Experimental Study on Heat Release Performance for Sorption Thermal Battery Based on Wave Analysis Method
Abstract
:1. Introduction
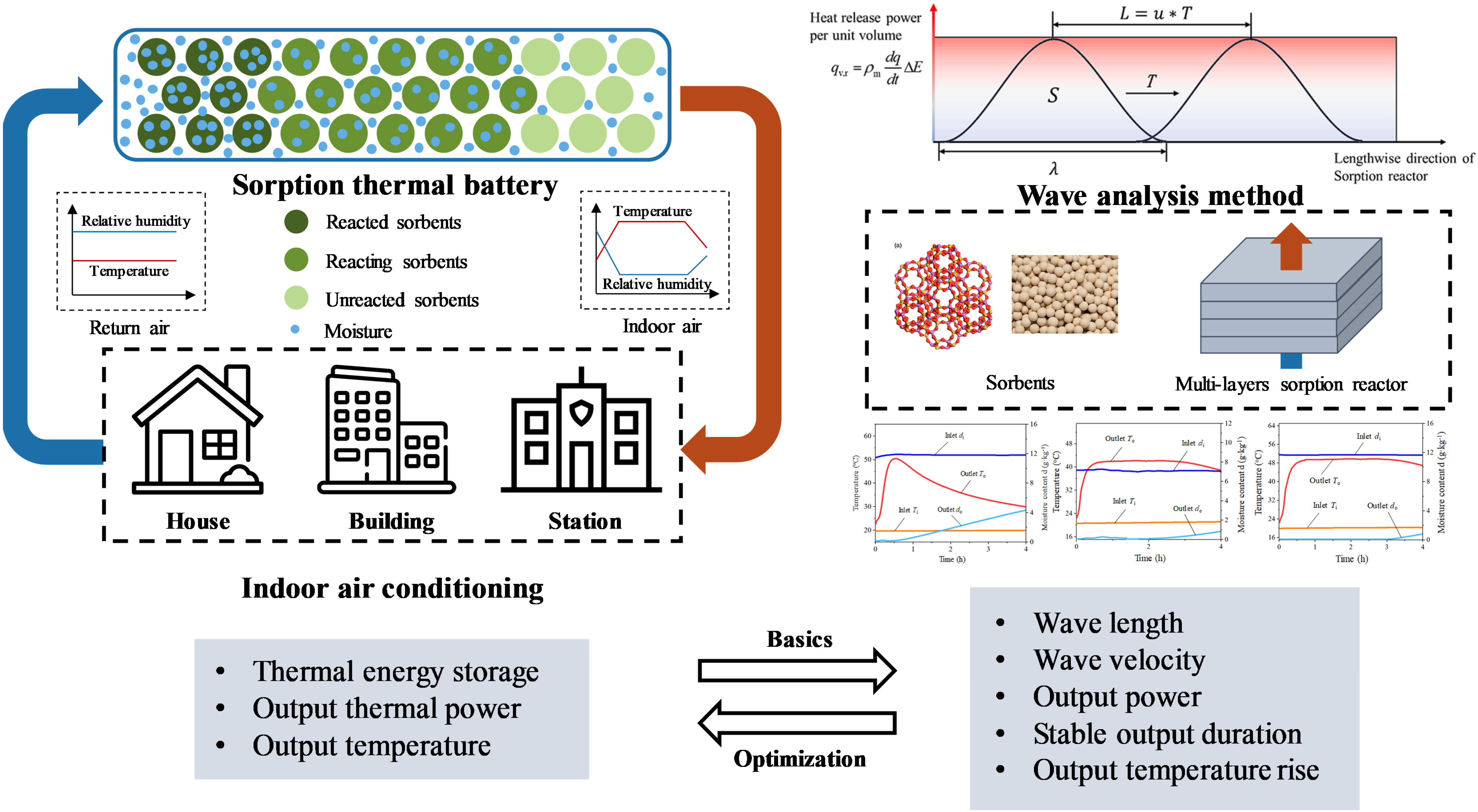
2. System Description and Methodology
2.1. Wave Analysis Method
2.2. Analysis of Variance (ANOVA)
2.3. Experimental Rig
3. Results and Discussion
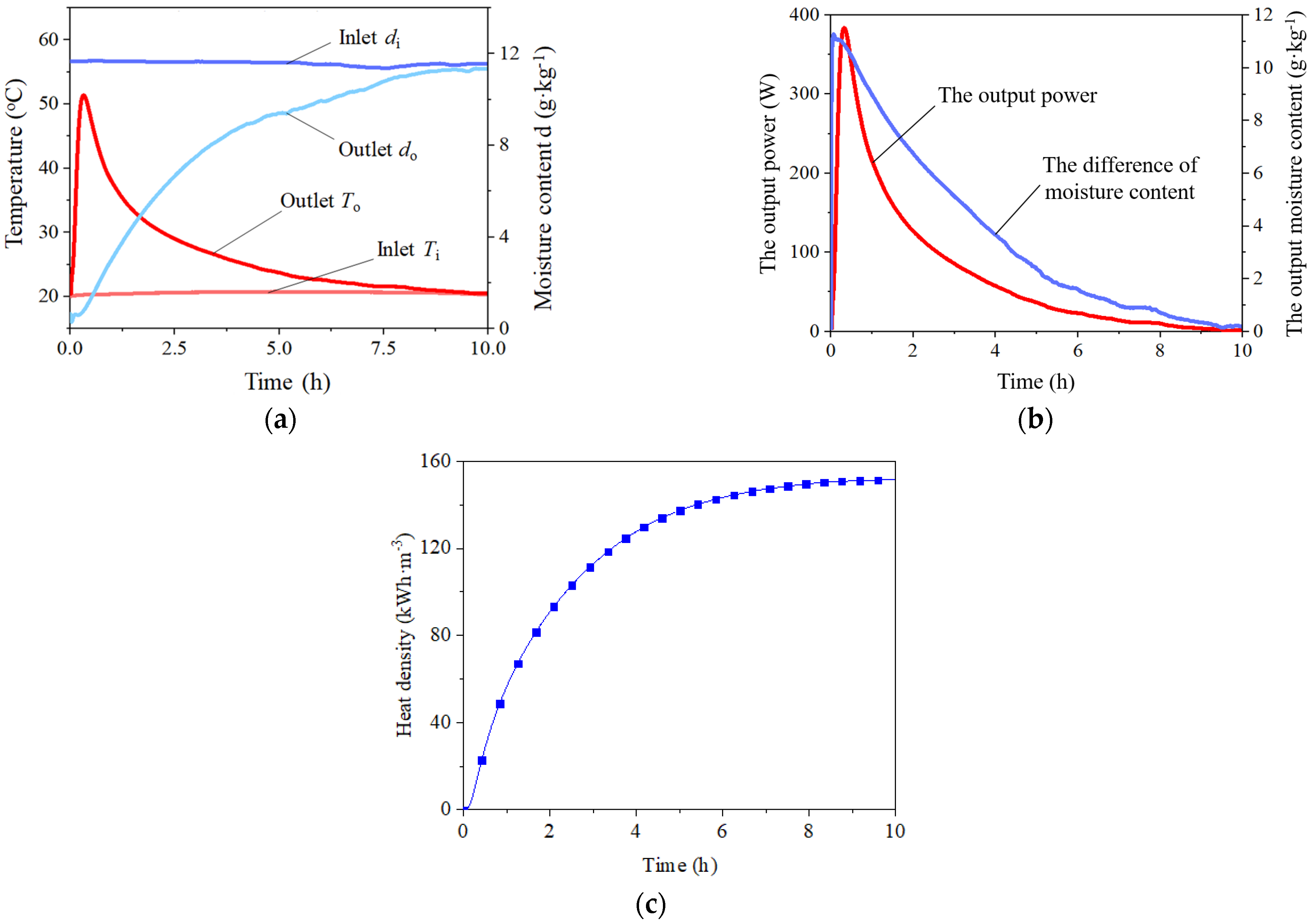
3.1. Pre-Experiment
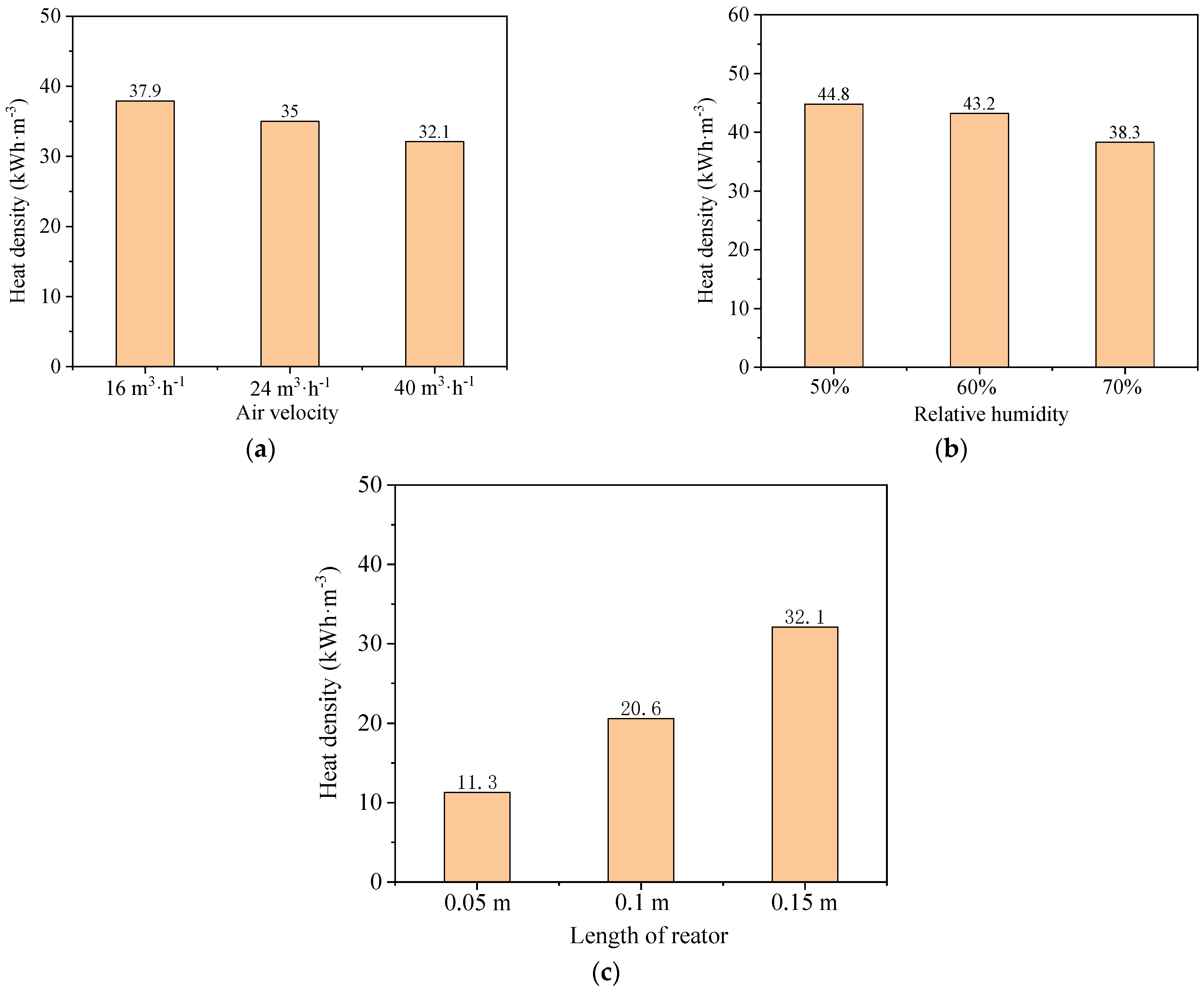
3.2. Parameteric Analysis
