Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration
Abstract
1. Introduction
1.1. Climate Change and Global Warming
1.2. Global Regulatory and Policy Frameworks: Recent COP Outcomes
1.3. Captured CO2 Storage and Utilization
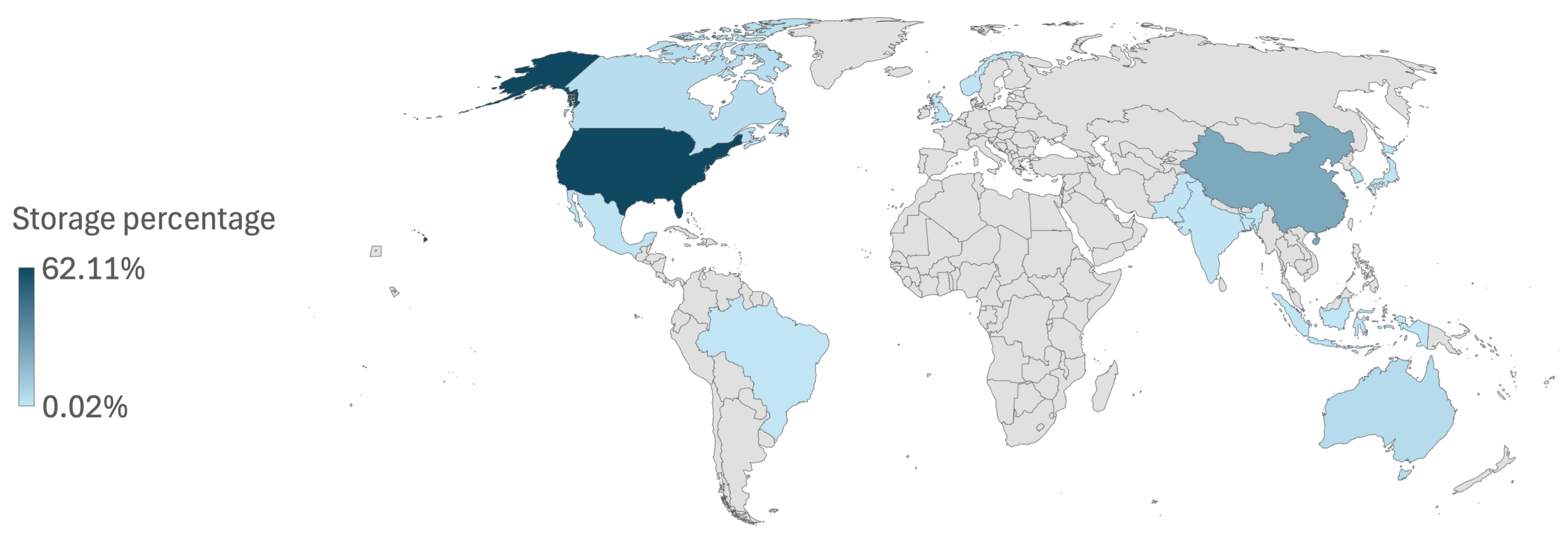
2. CO2 Storage
2.1. Enhanced Oil and Gas Recovery
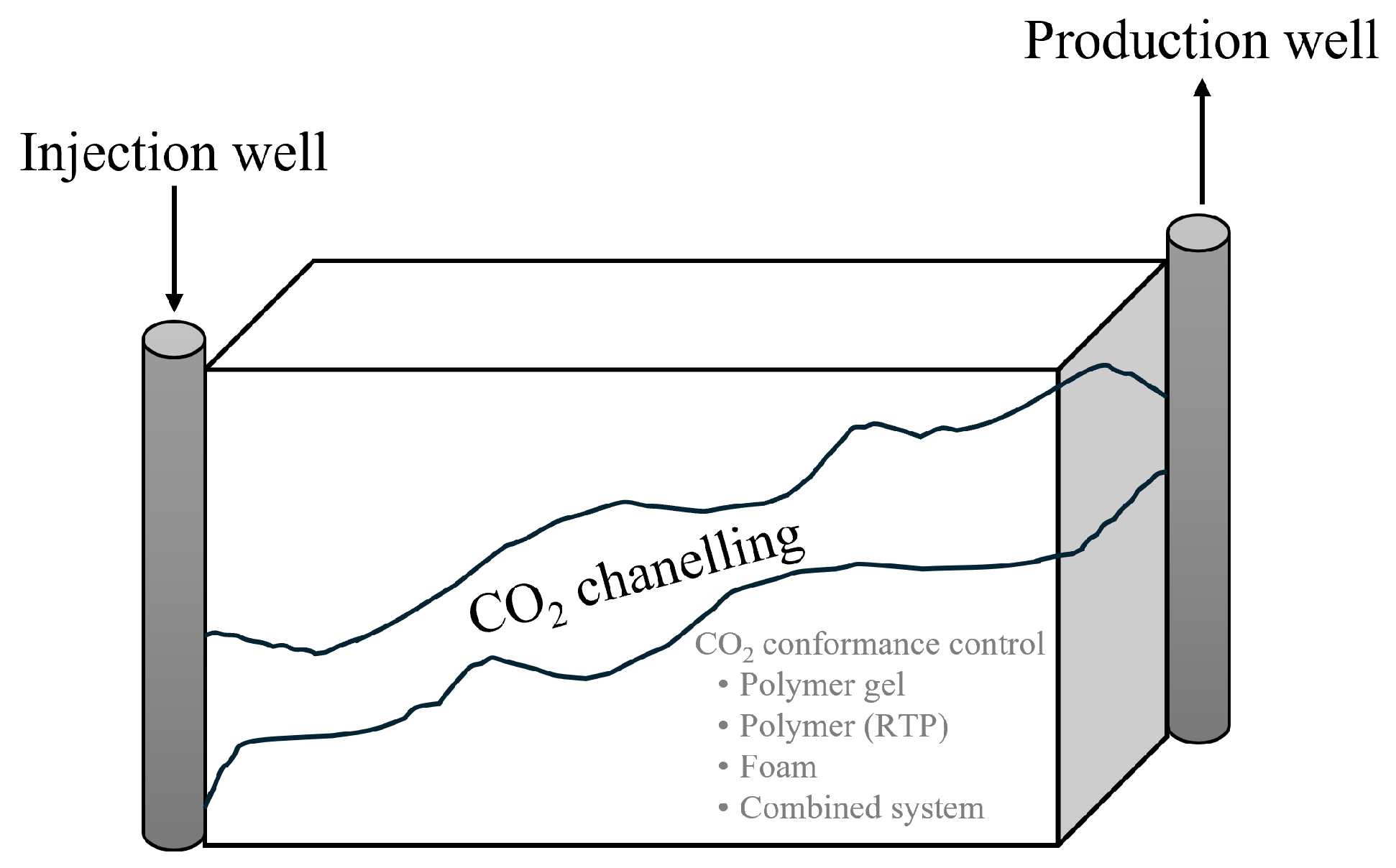
- Higher CAPEX and OPEX compared to the onshore CCS-EOR.
- Limited CO2 supply. Transport to the offshore facility might increase the overall cost.
- Limited space for retrofitting new equipment and pipelines. Replacing systems with new technology increases the cost since much more equipment needs to be replaced.
