Thermochemical Conversion of Biomass into Biochar: Enhancing Adsorption Kinetics and Pore Properties for Environmental Sustainability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Biochar Preparation
2.3. Biochar Characterization
2.4. Adsorption Performance Experiments
3. Results
3.1. Characterization of the Biochars
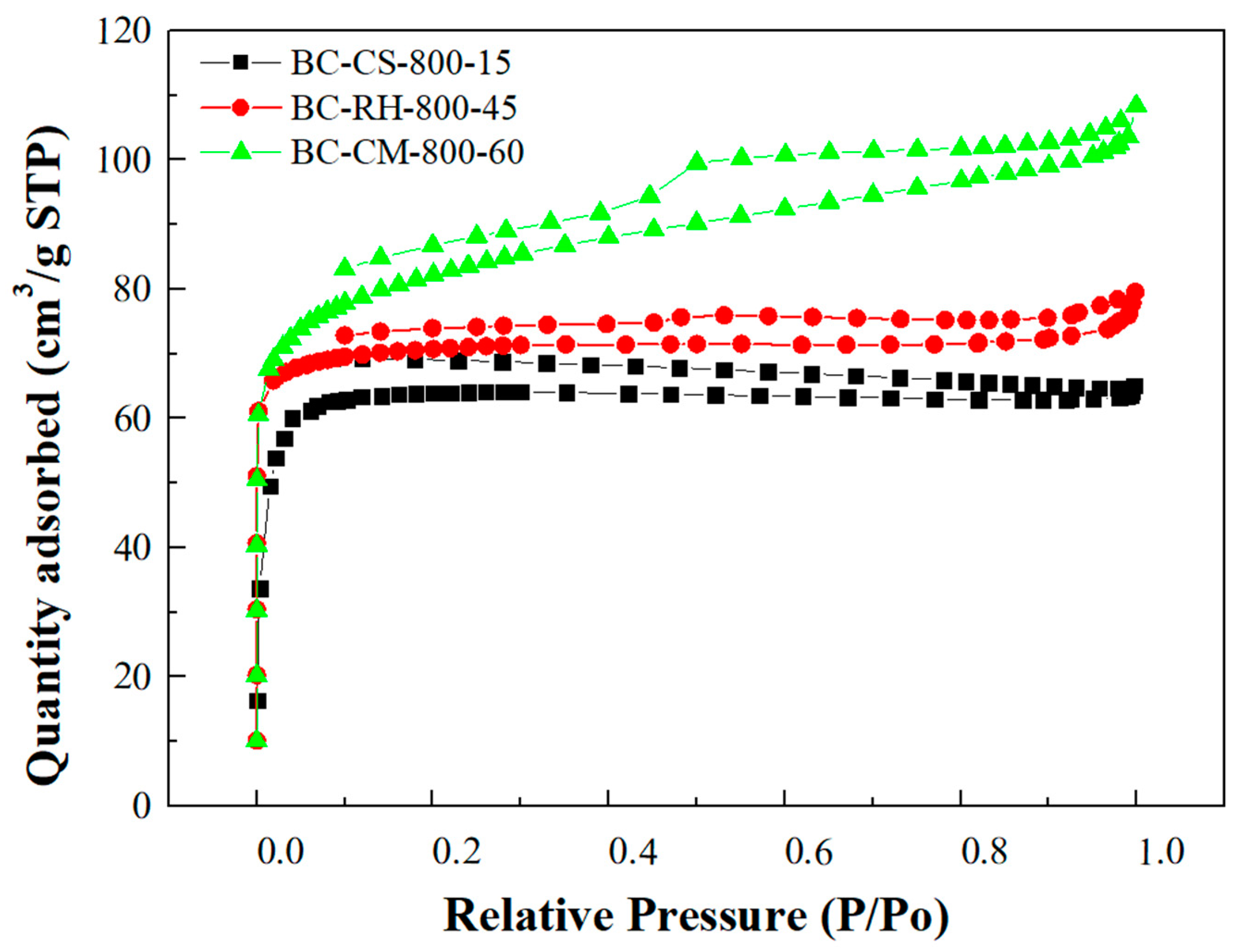
3.2. Pore Properties of the Resulting Biochars
3.3. Sorption Kinetic Performances of Resulting Biochar
3.4. Adsorption Kinetics Parameters
4. Conclusions
- This study demonstrates the significant impact of pyrolysis conditions on the pore structure and adsorption capabilities of biochar derived from coconut shell (BC-CS), rice husk (BC-RH), and cow manure (BC-CM).
