Abstract
Food waste contributes to hunger, poverty, and environmental pollution. Unfortunately, seafood, which provides high nutrient content, is significantly underutilized, with only 30% of high-value seafood consumed. This study addresses the urgent need to reuse these wastes, converting them into biofertilizers through solar drying. A solar drying plant was designed and built to produce fish powder as the base of fertilizer, achieving an equilibrium humidity of 400 kg in 11 h after sterilization by pre-cooking. The resulting biofertilizer was rigorously tested for its macronutrient composition, germination rate, presence of coliforms, and phytotoxicity. The findings indicate that fish waste can effectively replace synthetic fertilizers, fostering a circular economy and promoting sustainable agriculture. This research highlights the potential of using marine debris to produce biofertilizers, contributing to global sustainability efforts by harnessing marine debris and solar energy to offer an environmentally friendly alternative to chemical fertilizers.
1. Introduction
Food waste has economic, social, and environmental consequences. Rich countries waste 222 million tons of food, or about USD 680 billion. Reducing food loss is critical to reducing hunger and meeting ecological targets [1] because this lost food has a climate impact [2], especially animal-based food, which produces the highest greenhouse gas emissions [3]. Unfortunately, fish is among the most wasted foods (more than 35% of catches) [4].
Paradoxically, global fisheries consume about 30–40 million tons (Mt) of fuel and generate 180–200 Mt of CO2-equivalent GHGs annually [5]. Nevertheless, fish is an essential food because it contains vital proteins and polyunsaturated lipids, vitamin D, iodine, iron, calcium, zinc, phosphorus, potassium, and other micronutrients [6,7]. It accounts for about 17 percent of animal protein intake, and apparent per capita fish consumption is currently over 20 kg. Moreover, 200 million people are employed in fisheries and aquaculture worldwide [8]. However, trash fish, bones, intestines, heads, and tails (containing these nutritional components) are not used as food and constitute over 50% of caught fish [9], mainly because of overfishing, the low commercial value of some fish, and unused fish parts.
Plentiful studies have proposed using this nutrient-rich waste in various co-products, rescuing all the resources already used. For example, composting increases biomass conversion efficiency for biodiesel production, as in [10]. Also, it is proposed to be used for animal feed as a farmed fish diet component [9]; to improve other foods such as fried fish nuggets’ sensory attributes; and to increase protein while decreasing carbohydrate levels [11]. In another study, natural pigments were produced; fertilized eggs and the fish species were used for carotenoid pigment recovery [12]. Finally, it is also used in industry and cosmetics [13,14], as shown by Aoki et al.,2004 [15], who partially purified proteases from northern waste shrimp to tenderize beef.
One of the most important co-products of unused fish is fertilizer formulations, as the world is facing the worst food crisis in a decade and also due to substantial fertilizer shortages because of the Ukraine war intensification in February 2022, which has caused an increase in volatile prices [16].
Fertilizers are essential for plant nutrition and soil fertility to serve the current needs of food production [17]. However, chemical fertilizers are related to health risks, environmental hazards, and costs [18]. On the other hand, biofertilizers help to improve agricultural production, reduce hunger in the world, and increase rural farmers’ income sustainably. The literature shows many proposals, such as in [19], where the phosphorus content in composts made from fish waste, sewage sludge, green waste, and horse manure was investigated. Jung and Kim [20] produced biofertilizers from brown seaweed, achieving chlorophyll and carotenoid contents in lettuce; and in [21], fishery wastes in a 150-L reactor were used to evaluate biodegradation and fermentation to produce liquid fertilizer. Shrimp waste with urea at different ratios was processed into liquid fertilizer [22], and fish waste, molasses, and grape marc mixtures were developed and underwent natural biotransformation using Saccharomyces cerevisiae to produce fertilizer. Unfortunately, most of these processes must be performed in a lab or by specialized personnel.
The rich nutritional content of marine flour has been demonstrated by many authors [11,23,24,25]; most of this content can provide the soil with the necessary elements to promote the rapid and healthy growth of crops that are increasingly in demand due to the rapid population growth [26,27,28].
On the other hand, typically, to produce fertilizer from fish waste, a drying operation is used to preserve its properties and increase its shelf life. Drying also helps reduce volume, facilitating handling and transport. Many techniques are used to complete drying, such as gas or electric ovens, microwaves, lyophilization, infrared radiation, and spray drying, which have shown great feasibility and effectiveness [29,30,31,32,33,34]. Nonetheless, technical solar drying has several advantages over others: it does not use complex and expensive installations; the equipment is easy to operate; it requires little maintenance; and it is energetically more economical and sustainable.
Several studies have explored using food and fish waste as fertilizers [35]. However, there is a lack of research on incorporating solar drying as an energy source in processing it and retaining essential nutrients for improving agricultural soils, leading to cost savings and fewer greenhouse gas emissions. Integrating solar drying in organic discarded processing supports the circular economy, promoting resource efficiency and waste reduction. This approach boosts sustainability, strengthens agricultural systems, and completes the loop in organic waste management [36].
As part of a project supported by the National Council for Humanities, Sciences, and Technologies (Consejo Nacional de Humanidades, Ciencias, y Tecnologías), in search of supporting small fishermen in Campeche, Mexico, a solar plant has been built with which around one ton of very low-value fish and fish remains, such as bones, viscera, and skin, will be processed daily to obtain by-products, including biofertilizer.
In the present work, solar drying of fish waste was carried out in the constructed plant to produce a biofertilizer. The necessary tests determined the components for creating a natural fertilizer from this dehydrated unwanted fish in direct solar dryers, addressing food waste and fertilizer shortages, and promoting eco-friendly agricultural practices, contributing to global sustainability efforts.
2. Materials and Methods
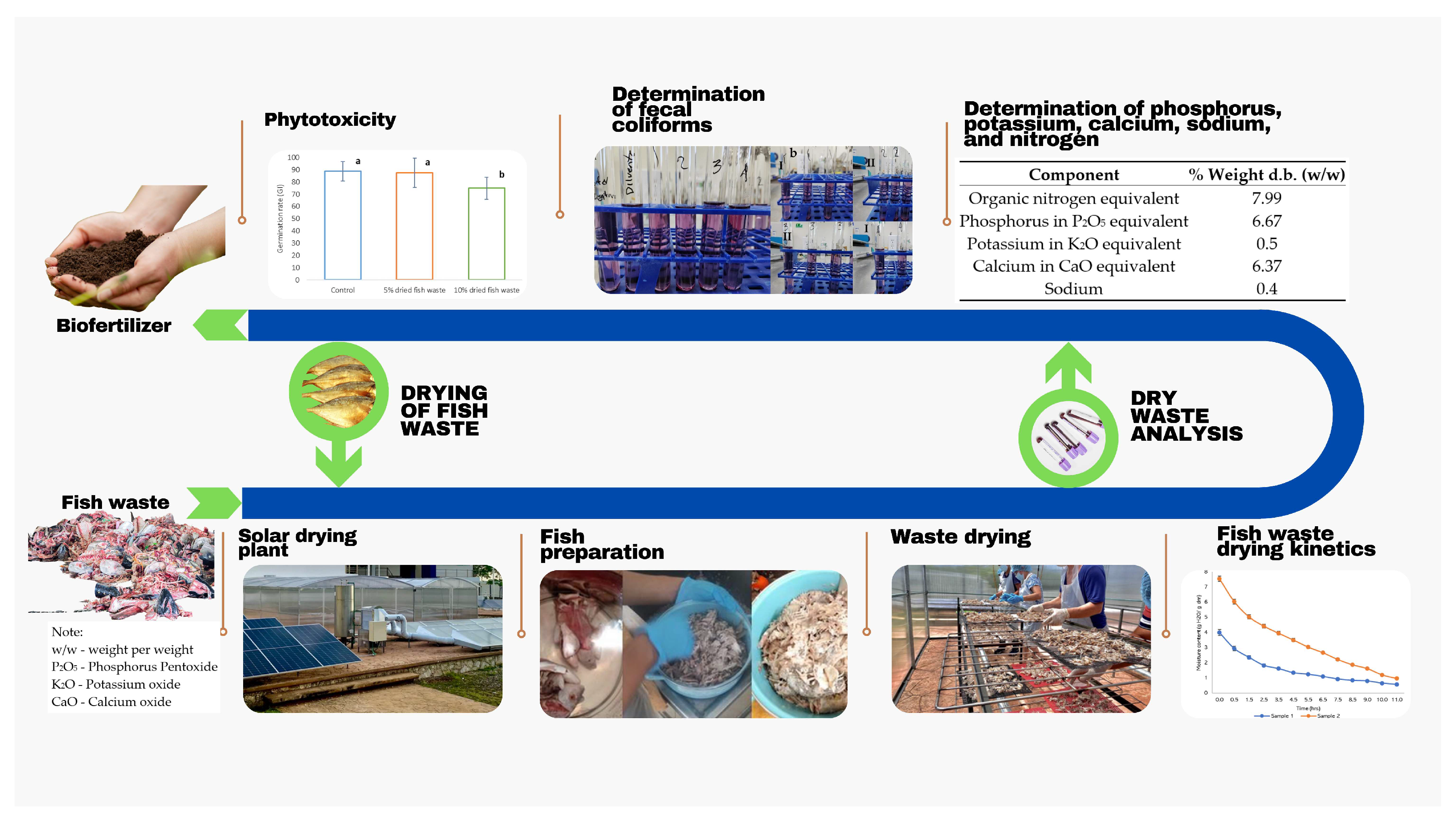
The methodology was developed in two stages: (1) the drying of the fish waste and (2) the analysis of this product to determine the essential macronutrient composition and feasibility of its use as a biofertilizer (Figure 1).
