Sustainable Ecological Non-Sintered Ceramsite (SENC) with Alkali Activators: Performance Regulation and Microstructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportion Design
2.3. Preparation Process
2.4. Test Methods of AABS
2.4.1. Compressive Strength Test
2.4.2. Nuclear Magnetic Resonance (NMR) Test
2.5. Test Methods of SENC
2.5.1. Physical Properties Test
2.5.2. Mechanical Properties Test
2.5.3. X-ray Diffraction (XRD) Test
2.5.4. Scanning Electron Microscopy (SEM) Test
3. Results and Discussion
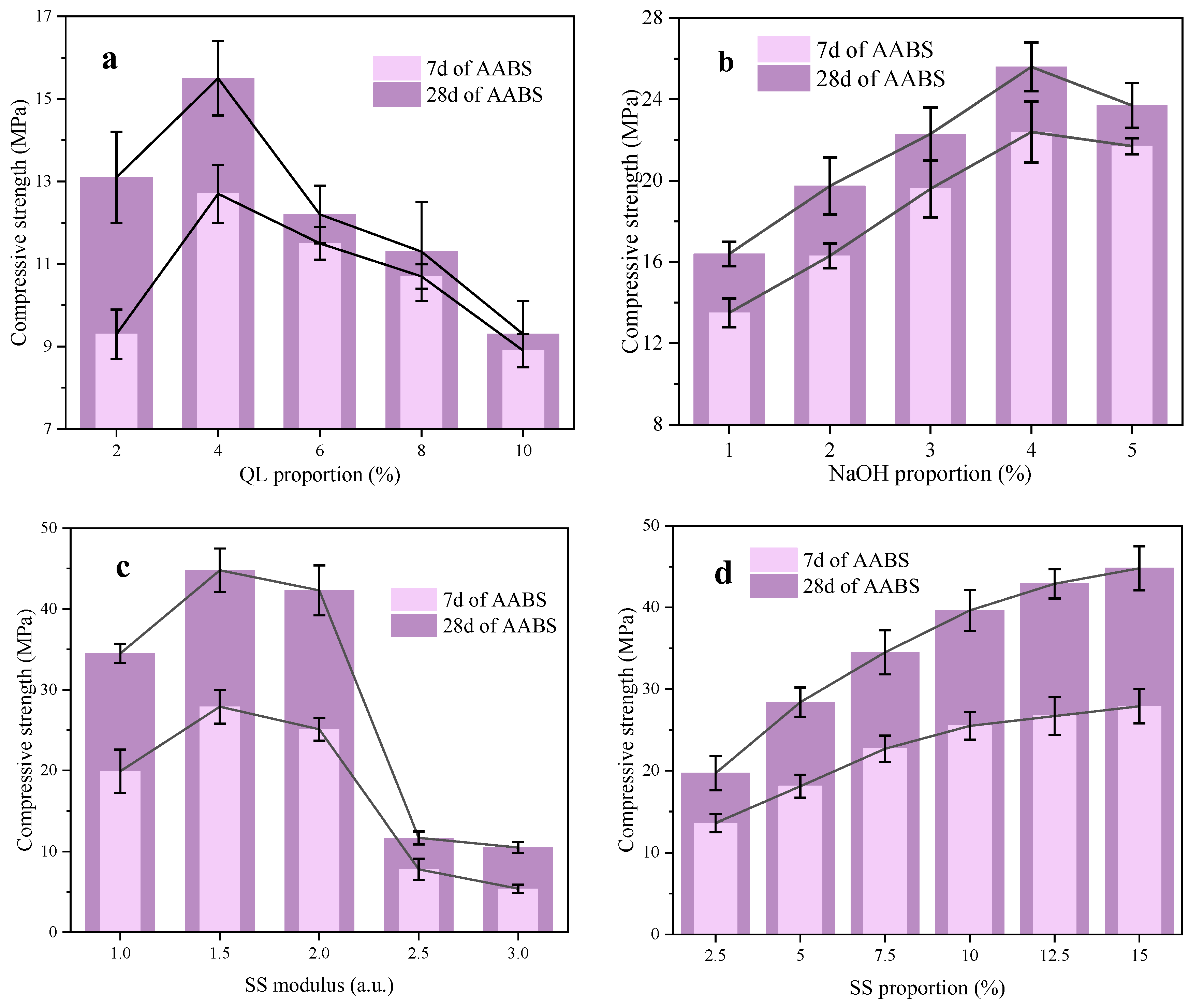