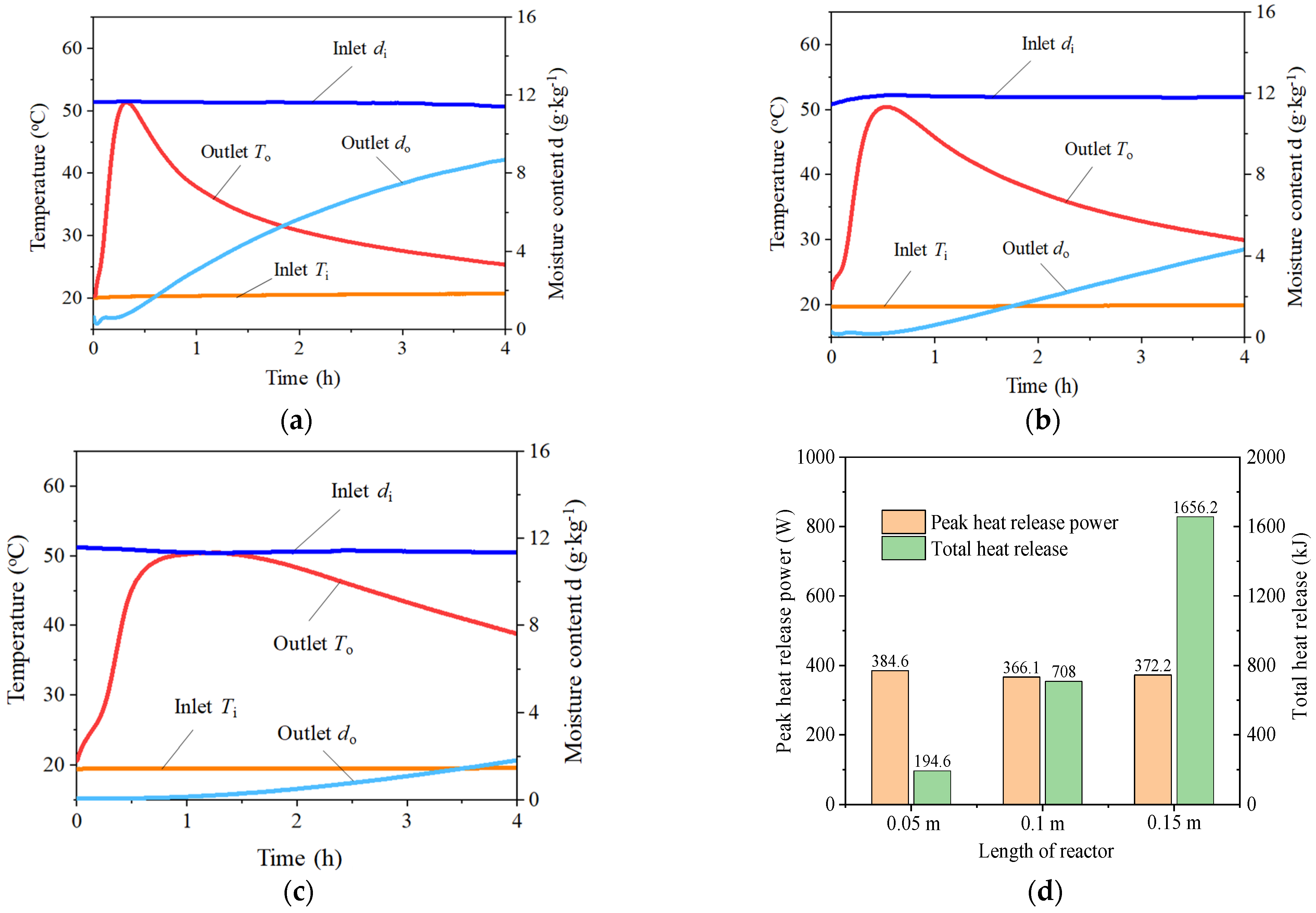
3.2.1. Adsorption Reactor Length
3.2.2. Relative Humidity
3.2.3. Working Time
3.3. Comparison of Heat Release Performance among Various Parameters
- (1)
- Under the experimental conditions, the length of the reactor is conducive to stable heat release time, while the contribution of relative humidity and air volume to stable heat release time is negatively correlated. This experimental result is similar to that using the wave analysis method.