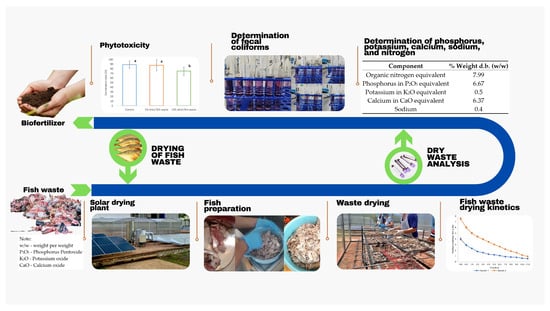
Figure 1.
Production of biofertilizer based on fish waste through solar drying.
2.1. Solar Drying
2.1.1. Solar Dying Plant
Two greenhouse-type polycarbonate solar panels were considered for better flexibility when constructing the solar drying plant (Figure 2). The total capacity is 1000 kg of fresh product. The main dryer (9 m × 6 m) has an approximate capacity of 700 kg of fresh product, and the secondary dryer (4 m × 2.5 m) has a 300 kg capacity.

Figure 2.
Solar dryer plant.
Auxiliary solar thermal energy systems provide the significant energy that water evaporation requires during dehydration. The selection and sizing of the solar equipment were technically and economically optimized through the dynamic simulation of a system covering the hot air energy demand for the dryer capacity (800 kg) using TRNSYS v18 software. Several factors were considered: climate, operating temperature, mass to dry, raw material characteristics, desired final humidity and cost of conventional energy used on-site, and valuable life-cycle cost analysis.
Two main options for heating air were evaluated: (a) a hybrid system combining solar air heaters with an auxiliary LP gas heater and (b) a system relying solely on LP gas for air heating. Both systems were analyzed regarding their economic and technical performance, including cost projections over 20 years, environmental impact, and optimization of parameters such as the number and arrangement of solar collectors.
Additionally, a photovoltaic solar system was installed to operate the extractor and fan for air circulation. This setup was complemented by an auxiliary thermal storage system comprising a thermal tank, a resistance, and a pump. This system allows the dehydration process to be carried out with hot air heated indirectly by solar thermal energy using an insulated hot water tank and a heat exchanger.
2.1.2. Measuring Instruments
The measuring instruments used for the process monitoring were the following:
Water activity and percentage of humidity: Portable Rotronic Hygropalm water activity meters, with a precision of ±0.01% mg, and Boeco brand model BMA 150 (accuracy of ±0.01% mg).
Weight Loss: A high-precision digital scale (Boeco model BPS 40 plus).
The instruments for monitoring climatic parameters are shown in Table 1.

Table 1.
Characteristics and description of weather station measuring instruments.
2.1.3. Drying of Fish Waste
The process began by washing the fish under running water to remove surface impurities; subsequently, the meat was separated from the waste (viscera, tail, head, spines, fins, and skin) and the scales. The product obtained in the previous stage was deposited in 40 L pots and boiled until a nutrient-rich broth was obtained (Figure 3).

Figure 3.
Waste cooking process.
Once the cooking was finished, the broth was separated from the solids and evenly distributed on plastic racks for drying. The racks were then placed on the trays provided and placed in the greenhouse-type dryer (Figure 4).

Figure 4.
Filling solar dryer with fish waste.
Throughout the process, measures were taken to ensure hygiene and food safety, avoiding cross-contamination and the growth of pathogenic microorganisms.
Periodic analyses of the broth and dry residues were carried out to determine their nutritional composition and potential applications.
2.1.4. Drying Kinetics
The cooked fish waste was drained and placed in meshes, distributing them homogeneously; these were placed inside the drying chamber. Two small samples were placed independently in a device that allows the weight loss to be monitored automatically. All experiments were repeated in triplicate.
The kinetic evolution was followed until it reached the moisture equilibrium. The product was removed from the greenhouse-type dryer, and the final humidity, water activity, and colorimetry were studied. All products were ground until we obtained flour, and the composition was analyzed.
2.2. Dried Fish Waste Analysis
The fish flour produced from dry fish was carefully examined for its potential benefits as a sustainable and eco-friendly natural fertilizer.
2.2.1. Nitrogen Determination
Based on NMX-AA-180-SCFI-2018 9/48 [37], organic nitrogen is determined by the Kjeldahl method [38] after removing ammoniacal nitrogen, digesting the sample, distilling, and titrating the ammonia in the distillate with titrated acid. One gram of dry residue was used, and two tablets of the Missouri catalyst were added to the digestion of 25 mL of concentrated sulfuric acid. Subsequently, the mixture was made up to 100 mL with distilled water, and distillation was carried out using 40% sodium hydroxide and collecting the ammonia by absorption in a 4% boric acid solution with 2 mL of Shiro Tashiro indicator. The titration of the absorbed ammonia was carried out with a titrated solution of 0.1 N HCl.
2.2.2. Determination of Sodium, Potassium, and Calcium
Na, K, and Ca alkali metals were prepared using flamometry with LP gas and potassium chloride, NaCl, and CaCl2 to prepare standard curves.
2.2.3. Determination of Phosphorus
A spectrophotometric method based on molybdenum blue was used to determine inorganic phosphorus using ascorbic acid as a reducing agent. A total of 50 mg of the residue was calcined in a crucible previously washed with distilled water and 0.1 N hydrochloric acid; an acid extraction was subsequently carried out accordingly [39]. The ash solubilize was tested with a reaction solution containing ten mM molybdate from ammonium molybdate, ten mM zinc acetate, and 5% ascorbic acid. Phosphate quantification was carried out using a standard curve read at 629 nm NaH2PO4 from 0 to 50 nmol equivalents of Pi.
2.2.4. Determination of Fecal Coliforms
The evaluation of the quality and efficiency of the treatment to obtain bi-solid waste free of pathogenic bacterial contamination, in particular fecal coliforms, was determined using the method for the quantification of fecal coliforms following NOM-004-SEMARNAT-2002 in its annex III [40], where the method for the quantification of fecal coliforms in sludge and biosolids is specified. Upon collection, the sample was refrigerated to maintain its integrity until analysis. The percentage of total solids (TS) in the sample was determined. Subsequently, a quantity equivalent to 4 g of TS was weighed for analysis. For the fecal coliform determination assay, lauryl tryptose broth with bromocresol purple was employed as the culture medium, prepared following the methodology described in NOM-004-SEMARNAT-2002 [40]. The sample was prepared with dilution water consisting of a sterile phosphate buffer in which serial decimal dilutions (10-n) were made. According to the standard, analyzing at least three serial dilutions is advisable to obtain optimal results. However, in the present work, four serial decimal dilutions were tested, consisting of 1 mL of the homogenized preparation in 9 mL of sterile dilution water.
Once the dilutions were obtained, 1 mL of the homogenized dilution was transferred into a tube containing 10 mL of lauryl tryptose broth and an inverted Durham tube. Finally, all tubes were incubated at 35 °C/24–48 h. The formula NMP/g TS = (MPN from tables) × (10/highest inoculated volume) was used to analyze and determine the most probable number (MPN/g TS). The entire assay was performed in triplicate.
2.2.5. Phytotoxicity
This test allows the prediction of the plant’s behavior against different substrates to determine a possible adverse effect of the product to be tested. A phytotoxicity bioassay determines the relationship between a dose of the substance under study and its reaction in test organisms to determine the presence of phytotoxic and genotoxic substances [39,41,42].
The germination index, the main bioindicator, combines seed germination with radicle growth. In this case, mixtures of previously sterilized soil with two percentages of dry fish residue were used in proportions of 10 and 5%, and 100% sterile soil was used as a negative control for phytotoxicity [39,41,42]. Due to their sensitivity to toxic effects, cucumber (Cucumis sativus) seeds were used for this purpose, with three repetitions of ten seeds each in a Petri dish for each treatment, placing the seeds at a depth of 2.5 cm in each case, using 50 g of total material in each treatment and 25 mL of distilled water. The seeds were incubated for five days at 28 °C in a Felisa incubator without forced convection. Then, the germinated seeds were counted, and the hypocotyl and epicotyl were measured [39,41,42].