3.1. Single Factor Effects of Alkali Activators
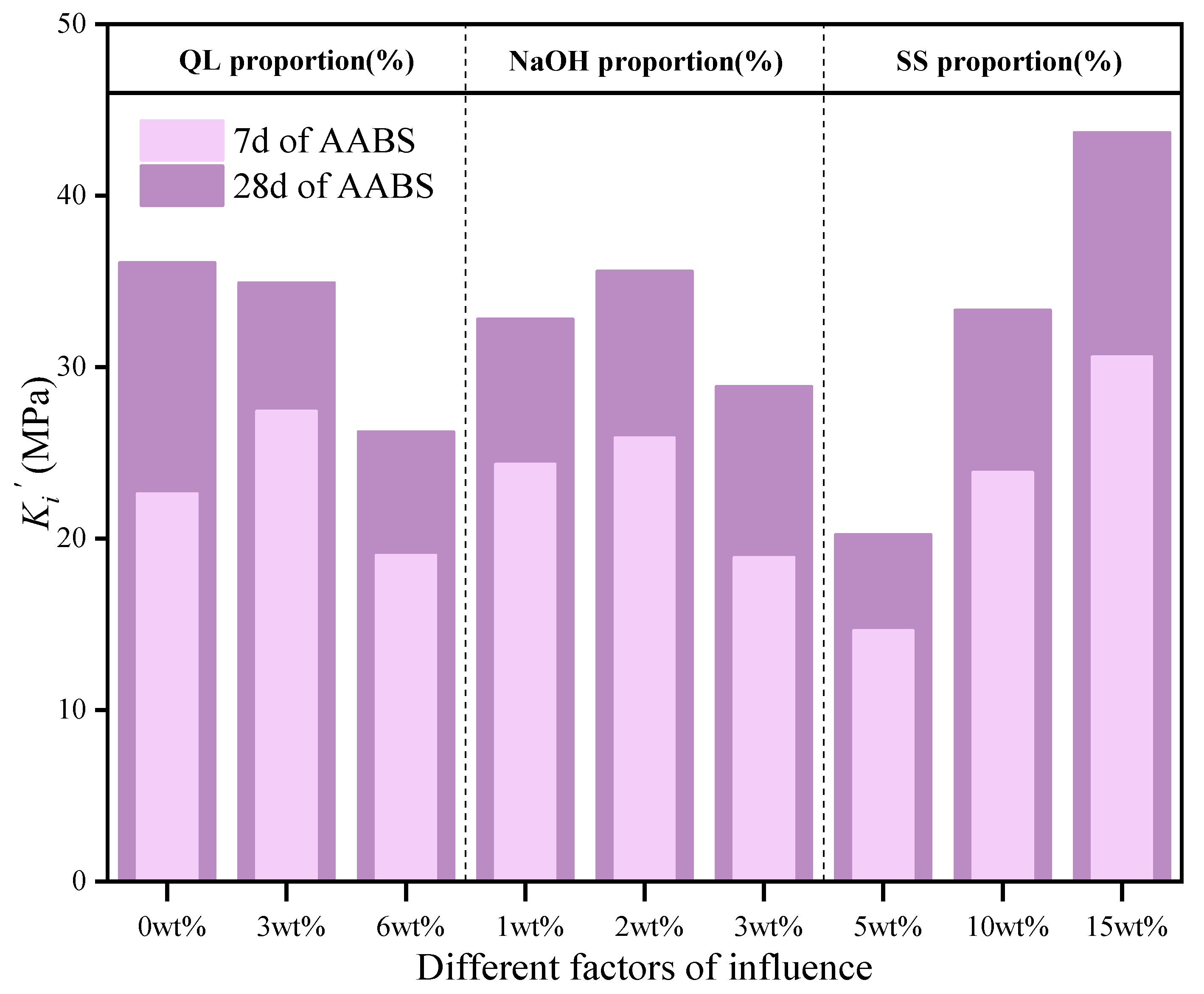
3.2. Multifactorial Effects of Alkali Activators
3.2.1. Mechanical Properties Analysis
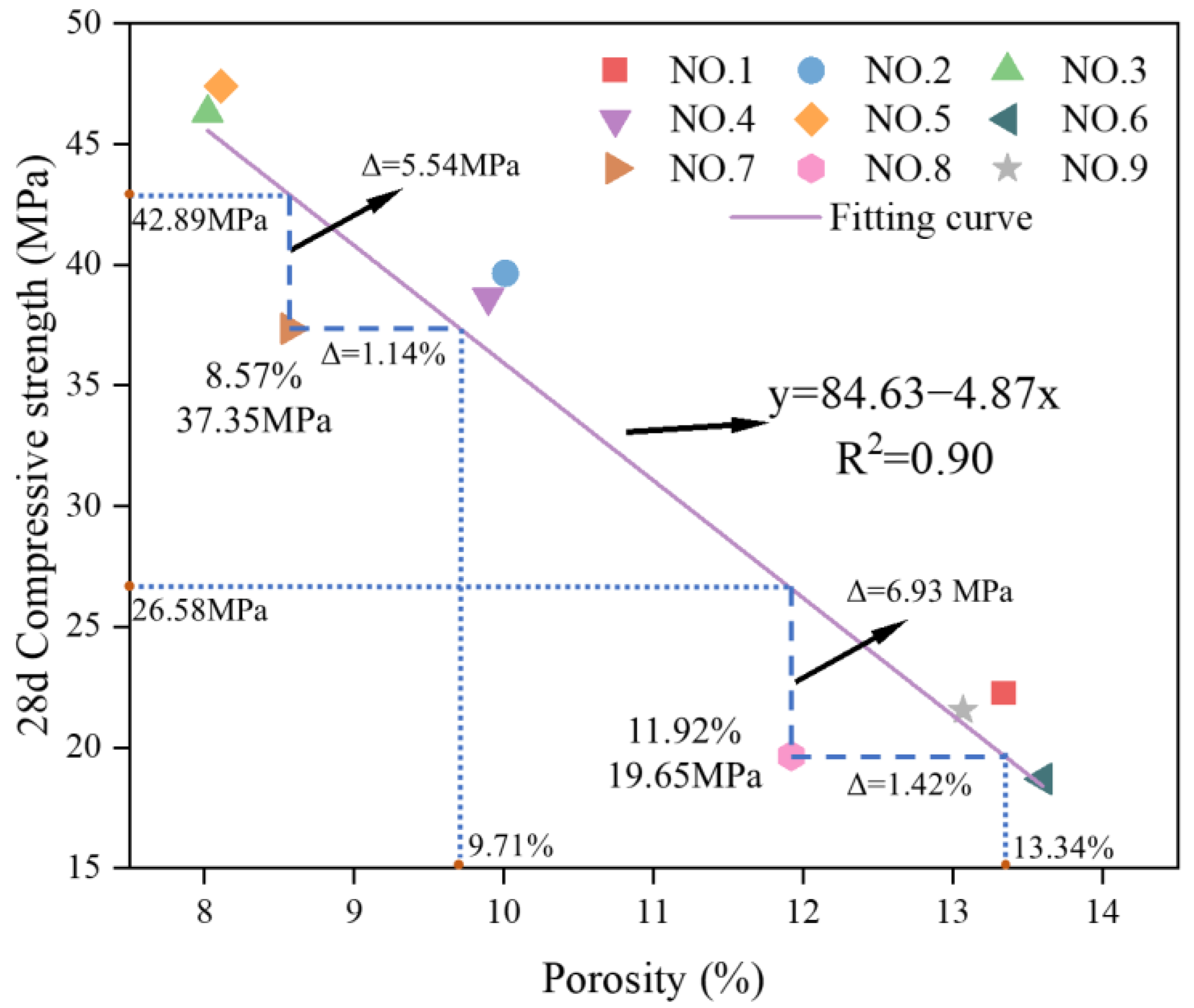
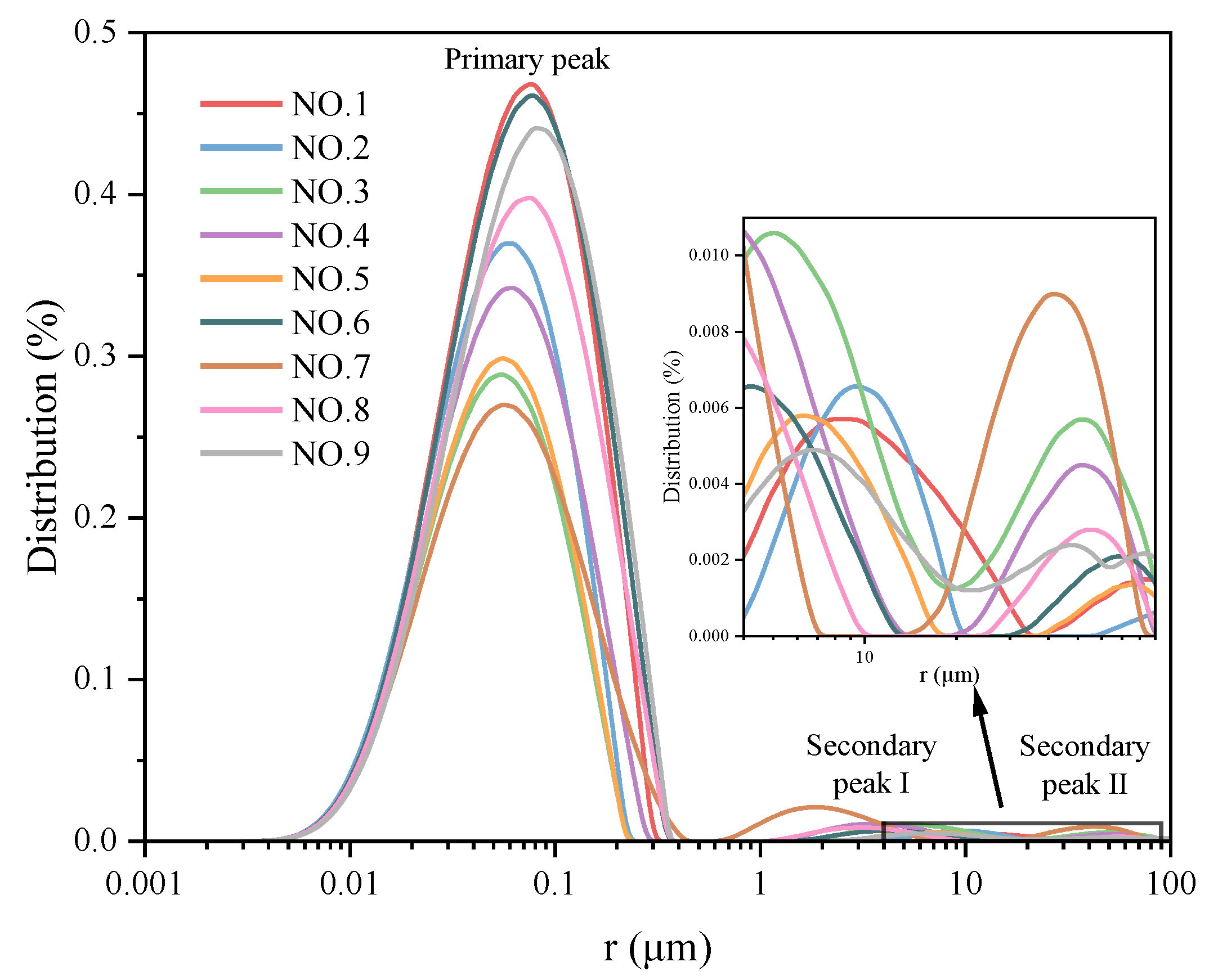
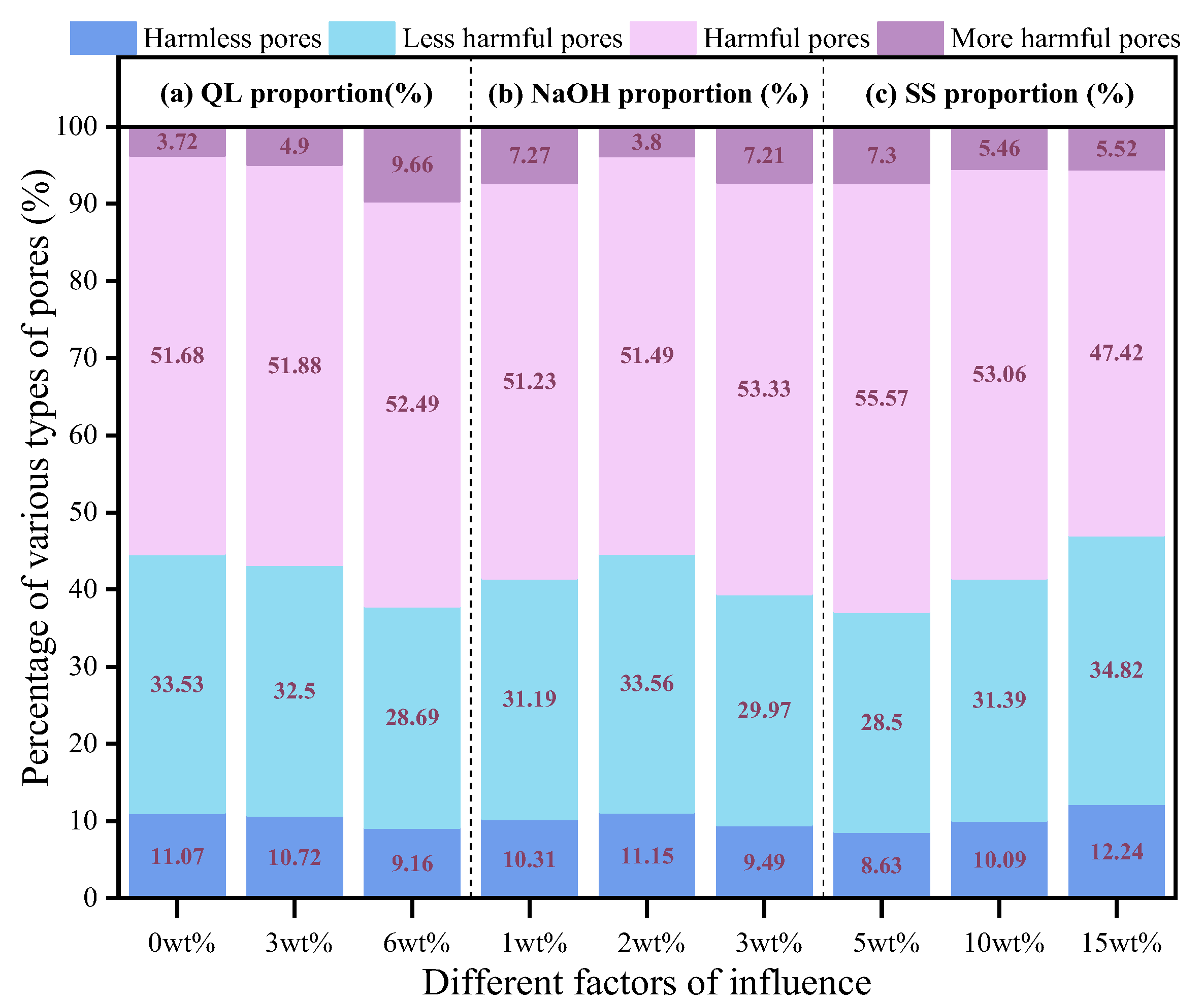
3.2.2. Pore Properties Analysis
3.3. Performance Analysis of SENC Based on the Optimal Composite Alkali Activators
3.3.1. Physical and Mechanical Properties
3.3.2. Microstructure
4. Conclusions
