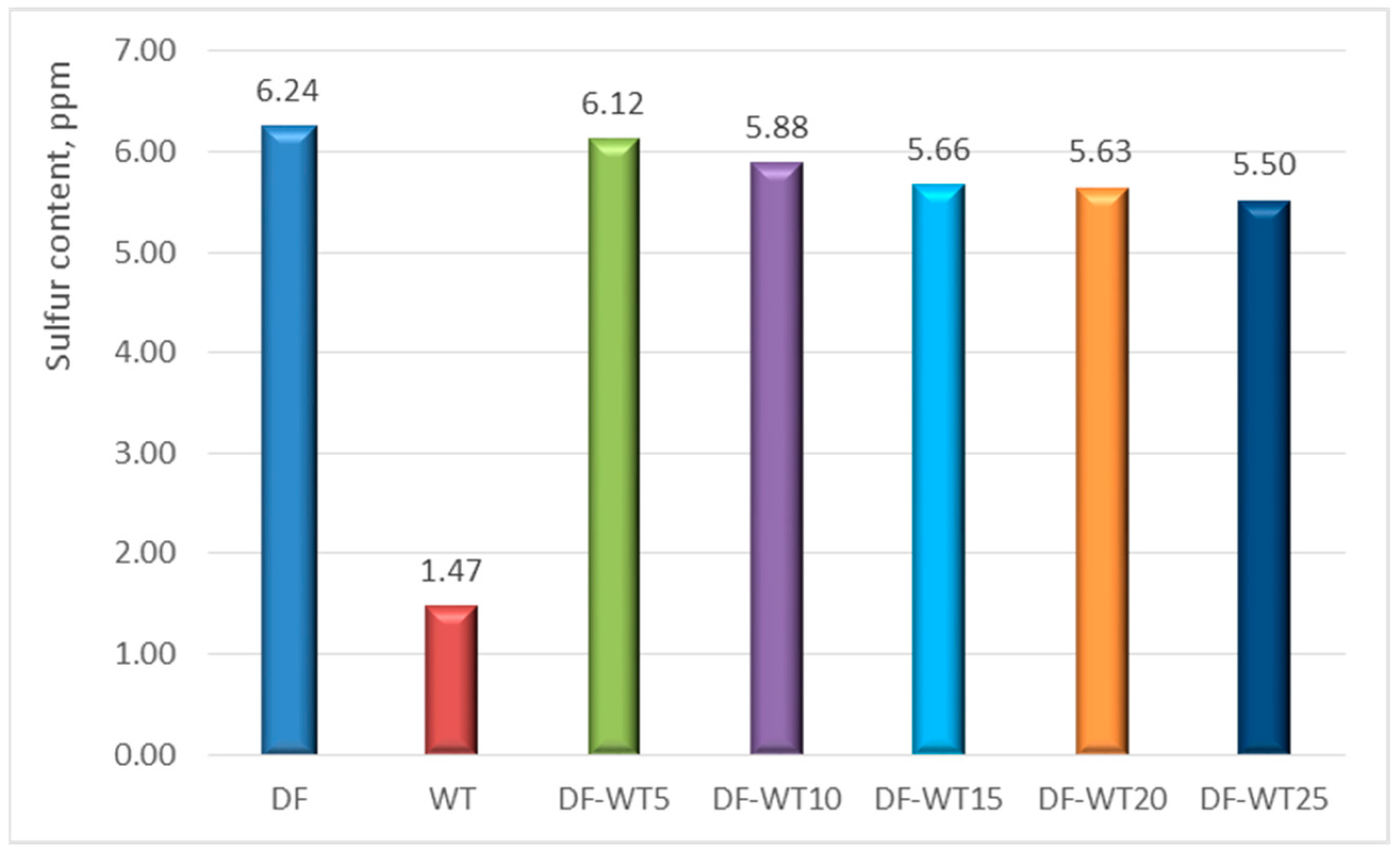
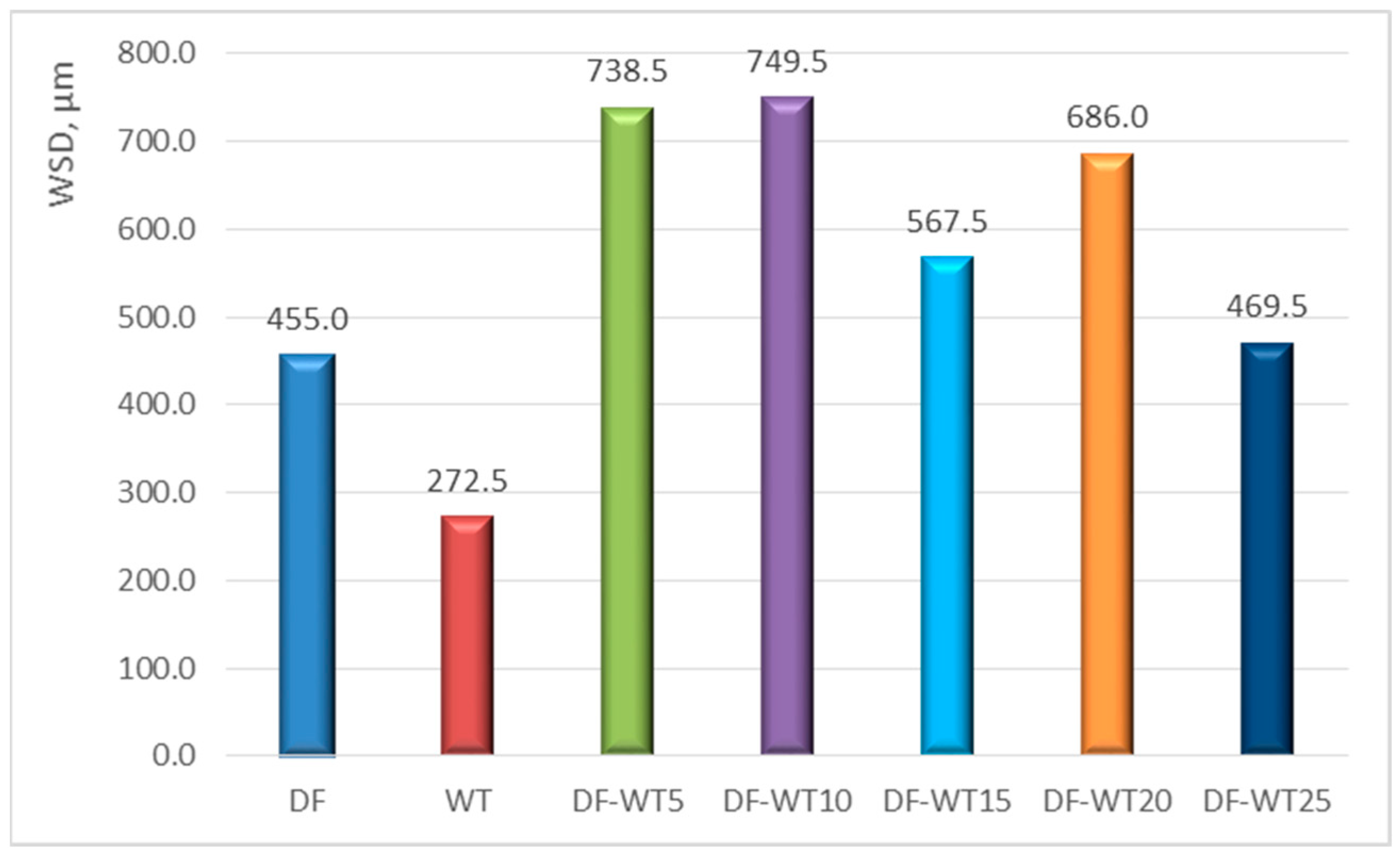
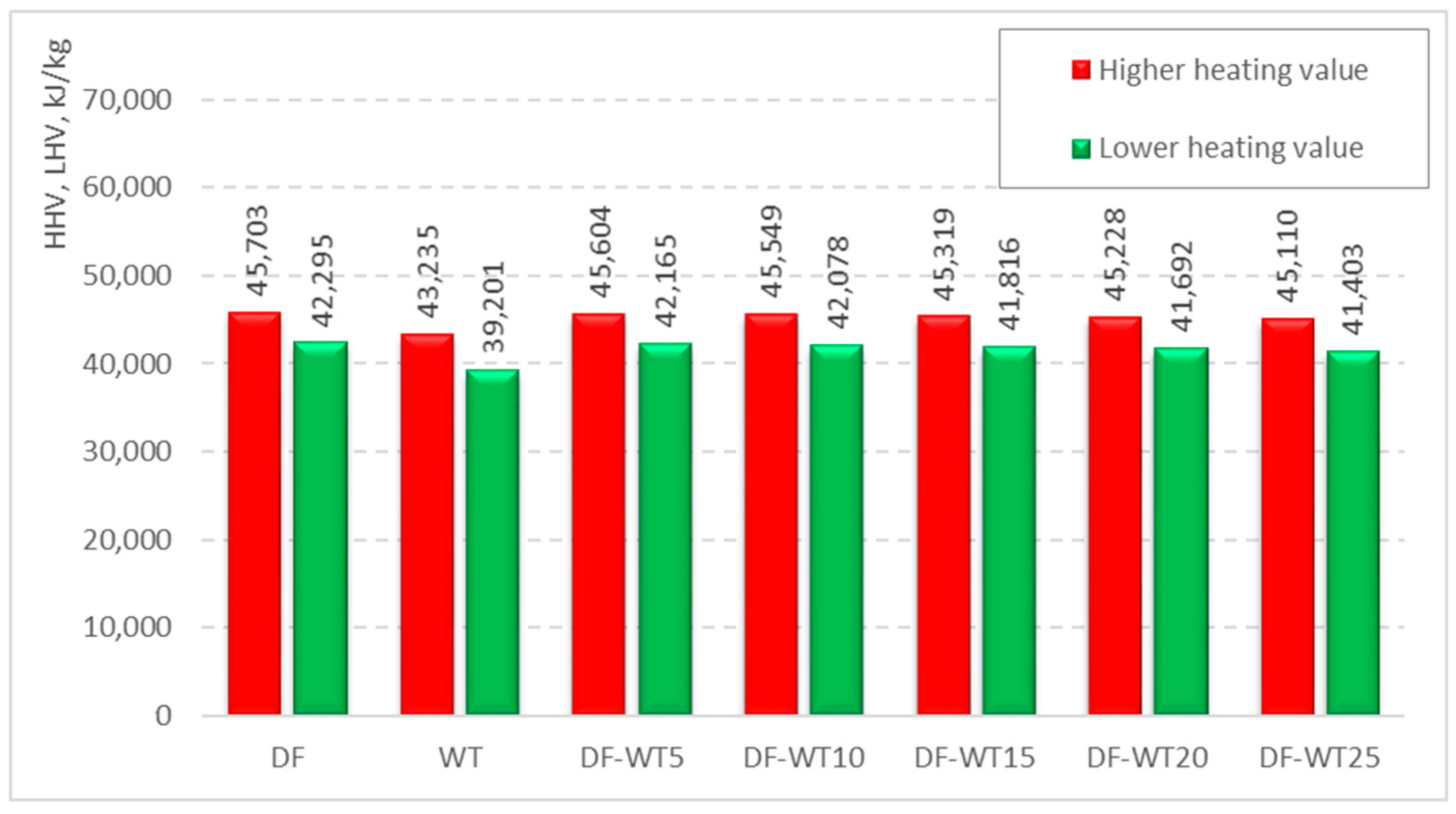
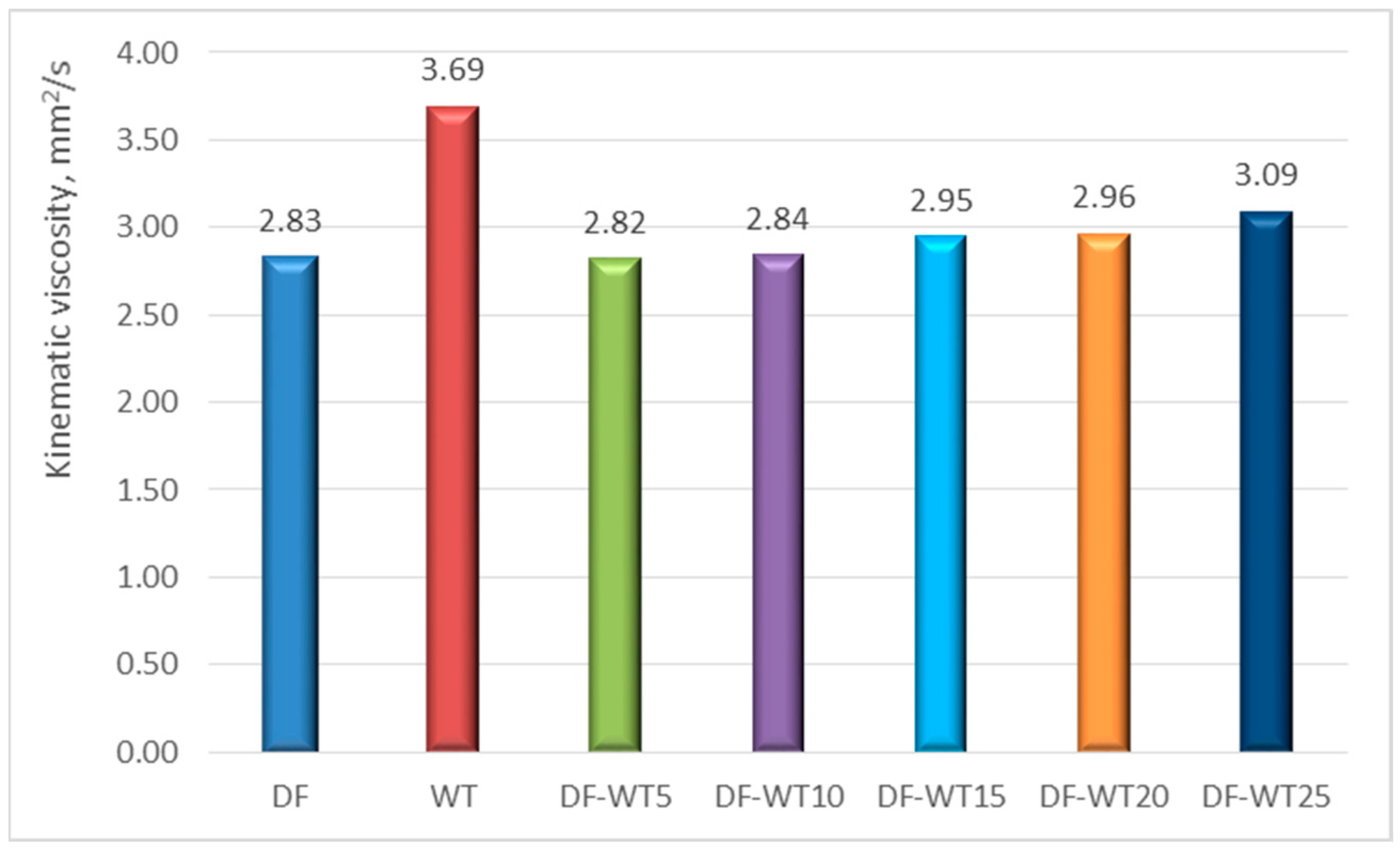
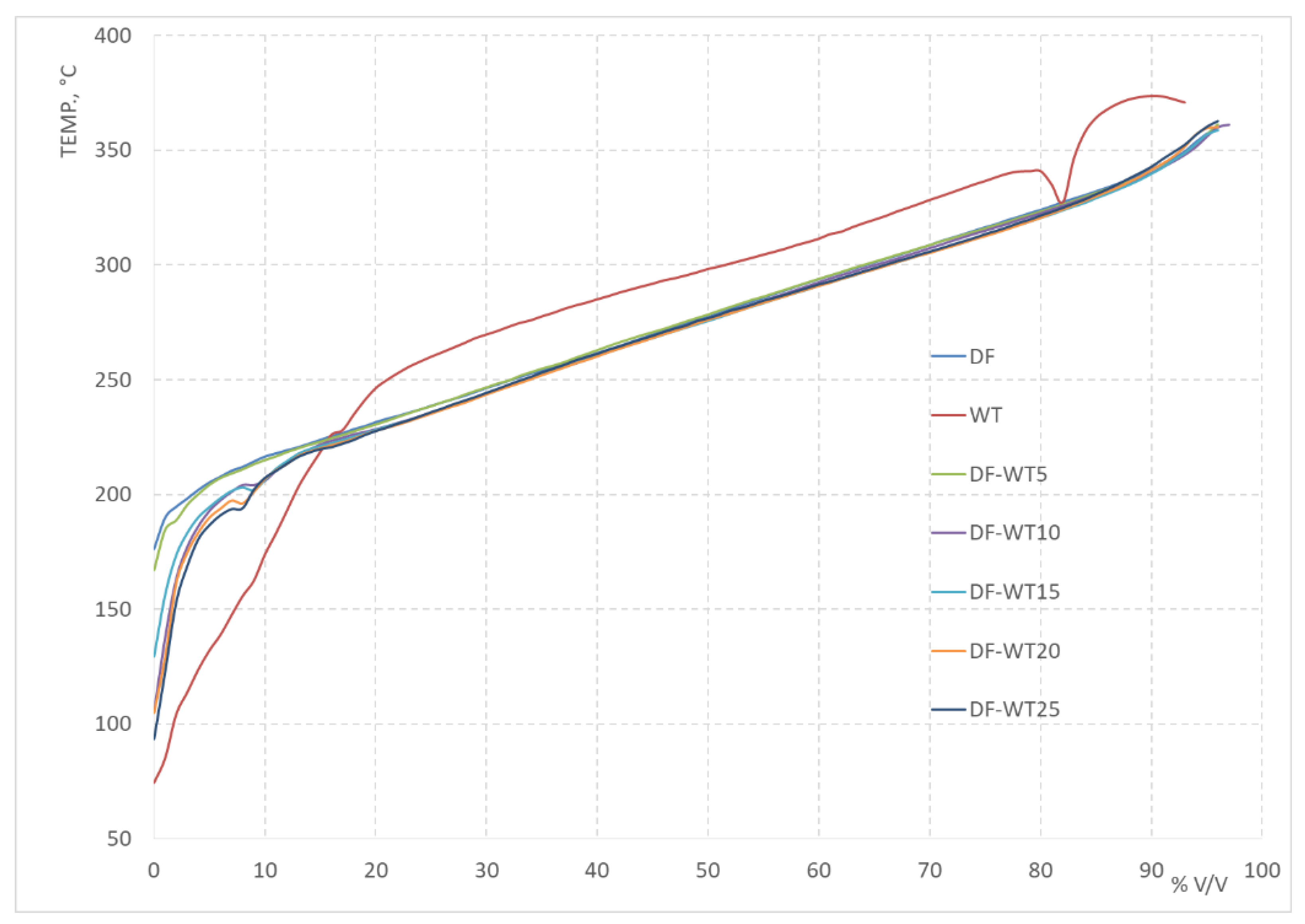
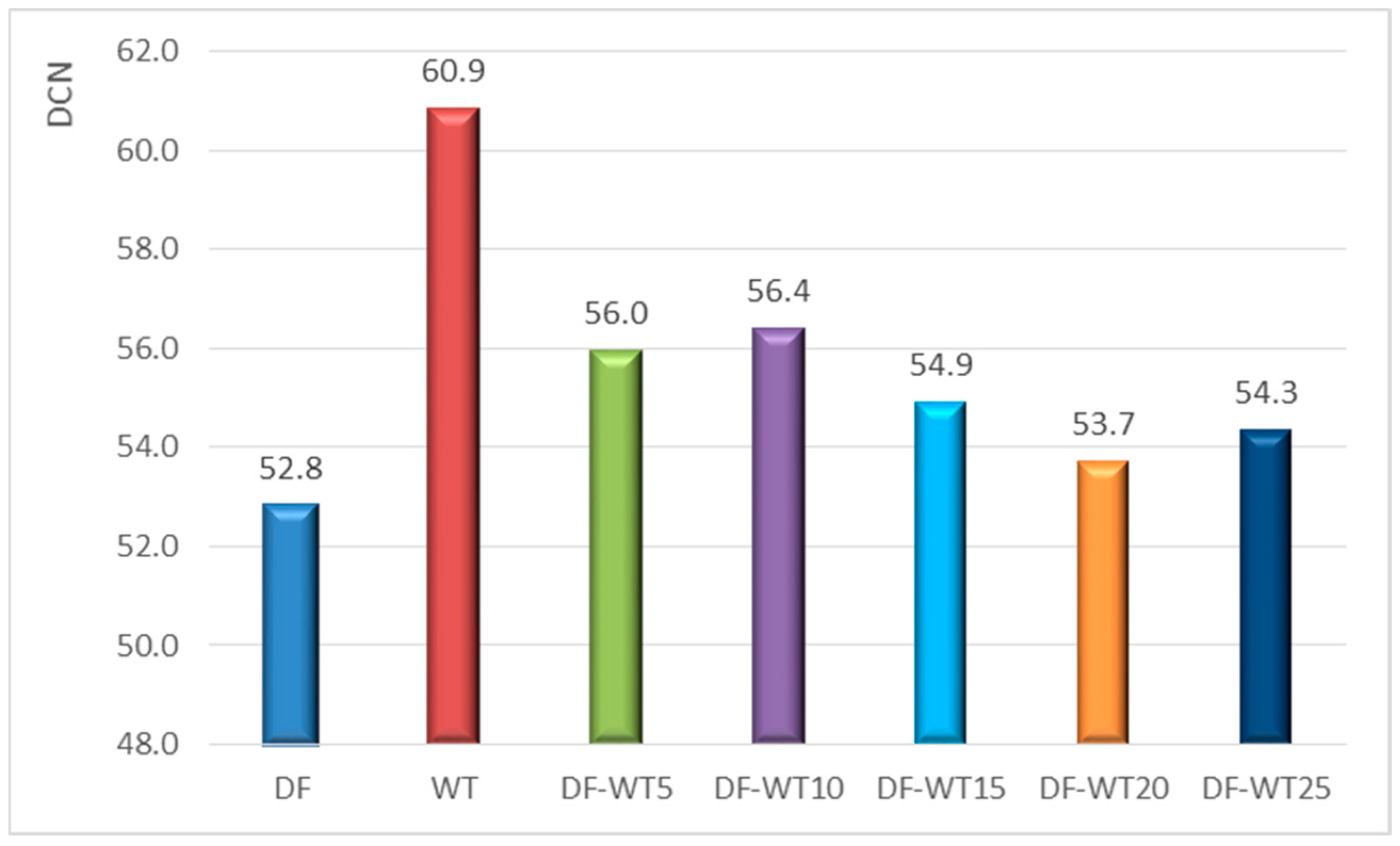
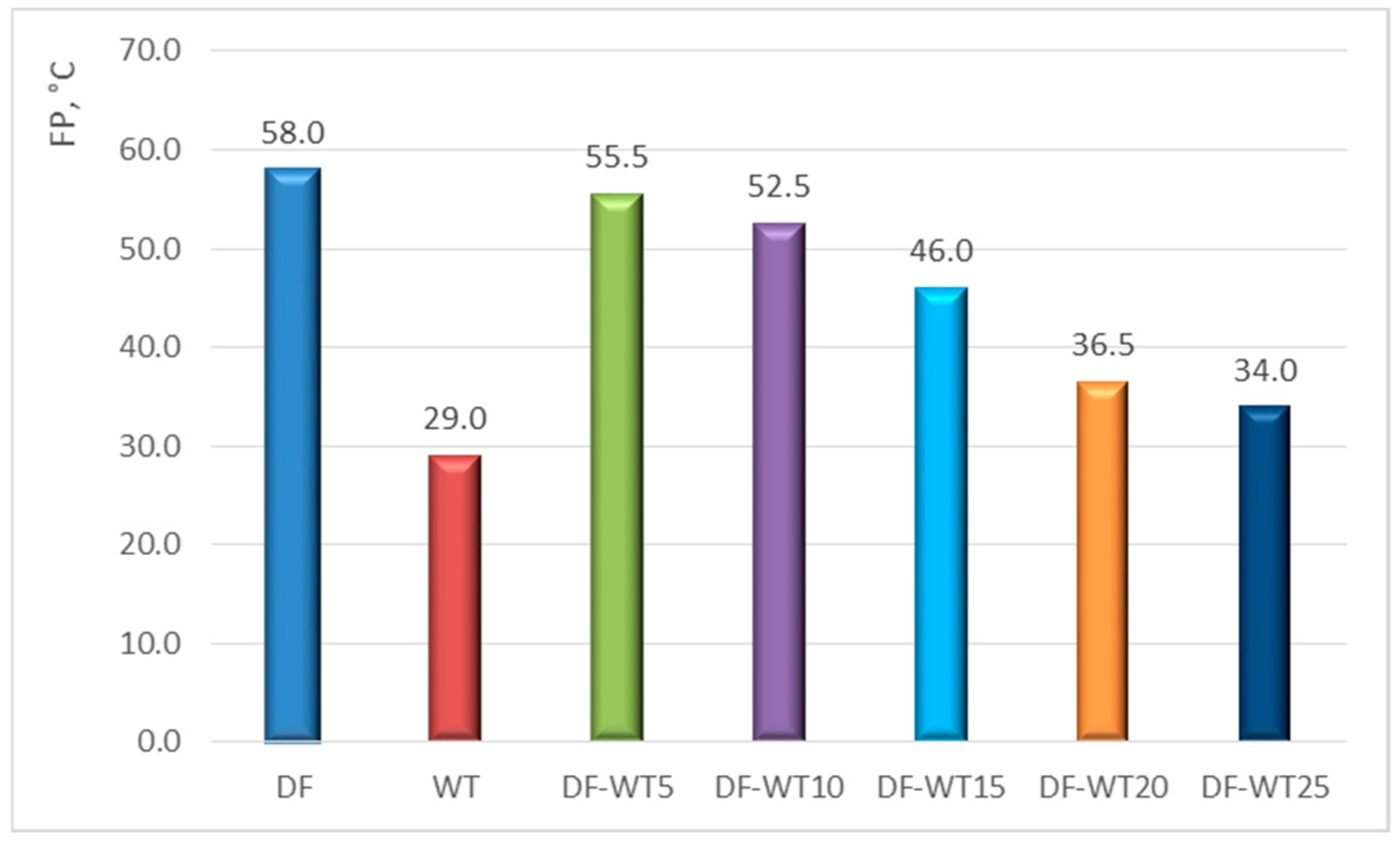
3.1. Results of Physicochemical Tests
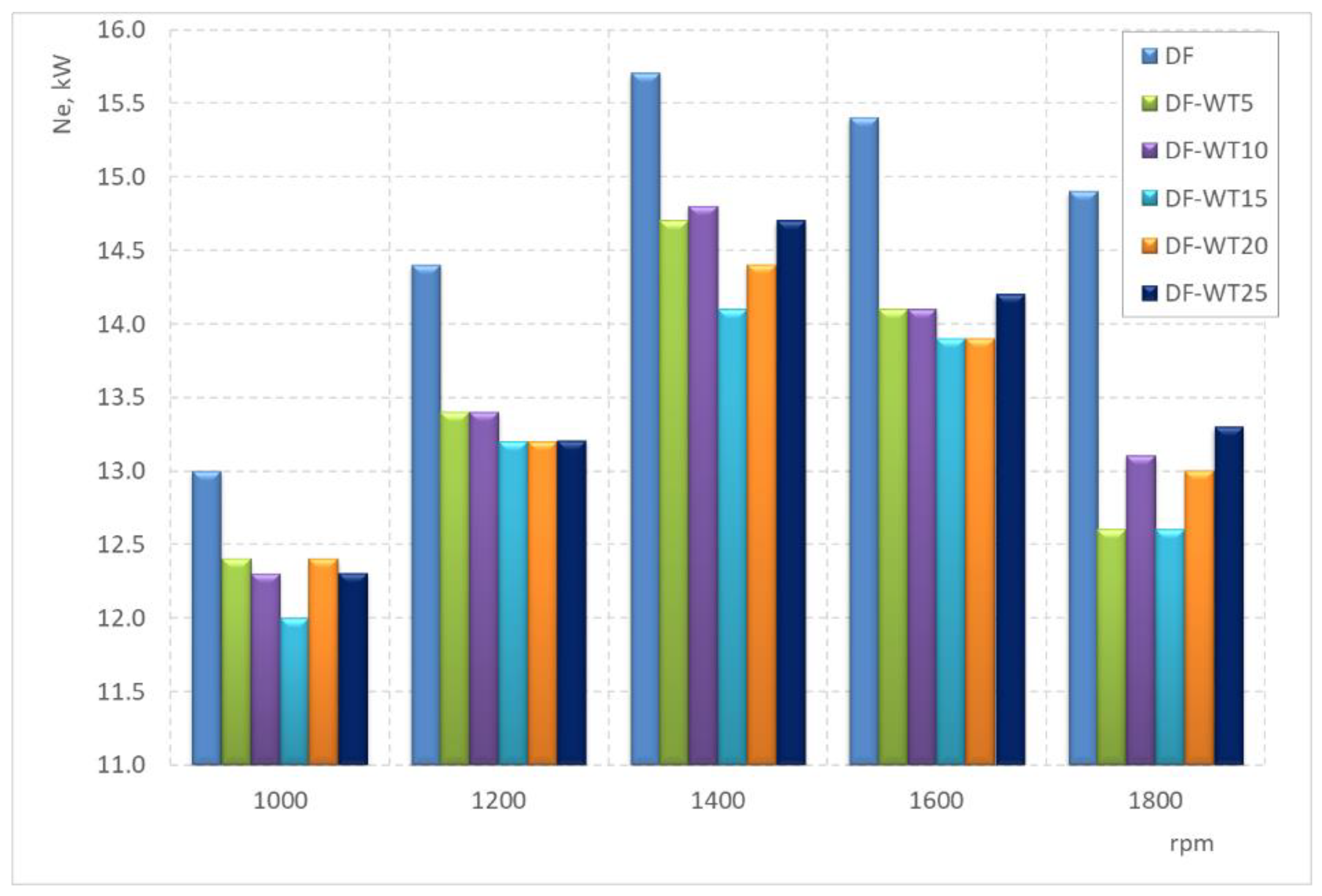
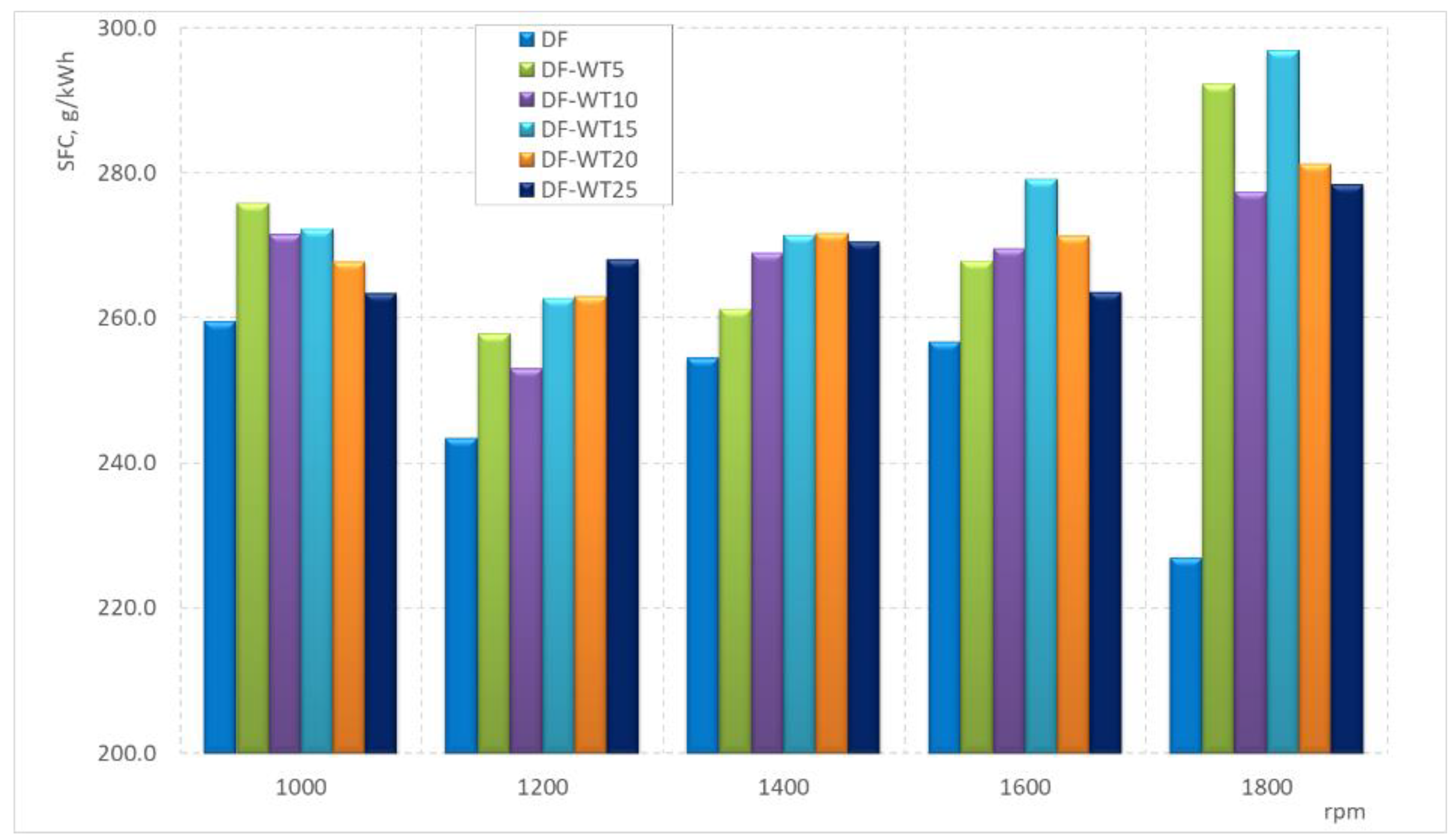
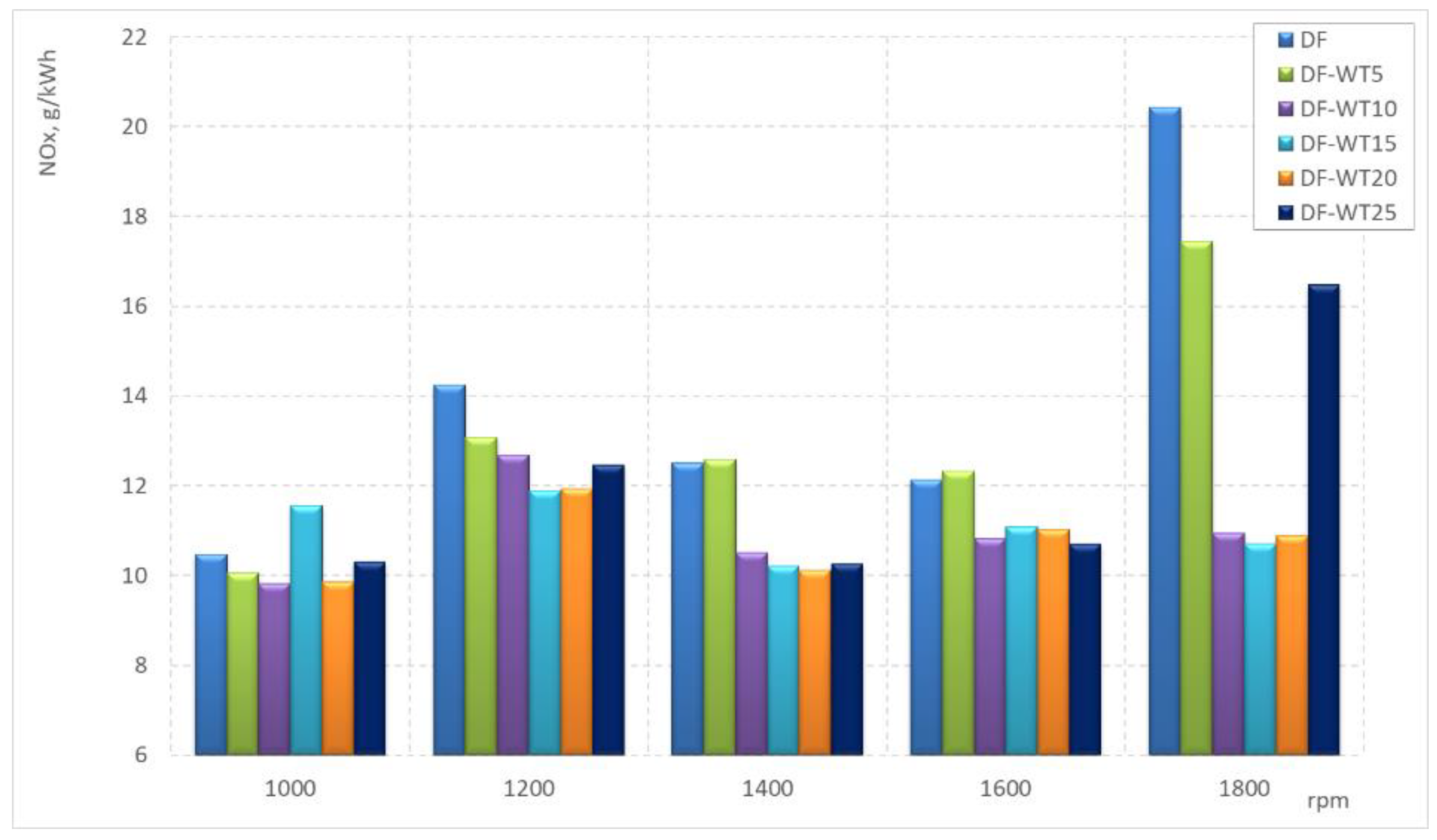
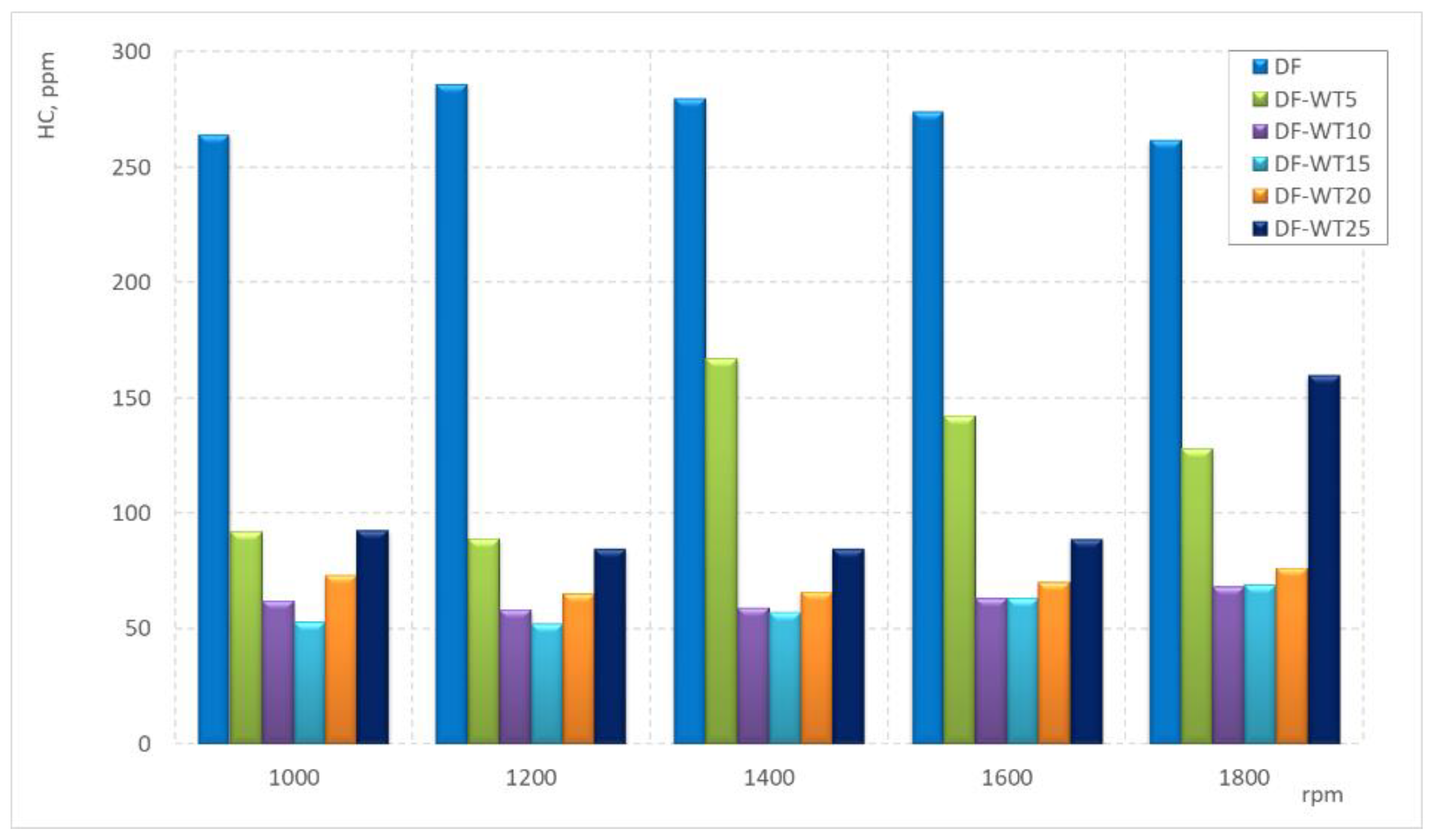
3.2. Results of Tests Carried Out on an Engine Dynamometer
4. Conclusions
- The physicochemical parameters of WT fuel mostly differ slightly from the corresponding parameters for DF; the biggest differences are in the derived cetane number DCN (about 61 for WT fuel and about 53 for DF); another parameter that significantly differentiates the two fuels is the CFPP (+3 °C for WT fuel and −18 °C for DF);
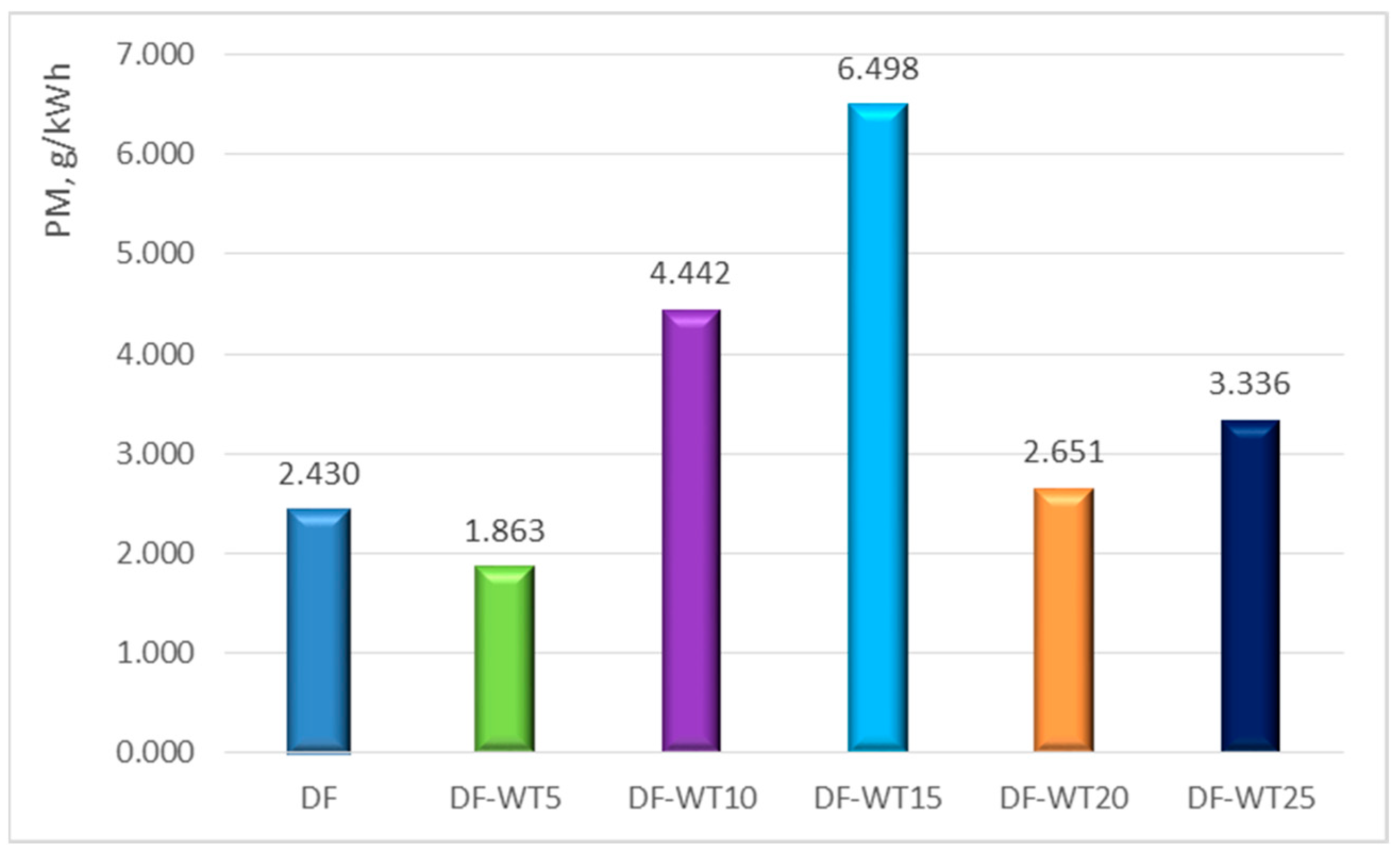
- The least favorable properties in terms of the obtained operational parameters are characterized by the DF-WT15 fuel; the biggest difference in the value of the obtained power is about 20%;
- Among the blends, DF-WT10 has the most favorable properties in terms of operational parameters;
- The NOx concentrations in the exhaust gas when the engine was fueled with DF-WT blends were about 15% lower (on average for all blends) than the NOx concentrations obtained when fueled with pure DF;