2.2. Enhanced Coal Bed Methane Recovery
2.3. Aquifer Storage

2.4. Salt Cavern Storage
2.5. Deep Ocean Storage
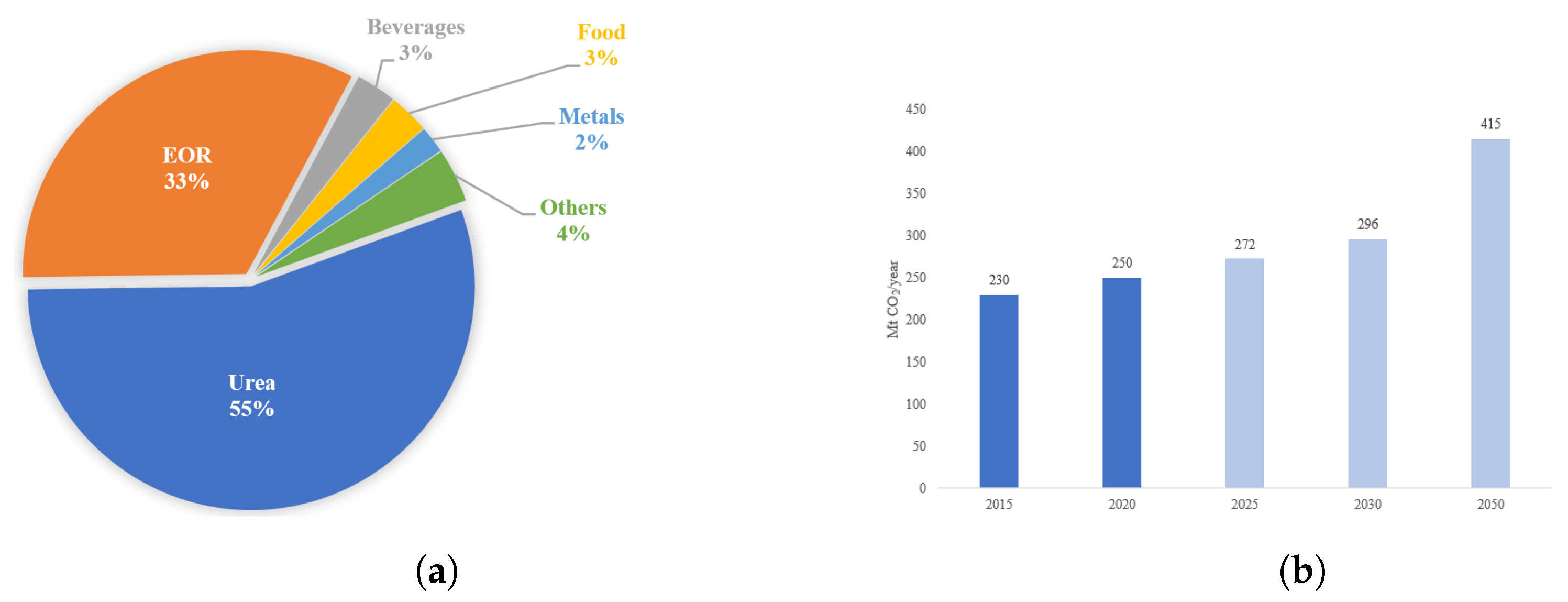
3. CO2 Utilization Technologies
3.1. Non-Energy Applications
| Potential | Process/Product | Sector |
|---|---|---|
| High | Oil extraction | Enhanced Oil, Gas and Coal Recovery |
| Stimulation/Fracturing of oil and gas | ||
| Polymer Processing | ||
| Chemicals and Fuels (Methanol, methane, CO, fertilizers and derivatives) | ||
| Medium | Food processing | Coffee decaffeinating |
| Wine production | ||
| Beverage carbonation | ||
| Dry ice production | ||
| Horticulture (greenhouses) | ||
| Food packaging and preservation | ||
| Medium | Mineralization | Calcium and magnesium carbonate for use in cement |
| Calcium bicarbonate | ||
| Bauxite residue treatment | ||
| CO2 concrete curing | ||
| Medium | Power | Working medium in CO2 cycles |
| Heat pumps | ||
| Low | Steel | Bottom stirring agent in basic oxygen furnaces |
| Injection to metal casting | ||
| Hardening sand cores and molds | ||
| Chilling medium | ||
