Modeling of Soil Cation Exchange Capacity Based on Chemometrics, Various Spectral Transformations, and Multivariate Approaches in Some Soils of Arid Zones
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Field Work and Lab Analysis
2.3. Acquisition of Spectral Data
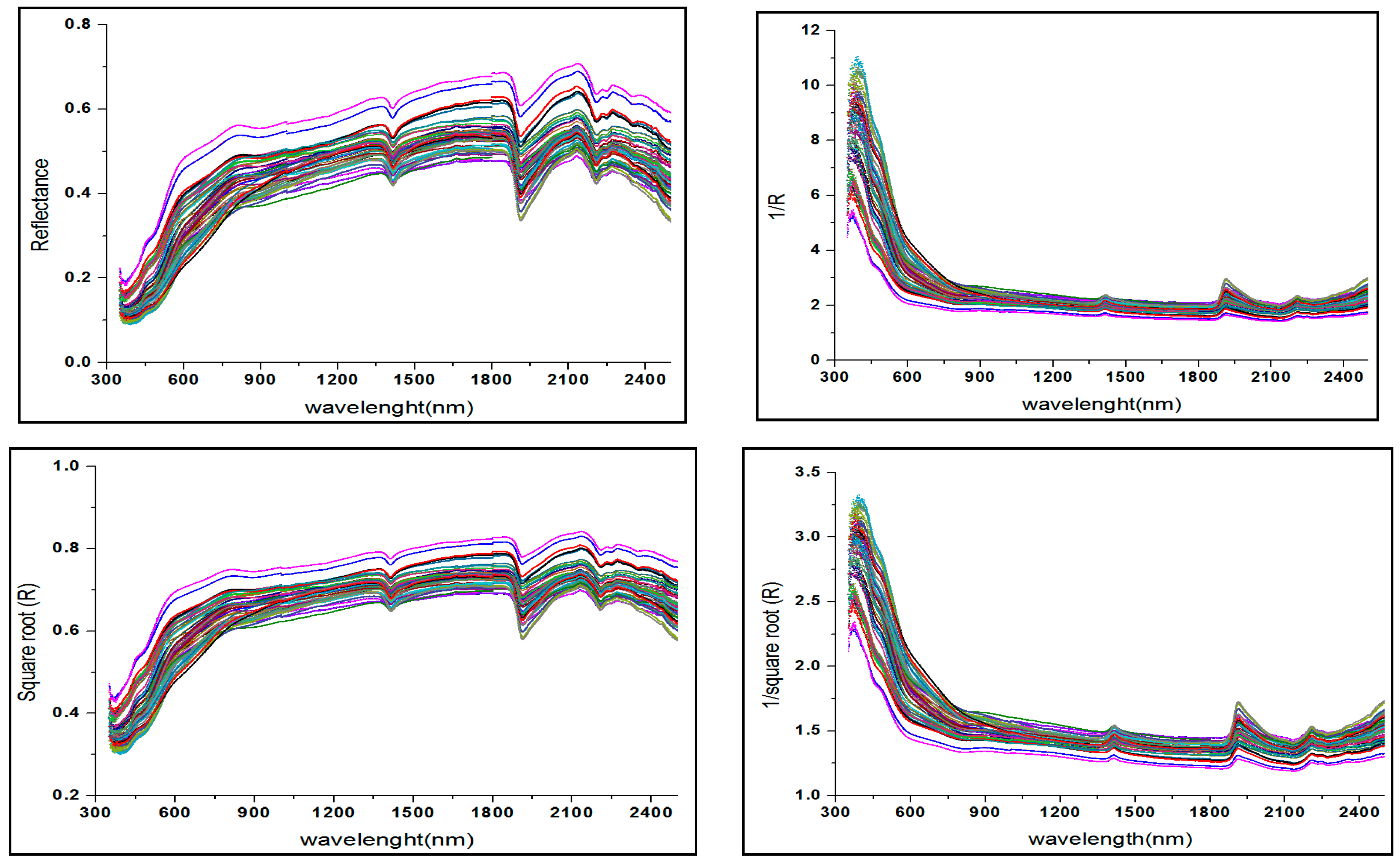
2.4. Data Preparation and Preprocessing Transformation
2.5. Multiple Variable Statistical Modeling
2.6. Assessment of the Developed Models’ Performance
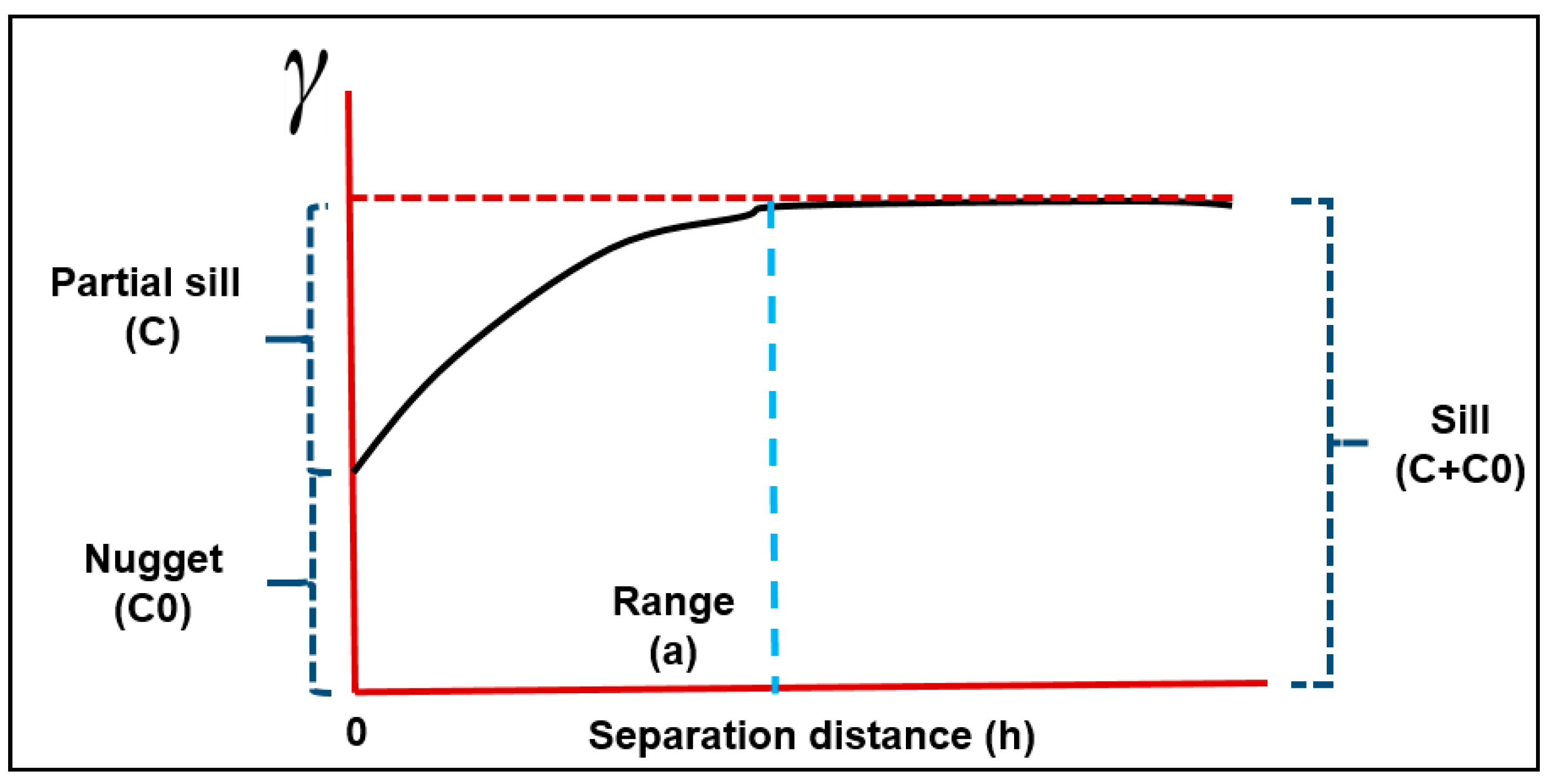