- Optimal pyrolysis at 800 °C with varying residence times notably influenced the BET surface area, total pore volume, and pore diameter, with BC-CM exhibiting the highest BET surface area (263.3 m2/g) and pore volume (0.164 cm3/g).
- Residence time at elevated temperatures affect the various types of biochars differently. The optimum conditions of residence time are biomass-specific. Too short or too long residence times can cause the biochar’s pores to clog or collapse, leading to a reduction in surface area and pore volume.
- Nitrogen adsorption–desorption isotherms indicated that these biochars exhibit Type I and IV isotherms, characteristic of microporous and mesoporous structures, with BC-CM demonstrating the highest adsorption capacity.
- The adsorption kinetics of methylene blue (MB) followed a pseudo-second-order model, suggesting some form of chemisorption as the primary mechanism. BC-CM showed the highest adsorption efficiency, followed by BC-RH and BC-CS, and increasing biochar doses enhanced MB removal efficiency.
- The adsorption capacity of the biochar is correlated with not only the physical properties, such as surface area and pore volume, but also the chemical characteristics of the biochar’s surface.
- The presence of functional groups such as the hydroxyl, carbonyl, and carboxyl groups further improved adsorption performance.
- While controlled experimental conditions provided valuable insights, real-world applications may present challenges due to varying environmental conditions. Future research should validate these findings in practical settings and refine pyrolysis protocols to ensure consistent biochar performance.
- This study underscores the need to optimize pyrolysis parameters and feedstock selection to enhance biochar’s effectiveness in environmental remediation applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kiran, B.R.; Mohan, S.V. Carbon farming: A circular framework to augment CO2 sinks and to combat climate change. Environ. Sci. Adv. 2024, 3, 522–542. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Saleh, H.M.; Hassan, A.I. Green conversion of carbon dioxide and sustainable fuel synthesis. Fire 2023, 6, 128. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Huang, H.J. Valorization of rice husk for the production of porous biochar materials. Fermentation 2021, 7, 70. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Zama, E.F.; Reid, B.J.; Arp, H.P.H.; Sun, G.X.; Yuan, H.Y.; Zhu, Y.G. Advances in research on the use of biochar in soil for remediation: A review. J. Soils Sediments 2018, 18, 2433–2450. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, R.; Ji, S.; Xie, L.; Zhang, H. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef]
- Wawra, A.; Friesl-Hanl, W.; Jäger, A.; Puschenreiter, M.; Soja, G.; Reichenauer, T.; Watzinger, A. Investigations of microbial degradation of polycyclic aromatic hydrocarbons based on 13 C-labeled phenanthrene in a soil co-contaminated with trace elements using a plant assisted approach. Environ. Sci. Pollut. Res. 2018, 25, 6364–6377. [Google Scholar] [CrossRef]
- Shen, Y. Biomass-derived porous carbons for sorption of Volatile organic compounds (VOCs). Fuel 2023, 336, 126801. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Sci. Total Environ. 2021, 753, 142249. [Google Scholar] [CrossRef]
- Puglla, E.P.; Guaya, D.; Tituana, C.; Osorio, F.; García-Ruiz, M.J. Biochar from agricultural by-products for the removal of lead and cadmium from drinking water. Water 2020, 12, 2933. [Google Scholar] [CrossRef]