| Low | Pharmaceutical | Chemical synthesis |
| Supercritical Fluid Extraction | ||
| Product transportation | ||
| Inerting | ||
| Low | Pulp and Paper | pH reduction during washing |
| Low | Energy crops | Algae cultivation |
| Low | Other | Electronics (printed circuit manufacture) |
| Pneumatic (working medium in hand tools and equipment) | ||
| Fire extinguishers, fire suspension | ||
| Urea production | ||
| Flavors, Fragrances | ||
| Blanket Products | ||
| Aerosols and propellants | ||
| Soda ash production for glass industry | ||
| Welding (shield gas) | ||
| Dry gas cleaning | ||
| Refrigerant gas | ||
| Water treatment |
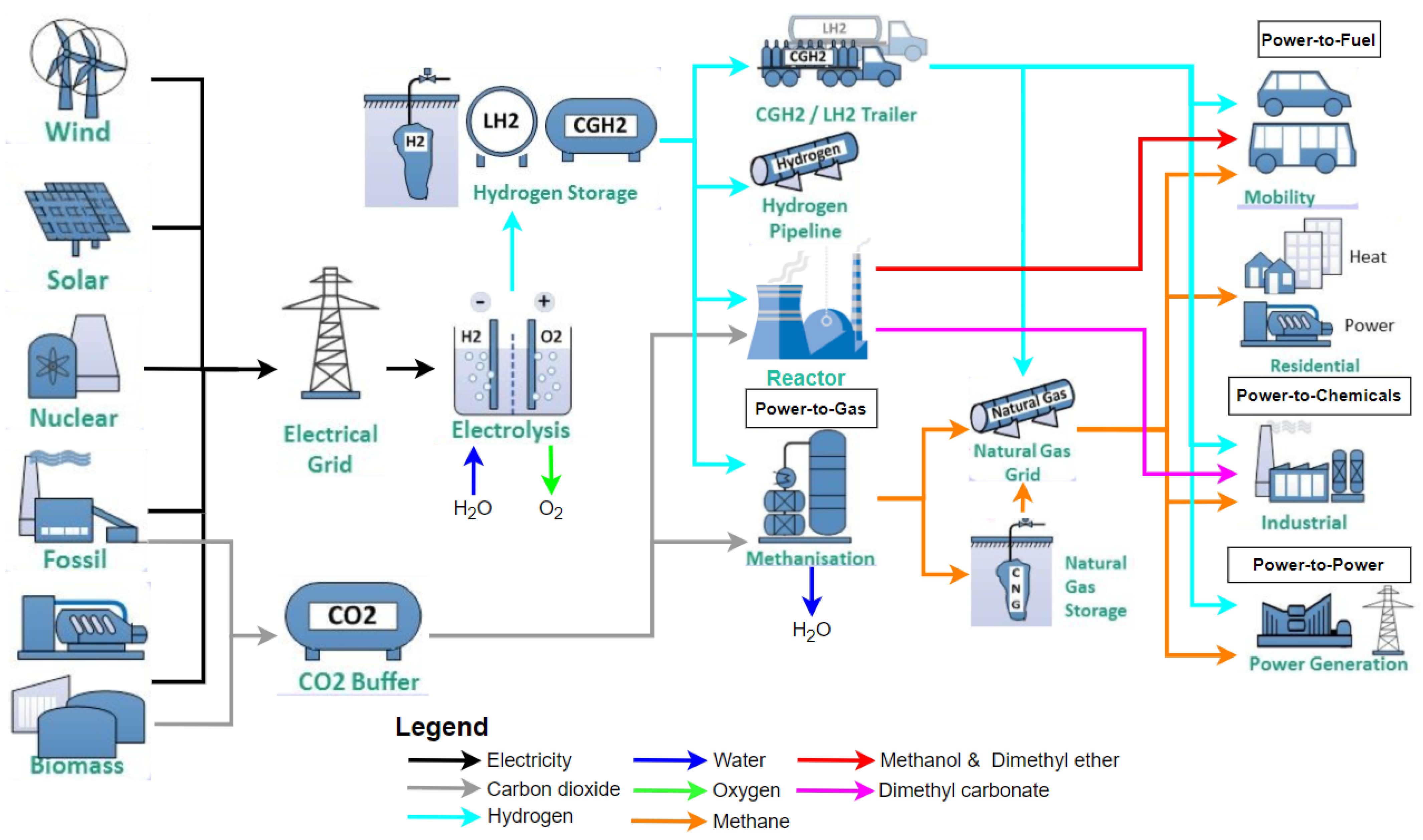
3.2. CO2 Applications in the Energy Sector
3.2.1. The Catalytic Sabatier Reaction
3.2.2. The Biological Sabatier Reaction
3.2.3. Dry CO2 Reforming
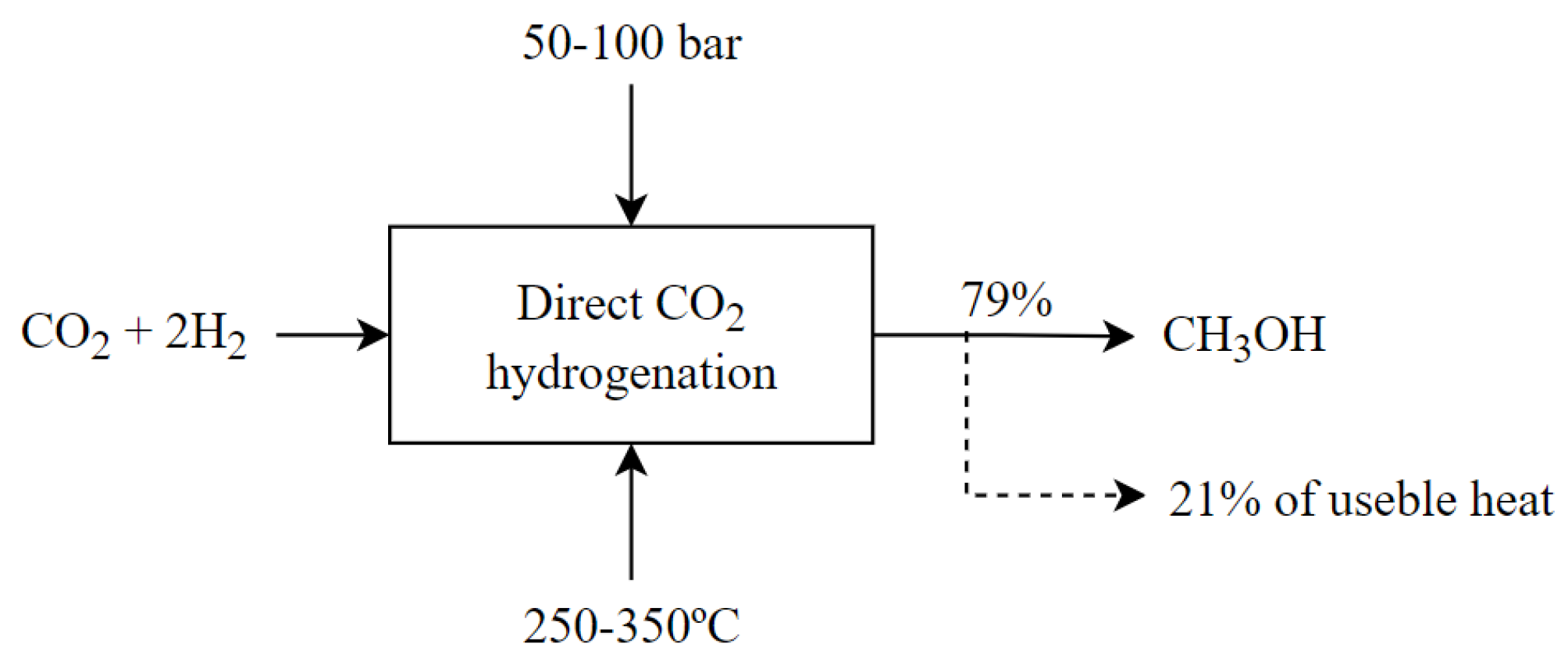
3.2.4. Methanol Production
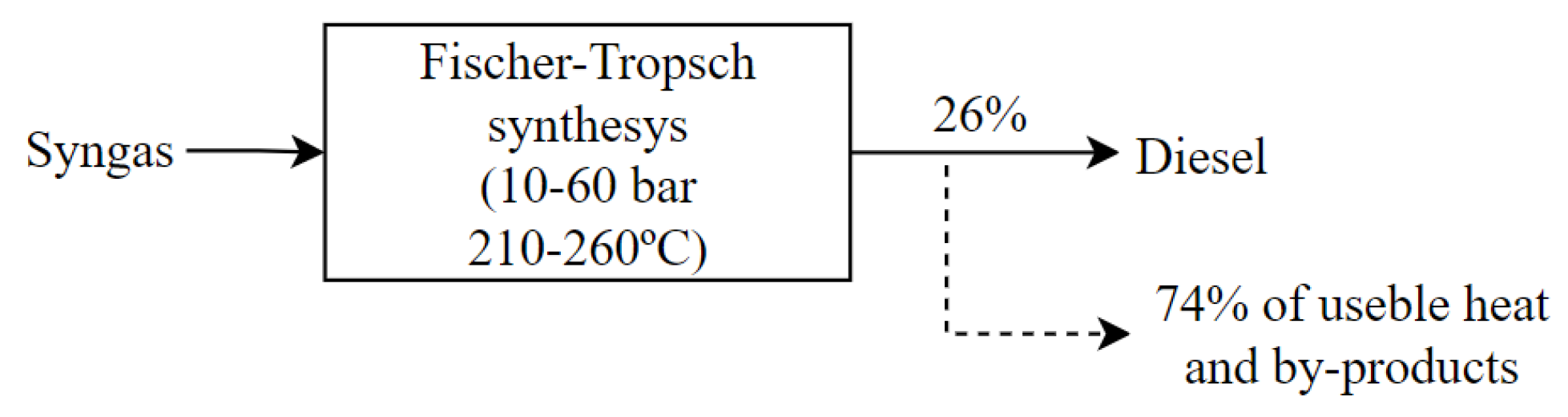
3.2.5. Fischer–Tropsch Process
3.2.6. CO2 Electrochemical Reduction
- (1)
- CO2 transport from gas phase to electrolyte bulk.