The germination index was determined by counting germinated seeds and the respective measurements. The relative germination percentage (PGR), relative root growth (RRC), and germination index (GI) were evaluated [42]. Statistical analysis was performed in SPSS-IBM v12.0.
3. Results and Discussion
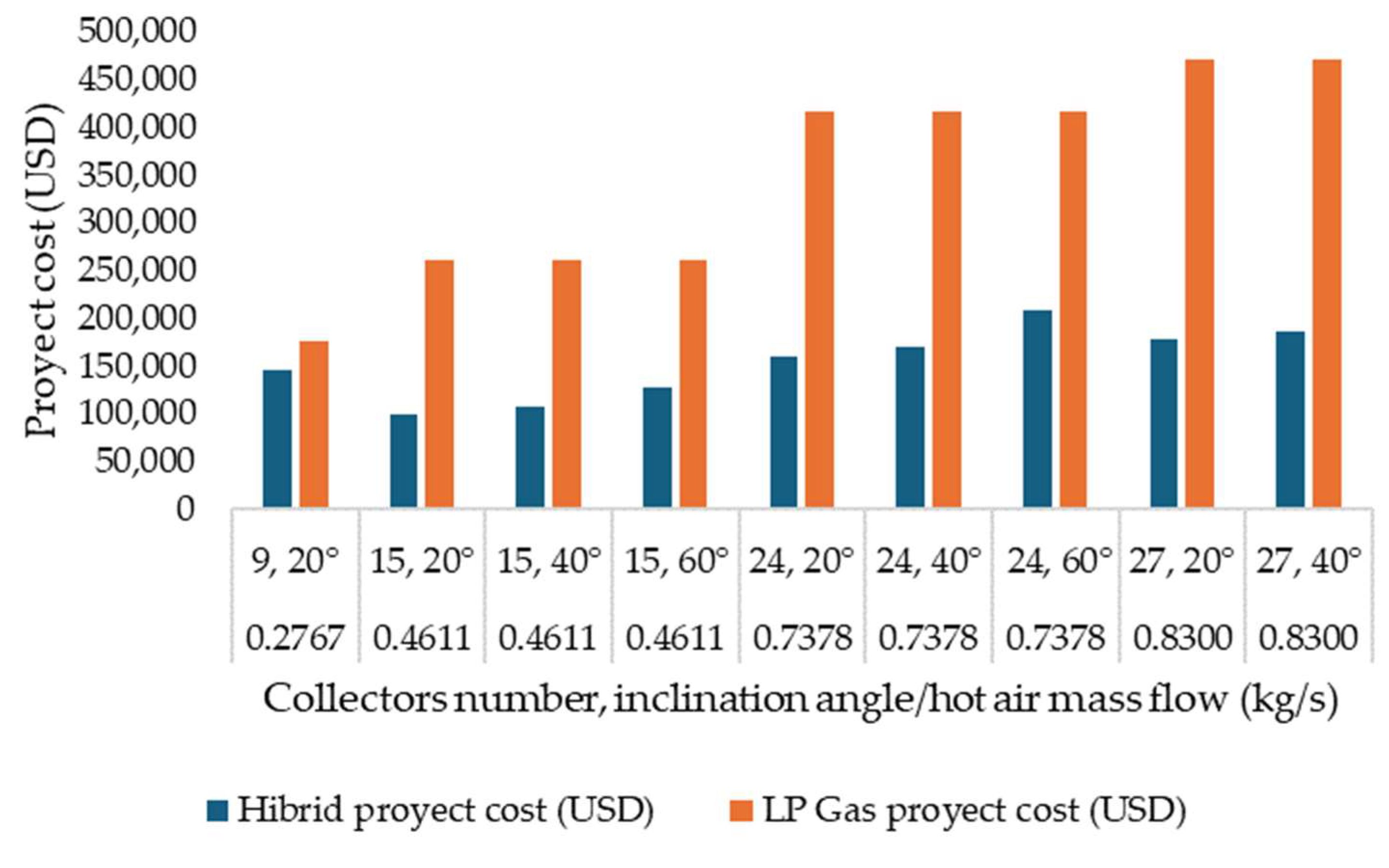
Using solar energy to dry fishery products in Campeche presents significant economic benefits compared to using fossil fuels such as LP gas. According to the technical report from the simulation, the total cost of a hybrid project that combines solar collectors and an auxiliary LP gas heater is considerably lower. In the optimal scenario, the project’s total cost is approximately USD 99,873, with a solar fraction of 64% and a payback period of 1.8 years. This result contrasts with the much higher cost of a project that exclusively uses LP gas, which can reach up to USD 470,000 in the worst-case scenario due to the absence of solar collectors (Figure 5).
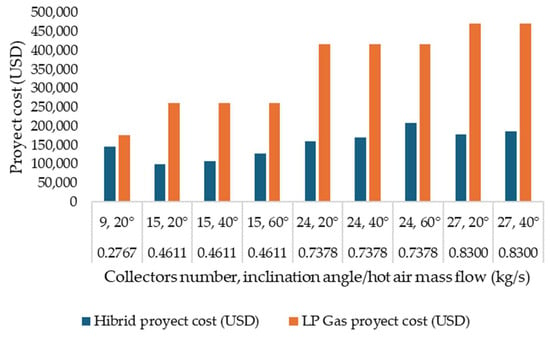
Figure 5.
Costs of various project configurations and energy sources.
In addition to the direct economic savings, solar energy significantly contributes to environmental sustainability by reducing greenhouse gas emissions. Solar technology, relying on the abundant solar resources available in Campeche, decreases the consumption of fossil fuels and their associated costs, which increase annually by an average of 10%. This strategy reduces environmental impact and provides stability and predictability in long-term operational costs.
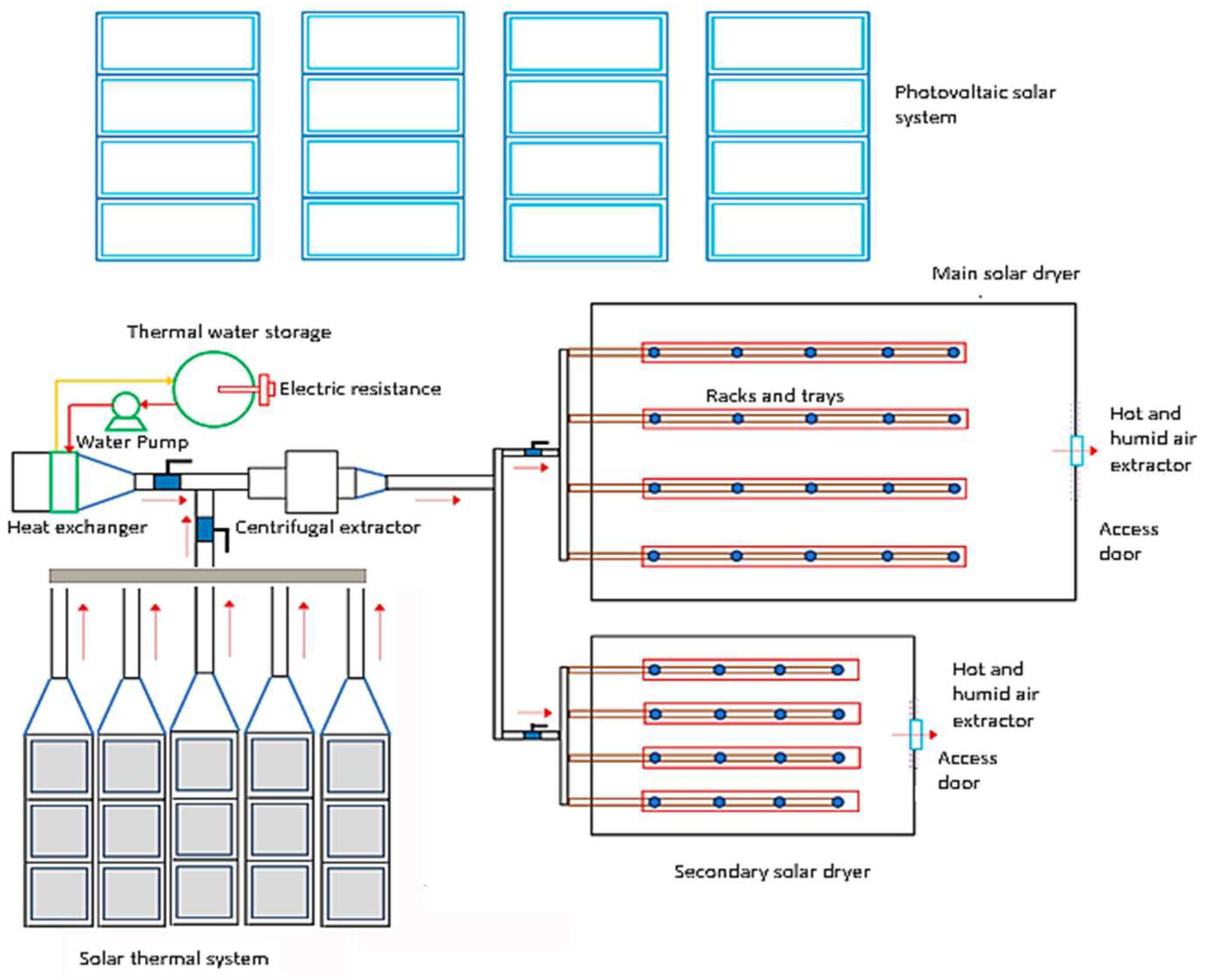
Figure 6 shows the final optimal configuration installed, which consists of 15 solar air-heating collectors in an arrangement of 3 collectors in series and five rows in parallel at an inclination angle of 20 degrees. Each collector has a collection area of 1.7 m2, which is approximately 25.5 m2 total collection area.