- (2)
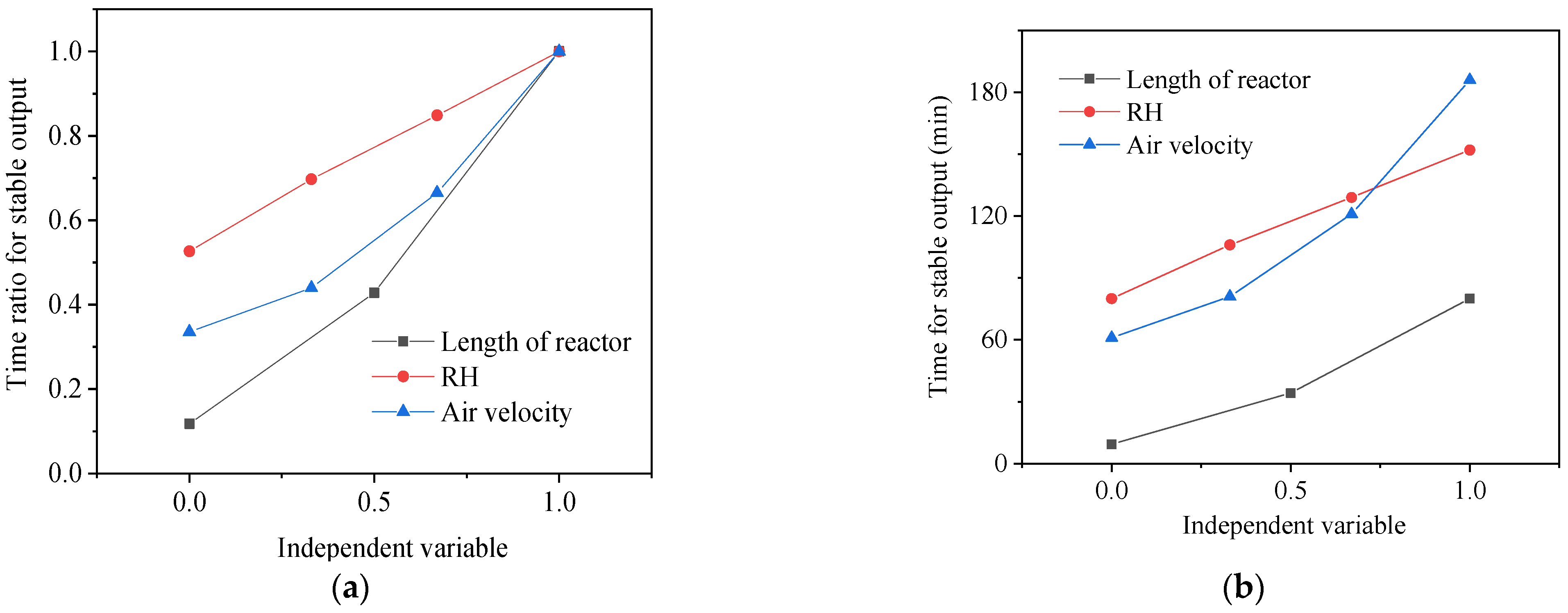
- In each experiment, the variation in RH is 10%, and the change in air volume is 8 m3·h−1. The variation in the reactor length is 0.05 m. Each parameter can have a different influence on stable heat release time. The relative increase ratio of three independent variable parameters to the stable heat release time of the STB is then compared, as shown in Figure 7a. It indicates that the variation in reactor length has the most positive effect on stable heat release time. The increase in the ratio is 0.9 of the maximum stable heat release time. Secondly, the variation in air velocity can also significantly change the stable heat release time. The increase ratio between various groups of air velocity is 0.66 of the maximum stable heat release time. Comparably, RH has the lowest increase ratio, and its stable heat release time has an increase ratio of 0.53.