- (2)
- CO2 diffusing through the electrolyte to the electrolyte/cathode interface.
- (3)
- CO2 adsorption at the cathode.
- (4)
- Intermediate product production at the cathode, such as CHO, COH, COOH, and CO.
- (5)
- Intermediate electrons coming from the cathode catalyst.
- (6)
- Product desorption from the electrode.
- (7)
- Reduced products transfer into liquid phases or bulk gas.
3.2.7. Photocatalytic CO2 Reduction
- (a)
- Carbon coordination.
- (b)
- O2 coordination.
- (c)
- Mixed coordination.
3.2.8. CO2 Fuel Cells
3.2.9. Dimethyl Ether and Dimethyl Carbonate Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APS | Announced Pledges Scenario |
| AUD | Australian dollars |
| BOEM | US Bureau of Ocean Energy Management |
| CAES | Compressed Air Energy Storage |
| CAPEX | Capital Expenditure |
| CBMR | Coal Bed Methane Recovery |
| CCS | Carbon Capture and Storage |
| CCU | Carbon Capture and Utilization |
| CEEP | Critical Excess Electricity Production |
| DMC | Dimethyl Carbonate |
| DME | Dimethyl Ether |
| ECBMR | Enhanced Coal bed Methane Recovery |
| EGR | Enhanced Gas Recovery |
| EOR | Enhanced Oil Recovery |
| ETS | European Emissions Trading Systems |
| IPCC | Intergovernmental Panel on Climate Change |
| IEA | International Energy Agency |
| LAES | Liquid Air Energy Storage |
| LCA | Life Cycle Asestment |
| LNG | Liquid Natural Gas |
| MCFCs | Molten Carbonate Fuel cells |
| NZD | New Zealand Dollar |
| NZE | Net Zero Emission scenario |
| OPEX | Operating Expediture |
| PHS | Pumped hydro storage |
| RES | Renewable Energy Sources |
| RWGS | Reverse Water Gas Shift |
| SEP | Surplus of Electricity Production |
| SNG | Synthetic Natural Gas |
| SOEC | Solid Oxide Electrolysis Cell |
| STEPS | Stated Policies Scenario |
| TRL | Technology Readiness Level |
| UNEP | UN Environment Program |
| UNFCCC | UN Framework Convention on Climate Change |
References
- Baker, H.S.; Millar, R.J.; Karoly, D.J.; Beyerle, U.; Guillod, B.P.; Mitchell, D.; Shiogama, H.; Sparrow, S.; Woollings, T. Higher CO2 concentrations increase extreme event risk in a 1.5 °C world. Nature 2018, 8, 604–608. [Google Scholar] [CrossRef]
- Brook, E.J.; Buizert, C. Antarctic and global climate history viewed from ice cores. Nature 2018, 558, 200–208. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Rogelj, J.; Séférian, R.; Wartenburger, R.; Allen, M.R.; Cain, M.; Millar, R.J.; Ebi, K.L.; Ellis, N.; Hoegh-Guldberg, O.; et al. The many possible climates from the Paris Agreement’s aim of 1.5 °C warming. Nature 2018, 558, 41–49. [Google Scholar] [CrossRef]
- European Commission. Paris Agreement, Climate Action; Technical Report 4; European Commission: Luxembourg, 2017. [Google Scholar]
- International Energy Agency. CO2 Emissions in 2023—A New Record High, but Is There Light at the End of the Tunnel? Licence: CC BY 4.0; Technical Report; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Huang, M.T.; Zhai, P.M. Achieving Paris Agreement temperature goals requires carbon neutrality by middle century with far-reaching transitions in the whole society. Adv. Clim. Chang. Res. 2021, 12, 281–286. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Technical Report; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Masood, E.; Tollefson, J. COP26 hasn’t solved the problem’: Scientists react to UN climate deal. Nature 2021, 599, 355–356. [Google Scholar] [CrossRef]
- Baylin-Stern, A.; Berghout, N. Is Carbon Capture too Expensive? Technical Report; International Energy Agency: Paris, France, 2021. [Google Scholar]
- United Nations Environment Programme. Emissions Gap Report 2022: The Closing Window—Climate Crisis Calls for Rapid Transformation of Societies; Technical Report; United Nations Environment Programme: Nairobi, Kenya, 2022. [Google Scholar]
- UNDP. Brief on COP27 Outcomes and Roadmap to COP28; Technical Report; United Nations Development Programme: New York, NY, USA, 2023. [Google Scholar]
- International Energy Agency. Supporting and Reporting Global Climate Progress. COP28: Tracking the Energy Outcomes; Technical Report; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Feron, P.H.M.; Hendriks, C.A. CO2 Capture Process Principles and Costs. Oil Gas Sci. Technol. 2005, 60, 451–459. [Google Scholar] [CrossRef]
- European-Commission. The Potential for CCS and CCU in Europe; Technical Report; European-Commission: Luxembourg, 2019. [Google Scholar]
- Luo, J.; Xie, Y.; Hou, M.Z.; Xiong, Y.; Wu, X.; Lüddeke, C.T.; Huang, L. Advances in subsea carbon dioxide utilization and storage. Energy Rev. 2023, 2, 100016. [Google Scholar] [CrossRef]
- Pollitt, M.G.; von der Fehr, N.H.M.; Willems, B.; Banet, C.; Coq, C.L.; Chyong, C.K. Recommendations for a future-proof electricity market design in Europe in light of the 2021–23 energy crisis. Energy Policy 2024, 188, 114051. [Google Scholar] [CrossRef]
- Lund, H.; Münster, E. Management of surplus electricity-production from a fluctuating renewable-energy source. Appl. Energy 2003, 76, 65–74. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Rajabloo, T.; Valee, J.; Marenne, Y.; Coppens, L.; De Ceuninck, W. Carbon capture and utilization for industrial applications. Energy Rep. 2023, 9, 111–116. [Google Scholar] [CrossRef]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Carbon-Credits. Live Carbon Prices Today; Carbon-Credits: Houston, TX, USA, 2024. [Google Scholar]
- Leonzio, G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical analysis of CO2 capture pathways and technologies. J. Environ. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- McLaughlin, H.; Littlefield, A.A.; Menefee, M.; Kinzer, A.; Hull, T.; Sovacool, B.K.; Bazilian, M.D.; Kim, J.; Griffiths, S. Carbon capture utilization and storage in review: Sociotechnical implications for a carbon reliant world. Renew. Sustain. Energy Rev. 2023, 177, 113215. [Google Scholar] [CrossRef]
- Malischek, R.; McCulloch, S. The World Has Vast Capacity to Store CO2: Net Zero Means We’ll Need It; IEA: Paris, France, 2021. [Google Scholar]
- Global-CCS-Institute. Global Status of CCS 2022; Technical Report; Global-CCS-Institute: Docklands, VIC, Australia, 2022. [Google Scholar]
- Solomon, S.; Carpenter, M.; Flach, T.A. Intermediate storage of carbon dioxide in geological formations: A technical perspective. Int. J. Greenh. Gas Control 2008, 2, 502–510. [Google Scholar] [CrossRef]
- Leonzio, G.; Shah, N. Recent advancements and challenges in carbon capture, utilization and storage. Curr. Opin. Green Sustain. Chem. 2024, 46, 100895. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and challenges in CO2 utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Ahmad, T.; Sahu, R.K.; Mandal, A.; Mubashir, M.; Ali, M.; Pal, N. Carbon capture and sequestration technology for environmental remediation: A CO2 utilization approach through EOR. Geoenergy Sci. Eng. 2024, 234, 212619. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Chen, S.; Qin, J.; Wang, G.; Yi, Y.; Zhang, L.; Wang, R.; Zhu, D. Experimental Evaluation of Combined Plugging System for CO2-Improved Oil Recovery and Storage. Energy Fuels 2023, 37, 4401–4412. [Google Scholar] [CrossRef]