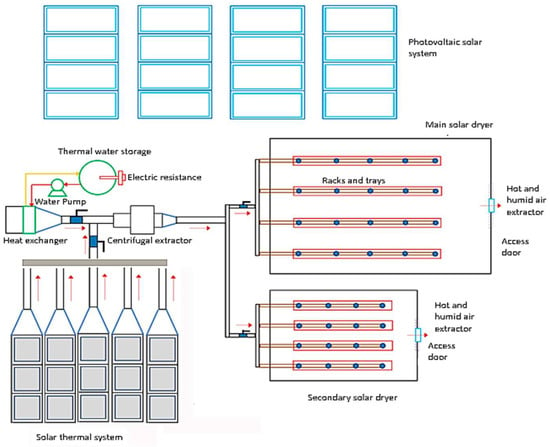
Figure 6.
Schematic design of the dryers and solar energy system.
The photovoltaic system is composed of 16 solar panels, which collectively cover an area of 2.56 m2, designed to generate a maximum electrical power output of 550 W, contributing to the system’s overall capacity of 8800 W. Additionally, the system is integrated with a set of eight 12-VDC 115 Ah batteries, enabling the solar dryer to function autonomously for a period of 3 h when fully charged. This operational autonomy can be extended to 6 h during overcast weather conditions, ensuring sustained functionality and performance regardless of variations in sunlight exposure.
3.1. Drying of Fish Waste
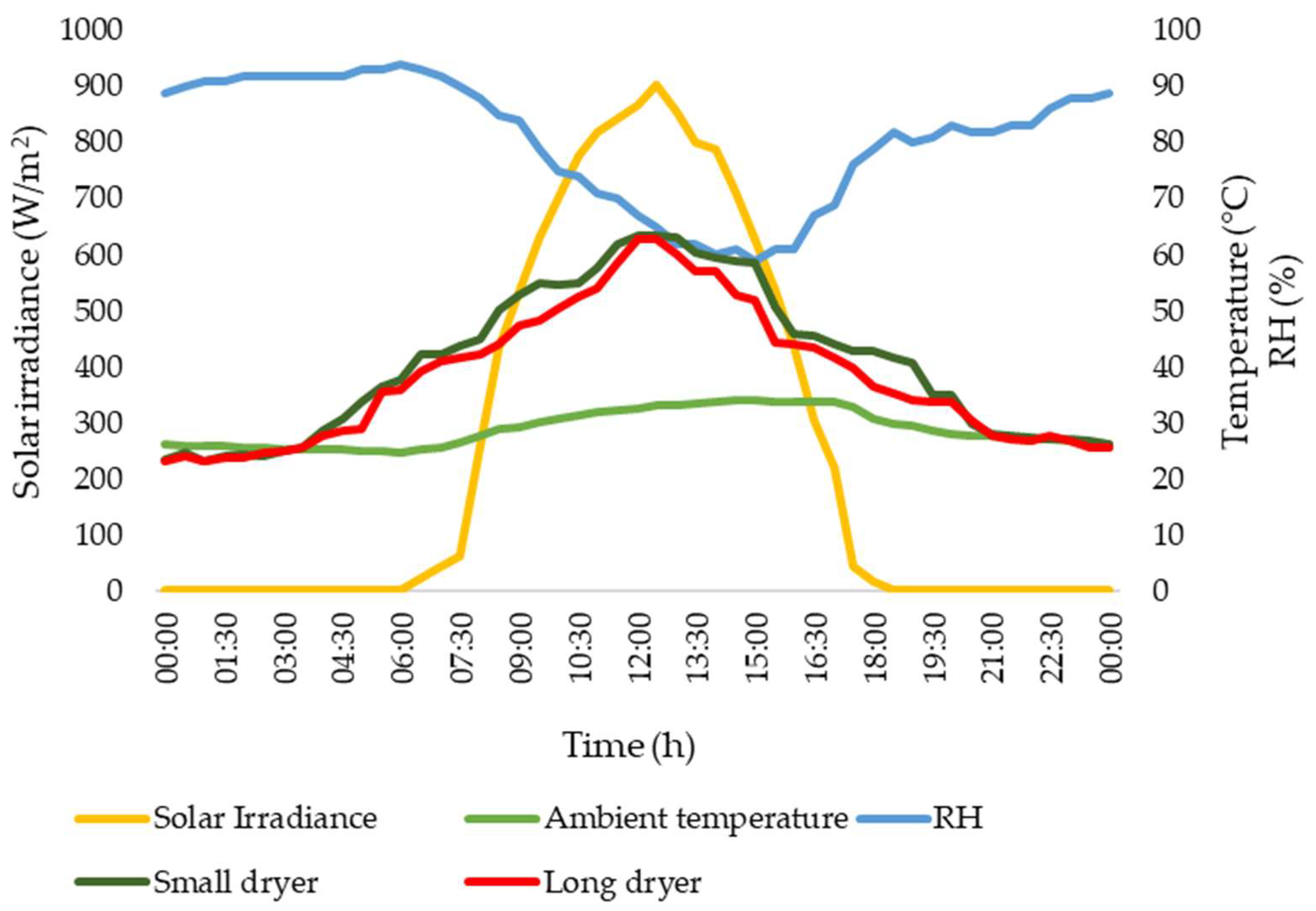
Figure 7 visually represents the standard climatic conditions and temperatures observed during the experiment. The environmental aspects and thermal levels play a vital role in the optimal functioning of solar drying systems.
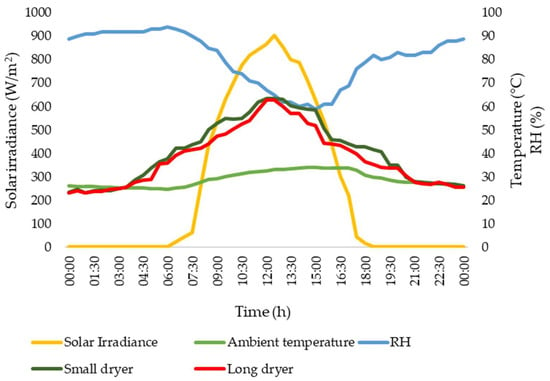
Figure 7.
Climate and temperatures in the dryers.