- (3)
- Between each group of experiments, a difference in the response degree of the stable heat release time to each parameter is indicated in Figure 7b. The stable heat release time is increased to about 80 min by increasing the length of the reactor. When reducing air velocity and inlet RH, the stable heat release time could be increased to 182 min and 152 min, respectively. From this perspective, increasing the length of the adsorption reactor and reducing inlet air velocity are more effective in extending the stable heat release time than reducing the RH of the inlet air.
4. Conclusions
- For the typical STB experiment, there would be a peak in the heat release process. The highest heat release temperature could reach 31 °C when the sorption time is 20 min. STBs using zeolite cannot completely absorb the moisture in the air, so the relative humidity of the outlet air in the whole process cannot be reduced below 5%. The adsorbents inside the reactor are weighed after the experiment, and the average sorption capacity is calculated to be 0.29 g·kg−1. The changing trend of the total heat storage capacity of STBs is similar to that of the total water sorption capacity. Total adsorption heat storage capacity could reach 150 kWh·m−3.
- For different reactor lengths, the outlet moisture content of the sorption reactor increases and stabilizes as the length of the sorption reactor increases from 0.05 m to 0.15 m, resulting in increased stable heat release time (from 9.4 min to 80 min) and total heat release (from 194.6 kJ to 1656.2 kJ). Varying the relative humidity (RH) impacts the stable heat release time, with reductions in RH leading to longer duration: 106 min at 70% RH, 129 min at 60% RH, and 152 min at 50% RH. When RHs decrease from 80% to 50%, the total heat amount increases from 1974.6 kJ to 2307.6 kJ due to the longer stable heat release time. By increasing inlet air velocity, the outlet moisture content rises faster, where an increase in air velocity from 16 m3·h−1 to 40 m3·h−1 reduces the stable heat release time from 182 min to 61 min. In comparison with the pre-experiment results, the heat release time increases by 2.3 times when the air velocity is reduced by 50%. Accordingly, the total heat release amount ranges from 1578.5 kJ to 1955.7 kJ.