- Sweatman, R.; Crookshank, S.; Edman, S. Outlook and Technologies for Offshore CO2 EOR/CCS Projects. Offshore Technol. Conf. Proc. 2011, 4, 2981–2993. [Google Scholar] [CrossRef]
- Bajpai, S.; Shreyash, N.; Singh, S.; Memon, A.R.; Sonker, M.; Tiwary, S.K.; Biswas, S. Opportunities, challenges and the way ahead for carbon capture, utilization and sequestration (CCUS) by the hydrocarbon industry: Towards a sustainable future. Energy Rep. 2022, 8, 111–116. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, E.; Li, B.; Kong, X.; Xu, J.; Peng, S.; Chen, Y. Laboratory experiments of CO2-enhanced coalbed methane recovery considering CO2 sequestration in a coal seam. Energy 2023, 262, 125473. [Google Scholar] [CrossRef]
- Liang, W.; Wang, J.; Sang, S.; Li, P. The influence of closed pores and stacked coal grains on gas transport in CO2 injection enhanced CH4 recovery process. J. Pet. Sci. Eng. 2022, 212, 110303. [Google Scholar] [CrossRef]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations—Coalbeds and Deep Saline Aquifers. J. Air Waste Manag. Assoc. 2012, 53, 645–715. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, X.; Lu, Y.; Li, H.; Feng, M. Fracture pattern and caprock integrity analyses via hydraulic fracturing for CO2 enhanced coal bed methane. Eng. Fract. Mech. 2020, 228, 106894. [Google Scholar] [CrossRef]
- Thomsen, S.; Flørning, J. CO2 Neutral Energy System Utilising the Subsurface; Technical Report; Energy Technology Development and Demonstration Programme (EUDP): Copenhagen, Denmark, 2019. [Google Scholar]
- Crotogino, F.; Prelicz, R.; Rudolph, T. Assessment of the potential, the actors and relevant business cases for large scale and seasonal storage of renewable electricity by hydrogen underground storage in Europe. In Overview on All Known Underground Storage Technologies for Hydrogen Status; Technical Report; 2014; D(4). Grant Agreement No. 303417. Funded under Specific Programme “Cooperation”: Joint Technology Initiatives; Available online: https://cordis.europa.eu/project/id/303417/reporting (accessed on 26 July 2024).
- Chen, Y.; Chen, S.; Li, D.; Jiang, X. Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors. Geotechnics 2023, 3, 70–103. [Google Scholar] [CrossRef]
- Yan, S.; Yadong, L.; Yushi, R.; Ze, B.; Hao, B.; Mingqi, L.; Qingchen, Z. Geochemical reaction of compressed CO2 energy storage using saline aquifer. Alex. Eng. J. 2023, 54, 679–689. [Google Scholar] [CrossRef]
- Anthonsen, K.; Aagaard, P.; Bergmo, P.; Erlström, M.; Fareide, J.; Gislason, S.; Mortensen, G.; Snæbjörnsdottir, S. CO2 storage potential in the Nordic region. Energy Procedia 2013, 37, 5080–5092. [Google Scholar] [CrossRef]
- Kaufmann, R.; Aavatsmark, I.; Nøkleby, P.H.; Aurdal, T.A. Using an aquifer as CO2 buffer storage. Int. J. Greenh. Gas Control 2016, 53, 106–116. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.; Mak, J. Handbook of Natural Gas Transmission and Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–35. [Google Scholar] [CrossRef]
- Bordenave, S.; Chatterjee, I.; Voordouw, G. Microbial community structure and microbial activities related to CO2 storage capacities of a salt cavern. Int. Biodeterior. Biodegrad. 2013, 81, 82–87. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Rackley, S.A. Carbon Capture and Storage, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Gunter, W.; Bachu, S.; Palombi, D.; Lakeman, B.; Sawchuk, W.; Bonner, D. Heartland Area Redwater reef saline aquifer CO2 storage project. Energy Procedia 2009, 1, 3943–3950. [Google Scholar] [CrossRef]
- Reichle, D.E. The Global Carbon Cycle and Climate Change—Scaling Ecological Energetics from Organism to the Biosphere; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Bergmo, P.S.; Grimstad, A.A.; Lindeberg, E.; Riis, F.; Johansen, W.T. Exploring geological storage sites for CO2 from Norwegian gas power plants: Utsira South. Energy Procedia 2009, 1, 2953–2959. [Google Scholar] [CrossRef][Green Version]
- Korbøl, R.; Kaddour, A. Sleipner vest CO2 disposal—Injection of removed CO2 into the utsira formation. Energy Convers. Manag. 1995, 36, 509–512. [Google Scholar] [CrossRef]
- Kongsjorden, H.; Karstad, O.; Torp, T.A. Saline aquifer storage of carbon dioxide in the Sleipner project. Waste Manag. 1998, 17, 303–308. [Google Scholar] [CrossRef]
- Hooper, B.N. The Latrobe Valley post combustion capture project. Energy Procedia 2009, 1, 1367–1372. [Google Scholar] [CrossRef][Green Version]
- Hjelm, L.; Anthonsen, K.L.; Dideriksen, K.; Nielsen, C.M.; Nielsen, L.H.; Mathiesen, A. Capture, Storage and Use of CO2 (CCUS)—Evaluation of the CO2 Storage Potential in Denmark; Technical Report; Geological Survey of Denmark and Greenland: København, Denmark, 2020. [Google Scholar]
- Mohammadian, E.; Hadavimoghaddam, F.; Kheirollahi, M.; Jafari, M.; Liu, B. Probing Solubility and pH of CO2 in aqueous solutions: Implications for CO2 injection into oceans. J. CO2 Util. 2023, 71, 102463. [Google Scholar] [CrossRef]
- Keil, R.G.; Nuwer, J.M.; Strand, S.E. Burial of agricultural byproducts in the deep sea as a form of carbon sequestration: A preliminary experiment. Mar. Chem. 2010, 122, 91–95. [Google Scholar] [CrossRef]
- Sheps, K.M.; Max, M.D.; Osegovic, J.P.; Tatro, S.R.; Brazel, L.A. A case for deep-ocean CO2 sequestration. Energy Procedia 2009, 1, 4961–4968. [Google Scholar] [CrossRef]
- Seibel, B.A.; Walsh, P.J. Carbon cycle: Potential impacts of CO2 injection on deep-sea biota. Science 2001, 294, 319–320. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Global-CCS-Institute. Global Status of CCS 2021—CCS Accelerating the Net Zero; Technical Report; Global-CCS-Institute: Docklands, VIC, Australia, 2021. [Google Scholar]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use—Creating Value from Emissions; Technical Report; IEA: Paris, France, 2019. [Google Scholar]
- Bocin-Dumitriu, A.; Perez-Fortes, M.; Tzimas, E.; Sveen, T. Carbon Capture and Utilization Workshop—Background and Proceedings; Technical Report; European Commission: Luxembourg, 2013. [Google Scholar] [CrossRef]
- Global CCS Institute. Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide; Technical Report; Global CCS Institute: Docklands, VIC, Australia, 2011. [Google Scholar]
- A Roadmap for the Global Implementation of Carbon Utilization Technologies—Transforming CO2 from a Liability to an Asset at Significant Market Scale; Technical Report; The Global CO2 Initiative (GCI) and CO2 Sciences. 2016. Available online: https://assets.ctfassets.net/xg0gv1arhdr3/5VPLtRFY3YAIasum6oYkaU/48b0f48e32d6f468d71cd80dbd451a3a/CBPI_Roadmap_Executive_Summary_Nov_2016_web.pdf (accessed on 15th June 2024).