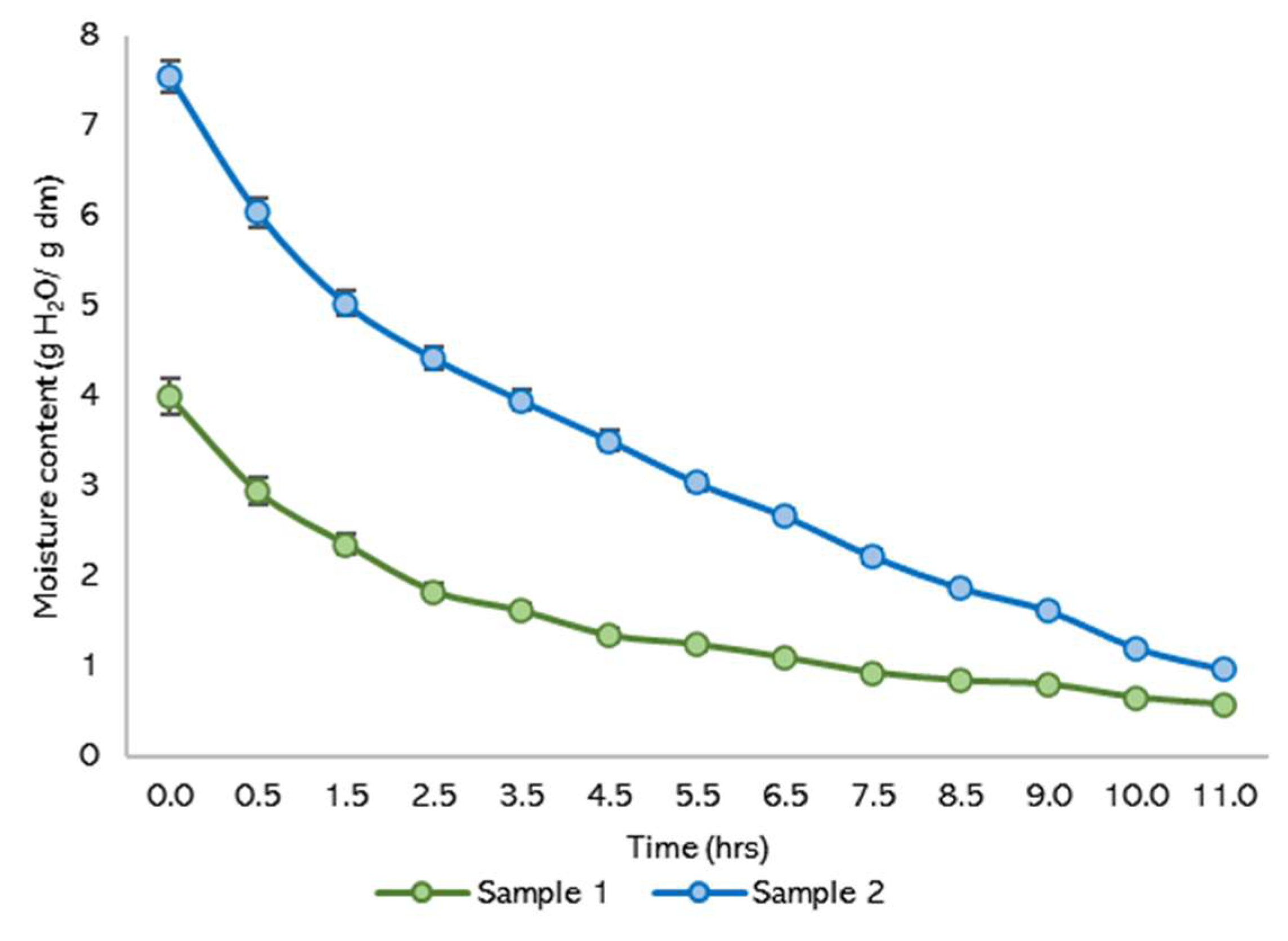
Figure 8 depicts moisture loss over time for two samples, demonstrating typical drying outcomes. The data suggest that around 11 h were required to stabilize the product and reach equilibrium moisture content, with all experiments conducted in triplicate. During the initial phase, a rapid decrease in moisture content is observed, aligning with free water removal. This phase is followed by a slower rate of moisture loss as the drying process progresses, indicating the transition to bound water removal.
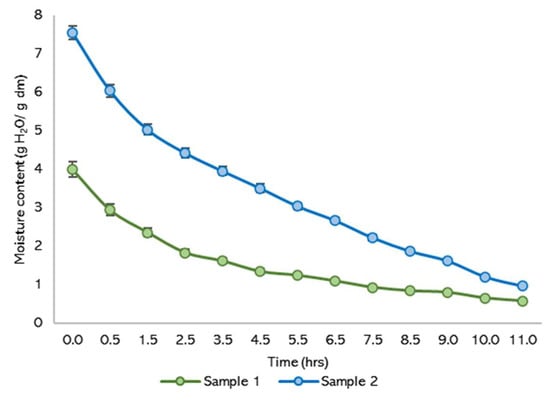
Figure 8.
Moisture content lost.
The extended duration required to reach equilibrium highlights the importance of monitoring and controlling drying parameters to ensure product stability and efficiency. The stabilization time and drying rates observed are critical for optimizing industrial drying processes and balancing energy consumption with effective moisture removal.
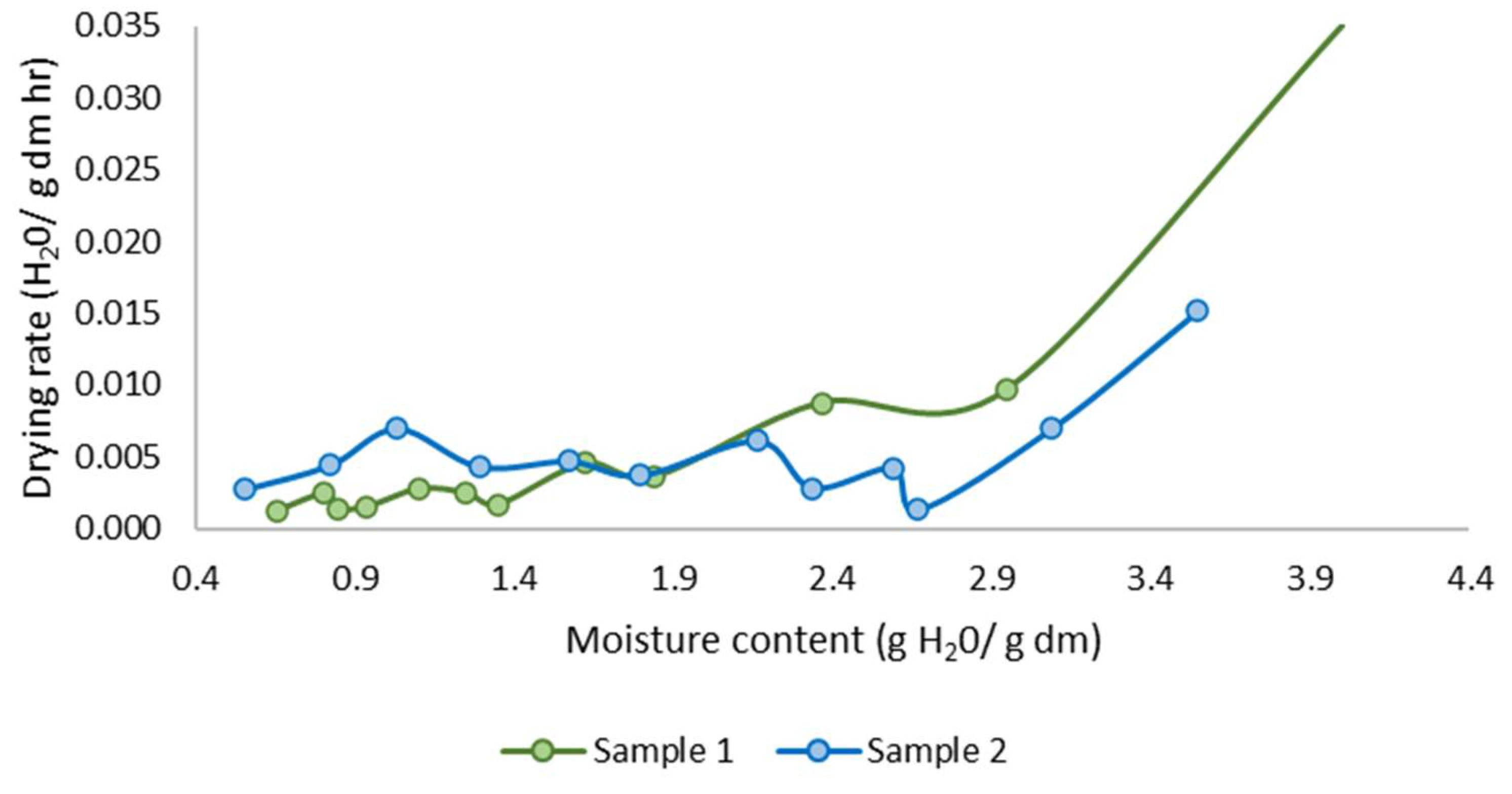
The drying kinetics reveal a typical pattern where the initial phase is characterized by a rapid loss of water, followed by a gradual decrease in the drying rate as the free water is eliminated (Figure 9). This behavior is consistent with established drying principles, where surface moisture removal occurs quickly. Subsequently, the rate of moisture loss diminishes as bound water within the material requires more energy to evaporate.
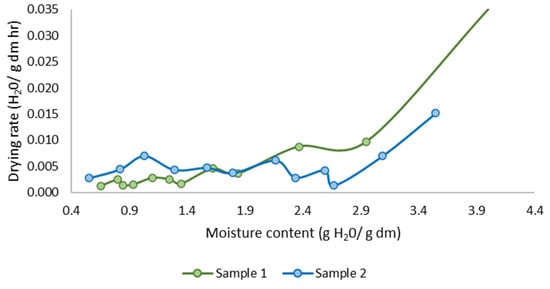
Figure 9.
Fish waste drying rate.