- The values of specific emissions of NOx, for average rotational speeds (1200–1600 rpm), when fed on DF were about 25% lower than for the blends; only the DF-WT5 fuel was characterized, at some measurement points (1400 and 1600 rpm), by specific emissions of NOx slightly higher than on a DF-powered engine;
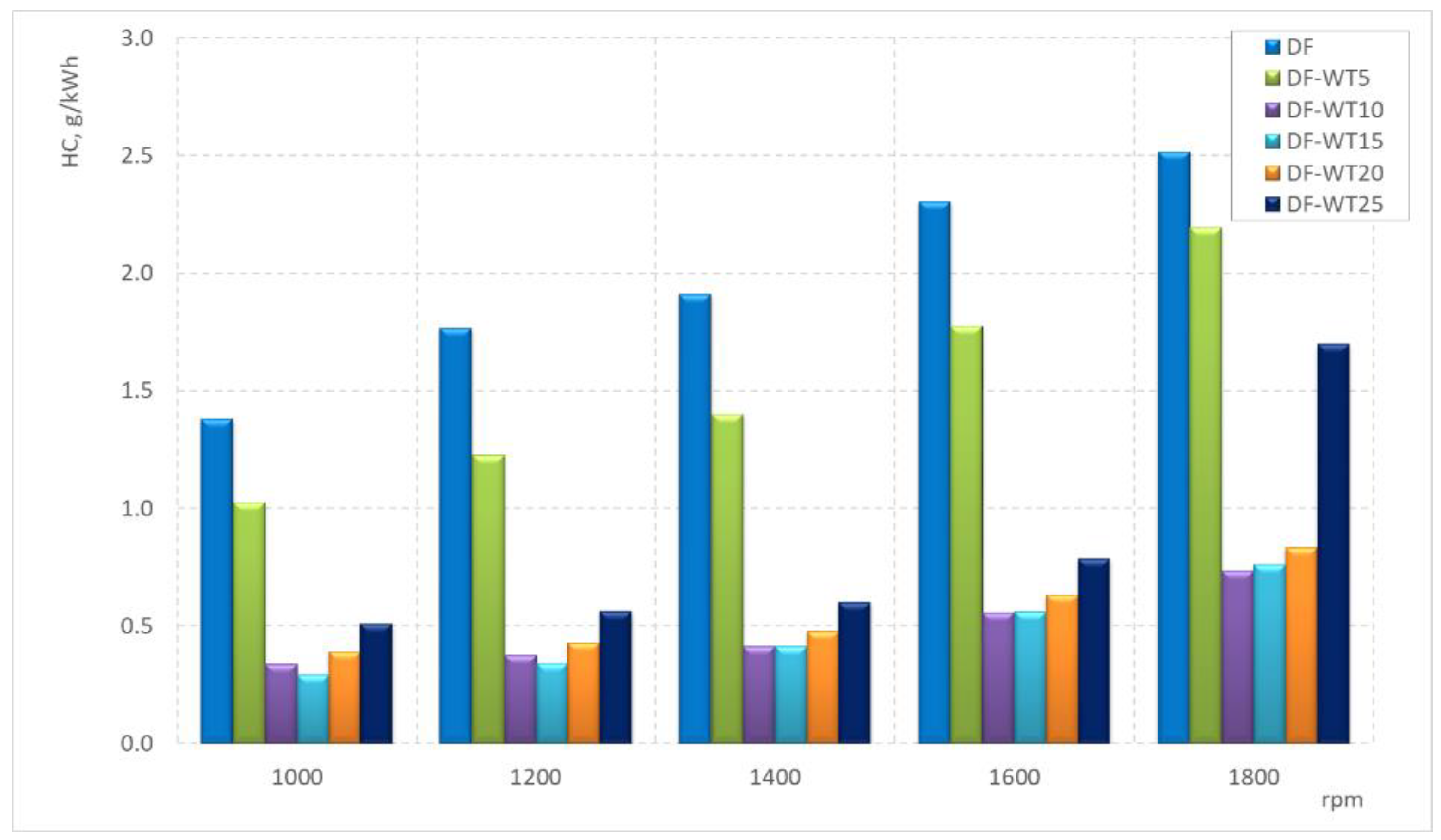
- The lowest concentration of HCs for the tested speeds was recorded when the engine was fueled with the DF-WT15 fuel;
- After exceeding 5% of WT fuel in the blend, there is a sharp reduction in specific HC emissions in the tested speed range;
- Knowledge of the selected parameters of pure synthetic WT fuel can be used for the design of fuel apparatus components, with this being both the appropriate selection of materials and the technology of the surface layer; some parameters of the new fuel (e.g., lubricity) differ significantly in value to commercial diesel fuel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFPP | Cold filter plugin point |
| cgas | The concentration of a pollutant in the exhaust gas |
| CLD | Chemi-luminescence detector |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CR | Common rail |
| DF | Diesel fuel |
| DTPO | Distilled tire pyrolysis oil |
| FID | Flame ionization detector |
| FP | Flash point |
| GDIL | Dilution air flow |
| GEFD | Diluted exhaust mass flow rate |
| GEXH | Mass flow rate of exhaust gas in the exhaust system |
| GTOT | Diluted exhaust gas flow |
| HC | Hydrocarbons |
| HHV | Higher heating value |
| HRT | Hartridge percentage scale of smoke |
| IC | Internal combustion engine |
| LHV | Lower heating value |