- The heat release time and heat storage efficiency are optimized in terms of reactor length, RH, and air velocity. Increasing the length of the adsorption reactor is the first choice for the optimization of STB system control. It can achieve stable temperature without affecting heat release power. Also, heat release time should be extended to increase the stable heat release amount per unit volume of adsorber and improve heat storage efficiency. Adjusting the RH of the inlet air for STBs is an effective method. Although reducing RH has an influence on heat release power, it can bring about a large increase in stable heat release time and heat storage per unit volume. However, air velocity regulation of the STB system has limited improvement for the system optimization. Although this method could increase stable heat release time, the increase in the total amount of heat storage is not obvious.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Greek symbols | |
| ANOVA | Analysis of variance |
| COP | Coefficient of performance |
| Cp | Specific heat, J·kg·K−1 |
| d | Moisture content of the air, g·kg−1 |
| ESD | Energy storage density |
| k | Kinetic coefficient, s−1 |
| L* | Stable output parameter |
| L1, L2 | Start and end position of sorption reaction, m |
| Lb | Sorption reactor length, m |
| LDF | Linear driving force |
| LHS | Latent heat storage |
| P | Air pressure, Pa |
| Ps | Saturated water vapor partial pressure of the air, Pa |
| PP | Polypropylene |
| PCM | Phase change material |
| q | Sorption capacity, g·kg−1 |
| qequ | Equilibrium orbed capacity, g·kg−1 |
| qv | Heat release power per unit volume, W·m−3 |
| Q | Output power, W |
| Qtot | Total heat output, J |
| RH | Relative humidity |
| s | Area, m2 |
| S | Wave area, W |
| SHS | Sensible heat storage |
| STB | Sorption thermal battery |
| STES | Sorption thermal energy storage |
| t | Stable output duration, s |
| t1, t2 | Start and end time of stable output stage, s |
| TES | Thermal energy storage |
| THS | Thermochemical heat storage |
| u | Wave velocity, m·s−1 |
| v | Velocity, m·s−1 |
| Other symbols | |
| ρ | Density, kg·m−3 |
| λ | Wave length, m |
| φ | Relative humidity of the air |
| ε | Reactor porosity |
| ΔT | Temperature difference, °C |
| ΔE | Sorption heat, J·kg−1 |
| Subscripts | |
| a | Air |
| t | Sorbent |
| in | Inlet of the reactor |
| out | Outlet of the reactor |
References
- Zhang, Z.; Zhang, J.; Yuan, H.; Zheng, X.; Mei, N. Design and experimental analysis of energy-saving and heat storage of a hot water tank based on the source-sink matching principle. Case Stud. Therm. Eng. 2023, 41, 102672. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, R.Y.; Shi, W.K.; Wu, E.Y.; Li, B.; Zhang, X.J. Water effect on adsorption carbon capture in metal-organic framework: A molecular simulation. Carbon Capture Sci. Technol. 2022, 4, 100061. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A review of hydrogen/rock/brine interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, 101066. [Google Scholar] [CrossRef]
- Vecchi, A.; Sciacovelli, A. Long-duration thermo-mechanical energy storage—Present and future techno-economic competitiveness. Appl. Energy 2023, 334, 120628. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ramadan, M.; Olabi, A.-G.; Pullen, K.; Naher, S. A review of mechanical energy storage systems combined with wind and solar applications. Energy Convers. Manag. 2020, 210, 112670. [Google Scholar] [CrossRef]
- Steinmann, W.-D. Thermo-mechanical concepts for bulk energy storage. Renew. Sustain. Energy Rev. 2017, 75, 205–219. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X. Progress of research on phase change energy storage materials in their thermal conductivity. J. Energy Storage 2023, 61, 106772. [Google Scholar] [CrossRef]
- Hua, W.; Lv, X.; Zhang, X.; Ji, Z.; Zhu, J. Research progress of seasonal thermal energy storage technology based on supercooled phase change materials. J. Energy Storage 2023, 67, 107378. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelghafar, A.A.; Maghrabie, H.M.; Sayed, E.T.; Rezk, H.; Radi, M.A.; Obaideen, K.; Abdelkareem, M.A. Application of artificial intelligence for prediction, optimization, and control of thermal energy storage systems. Therm. Sci. Eng. Prog. 2023, 39, 101730. [Google Scholar] [CrossRef]
- Lyden, A.; Brown, C.S.; Kolo, I.; Falcone, G.; Friedrich, D. Seasonal thermal energy storage in smart energy systems: District-level applications and modelling approaches. Renew. Sustain. Energy Rev. 2022, 167, 112760. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Khan, H.A.; Al-Othman, A.; Almomani, F.; Ajith, S. A comprehensive review on the recent advances in materials for thermal energy storage applications. Int. J. Thermofluids 2023, 18, 100326. [Google Scholar] [CrossRef]