- Fseha, Y.H.; Sizirici, B.; Yildiz, I. Phoenix dactylifera (date palm)-derived biochar application for the adsorptive removal of multiple inorganics from groundwater for drinking water purposes. Arab. J. Sci. Eng. 2023, 48, 12725–12740. [Google Scholar] [CrossRef]
- Zubair, M.; Mu’azu, N.D.; Jarrah, N.; Blaisi, N.I.; Aziz, H.A.; Al-Harthi, M.A. Adsorption behavior and mechanism of methylene blue, crystal violet, eriochrome black T, and methyl orange dyes onto biochar-derived date palm fronds waste produced at different pyrolysis conditions. Water Air Soil Pollut. 2020, 231, 240. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio-based adsorbent: A critical review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef]
- Akhtar, M.; Iqbal, S.; Kausar, A.; Bhanger, M.I.; Shaheen, M.A. An economically viable method for the removal of selected divalent metal ions from aqueous solutions using activated rice husk. Colloids Surf. B Biointerfaces 2010, 75, 149–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, R.; Zhao, J.; Ma, F.; Zhang, Y.; Meng, Q. Characterization of H3PO4-treated rice husk adsorbent and adsorption of copper (II) from aqueous solution. Biomed. Res. Int. 2014, 2014, 496878. [Google Scholar]
- Farhan, A.T.A.; Ong, K.K.; Yunus, W.W.; Jabit, M.L.; Fitrianto, A.; Hussin, A.G.A.; Teoh, C.C. Kinetics study of nickel (II) ions sorption by thermally treated rice husk. Nat. Environ. Pollut. Technol. 2017, 16, 889. [Google Scholar]
- Azadi, F.; Saadat, S.; Karimi-Jashni, A. Experimental investigation and modeling of nickel removal from wastewater using modified rice husk in continuous reactor by response surface methodology. Iran. J. Sci. Technol. Trans. Civ. Eng. 2018, 42, 315–323. [Google Scholar] [CrossRef]
- Xia, Y.; Zuo, H.; Lv, J.; Wei, S.; Yao, Y.; Liu, Z.; Lin, Q.; Yu, Y.; Yu, W.; Huang, Y. Preparation of multi-layered microcapsule-shaped activated biomass carbon with ultrahigh surface area from bamboo parenchyma cells for energy storage and cationic dyes removal. J. Clean. Prod. 2023, 396, 136517. [Google Scholar] [CrossRef]
- Acharya, J.; Kumar, U.; Rafi, P.M. Removal of heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-a review. Int. J. Curr. Eng. Technol. 2018, 8, 526–530. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, P.C.; Lin, Y.Q. Reusing cow manure for the production of activated carbon using potassium hydroxide (KOH) activation process and its liquid-phase adsorption performance. Processes 2019, 7, 737. [Google Scholar] [CrossRef]
- Tsai, W.T.; Hsu, C.H.; Lin, Y.Q. Highly porous and nutrients-rich biochar derived from dairy cattle manure and its potential for removal of cationic compound from water. Agriculture 2019, 9, 114. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Ajien, A.; Idris, J.; Md Sofwan, N.; Husen, R.; Seli, H. Coconut shell and husk biochar: A review of production and activation technology, economic, financial aspect and application. Waste Manag. Res. 2023, 41, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 1–13. [Google Scholar]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2018, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, T.; Gurav, R.; Bhatia, S.; Park, Y.; Kim, H.; Kan, E.; Yang, Y. Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2019, 710, 136282. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, M.; Wang, Y.; Chen, J.; Zhang, J. Biochars prepared from rabbit manure for the adsorption of rhodamine B and Congo red: Characterisation, kinetics, isotherms and thermodynamic studies. Water Sci. Technol. 2020, 81, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, K. Effect of nitric acid pre-oxidation concentration on pore structure and nitrogen/oxygen active decoration sites of ethylenediamine-modified biochar for mercury(II) adsorption and the possible mechanism. Chemosphere 2019, 220, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C. Considerations in biochar characterization. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 87–100. [Google Scholar]