- Stolaroff, J.K.; Lowry, G.V.; Keith, D.W. Using CaO- and MgO-rich industrial waste streams for carbon sequestration. Energy Convers. Manag. 2005, 46, 687–699. [Google Scholar] [CrossRef]
- Stolaroff, J.K.; Keith, D.W.; Lowry, G.V. Carbon Dioxide Capture from Atmospheric Air Using Sodium Hydroxide Spray. Environ. Sci. Technol. 2008, 42, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S. The thermodynamics of direct air capture of carbon dioxide. Energy 2013, 50, 38–46. [Google Scholar] [CrossRef]
- Zeman, F. Energy and Material Balance of CO2 Capture from Ambient Air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Storti, G.; Mazzotti, M. Process design and energy requirements for the capture of carbon dioxide from air. Chem. Eng. Process. Process Intensif. 2006, 45, 1047–1058. [Google Scholar] [CrossRef]
- Ranjan, M.; Herzog, H.J. Feasibility of air capture. Energy Procedia 2011, 4, 2869–2876. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory. Carbon Transport & Storage Program; Technical Report; US Department of Energy: Washington, DC, USA, 2013.
- Georgakaki, A.; Sales Agut, C.; Tzimas, E.; Perez Fortes, M.d.M. SETIS Magazine: Carbon Capture Utilisation and Storage; Technical Report; European Commission: Luxembourg, 2016. [Google Scholar]
- Schüwer, D.; Arnold, K.; Bienge, K.; Bringezu, S.; Echternacht, L.; Esken, A.; Fischedick, M.; von Geibler, J.; Höller, S.; Merten, F.; et al. CO2 Reuse NRW: Evaluating Gas Sources, Demand and Utilization for CO2 and H2 within the North Rhine-Westphalia Area with Respect to Gas Qualities; Technical Report; Wuppertal Institute for Climate, Environment and Energy: Wuppertal, Germany, 2015. [Google Scholar]
- Styring, P.; Jansen, D.; de Coninck, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy Using CO2 to Manufacture Fuel, Chemicals and Materials; Technical Report; The Centre for Low Carbon Futures & CO2Chem: New York, NY, USA, 2011. [Google Scholar]
- Vilbergsson, K.V.; Dillman, K.; Emami, N.; Ásbjörnsson, E.J.; Heinonen, J.; Finger, D.C. Can remote green hydrogen production play a key role in decarbonizing Europe in the future? A cradle-to-gate LCA of hydrogen production in Austria, Belgium, and Iceland. Int. J. Hydrogen Energy 2023, 48, 17711–17728. [Google Scholar] [CrossRef]
- Billig, E.; Decker, M.; Benzinger, W.; Ketelsen, F.; Pfeifer, P.; Peters, R.; Stolten, D.; Thrän, D. Non-fossil CO2 recycling—The technical potential for the present and future utilization for fuels in Germany. J. CO2 Util. 2019, 30, 130–141. [Google Scholar] [CrossRef]
- Nielsen, S.; Skov, I.R. Investment screening model for spatial deployment of power-to-gas plants on a national scale—A Danish case. Int. J. Hydrogen Energy 2019, 44, 9544–9557. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef]
- Müller, K.; Städter, M.; Rachow, F.; Hoffmannbeck, D.; Schmeißer, D. Sabatier-based CO2-methanation by catalytic conversion. Environ. Earth Sci. 2013, 70, 3771–3778. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Benjaminsson, G.; Benjaminsson, J.; Rudberg, R.B. Power-to-Gas—A Technical Review. 2013. Available online: https://www.semanticscholar.org/paper/Power-to-Gas-%E2%80%93-A-technical-review-Benjaminsson-Benjaminsson/c87e86c42959ed1879f85e8f78c715aaf78b7e23 (accessed on 15th June 2024).