- Mahon, H.; O’Connor, D.; Friedrich, D.; Hughes, B. A review of thermal energy storage technologies for seasonal loops. Energy 2022, 239, 122207. [Google Scholar] [CrossRef]
- Lingayat, A.; Das, P.; Gilago, M.C.; Chandramohan, V.P. A detailed assessment of paraffin waxed thermal energy storage medium for solar dryers. Sol. Energy 2023, 261, 14–27. [Google Scholar] [CrossRef]
- Crespo, A.; Fernández, C.; Vérez, D.; Tarragona, J.; Borri, E.; Frazzica, A.; Cabeza, L.F.; de Gracia, A. Thermal performance assessment and control optimization of a solar-driven seasonal sorption storage system for residential application. Energy 2023, 263, 125382. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, W. A novel double-effect compression-assisted absorption thermal battery with high storage performance for thermal energy storage. Renew. Energy 2022, 191, 902–918. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, C.Y.; Yan, J. Investigation on the Ca(OH)2/CaO thermochemical energy storage system with potassium nitrate addition. Sol. Energy Mater. Sol. Cells 2020, 215, 110646. [Google Scholar] [CrossRef]
- Wang, K.W.; Yan, T.; Pan, W.G. Optimization strategies of microencapsulated phase change materials for thermal energy storage. J. Energy Storage 2023, 68, 107844. [Google Scholar] [CrossRef]
- Yan, J.; Pan, Z.H.; Zhao, C.Y. Experimental study of MgO/Mg(OH)2 thermochemical heat storage with direct heat transfer mode. Appl. Energy 2020, 275, 115356. [Google Scholar] [CrossRef]
- Humphries, T.D.; Paskevicius, M.; Alamri, A.; Buckley, C.E. Thermodynamic destablization of SrH2 using Al for the next generation of high temperature thermal batteries. J. Alloys Compd. 2022, 894, 162404. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Li, X.; Qu, W.; Zhou, T.; Dong, T.; Deng, L.; Zhang, J.; Zhao, J. A high energy density 3D nano-carbon based magnesium hydroxide reversible chemical reaction heat storage material synthesis and heat transfer performance investigation. J. Energy Storage 2022, 50, 104260. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, B.; Wang, R. Water based adsorption thermal battery: Sorption mechanisms and applications. Energy Storage Mater. 2023, 54, 794–821. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, W. Type II absorption thermal battery for temperature upgrading: Energy storage heat transformer. Appl. Energy 2022, 324, 119748. [Google Scholar] [CrossRef]
- Girnik, I.S.; Grekova, A.D.; Li, T.X.; Wang, R.Z.; Dutta, P.; Murthy, S.S.; Aristov, Y.I. Composite “LiCl/MWCNT/PVA” for adsorption thermal battery: Dynamics of methanol sorption. Renew. Sustain. Energy Rev. 2020, 123, 109748. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Wang, R.; Zhu, F.; Lu, Y.; Roskilly, A.P. Experimental investigation on an innovative resorption system for energy storage and upgrade. Energy Convers. Manag. 2017, 138, 651–658. [Google Scholar] [CrossRef]
- Jiang, L.; Roskilly, A.P.; Wang, R.Z.; Wang, L.W. Analysis on innovative resorption cycle for power and refrigeration cogeneration. Appl. Energy 2018, 218, 10–21. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, W.; Lin, Y.C.; Wang, R.Q.; Zhang, X.J.; Hu, M.K. Hybrid thermochemical sorption seasonal storage for ultra-low temperature solar energy utilization. Energy 2022, 239, 122068. [Google Scholar] [CrossRef]
- Sharma, R.; Anil Kumar, E.; Dutta, P.; Srinivasa Murthy, S.; Aristov, Y.I.; Tokrev, M.M.; Li, T.; Wang, R. Ammoniated salt based solid sorption thermal batteries: A comparative study. Appl. Therm. Eng. 2021, 191, 116875. [Google Scholar] [CrossRef]
- Chao, J.; Xu, J.; Bai, Z.; Wang, P.; Wang, R.; Li, T. Integrated heat and cold storage enabled by high-energy-density sorption thermal battery based on zeolite/MgCl2 composite sorbent. J. Energy Storage 2023, 64, 107155. [Google Scholar] [CrossRef]
- ElBahloul, A.A.; Zeidan, E.-S.B.; El-Sharkawy, I.I.; Hamed, A.M.; Radwan, A. Recent advances in multistage sorption thermal energy storage systems. J. Energy Storage 2022, 45, 103683. [Google Scholar] [CrossRef]
- Wu, S.-F.; Wang, L.-W.; An, G.-L.; Zhang, B. Excellent ammonia sorption enabled by metal-organic framework nanocomposites for seasonal thermal battery. Energy Storage Mater. 2023, 54, 822–835. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Meng, B.; Cui, F.; Loonen, R.; Nouar, F.; Serre, C.; Hensen, J. Moisture-participating MOF thermal battery for heat reallocation between indoor environment and building-integrated photovoltaics. Nano Energy 2021, 87, 106224. [Google Scholar] [CrossRef]
- Mileo, P.G.M.; Ho Cho, K.; Park, J.; Devautour-Vinot, S.; Chang, J.-S.; Maurin, G. Unraveling the Water Adsorption Mechanism in the Mesoporous MIL-100(Fe) Metal–Organic Framework. J. Phys. Chem. C 2019, 123, 23014–23025. [Google Scholar] [CrossRef]