- Tan, X.F.; Zhu, S.S.; Wang, R.P.; Chen, Y.D.; Show, P.L.; Zhang, F.F.; Ho, S.H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, C.; Xu, J. Enhanced adsorption of Cd (II) from aqueous solution by a shrimp bran modified Typha orientalis biochar. Environ. Sci. Pollut. Res. 2019, 26, 37092–37100. [Google Scholar] [CrossRef] [PubMed]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; RT, R.K. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and π–π interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Herath, I.; Bundschuh, J.; Al-Juboori, R.A.; Vithanage, M.; Mohan, D. Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: What needs to be done in future research? Environ. Int. 2019, 127, 52–69. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Ahmad, J.; Patuzzi, F.; Rashid, U.; Shahabz, M.; Ngamcharussrivichai, C.; Baratieri, M. Exploring untapped effect of process conditions on biochar characteristics and applications. Environ. Technol. Innov. 2021, 21, 101310. [Google Scholar] [CrossRef]
- Xi, J.; Li, H.; Xi, J.; Tan, S.; Zheng, J.; Tan, Z. Preparation of high porosity biochar materials by template method: A review. Environ. Sci. Pollut. Res. 2020, 27, 20675–20684. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.; Bai, L.; Chen, X.; Li, B. High effective adsorption of Pb(II) from solution by biochar derived from torrefaction of ammonium persulphate pretreated bamboo. Bioresour. Technol. 2020, 323, 124616. [Google Scholar] [CrossRef] [PubMed]
- Maziarka, P.; Wurzer, C.; Arauzo, P.J.; Dieguez-Alonso, A.; Mašek, O.; Ronsse, F. Do you BET on routine? The reliability of N2 physisorption for the quantitative assessment of biochar’s surface area. Chem. Eng. J. 2021, 418, 129234. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption surface area and porosity. J. Electrochem. Soc. 1967, 114, 279Ca. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.; Bibi, I.; Wang, H.; Tsang, D.C.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Iwuozor, K.; Ighalo, J.; Ogunfowora, L.; Adeniyi, A.; Igwegbe, C. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. J. Environ. Chem. Eng. 2021, 9, 105658. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Khan, I. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Sah, M.K.; Edbey, K.; EL-Hashani, A.; Almshety, S.; Mauro, L.; Alomar, T.S.; Bhattarai, A. Exploring the biosorption of methylene blue dye onto agricultural products: A critical review. Separations 2022, 9, 256. [Google Scholar] [CrossRef]





| Sample | Carbon (wt%) | Hydrogen (wt%) | Nitrogen (wt%) | Sulfur (wt%) | Oxygen (wt%) | H/C Ratio | O/C Ratio |
|---|---|---|---|---|---|---|---|
| BC-CS-800-0 | 90.6 | 1.3 | 0.8 | 0.1 | 4.2 | 0.17 | 0.03 |
| BC-CS-800-15 | 91.8 | 1.1 | 0.8 | 0.1 | 10.3 | 0.14 | 0.08 |
| BC-CS-800-30 | 93.4 | 1.2 | 0.9 | 0.0 | 7.5 | 0.15 | 0.06 |
| BC-CS-800-45 | 93.8 | 1.2 | 0.9 | 0.1 | 4.0 | 0.15 | 0.03 |
| BC-CS-800-60 | 97.3 | 1.2 | 0.4 | 0.1 | 1.1 | 0.15 | 0.01 |
| BC-RH-800-0 | 56.0 | 1.1 | 0.0 | 0.1 | 42.9 | 0.24 | 0.57 |
| BC-RH-800-15 | 58.6 | 1.3 | 0.5 | 0.2 | 39.5 | 0.27 | 0.51 |
| BC-RH-800-30 | 61.3 | 1.2 | 0.6 | 0.2 | 39.7 | 0.23 | 0.49 |
| BC-RH-800-45 | 64.9 | 1.1 | 0.5 | 0.1 | 41.3 | 0.20 | 0.48 |