- Leonzio, G. Process analysis of biological Sabatier reaction for bio-methane production. Chem. Eng. J. 2016, 290, 490–498. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Schlereth, D.; Hinrichsen, O. A fixed-bed reactor modeling study on the methanation of CO2. Chem. Eng. Res. Design 2014, 92, 702–712. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Peña, B.; Alarcón, A.; Guilera, J.; Perpiñán, J.; Romeo, L.M. Synthetic natural gas production in a 1 kW reactor using Ni–Ce/Al2O3 and Ru–Ce/Al2O3: Kinetics, catalyst degradation and process design. Energy 2022, 256, 124720. [Google Scholar] [CrossRef]
- Guilera, J.; Ramon Morante, J.; Andreu, T. Economic viability of SNG production from power and CO2. Energy Convers. Manag. 2018, 162, 218–224. [Google Scholar] [CrossRef]
- Sveinbjörnsson, D.; Münster, E.; Aryal, N.; Bo, R.; Pedersen, B. WP1 Gas Conditioning and Grid Operation—Upgrading of Biogas to Biomethane with the Addition of Hydrogen from Electrolysis; Technical Report; PlanEnergi: København, Denmark, 2017. [Google Scholar]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid biogas upgrading in a two-stage thermophilic reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane: Effect of pretreatments. Appl. Catal. A Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Kim, J.H.; Suh, D.J.; Park, T.J.; Kim, K.L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Kaydouh, M.N.; El Hassan, N.; Davidson, A.; Casale, S.; El Zakhem, H.; Massiani, P. Effect of the order of Ni and Ce addition in SBA-15 on the activity in dry reforming of methane. C. R. Chim. 2015, 18, 293–301. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113–114, 19–30. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.F. Syngas Production by Methane Reforming with Carbon Dioxide on Noble Metal Catalysts. J. Nat. Gas Chem. 2006, 15, 327–334. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Tailoring Hybrid Nonstoichiometric Ceria Redox Cycle for Combined Solar Methane Reforming and Thermochemical Conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. A thermochemical study of ceria: Exploiting an old material for new modes of energy conversion and CO2 mitigation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3269–3294. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Takalkar, G.D. Nanostructured co-precipitated Ce0.9Ln0.1O2 (Ln = La, Pr, Sm, Nd, Gd, Tb, Dy, or Er) for thermochemical conversion of CO2. Ceram. Int. 2018, 44, 16688–16697. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C.J. Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over Cu·ZnO supported on Al2O3 and Nb2O5. J. CO2 Util. 2016, 15, 83–88. [Google Scholar] [CrossRef]
- Schemme, S.; Breuer, J.L.; Samsun, R.C.; Peters, R.; Stolten, D. Promising catalytic synthesis pathways towards higher alcohols as suitable transport fuels based on H2 and CO2. J. CO2 Util. 2018, 27, 223–237. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Danish Energy Agency. Technology Data for Renewable Fuels; Technical Report; Danish Energy Agency: København, Denmark, 2017. [Google Scholar]
- Bargiacchi, E.; Antonelli, M.; Desideri, U. A comparative assessment of Power-to-Fuel production pathways. Energy 2019, 183, 1253–1265. [Google Scholar] [CrossRef]
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-based synthetic fuels: A techno-economic comparison of alcohol, ether and hydrocarbon production. Int. J. Hydrogen Energy 2020, 45, 5395–5414. [Google Scholar] [CrossRef]
- Ridjan, I.; Mathiesen, B.V.; Connolly, D.; Duić, N. The feasibility of synthetic fuels in renewable energy systems. Energy 2013, 57, 76–84. [Google Scholar] [CrossRef]
- Decker, M.; Schorn, F.; Samsun, R.C.; Peters, R.; Stolten, D. Off-grid power-to-fuel systems for a market launch scenario—A techno-economic assessment. Appl. Energy 2019, 250, 1099–1109. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Schmidt, P.; Weindorf, W.; Roth, A.; Batteiger, V.; Riegel, F. Power-to-Liquids Potentials and Perspectives for the Future Supply of Renewable Aviation Fuel; Technical Report; German Environment Agency: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- CRI. Projects: Emissions-to-Liquids Technology; CRI: Tokyo, Japan, 2021. [Google Scholar]
- Becker, W.L.; Braun, R.J.; Penev, M.; Melaina, M. Production of Fischer–Tropsch liquid fuels from high temperature solid oxide co-electrolysis units. Energy 2012, 47, 99–115. [Google Scholar] [CrossRef]
- Schemme, S.; Samsun, R.C.; Peters, R.; Stolten, D. Power-to-fuel as a key to sustainable transport systems—An analysis of diesel fuels produced from CO2 and renewable electricity. Fuel 2017, 205, 198–221. [Google Scholar] [CrossRef]
- Ail, S.S.; Dasappa, S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis —Technology review and current scenario. Renew. Sustain. Energy Rev. 2016, 58, 267–286. [Google Scholar] [CrossRef]
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst: Effect of process conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef]
- Zhao, K.; Bkour, Q.; Hou, X.; Kang, S.W.; Park, J.C.; Norton, M.G.; Yang, J.I.; Ha, S. Reverse water gas shift reaction over CuFe/Al2O3 catalyst in solid oxide electrolysis cell. Chem. Eng. J. 2018, 336, 20–27. [Google Scholar] [CrossRef]
- Shafer, W.D.; Gnanamani, M.K.; Graham, U.M.; Yang, J.; Masuku, C.M.; Jacobs, G.; Davis, B.H. Fischer—Tropsch: Product Selectivity—The Fingerprint of Synthetic Fuels. Catalysts 2019, 9, 259. [Google Scholar] [CrossRef]
- International Energy Agency-AMF. Diesel and Gasoline; International Energy Agency-AMF: Paris, France, 2021. [Google Scholar]
- Gill, S.S.; Tsolakis, A.; Dearn, K.D.; Rodríguez-Fernández, J. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Dry, M.E. High quality diesel via the Fischer–Tropsch process—A review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yang, C.W.; Kim, B.J.; Moon, J.H.; Jeong, J.Y.; Jeong, S.H.; Lee, S.H.; Kim, J.H.; Seo, M.W.; Lee, S.B.; et al. Fischer–tropsch diesel production and evaluation as alternative automotive fuel in pilot-scale integrated biomass-to-liquid process. Appl. Energy 2016, 180, 301–312. [Google Scholar] [CrossRef]
- Rane, S.; Borg, Ø.; Yang, J.; Rytter, E.; Holmen, A. Effect of alumina phases on hydrocarbon selectivity in Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2010, 388, 160–167. [Google Scholar] [CrossRef]
- Sage, V.; Sun, Y.; Hazewinkel, P.; Bhatelia, T.; Braconnier, L.; Tang, L.; Chiang, K.; Batten, M.; Burke, N. Modified product selectivity in Fischer-Tropsch synthesis by catalyst pre-treatment. Fuel Process. Technol. 2017, 167, 183–192. [Google Scholar] [CrossRef]
- Mißbach, H.; Schmidt, B.C.; Duda, J.P.; Lünsdorf, N.K.; Goetz, W.; Thiel, V. Assessing the diversity of lipids formed via Fischer-Tropsch-type reactions. Org. Geochem. 2018, 119, 110–121. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Stranges, A. Germany’s synthetic fuel industry, 1927–1945. In The German Chemical Industry in the Twentieth Century; Springer: Dordrecht, The Netherlands, 2000; pp. 147–216. [Google Scholar] [CrossRef]
- Jarvis, S.M.; Samsatli, S. Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis. Renew. Sustain. Energy Rev. 2018, 85, 46–68. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Miao, Y.; Zhang, Y.; Wang, J. New trends in the development of CO2 electrochemical reduction electrolyzer. J. Environ. Chem. Eng. 2024, 12, 112369. [Google Scholar] [CrossRef]