The observed highest drying rate was 0.035 H2O/g dm min, reflecting the rapid initial phase, while the final drying rate decreased significantly to 0.0013 H2O/g dm min. This decline in drying rate can be attributed to the transition from the constant rate period, dominated by surface evaporation, to the falling rate period, where internal moisture diffusion becomes the limiting factor. Such a trend underscores the importance of optimizing drying parameters to balance energy consumption and drying efficiency in practical applications.
3.2. Dried Fish Waste Analysis
The results of the NPK characterization of discarded fish from solar drying are shown below. The macronutrient determinations of the waste obtained by solar drying yielded an approximate NPK ratio of 8-7-0.5, as seen in Table 2.

Table 2.
Percentage of macronutrient composition of dried fish.
The product obtained has similar parameters to fish flour obtained by conventional drying procedures [43]. These proportions allow dosing for various crops, mainly vegetables, that require smaller quantities of fertilizers than cereal crops. One advantage is the contribution of phosphorus, since synthetic fertilizers are the most expensive and inaccessible to small producers.
Currently, at the national level, few regulations refer to organic waste’s characterization and quality standards for composting. For instance, NMX-AA-180-SCFI-2018 outlines the methods and procedures for the aerobic treatment of the organic fraction waste of Urban Solid Waste and Special Management, as well as the commercial information and quality parameters of the final products. It indicates that a finished compost with less than 7% of the sum of macronutrients is considered a soil improver. In contrast, if the sum exceeds this threshold, it must be labeled as an organic fertilizer, and the percentages must be specified.
There is no reference to sodium being considered a contaminant within the standards. However, Annex AS-05 Soil moisture content of NOM-021-RECNAT-2000, which establishes the fertility, salinity, and soil classification specifications, recommends evaluating salinity indices and soil mixtures for assessment.
The calcium content should be assessed based on the pH of the soil type where the waste will be used and the type of crop to be established. Combining alkaline soils with other organic matter is advisable to support pH buffering.
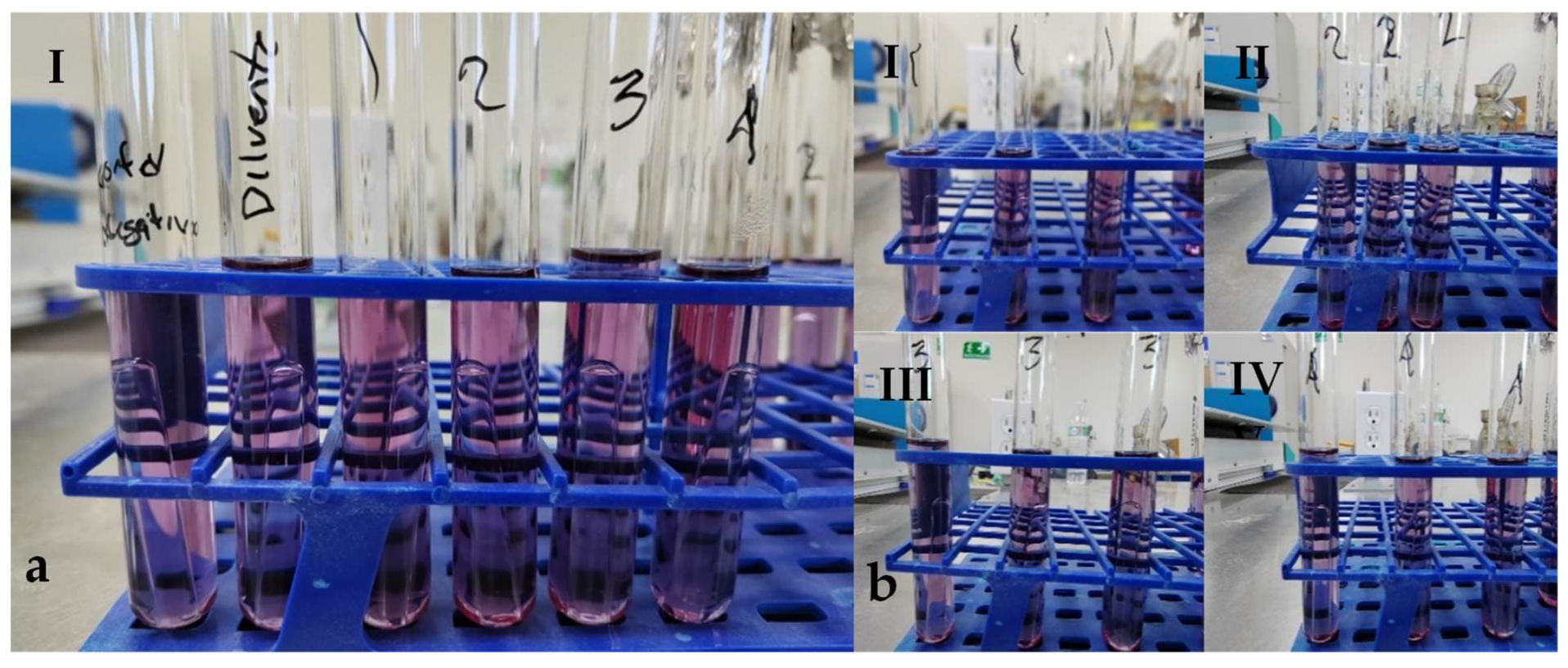
Regarding total and fecal coliforms, after incubating the samples for 24 h, no color change in the medium or gas production was observed, so an additional 24 h incubation was conducted. After 48 h, the sample was reanalyzed. Still, no gas production or color change was detected in the dilutions, indicating that the sample was negative for total and fecal coliforms (Figure 10).
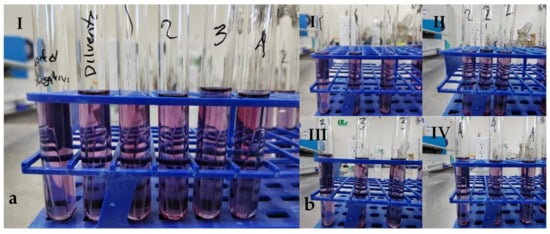
Figure 10.
Determination of total coliforms on fish meal obtained by solar drying. (a) (I) Tubes containing, from left to right: negative control, diluent control, and serial dilutions of 10-2, 10-3, 10-4 and 10-5. (b) Triplicate serial dilutions of 10-2, 10-3, 10-4, and 10-5 (I–IV), showing no visible color change or gas production in the medium.
In panels a and b, no gas production is observed, and no change in the color of the medium either, so the presence of fecal coliforms was not detectable. In panel a, the negative control tube, the diluent control, the 10-2 dilution (1), the 10-3 dilution (2), the 10-4 dilution (3), and the 10-5 dilution (4) are observed from left to right. In panel b, serial dilutions are observed in triplicate, corresponding to the 10-2 dilution (1), the 10-3 dilution (2), the 10-4 dilution (3), and the 10-5 dilution (4); no change in color or gas production was observed in the medium.
This result is considered positive regarding the potential use of the obtained residue, as it suggests there would be no contamination by fecal coliforms in the crops if the residue were used as fertilizer.
The analyzed samples did not exhibit gas production or color change in the medium, which was consistent with the control group. Therefore, they are classified as negative for fecal coliforms. The Most Probable Number (MPN) is expressed in units of MPN per gram of total solids (MPN/g TS).