| mgas | Emission mass flow rates of individual pollutants |
| MPM | Mass of particulate matter accumulated on the measurement filter |
| MSAM | Mass of diluted exhaust gas that flowed through the filter in the test |
| NDIR | Non-dispersive infrared |
| Ne | Brake power of the engine |
| NOx | Nitrogen oxides |
| PM | Particulate matter |
| PMmass | Particulate mass flow rate |
| q | Degree of dilution |
| SOX | Sulfur oxides |
| TDO | Tire-derived oil |
| THC | Total hydrocarbons |
| TPO | Tire pyrolysis oil |
| ugas | The ratio between the density of the exhaust gas component and the density of the exhaust gas |
| WT | Waste tires |
References
- Tutak, W.; Jamrozik, A. Comparative Analysis of Combustion Stability of Diesel/Ethanol Utilization by Blend and Dual Fuel. Processes 2019, 7, 946. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jaworski, A.; Mądziel, M.; Woś, P. The investigation of auto-ignition properties of 1-butanol–biodiesel blends under various temperatures conditions. Fuel 2023, 346, 128388. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ge, J.C.; Choi, N.J. Effects of Ethanol–Diesel on the Combustion and Emissions from a Diesel Engine at a Low Idle Speed. Appl. Sci. 2020, 10, 4153. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jaworski, A.; Ustrzycki, A.; Lejda, K.; Balawender, K.; Woś, P. Use of the constant volume combustion chamber to examine the properties of autoignition and derived cetane number of mixtures of diesel fuel and ethanol. Fuel 2017, 200, 564–575. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. The Effect of RME-1-Butanol Blends on Combustion, Performance and Emission of a Direct Injection Diesel Engine. Energies 2021, 14, 2941. [Google Scholar] [CrossRef]