- QL could effectively enhance the early age compressive strength of AABS, but lower addition is not enough to fully activate the pozzolanic activity of MCA and FS, and higher addition is harmful to the structure of AABS. NaOH could significantly improve the compressive strength of AABS, but it should be noted that the addition of NaOH should not be too high to avoid expansion. Controlling the SS modulus between 1 a.u. and 1.5 a.u. is beneficial for compressive strength. Although SS was not conducive to the formation of early strength of AABS, it was beneficial to the pore refinement and the improvement of 28 d compressive strength.
- Based on the single factor test, it was found that there was a complementary effect between the alkali activators. NaOH enhanced the reinforcing effect of QL on compressive strength at early ages. NaOH and SS was directly related to the development of 28 d compressive strength and compensated for the limitation of QL on the compressive strength. Therefore, the use of a composite alkali activator was recommended. Targeted at the compressive strength and porosity, the optimal composite alkali activator incorporated the additions of QL, NaOH, and SS at 3%, 2% and 15%, respectively.
- The bulk density and the apparent density of SENC were 971.41 kg/m3 and 1852.61 kg/m3, respectively. The 7 d and 28 d numerical tube pressure could reach 8.7 MPa and 12.2 MPa, respectively. The 1440 min water absorption at 28 d was 9.8%. SENC showed excellent physical and mechanical properties, and had the potential to be used as lightweight concrete aggregate, bionic material, and so on.