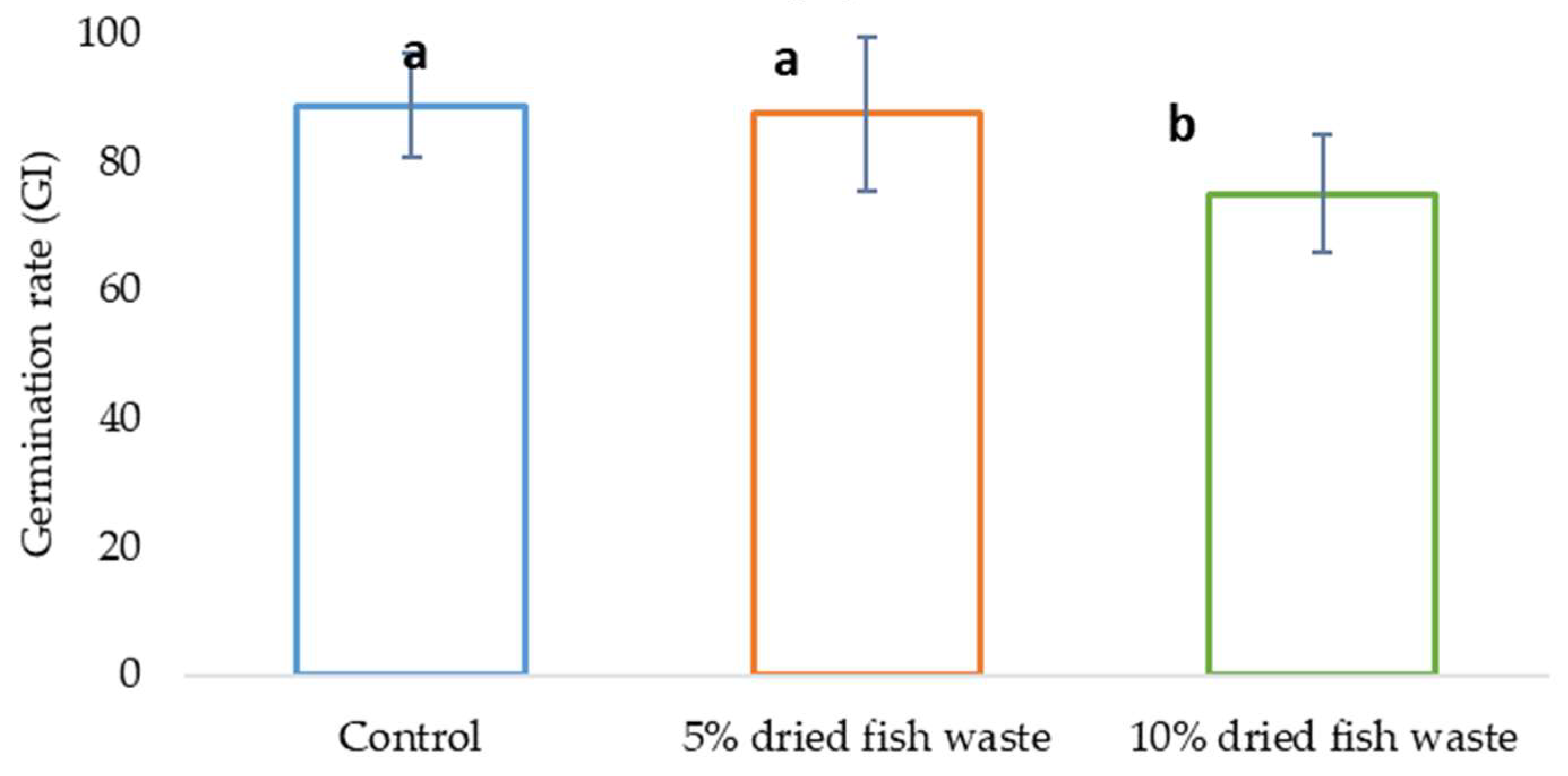
In the phytotoxicity assay using cucumber (Cucumis sativus) seeds as the indicator species, a germination index (GI) analysis for each treatment revealed that while a 5% fish waste concentration did not negatively impact germination, a 10% concentration did, as illustrated in Figure 11. Although the 5% fish concentration did not significantly enhance germination, it is essential to note that it did not inhibit it, suggesting that fish flour biofertilizer at this concentration does not exhibit phytotoxic effects.
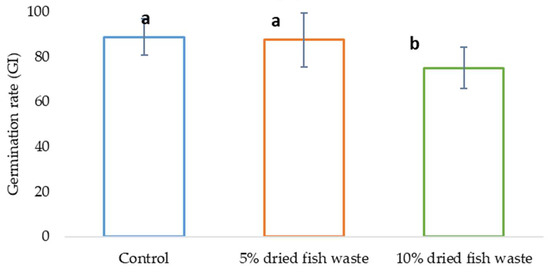
Figure 11.
GI test results.
Figure 11 presents the germination index (GI) test results. The bars labeled with the same letter indicate homogeneity between groups at a significance level of 0.05 (α = 0.05), according to the ANOVA test.
There is limited information on studies explicitly addressing the cytotoxicity or effects on seed germination of fishmeal or fish waste biofertilizers [28,44,45]. Most research focuses on the positive impacts of fishmeal-based biofertilizers on plant growth and development. For instance, Radziemska et al. (2019) [26] demonstrated that a fish waste compost composed of 80% fish and 20% pine bark, with a final NPK ratio of 11.1-2.61-3.07, is a viable agricultural fertilizer. Their study found that mature compost did not exhibit phytotoxicity and even stimulated root growth at a 25% concentration using white mustard seeds (Sinapis alba L.). The compost did not negatively affect seed germination, even at the highest concentration of 100%. The 25% compost concentration stimulated root growth compared to the control and higher compost concentrations, and it also increased the yield and nutrient uptake of ice lettuce, highlighting its potential as a valuable resource for improving plant growth and soil fertility.
Overall, the results suggest that caution should be exercised when using excessive amounts of fish waste biofertilizer without proper composting or humification. While mature fertilizers can be beneficially applied, the effects of using crude fish meal need a thorough evaluation. More research is necessary to understand these products’ potential risks and benefits fully, ensuring their safe and effective use in agricultural applications.
The decrease in the GI using 10% fish residue could be attributed to the fat content, as fats and oils are recalcitrant soil contaminants that can negatively impact fertility when soil flora cannot neutralize lipids and free fatty acids. These substances can contaminate water sources if not managed carefully [46].
Figure 12 shows a cucumber seed from an experiment using dried fish as fertilizer.

Figure 12.
Germinated cucumber seed with 5% dry fish waste.
4. Conclusions
This study successfully converted marine waste and low-value fish into a nutrient-rich biofertilizer through solar drying. The process produced a biofertilizer with a NPK ratio of approximately 8-7-0.5, suitable for various crops. The absence of fecal coliforms and the positive impact on seed germination, particularly with a 5% soil mixture, prove the safety and effectiveness of the product. In addition, solar energy was incorporated into the drying process, highlighting the environmental and economic benefits compared with gas use. The findings suggest that unused sun-dried fish can effectively replace synthetic fertilizers, offering a sustainable solution to nutrient recycling in agriculture. This approach supports circular economy principles, reduces greenhouse gas emissions, and provides a profitable alternative for smallholder farmers, especially in regions facing fertilizer shortages.
Author Contributions
Conceptualization, B.C.-T. and M.C.T.; methodology, B.C.-T. and M.F.M.d.C.; validation, E.O.Z.G., B.C.-T., and M.C.T.; formal analysis, G.A.M.-P.; investigation, A.D.N., M.F.M.d.C., and M.C.T.; data curation, A.D.N. and G.A.M.-P.; writing—original draft preparation, B.C.-T. and M.C.T.; writing—review and editing, G.A.M.-P., B.C.-T. and M.C.T.; visualization, M.F.M.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Strategic Energy and Climate Change Program from CONAHCYT for the FOP04-2021-03-319524 project. A community plant for drying fishery products operated with solar thermal energy is needed for integration into rural communities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAO. Infographics: The High Cost of Food Waste—Global Landscapes Forum. Available online: https://www.globallandscapesforum.org/infographic/infographic-high-cost-of-food-waste/?gclid=Cj0KCQiA8t2eBhDeARIsAAVEga0HqOsvU3big_e6MoxwO-lMWfwmhp5anVTl4Pu98asPmT5yxi6rR8waAncoEALw_wcB (accessed on 31 January 2023).
- UN. Food and Climate Change: Healthy Diets for a Healthier Planet|United Nations. Available online: https://www.un.org/en/climatechange/science/climate-issues/food?gclid=Cj0KCQiA8t2eBhDeARIsAAVEga0QmfDoXjA0UHfwhDryfOTu_KDUfegyjp8DwhY0e4lh2U8R0JaIXZwaAmEeEALw_wcB (accessed on 31 January 2023).
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. To Feed the World, We Need to Waste Less Fish. Available online: https://www.weforum.org/agenda/2022/09/feed-world-sustainably-reduce-fish-waste/ (accessed on 7 April 2023).
- Chassot, E.; Antoine, S.; Guillotreau, P.; Lucas, J.; Assan, C.; Marguerite, M.; Bodin, N. Fuel consumption and air emissions in one of the world’s largest commercial fisheries. Environ. Pollut. 2021, 273, 116454. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Lall, S.P.; Anderson, D.M.; McNiven, M.A. Availability of amino acids from various fish meals fed to Atlantic salmon (Salmo solar). Aquaculture 1995, 138, 291–301. [Google Scholar] [CrossRef]
- Muscolo, A.; Mauriello, F.; Marra, F.; Calabrò, P.S.; Russo, M.; Ciriminna, R.; Pagliaro, M. AnchoisFert: A New Organic Fertilizer from Fish Processing Waste for Sustainable Agriculture. Glob. Chall. 2022, 6, 2100141. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Loss and Waste in Fish Value Chains. Available online: https://www.fao.org/flw-in-fish-value-chains/overview/objective/en/ (accessed on 7 April 2023).