- Corsini, A.; Marchegiani, A.; Rispoli, F.; Sciulli, F.; Venturini, P. Vegetable Oils as Fuels in Diesel Engine. Engine Performance and Emissions. Energy Procedia 2015, 81, 942–949. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, Y.; Kumar, V.; Singh, B. Biodiesel as a potential replacement fuel for CI engine to meet the sustainability criteria: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Hunicz, J.; Matijošius, J.; Rimkus, A.; Kilikevičius, A.; Kordos, P.; Mikulski, M. Efficient hydrotreated vegetable oil combustion under partially premixed conditions with heavy exhaust gas recirculation. Fuel 2020, 268, 117350. [Google Scholar] [CrossRef]
- Jaworski, A.; Kuszewski, H.; Longwic, R.; Sander, P. Assessment of Self-Ignition Properties of Canola Oil–n-Hexane Blends in a Constant Volume Combustion Chamber and Compression Ignition Engine. Appl. Sci. 2023, 13, 10558. [Google Scholar] [CrossRef]
- Sudharshan Ravi, S.; Mazumder, J.; Sun, J.; Brace, C.; Turner, J.W.G. Techno-Economic assessment of synthetic E-Fuels derived from atmospheric CO2 and green hydrogen. Energy Convers. Manag. 2023, 291, 117271. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Jaworski, A.; Balawender, K.; Kuszewski, H.; Jaremcio, M. The Assessment of PM2.5 and PM10 Immission in Atmospheric Air in a Climate Chamber during Tests of an Electric Car on a Chassis Dynamometer. Atmosphere 2024, 15, 270. [Google Scholar] [CrossRef]