| BC-RH-800-60 | 66.3 | 1.1 | 0.5 | 0.1 | 41.9 | 0.20 | 0.47 |
| BC-CM-800-0 | 51.0 | 1.0 | 1.8 | 0.4 | 45.8 | 0.24 | 0.67 |
| BC-CM-800-15 | 60.4 | 1.3 | 1.3 | 0.3 | 36.8 | 0.26 | 0.46 |
| BC-CM-800-30 | 63.8 | 1.3 | 1.1 | 0.3 | 33.5 | 0.24 | 0.39 |
| BC-CM-800-45 | 64.8 | 1.3 | 1.3 | 0.3 | 32.4 | 0.24 | 0.38 |
| BC-CM-800-60 | 66.6 | 1.2 | 1.0 | 0.3 | 30.9 | 0.22 | 0.35 |
| Biochar Product | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) | |||
|---|---|---|---|---|---|---|
| SBET | Smicro | Sext | Vt | Vmicro | 4Vt/SBET | |
| BC-CS-800-0 | 81.56 | 69.79 | 11.76 | 0.035 | 0.003 | 2.5 |
| BC-CS-800-15 | 197.38 | 179.54 | 17.84 | 0.099 | 0.09 | 2.01 |
| BC-CS-800-30 | 90.32 | 78.35 | 11.978 | 0.040 | 0.041 | 2.11 |
| BC-CS-800-45 | 174.51 | 162.27 | 12.23 | 0.087 | 0.081 | 1.99 |
| BC-CS-800-60 | 149 | 147.23 | 1.77 | 0.076 | 0.076 | 2.04 |
| BC-RH-800-0 | 64.34 | 52.88 | 11.46 | 0.049 | 0.031 | 2.77 |
| BC-RH-800-15 | 197.26 | 178.66 | 18.6 | 0.119 | 0.095 | 2.08 |
| BC-RH-800-30 | 187.8 | 176.77 | 11.03 | 0.142 | 0.093 | 2.18 |
| BC-RH-800-45 | 220.62 | 196.62 | 23.99 | 0.127 | 0.014 | 2.18 |
| BC-RH-800-60 | 194.4 | 171.37 | 23.03 | 0.114 | 0.092 | 2.14 |
| BC-CM-800-0 | 195.64 | 165.4 | 30.24 | 0.113 | 0.086 | 2.31 |
| BC-CM-800-15 | 251.96 | 200.64 | 51.31 | 0.145 | 0.102 | 2.31 |
| BC-CM-800-30 | 233.52 | 177.64 | 55.88 | 0.138 | 0.092 | 2.36 |
| BC-CM-800-45 | 118.31 | 91.74 | 26.58 | 0.089 | 0.048 | 2.71 |
| BC-CM-800-60 | 263.3 | 169.55 | 93.75 | 0.164 | 0.086 | 2.49 |
| Initial MB Concentration (mg/L) |
k
(g/mg·min) | qe (mg/g) | Correlation Coefficient |
t
1/2
(min) | |
|---|---|---|---|---|---|
| BC-CS-800-15 | 1 mg/L | 0.725 | 0.108 | 0.998 | 12.77 |
| 5 mg/L | 0.253 | 0.220 | 0.968 | 17.99 | |
| 10 mg/L | 0.118 | 0.340 | 0.991 | 24.97 | |
| BC-RH-800-45 | 1 mg/L | 0.024 | 1.552 | 0.994 | 27.19 |
| 5 mg/L | 0.077 | 1.200 | 0.998 | 10.85 | |
| 10 mg/L | 0.0601 | 1.145 | 0.956 | 14.54 | |
| BC-CM-800-60 | 1 mg/L | 0.171 | 0.378 | 0.996 | 15.46 |
| 5 mg/L | 0.935 | 1.042 | 0.955 | 1.03 | |
| 10 mg/L | 0.101 | 2.180 | 0.997 | 4.53 |
|
Adsorbent Dosage (g/0.1 L) |
k
(g/mg·min) | qe (mg/g) | Correlation Coefficient |
t
1/2
(min) | |
|---|---|---|---|---|---|
| BC-CS-800-15 | 0.1 g | 0.017 | 1.049 | 0.683 | 56.40 |
| 0.3 g | 0.176 | 0.330 | 0.979 | 17.24 | |
| 0.5 g | 0.253 | 0.216 | 0.968 | 18.32 | |
| BC-RH-800-45 | 0.1 g | 0.276 | 2.472 | 0.987 | 1.47 |
| 0.3 g | 0.020 | 2.767 | 0.997 | 17.89 | |
| 0.5 g | 0.058 | 4.189 | 1.000 | 4.09 | |
| BC-CM-800-60 | 0.1 g | 0.007 | 3.946 | 0.989 | 38.99 |
| 0.3 g | 0.011 | 2.107 | 0.979 | 42.00 | |
| 0.5 g | 0.007 | 2.800 | 0.955 | 51.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.-J.; Morgan, H.M., Jr.; Tsai, W.-T.; Chien, H.; Yen, T.-B.; Lee, Y.-R. Thermochemical Conversion of Biomass into Biochar: Enhancing Adsorption Kinetics and Pore Properties for Environmental Sustainability. Sustainability 2024, 16, 6623. https://doi.org/10.3390/su16156623
Jiang T-J, Morgan HM Jr., Tsai W-T, Chien H, Yen T-B, Lee Y-R. Thermochemical Conversion of Biomass into Biochar: Enhancing Adsorption Kinetics and Pore Properties for Environmental Sustainability. Sustainability. 2024; 16(15):6623. https://doi.org/10.3390/su16156623
Chicago/Turabian StyleJiang, Tasi-Jung, Hervan Marion Morgan, Jr., Wen-Tien Tsai, Herlin Chien, Tsair-Bor Yen, and Yu-Ru Lee. 2024. "Thermochemical Conversion of Biomass into Biochar: Enhancing Adsorption Kinetics and Pore Properties for Environmental Sustainability" Sustainability 16, no. 15: 6623. https://doi.org/10.3390/su16156623