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties; Taylor & Francis: Abingdon, UK, 2000; Volume 40, pp. 43–81. ISBN 1040869009. [Google Scholar]
- Isibika, A.; Vinnerås, B.; Kibazohi, O.; Zurbrügg, C.; Lalander, C. Co-composting of banana peel and orange peel waste with fish waste to improve conversion by black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae. J. Clean. Prod. 2021, 318, 128570. [Google Scholar] [CrossRef]
- de Carvalho Bonfim, B.; Monteiro, M.L.G.; dos Santos, A.F.G.N.; dos Santos Vilar, J.; Conte-Junior, C.A. Nutritional Improvement and Consumer Perspective of Fish Nuggets with Partial Substitution of Wheat Flour Coating by Fish (Priacanthus arenatus, Cuvier, 1829) Waste Flour. J. Aquat. Food Prod. Technol. 2020, 29, 28–42. [Google Scholar] [CrossRef]
- Czeczuga, B. Carotenoids in fish. XXVIII. Carotenoids in Micropterus salmoides (Lalépéde) Centrarchidae. Hydrobiologia 1981, 78, 45–48. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Ruttanapornvareesakul, Y.; Ikeda, M.; Hara, K.; Osako, K.; Kongpun, O.; Nozaki, Y. Effect of shrimp head protein hydrolysates on the state of water and denaturation of fish myofibrils during dehydration. Fish. Sci. 2005, 71, 220–228. [Google Scholar] [CrossRef]
- Aoki, H.; Ahsan, M.N.; Matsuo, K.; Hagiwara, T.; Watabe, S. Partial purification of proteases that are generated by processing of the Northern shrimp Pandalus borealis and which can tenderize beef. Int. J. Food Sci. Technol. 2004, 39, 471–480. [Google Scholar] [CrossRef]
- World Bank. How to Manage the World’s Fertilizers to Avoid a Prolonged Food Crisis. Available online: https://blogs.worldbank.org/voices/how-manage-worlds-fertilizers-avoid-prolonged-food-crisis (accessed on 7 April 2023).
- FAO. Plant Nutrition for Food Security; FAO: Rome, Italy, 2006; ISBN 9251054908. [Google Scholar]
- Sharma, V.; Kaur, J.; Sharma, S. Plant Growth Promoting Rhizobacteria: Potential microbes for sustainable agriculture. Biotecnol. Veg. 2020, 20, 157–166. [Google Scholar] [CrossRef]
- Lanno, M.; Kriipsalu, M.; Shanskiy, M.; Silm, M.; Kisand, A. Distribution of phosphorus forms depends on compost source material. Resources 2021, 10, 102. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, J.K. Complete reutilisation of mixed mackerel and brown seaweed wastewater as a high-quality biofertiliser in open-flow lettuce hydroponics. J. Clean. Prod. 2020, 247, 119081. [Google Scholar] [CrossRef]
- Kang, K.H.; Lee, H.; Park, T.; Sim, J.H.; Kim, S.-Y. Biodegradation of High Salinity Fishery Wastes in a 150-L Reactor by Bacillus licheniformis TK3-Y for Reutilization as Liquid Fertilizer. KSBB J. 2020, 35, 288–293. [Google Scholar] [CrossRef]
- Hidayati, P.A.; Mubarak, A.S. Sudarno The optimal n/p ratio of shrimp culture waste liquid fertilizer on growth of Chlorella vulgaris. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012090. [Google Scholar] [CrossRef]
- Brasileiro, O.L.; Cavalheiro, J.M.O.; de Sousa Prado, J.P.; dos Anjos, A.G.; Cavalheiri, T.T.B. Determination of the chemical composition and functional properties of shrimp waste protein concentrate and lyophilized flour. Ciência Agrotecnologia 2012, 36, 189–194. [Google Scholar] [CrossRef]
- Zebib, H.; Teame, T.; Aregawi, T.; Meresa, T. Nutritional and sensory acceptability of wheat bread from fish flour. Cogent Food Agric. 2020, 6, 1714831. [Google Scholar] [CrossRef]
- de Oliveira, I.S.; Lourenço, L.d.F.H.; Sousa, C.L.; Joele, M.R.S.P.; do Amaral Ribeiro, S.d.C. Composition of MSM from Brazilian catfish and technological properties of fish flour. Food Control 2015, 50, 38–44. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Adamcová, D.; Brtnický, M.; Mazur, Z. Valorization of Fish Waste Compost as a Fertilizer for Agricultural Use. Waste Biomass Valorization 2019, 10, 2537–2545. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Chapter 13—Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–245. ISBN 978-0-12-819555-0. [Google Scholar]
- Illera-Vives, M.; Seoane Labandeira, S.; Brito, L.M.; López-Fabal, A.; López-Mosquera, M.E. Evaluation of compost from seaweed and fish waste as a fertilizer for horticultural use. Sci. Hortic. 2015, 186, 101–107. [Google Scholar] [CrossRef]
- Marzuki, S.U.; Pranoto, Y.; Khumsap, T.; Nguyen, L.T. Effect of blanching pretreatment and microwave-vacuum drying on drying kinetics and physicochemical properties of purple-fleshed sweet potato. J. Food Sci. Technol. 2021, 58, 2884–2895. [Google Scholar] [CrossRef]
- Saengrayap, R.; Tansakul, A.; Mittal, G.S. Effect of far-infrared radiation assisted microwave-vacuum drying on drying characteristics and quality of red chilli. J. Food Sci. Technol. 2015, 52, 2610–2621. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, M.; Hu, Q.; Sun, J. Characteristics of Microwave Drying of Bighead Carp. Dry. Technol. 2005, 23, 637–643. [Google Scholar] [CrossRef]
- Reineccius, G.A. The Spray Drying of Food Flavors. Dry. Technol. 2004, 22, 1289–1324. [Google Scholar] [CrossRef]
- Bajgai, T.R.; Hashinaga, F. High Electric Field Drying of Japanese Radish. Dry. Technol. 2001, 19, 2291–2302. [Google Scholar] [CrossRef]
- Batista, J.T.S.; da Silva Araújo Matias, C.; da Silva Martins, L.H.; Cardoso, D.N.P.; Joele, MRSP; de Fátima Henriques Lourenço, L. Effect of convection drying and lyophilization of fish myofibrillar proteins on the technological properties of biodegradable films. Dry. Technol. 2022, 40, 1673–1687. [Google Scholar] [CrossRef]
- Mandal, M.; Roy, A.; Das, S.; Rakwal, R.; Agrawal, G.K.; Singh, P.; Awasthi, A.; Sarkar, A. Food waste-based bio-fertilizers production by bio-based fermenters and their potential impact on the environment. Chemosphere 2024, 353, 141539. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, M.M.; Buitrago, E.M.; Yñiguez, R.; Puig-Cabrera, M. Circular economy and agriculture: Mapping circular practices, drivers, and barriers for traditional table-olive groves. Sustain. Prod. Consum. 2024, 46, 430–441. [Google Scholar] [CrossRef]
- NMX-AA-180-SCFI-2018; Que establece los métodos y procedimientos para el tratamiento aerobio de la fracción orgánica de los residuos sólidos urbanos y de manejo especial, así como la información comercial y de sus parámetros de calidad de los productos finales. Dirección General de Normas Mexicanas: Mexico City, Mexico, 2018; p. 52.
- Licon, C. Proximate and Other Chemical Analyses☆. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 521–529. ISBN 978-0-12-818767-8. [Google Scholar]
- Knowles, V.; Plaxton, W. Quantification of Total and Soluble Inorganic Phosphate. Bio-Protocol 2013, 3, e890. [Google Scholar] [CrossRef]
- DOF—Diario Oficial de la Federación NOM-004-SEMARNAT-2002. Available online: https://dof.gob.mx/nota_detalle.php?codigo=691939&fecha=15/08/2003#gsc.tab=0 (accessed on 17 July 2024).
- Gerber, M.D.; Arsand, D.R.; Lucia, T.; Correa, É.K. Phytotoxicity Evaluation of Wastewater from Rice Parboiling. Bull. Environ. Contam. Toxicol. 2018, 101, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, G.; Chen, W.; Yang, Y.; Ma, R.; Li, D.; Shen, Y.; Li, G.; Yuan, J. Phytotoxicity of farm livestock manures in facultative heap composting using the seed germination index as indicator. Ecotoxicol. Environ. Saf. 2022, 247, 114251. [Google Scholar] [CrossRef] [PubMed]
- Blas, C.; Mateos, G.; García, P. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos para la Fabricación de Piensos Compuestos; Blas, C., García-Rebollar, P., Gorrachategui, M., Mateos, G., Eds.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2019; Volume 1, ISBN 978-8409156887. [Google Scholar]
- Illera-Vives, M.; Seoane Labandeira, S.; Iglesias Loureiro, L.; López-Mosquera, M.E. Agronomic assessment of a compost consisting of seaweed and fish waste as an organic fertilizer for organic potato crops. J. Appl. Phycol. 2017, 29, 1663–1671. [Google Scholar] [CrossRef]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.-K. Fish and fish waste-based fertilizers in organic farming—With status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).