- The composite alkali activator was able to effectively activate the pozzolanic activity of MCA and FS to increase the numerical tube pressure of SENC, as well as significantly refine the pores of SENC. The densification of the SENC structure was improved by the hydration products of MCA and FS (C-A-S-H, calcium hemicarboaluminate, hydrotalcite, etc.).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Liu, J.; Klaar, M.; Chen, A.; Gudmundsson, L.; Holden, J. Anthropogenic climate change has influenced global river flow seasonality. Science 2024, 383, 1009–1014. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A. Comparison of energy consumption and carbon emissions from clinker and recycled cement production. J. Clean. Prod. 2021, 306, 127277. [Google Scholar] [CrossRef]
- Liu, J.; Bawa, K.S.; Seager, T.P.; Mao, G.; Ding, D.; Lee, J.S.H.; Swim, J.K. On knowledge generation and use for sustainability. Nat. Sustain. 2019, 2, 80–82. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Ma, H.; Yang, H.; Zhao, Y.; Liu, B. Effect of albite on shrinkage and carbonation resistance of alkali-activated slag. Constr. Build. Mater. 2023, 409, 134141. [Google Scholar] [CrossRef]
- Wi, K.; Lee, H.; Lim, S.; Song, H.; Hussin, M.W.; Ismail, M.A. Use of an agricultural by-product, nano sized Palm Oil Fuel Ash as a supplementary cementitious material. Constr. Build. Mater. 2018, 183, 139–149. [Google Scholar] [CrossRef]
- Bondar, D.; Ma, Q.; Soutsos, M.; Basheer, M.; Provis, J.L.; Nanukuttan, S. Alkali activated slag concretes designed for a desired slump, strength and chloride diffusivity. Constr. Build. Mater. 2018, 190, 191–199. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Wu, T.; Liu, K.; Cheng, X.; Zhang, J. Analysis of energy, carbon emissions and economics during the life cycle of biomass power generation: Case comparison from China. Biomass Bioenerg. 2024, 182, 107098. [Google Scholar] [CrossRef]
- Šupić, S.; Malešev, M.; Radonjanin, V.; Bulatović, V.; Milović, T. Reactivity and pozzolanic properties of biomass ashes generated by wheat and soybean straw combustion. Materials 2021, 14, 1004. [Google Scholar] [CrossRef] [PubMed]
- Olatoyan, O.J.; Kareem, M.A.; Adebanjo, A.U.; Olawale, S.O.A.; Alao, K.T. Potential use of biomass ash as a sustainable alternative for fly ash in concrete production: A review. Hybrid Adv. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Sunita, A. Effect of biomass ash, foundry sand and recycled concrete aggregate over the strength aspects of the concrete. Mater. Today Proc. 2021, 50, 2044–2051. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Camões, A.; Branco, F.G.; Aguiar, J.B.; Fangueiro, R. Recycling of biomass and coal fly ash as cement replacement material and its effect on hydration and carbonation of concrete. Waste Manag. 2019, 94, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Yurt, Ü.; Bekar, F. Comparative study of hazelnut-shell biomass ash and metakaolin to improve the performance of alkali-activated concrete: A sustainable greener alternative. Constr. Build. Mater. 2022, 320, 126230. [Google Scholar] [CrossRef]
- Velay-Lizancos, M.; Azenha, M.; Martínez-Lage, I.; Vázquez-Burgo, P. Addition of biomass ash in concrete: Effects on E-Modulus, electrical conductivity at early ages and their correlation. Constr. Build. Mater. 2017, 157, 1126–1132. [Google Scholar] [CrossRef]
- Wu, X.; Gu, F.; Su, C.; Wang, W.; Pu, K.; Shen, D.; Long, Y. Preparing high-strength ceramsite from ferronickel slag and municipal solid waste incineration fly ash. Ceram. Int. 2022, 48, 34265–34272. [Google Scholar] [CrossRef]
- Frankovič, A.; Bosiljkov, V.B.; Ducman, V. Lightweight aggregates made from fly ash using the cold-bond process and their use in lightweight concrete. Mater. Tehnol. 2017, 51, 267–274. [Google Scholar] [CrossRef]
- Hu, G.; Yan, K.; Gao, J.; Cheng, F.; Huo, X.; Guo, M.; Zhang, M. A non-fired ceramsite construction material with enhanced lightweight high strength properties. Constr. Build. Mater. 2023, 371, 130771. [Google Scholar] [CrossRef]
- Pan, H.; Si, X.; Wang, S.; Wang, J.; Bao, K.; Zhao, Q. Preparation of low-carbon and environmentally friendly non-sintered ceramsite (NSC) employing steel slag, GGBS and fly ash: Experiment and performance regulation. Constr. Build. Mater. 2024, 411, 134438. [Google Scholar] [CrossRef]
- Yu, Q.; Li, C.; Wang, Z.; Deng, A. Study on preparation and properties of unburned ceramsite with steel slag. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022067. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, W.; Jia, Y.; Xue, C.; Qiu, Y.; Zhao, Q.; Wang, D. Preparation of non-sintered lightweight aggregate ceramsite based on red mud-carbide slag-fly ash: Strength and curing method optimization. J. Clean. Prod. 2022, 372, 133788. [Google Scholar] [CrossRef]
- Ma, C.; Bao, S.; Zhang, Y.; Luo, Y.; Gui, Y.; Ren, Y. Preparation of non-sintered sewage sludge based ceramsite by alkali-thermal activation and hydration mechanism. Ceram. Int. 2022, 48, 31606–31613. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Academy of Building Research: Beijing, China, 2021.
- GB/T 17431.2-2010; Lightweight Aggregates and Its Test Methods—Part 2: Test Method for Lightweight Aggregates. Academy of Building Research: Beijing, China, 2010.