- Yan, T.; Li, T.; Xu, J.; Chao, J.; Wang, R.; Aristov, Y.I.; Gordeeva, L.G.; Dutta, P.; Murthy, S.S. Ultrahigh-Energy-Density Sorption Thermal Battery Enabled by Graphene Aerogel-Based Composite Sorbents for Thermal Energy Harvesting from Air. ACS Energy Lett. 2021, 6, 1795–1802. [Google Scholar] [CrossRef]
- Strelova, S.V.; Gordeeva, L.G.; Grekova, A.D.; Salanov, A.N.; Aristov, Y.I. Composites “lithium chloride/vermiculite” for adsorption thermal batteries: Giant acceleration of sorption dynamics. Energy 2023, 263, 125733. [Google Scholar] [CrossRef]
- Li, W.; Jaromír Klemeš, J.; Wang, Q.; Zeng, M. Efficient thermal management strategy of Li-ion battery pack based on sorption heat storage. Energy Convers. Manag. 2022, 256, 115383. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Salt hydrate–based gas-solid thermochemical energy storage: Current progress, challenges, and perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111846. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Chen, X.; Riffat, S. Novel “open-sorption pipe” reactor for solar thermal energy storage. Energy Convers. Manag. 2016, 121, 321–334. [Google Scholar] [CrossRef]
- Malley-Ernewein, A.; Lorente, S. Analysis of thermochemical energy storage in an elemental configuration. Sci. Rep. 2019, 9, 15875. [Google Scholar] [CrossRef]
- Michel, B.; Mazet, N.; Neveu, P. Experimental investigation of an open thermochemical process operating with a hydrate salt for thermal storage of solar energy: Local reactive bed evolution. Appl. Energy 2016, 180, 234–244. [Google Scholar] [CrossRef]
- Hanikel, N.; Prévot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid Cycling and Exceptional Yield in a Metal-Organic Framework Water Harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Fisher, R.; Ding, Y.; Sciacovelli, A. Hydration kinetics of K2CO3, MgCl2 and vermiculite-based composites in view of low-temperature thermochemical energy storage. J. Energy Storage 2021, 38, 102561. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, R.; Feng, P. Air humidity assisted sorption thermal battery governed by reaction wave model. Energy Storage Mater. 2020, 27, 9–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Sorption thermal energy storage: Concept, process, applications and perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- Lin, Y.C.; Fan, Y.B.; Chen, S.; Zhang, X.J.; Frazzica, A.; Jiang, L. Wave analysis method for air humidity assisted sorption thermal battery: A new perspective. Energy Convers. Manag. 2023, 277, 116638. [Google Scholar] [CrossRef]



| Method | Sensible | Latent | Chemical |
|---|---|---|---|
| Energy density | Small (0.2 GJ·m−3) | Medium (0.3–0.5 GJ·m−3) | Large (0.7–3.0 GJ·m−3) |
| Medium | Water, pebble, soil, etc. | Organics, inorganics | Metal chlorides, metal hydrides |
| Lifespan | Long | Limited by PCM | Limited by reactants |
| Advantages | Low cost, simple, reliable, environmentally friendly | Higher energy density, constant temperature | Highest energy density, negligible heat loss |
| Disadvantages | Large heat loss, high cost of construction | Crystallization, corrosion | Complex system, high initial investment |
| State of development | Large-scale demonstration plants | Lab-scale prototypes, several commercial products | Lab-scale prototypes |
| Influence Parameters | Wave Parameters | ||||
|---|---|---|---|---|---|
| Wave Length, L | Wave Velocity, v | Output Power, S | Stable Output Duration, t | Output Temperature Rise, ΔT | |
| Sorption reactor length, Lb | √ | × | √ | ○ | √ |
| Air velocity, va | ○ | ○ | ○ | √ | × |
| Kinetic coefficient, k | √ | × | × | × | × |
| Reactor porosity, ε | √ | √ | √ | √ | ○ |
| Air relative humidity, RH | √ | √ | ○ | ○ | ○ |
| Testing Apparatus/Type | Measurement Accuracy |
|---|---|
| Temperature and humidity sensors VAISALA HTM100 | ±1.5% RH ±0.25 °C |
| Agilent 349370A | 22-bit resolution |
| Pressure transducers | ±1.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Liu, W.; Lin, Y.; Gao, N.; Zhang, X.; Jiang, L. Experimental Study on Heat Release Performance for Sorption Thermal Battery Based on Wave Analysis Method. Sustainability 2024, 16, 6654. https://doi.org/10.3390/su16156654
Yu M, Liu W, Lin Y, Gao N, Zhang X, Jiang L. Experimental Study on Heat Release Performance for Sorption Thermal Battery Based on Wave Analysis Method. Sustainability. 2024; 16(15):6654. https://doi.org/10.3390/su16156654
Chicago/Turabian StyleYu, Meng, Wei Liu, Yuchen Lin, Neng Gao, Xuejun Zhang, and Long Jiang. 2024. "Experimental Study on Heat Release Performance for Sorption Thermal Battery Based on Wave Analysis Method" Sustainability 16, no. 15: 6654. https://doi.org/10.3390/su16156654