- Zerin, N.H.; Rasul, M.G.; Jahirul, M.I.; Sayem, A.S.M. End-of-life tyre conversion to energy: A review on pyrolysis and activated carbon production processes and their challenges. Sci. Total Environ. 2023, 905, 166981. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. Properties of high strength concrete containing scrap tire rubber. J. Clean. Prod. 2016, 113, 86–92. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-C.; Bai, C.-J.; Lin, C.-T.; Prakash, S. Alternative fuel produced from thermal pyrolysis of waste tires and its use in a DI diesel engine. Appl. Therm. Eng. 2015, 93, 330–338. [Google Scholar] [CrossRef]
- Kapilan, N.; Jullya, N. Studies on Improvement of Performance of Compression Ignition Engine Fuelled with Mixture of Honge Biodiesel and Tire Pyrolysis Oil, Strojnícky časopis. J. Mech. Eng. 2018, 68, 15–24. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of tire pyrolysis oil as an alternate fuel for diesel engines: A review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Islam, M.N.; Nahian, M.R. Improvement of Waste Tire Pyrolysis Oil and Performance Test with Diesel in CI Engine. J. Renew. Energy 2016, 2016, 5137247. [Google Scholar] [CrossRef]
- Khairil; Rickansvar, M.; Mubarak, A.; Sofyan, S. Experimental study the effect of diesel engine performance fuelled by waste tire oil- dexlite blends. BIO Web Conf. 2023, 62, 04003. [Google Scholar] [CrossRef]