- Sha, F.; Li, S.; Liu, R.; Li, Z.; Zhang, Q. Experimental study on performance of cement-based grouts admixed with fly ash, bentonite, superplasticizer and water glass. Constr. Build. Mater. 2018, 161, 282–291. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Chemical activation of blended cements made with lime and natural Pozzolans. Cem. Concr. Res. 1993, 23, 1389–1396. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, X.; Su, Y.; Strnadel, B.; Miao, W. Hydration and compressive strength of supersulfated cement with low-activity high alumina ferronickel slag. Cem. Concr. Compos. 2023, 136, 104892. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, W.; Lu, Z.; Chen, H. Shrinkage compensation of alkali-activated slag concrete and microstructural analysis. Constr. Build. Mater. 2014, 66, 422–428. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Huang, H.; Yin, S. Difference in the reaction process of slag activated by waterglass solution and NaOH solution. Struct. Concr. 2019, 20, 1528–1540. [Google Scholar] [CrossRef]
- Che, J.; Yang, R.; Wang, J.; Liu, H.; Hu, Y.; Doh, S.I. Research on basic mechanical properties of different modulus alkaline excited ECC. Phys. Chem. Earth. Part A/B/C 2022, 128, 103233. [Google Scholar] [CrossRef]
- Li, M.; Huang, G.; Cui, Y.; Wang, B.; Chang, B.; Yin, Q.; Zhang, S.; Wang, Q.; Feng, J.; Ge, M. Coagulation mechanism and compressive strength characteristics analysis of high-strength alkali-activated slag grouting material. Polymers 2022, 14, 3980. [Google Scholar] [CrossRef]
- Luo, L.; Yao, W.; Liang, G.; Luo, Y. Workability, autogenous shrinkage and microstructure of alkali-activated slag/fly ash slurries: Effect of precursor composition and sodium silicate modulus. J. Build. Eng. 2023, 73, 106712. [Google Scholar] [CrossRef]
- Zidan, I.; Khalaf, M.A.; Helmy, A.I.I. Properties of alkali-activated slag mortar and prediction of its compressive strength. Ain. Shams Eng. J. 2023, 14, 102536. [Google Scholar] [CrossRef]
- Dai, X.; Ren, Q.; Aydin, S.; Yardimci, M.Y.; Schutter, G.D. Accelerating the reaction process of sodium carbonate-activated slag mixtures with the incorporation of a small addition of sodium hydroxide/sodium silicate. Cem. Concr. Compos. 2023, 141, 105118. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, Y.; Zheng, W.; Huang, W. Effect of dosage of sodium carbonate on the strength and drying shrinkage of sodium hydroxide based alkali-activated slag paste. Constr. Build. Mater. 2018, 179, 11–24. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Bie, Y.; Duan, P.; Wang, L. Multi-scale performance study of concrete with recycled aggregate from tannery sludge. Case Stud. Constr. Mater. 2022, 17, e01698. [Google Scholar] [CrossRef]
- GB/T 17431.1-2010; Lightweight Aggregates and Its Test Methods—Part 1: Lightweight Aggregates. China Academy of Building Research: Beijing, China, 2010.
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; Nicolas, R.S.; Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2015, 48, 517–529. [Google Scholar] [CrossRef]








| Material | Percentage Composition (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | TiO2 | Na2O | MnO | K2O | P2O5 | LOI | |
| FS | 41 | 28 | 13.9 | 0.369 | 9.78 | 2.85 | 1.5 | 1.17 | 0.543 | 0.519 | / | 0.369 |
| MCA | 32.3 | 28.4 | 8.24 | 5.61 | 7.47 | 5.78 | 0.836 | 1.69 | 0.281 | 4.28 | 3.7 | 1.413 |
| Levels | Factors | ||
|---|---|---|---|
| (A) QL (%) | (B) NaOH (%) | (C) SS (%) | |
| 1 | 0 | 1 | 5 |
| 2 | 3 | 2 | 10 |
| 3 | 6 | 3 | 15 |
| Number | Groups | MCA (g) | FS (g) | Factor | Water (g) | ||