- Nabil, S.K.; McCoy, S.; Kibria, M.G. Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks. Green Chem. 2021, 23, 867–880. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, C.; Zheng, G. Coupling Value-Added Anodic Reactions with Electrocatalytic CO2 Reduction. Chem.-A Eur. J. 2023, 29, e202203147. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xia, C.; Zhu, P.; Lu, Y.; Wang, H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 2020, 11, 3633. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.; Bardow, A.; Bongartz, D.; Burre, J.; Chung, W.; Deutz, S.; Han, D.; Heßelmann, M.; Kohlhaas, Y.; König, A.; et al. Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels. Green Chem. 2020, 22, 3842–3859. [Google Scholar] [CrossRef]
- Arora, I.; Garg, S.; Sapi, A.; Ingole, P.P.; Chandra, A. Insights into photocatalytic CO2 reduction reaction pathway: Catalytic modification for enhanced solar fuel production. J. Ind. Eng. Chem. 2024, 137, 1–28. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Call, A.; Cibian, M.; Yamamoto, K.; Nakazono, T.; Yamauchi, K.; Sakai, K. Highly Efficient and Selective Photocatalytic CO2 Reduction to CO in Water by a Cobalt Porphyrin Molecular Catalyst. ACS Catal. 2019, 9, 4867–4874. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Hasan, M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Lootah, M.A.; Sayed, E.T.; Wilberforce, T.; Alawadhi, H.; Yousef, B.A.; Olabi, A.G. Fuel cells for carbon capture applications. Sci. Total Environ. 2021, 769, 144243. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L. Energy Efficiency Management of Coupling System for Molten Carbonate Fuel Cells. Int. J. Electrochem. Sci. 2022, 17, 220945. [Google Scholar] [CrossRef]
- Suzanne, F.; Anthony, T. Molten Carbonate Fuel Cells for 90% Post Combustion CO2 Capture from a New Build CCGT. Front. Energy Res. 2021, 9, 359. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Michailos, S.; McCord, S.; Sick, V.; Stokes, G.; Styring, P. Dimethyl ether synthesis via captured CO2 hydrogenation within the power to liquids concept: A techno-economic assessment. Energy Convers. Manag. 2019, 184, 262–276. [Google Scholar] [CrossRef]
- Vad Mathiesen, B.; Johannsen, R.M.; Kermeli, K.; Crijns-Graus, W.; Lund, H.; Skov, I.R. The green transition of industry—An introduction to IndustryPLAN. Smart Energy 2023, 11, 100111. [Google Scholar] [CrossRef]
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME—Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Xiao, M.; Wang, S.J.; Han, D.M.; Lu, Y.X.; Meng, Y.Z. Effects of Mo promoters on the Cu–Fe bimetal catalysts for the DMC formation from CO2 and methanol. Chin. Chem. Lett. 2013, 24, 307–310. [Google Scholar] [CrossRef]
- Zhang, M.; Alferov, K.A.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. Continuous Dimethyl Carbonate Synthesis from CO2 and Methanol Using Cu-Ni@VSiO as Catalyst Synthesized by a Novel Sulfuration Method. Catalysts 2018, 8, 142. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Xiao, M.; Han, D.; Lu, Y.; Meng, Y. Novel Cu-Fe bimetal catalyst for the formation of dimethyl carbonate from carbon dioxide and methanol. RSC Adv. 2012, 2, 6831–6837. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Z.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Electrochemical synthesis of dimethyl carbonate from CO2 and methanol over carbonaceous material supported DBU in a capacitor-like cell reactor. RSC Adv. 2016, 6, 40010–40016. [Google Scholar] [CrossRef]
- Selva, M.; Marques, C.A.; Tundo, P. The addition reaction of dialkyl carbonates to ketones. Gazz. Chim. Ital. 1994, 123, 515–518. [Google Scholar] [CrossRef]
- Bortnick, N.; Luskin, L.S.; Hurwitz, M.D.; Rytina, A.W. t-Carbinamines, RR′R″CNH2. III. The Preparation of Isocyanates, Isothiocyanates and Related Compounds1. J. Am. Chem. Soc. 2002, 78, 4358–4361. [Google Scholar] [CrossRef]
- Vauthey, I.; Valot, F.; Gozzi, C.; Fache, F.; Lemaire, M. An environmentally benign access to carbamates and ureas. Tetrahedron Lett. 2000, 41, 6347–6350. [Google Scholar] [CrossRef]
- Selva, M.; Tundo, P.; Perosa, A. The synthesis of alkyl carbamates from primary aliphatic amines and dialkyl carbonates in supercritical carbon dioxide. Tetrahedron Lett. 2002, 43, 1217–1219. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, H.; Ma, L.; Liu, F.; Liu, Z. Research Progress in the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Catal. Surv. Asia 2012, 16, 138–147. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Highly selective and multifunctional chitosan/ionic liquids catalyst for conversion of CO2 and methanol to dimethyl carbonates at mild reaction conditions. Fuel 2017, 166, 495–501. [Google Scholar] [CrossRef]
- Hong, H.N.; Samsun, R.C.; Peters, R.; Stolten, D. Greener production of dimethyl carbonate by the Power-to-Fuel concept: A comparative techno-economic analysis. Electrochem. Process. Eng. 2021, 23, 1734–1747. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Kearns, D.; Liu, H.; Consoli, C. Technology Readiness and Costs of CCS; Global CCS Institure: Docklands, VIC, Australia, 2021. [Google Scholar]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Bhavsar, A.; Hingar, D.; Ostwal, S.; Thakkar, I.; Jadeja, S.; Shah, M. The current scope and stand of carbon capture storage and utilization—A comprehensive review. Case Stud. Chem. Environ. Eng. 2023, 8, 100368. [Google Scholar] [CrossRef]
- Ahmad, T.; Liu, S.; Sajid, M.; Li, K.; Ali, M.; Liu, L.; Chen, W. Electrochemical CO2 reduction to C2+ products using Cu-based electrocatalysts: A review. Nano Res. Energy 2022, 1, 9120021. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Zhang, Z.; Hu, W. Recent advances in carbon-based materials for electrochemical CO2 reduction reaction. Chin. Chem. Lett. 2022, 33, 2270–2280. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2022; Technical Report; IEA: Paris, France, 2022. [Google Scholar]




| Markets | Carbon Price |
|---|---|
| EU ETS | EUR 72.4/t |
| New Zealand (NZD) | NZD 51.5/t |
| Australia (AUD) | AUD 33.5/t |
| California (USA) | USD 44.0/t |
| South Korea | USD 6.5/t |
| China | USD 12.6/t |
| Mechanisms | CO2 Trapping Phase | Description |
|---|---|---|
| Mineral | Reacted solid phase | Dissolved CO2 reacts with minerals based on Fe, Ca or Mg to form carbonates |
| Hydrodynamic | Supercritical fluid | Undissolved CO2 is trapped by cap rocks with low permeability |
| Solubility | Dissolved liquid phase | CO2 is dissolved in the brine water |
| Residual | Gas phase | CO2 displaces water from the rock pores |
| Year | 2030 | 2050 | ||||
|---|---|---|---|---|---|---|
| Scenarios | STEPS | APS | NZE | STEPS | APS | NZE |
| Gas demand (bcm) | 4456 | 4069 | 3666 | 4661 | 3568 | 2681 |
| SNG share (%) | 1.12 | 1.22 | 1.36 | 1.50 | 1.96 | 2.61 |
| Gasoline demand (mb/d) | 23.2 | 20.6 | - | 19.3 | 8.2 | - |
| Methanol share (%) | 6.6 | 7.4 | - | 11.1 | 26.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration. Sustainability 2024, 16, 6639. https://doi.org/10.3390/su16156639
Garcia JA, Villen-Guzman M, Rodriguez-Maroto JM, Paz-Garcia JM. Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration. Sustainability. 2024; 16(15):6639. https://doi.org/10.3390/su16156639
Chicago/Turabian StyleGarcia, Jose Antonio, Maria Villen-Guzman, Jose Miguel Rodriguez-Maroto, and Juan Manuel Paz-Garcia. 2024. "Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration" Sustainability 16, no. 15: 6639. https://doi.org/10.3390/su16156639
APA StyleGarcia, J. A., Villen-Guzman, M., Rodriguez-Maroto, J. M., & Paz-Garcia, J. M. (2024). Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration. Sustainability, 16(15), 6639. https://doi.org/10.3390/su16156639