- Akhil, M.; Saikat, D.; Saravanan, B.; Vasudeva, M. Liquid fuel from waste tires: Novel refining, advanced characterization and utilization in engines with ethyl levulinate as an additive. RSC Adv. 2021, 11, 9807–9826. [Google Scholar] [CrossRef]
- İlkılıç, C.; Aydın, H. Fuel production from waste vehicle tires by catalytic pyrolysis and its application in a diesel engine. Fuel Process. Technol. 2011, 92, 1129–1135. [Google Scholar] [CrossRef]
- Kapilan, N.; Kumar, K.A.; Chandra, B.P.H. Studies on Pyrolysis Oil derived from waste Tires as Fuel for Diesel Engine. J. Phys. Conf. Ser. 2021, 2070, 012232. [Google Scholar] [CrossRef]
- Doğan, O.; Çelik, M.B.; Özdalyan, B. The effect of tire derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 2012, 95, 340–346. [Google Scholar] [CrossRef]
- Marshal, S.J.J.; Samuel, S.Y.; Sundari, K.G.; Rajamohan, S. 4—Diesel engine performance and emissions with fuels derived from waste tyres. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals; Khan, A., Pizzi, A., Jawaid, M., Azum, N., Asiri, A., Isa, I., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 69–92. ISBN 9780323901505. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies 2021, 14, 3837. [Google Scholar] [CrossRef]
- Mello, M.; Rutto, H.; Seodigeng, T. Waste tire pyrolysis and desulfurization of tire pyrolytic oil (TPO)—A review. J. Air Waste Manag. Assoc. 2023, 73, 159–177. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nabi, M.N.; Rainey, T.J.; Bodisco, T.; Bayley, T.; Randall, D.; Ristovski, Z.; Brown, R.J. Novel biofuels derived from waste tyres and their effects on reducing oxides of nitrogen and particulate matter emissions. J. Clean. Prod. 2020, 242, 118463. [Google Scholar] [CrossRef]