|---|---|---|---|---|---|---|---|
| (A) QL (g) | (B) NaOH (g) | (C) SS (g) | |||||
| NO. 1 | A (0 wt%) B (1 wt%) C (5 wt%) | 50 | 50 | 0 | 1 | 5 | 31.79 |
| NO. 2 | A (0 wt%) B (2 wt%) C (10 wt%) | 0 | 2 | 10 | 28.58 | ||
| NO. 3 | A (0 wt%) B (3 wt%) C (15 wt%) | 0 | 3 | 15 | 25.38 | ||
| NO. 4 | A (3 wt%) B (1 wt%) C (10 wt%) | 3 | 1 | 10 | 28.58 | ||
| NO. 5 | A (3 wt%) B (2 wt%) C (15 wt%) | 3 | 2 | 15 | 25.38 | ||
| NO. 6 | A (3 wt%) B (3 wt%) C (5 wt%) | 3 | 3 | 5 | 31.79 | ||
| NO. 7 | A (6 wt%) B (1 wt%) C (15 wt%) | 6 | 1 | 15 | 25.38 | ||
| NO. 8 | A (6 wt%) B (2 wt%) C (5 wt%) | 6 | 2 | 5 | 31.79 | ||
| NO. 9 | A (6 wt%) B (3 wt%) C (10 wt%) | 6 | 3 | 10 | 28.58 | ||
| MCA (g) | FS (g) | QL Proportion (g) | NaOH Proportion (g) | SS Proportion (g) | Water (g) |
|---|---|---|---|---|---|
| 50 | 50 | 3 | 2 | 15 | 32.24 |
| Ranges of Particle Sizes | Particle Shape Factor | Apparent Density (kg/m3) | Bulk Density (kg/m3) | Water Absorption Rate (%) | Numerical Tube Pressure (MPa) | Softening Coefficient | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Average | 7 d | 28 d | 7 d | 28 d | ||||
| 10~16 mm | 1.58 | 1.03 | 1.37 | 1852.61 | 971.41 | 9.80 | 8.7 | 12.2 | 0.93 | 0.96 |
| Raw Materials | Other Materials | Curing Methods | Bulk Density (kg/m3) | Apparent Density (kg/m3) | Numerical tube Pressure (MPa) | Reference |
|---|---|---|---|---|---|---|
| 90% Fly ash 10% Cement | / | Curing in standard curing room | 919.5 | 2248.7 | 0.96 | Frankovič et al. [16] |
| 85% Low-activity circulating fluidized bed fly ash 15% Cement | 6% Quicklime 6% Desulfurization gypsum 2% Hydrogen peroxide | Steam curing at 70 °C for 12 h | 695.34 | 1091.88 | 5.5 | Hu et al. [17] |
| 30% Steel slag 30% Ground granulated blast-furnace slag 30% Fly ash 10% Cement | 3% Sodium silicate solution 5% CaSO4•2H2O | Steam curing at 60 °C for 12 h | 960.3 | / | 12.4 | Pan et al. [18] |
| 40% Steel slag 40% Fly ash 10% Clay 10% Cement | 2% Expanded perlite 1% grade-A cellulose | Natural curing | 944 | / | 8.15 | Yu et al. [19] |
| 30% Red mud 30% Carbide slag 40% Fly ash | Wastewater with NaOH | Steam curing at 80 °C for 12 h | 770 | 1400 | 4.5 | Shi et al. [20] |
| 80% Sewage sludge 20% Fly ash | 6% CaO 9% NaOH | 600 °C thermal activation | 852 | 2603 | 7.43 | Ma et al. [21] |
| 50% Furnace slag 50% Ash of mushroom and corn straw | 3% Quicklime powder 2% NaOH 15% Sodium silicate solution | Curing in standard curing room | 971.41 | 1852.61 | 12.2 | SENC (this study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhou, J.; Bie, Y.; Zhao, M.; Duan, P.; Hu, Q. Sustainable Ecological Non-Sintered Ceramsite (SENC) with Alkali Activators: Performance Regulation and Microstructure. Sustainability 2024, 16, 6634. https://doi.org/10.3390/su16156634
Chen S, Zhou J, Bie Y, Zhao M, Duan P, Hu Q. Sustainable Ecological Non-Sintered Ceramsite (SENC) with Alkali Activators: Performance Regulation and Microstructure. Sustainability. 2024; 16(15):6634. https://doi.org/10.3390/su16156634
Chicago/Turabian StyleChen, Shoukai, Jingfei Zhou, Yajing Bie, Mengdie Zhao, Peidong Duan, and Qingfeng Hu. 2024. "Sustainable Ecological Non-Sintered Ceramsite (SENC) with Alkali Activators: Performance Regulation and Microstructure" Sustainability 16, no. 15: 6634. https://doi.org/10.3390/su16156634
APA StyleChen, S., Zhou, J., Bie, Y., Zhao, M., Duan, P., & Hu, Q. (2024). Sustainable Ecological Non-Sintered Ceramsite (SENC) with Alkali Activators: Performance Regulation and Microstructure. Sustainability, 16(15), 6634. https://doi.org/10.3390/su16156634