- Pote, R.N.; Patil, R.K. Combustion and emission characteristics analysis of waste tyre pyrolysis oil. SN Appl. Sci. 2019, 1, 294. [Google Scholar] [CrossRef]
- Mikulski, M.; Ambrosewicz-Walacik, M.; Hunicz, J.; Nitkiewicz, S. Combustion engine applications of waste tyre pyrolytic oil. Prog. Energy Combust. Sci. 2021, 85, 100915. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Kumaravel, A. Tyre pyrolysis oil as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2016, 60, 1678–1685. [Google Scholar] [CrossRef]
- Directive 2005/55/EC; Directive 2005/55/EC of the European Parliament and of the Council of 28 September 2005 on the Approximation of the Laws of the Member States Relating to the Measures to Be Taken against the Emission of Gaseous and Particulate Pollutants from Compression-Ignition Engines for Use in Vehicles, and the Emission of Gaseous Pollutants from Positive-Ignition Engines Fuelled with Natural Gas or Liquefied Petroleum Gas for Use in Vehicles. European Union: Maastricht, The Netherlands, 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005L0055 (accessed on 8 July 2024).



| Fuel Description | Volume Fraction [%] | |
|---|---|---|
| Diesel Fuel | Waste Tire-Derived Fuel | |
| DF | 100 | 0 |
| WT | 0 | 100 |
| DF-WT5 | 95 | 5 |
| DF-WT10 | 90 | 10 |
| DF-WT15 | 85 | 15 |
| DF-WT20 | 80 | 20 |
| DF-WT25 | 75 | 25 |
| Parameter | Value |
|---|---|
| Number of cylinders | 1 |
| Number of strokes | 4 |
| Cooling type | Liquid |
| Piston stroke | 146 mm |
| Cylinder diameter | 127 mm |
| Displacement capacity | 1850 cm3 |
| Compression ratio | 15.75 |
| Nominal RPM | 2200 rpm |
| Oiling system | circulation under pressure |
| L.p. | n [rpm] | αww [deg CA before TDC] | tis [µs] | pinj [MPa] |
|---|---|---|---|---|
| 1 | 1000 | 18 | 3500 | 80 |
| 2 | 1200 | 20 | 3100 | 80 |
| 3 | 1400 | 22 | 2950 | 80 |
| 4 | 1600 | 22 | 2500 | 80 |
| 5 | 1800 | 22 | 2100 | 80 |
| Sample Name | Component | Number of Analyses | Average Mass Fraction [%] |
|---|---|---|---|
| DF | C | 7 | 81.51 |
| H | 18.48 | ||
| N | 0.00 * | ||
| S | 0.00 * | ||
| WT | C | 7 | 84.38 |
| H | 16.61 | ||
| N | 0.00 * | ||
| S | 0.00 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowski, M.; Jaworski, A.; Kuszewski, H.; Balawender, K. Performance of a Diesel Engine Fueled by Blends of Diesel Fuel and Synthetic Fuel Derived from Waste Car Tires. Sustainability 2024, 16, 6404. https://doi.org/10.3390/su16156404
Jakubowski M, Jaworski A, Kuszewski H, Balawender K. Performance of a Diesel Engine Fueled by Blends of Diesel Fuel and Synthetic Fuel Derived from Waste Car Tires. Sustainability. 2024; 16(15):6404. https://doi.org/10.3390/su16156404
Chicago/Turabian StyleJakubowski, Mirosław, Artur Jaworski, Hubert Kuszewski, and Krzysztof Balawender. 2024. "Performance of a Diesel Engine Fueled by Blends of Diesel Fuel and Synthetic Fuel Derived from Waste Car Tires" Sustainability 16, no. 15: 6404. https://doi.org/10.3390/su16156404