2.7. Semivariogram Analysis
3. Results and Discussion
3.1. Descriptive Statistics of Soil Samples
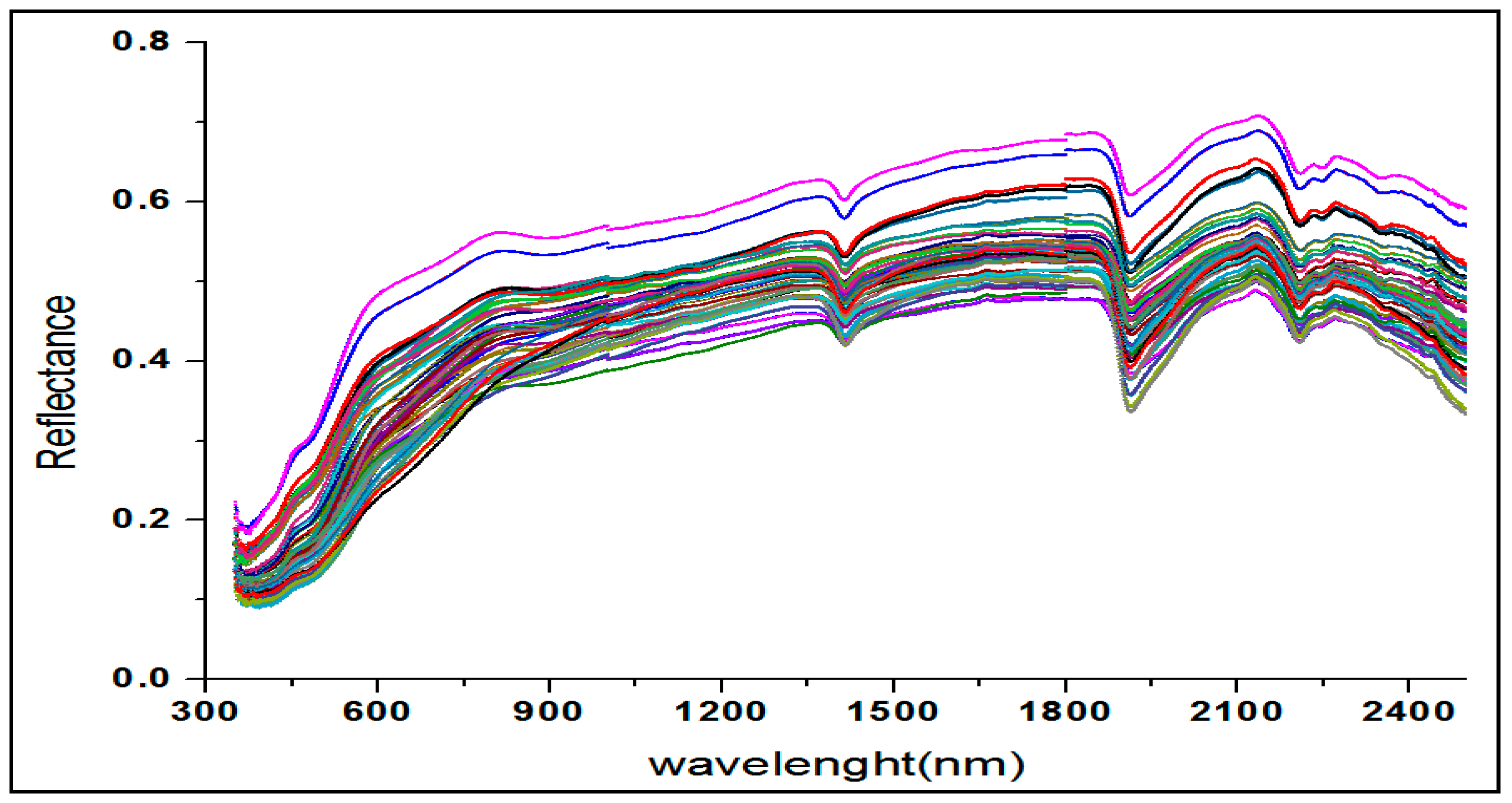
3.2. Qualitative Description of the Soil Spectra
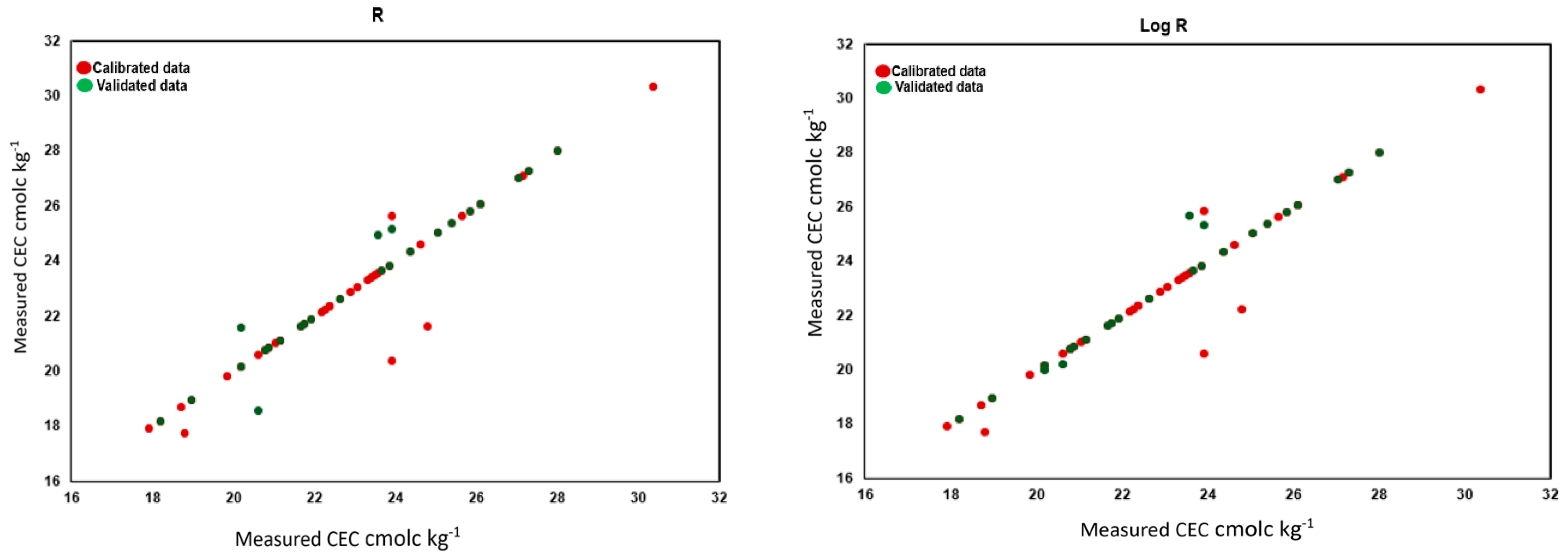
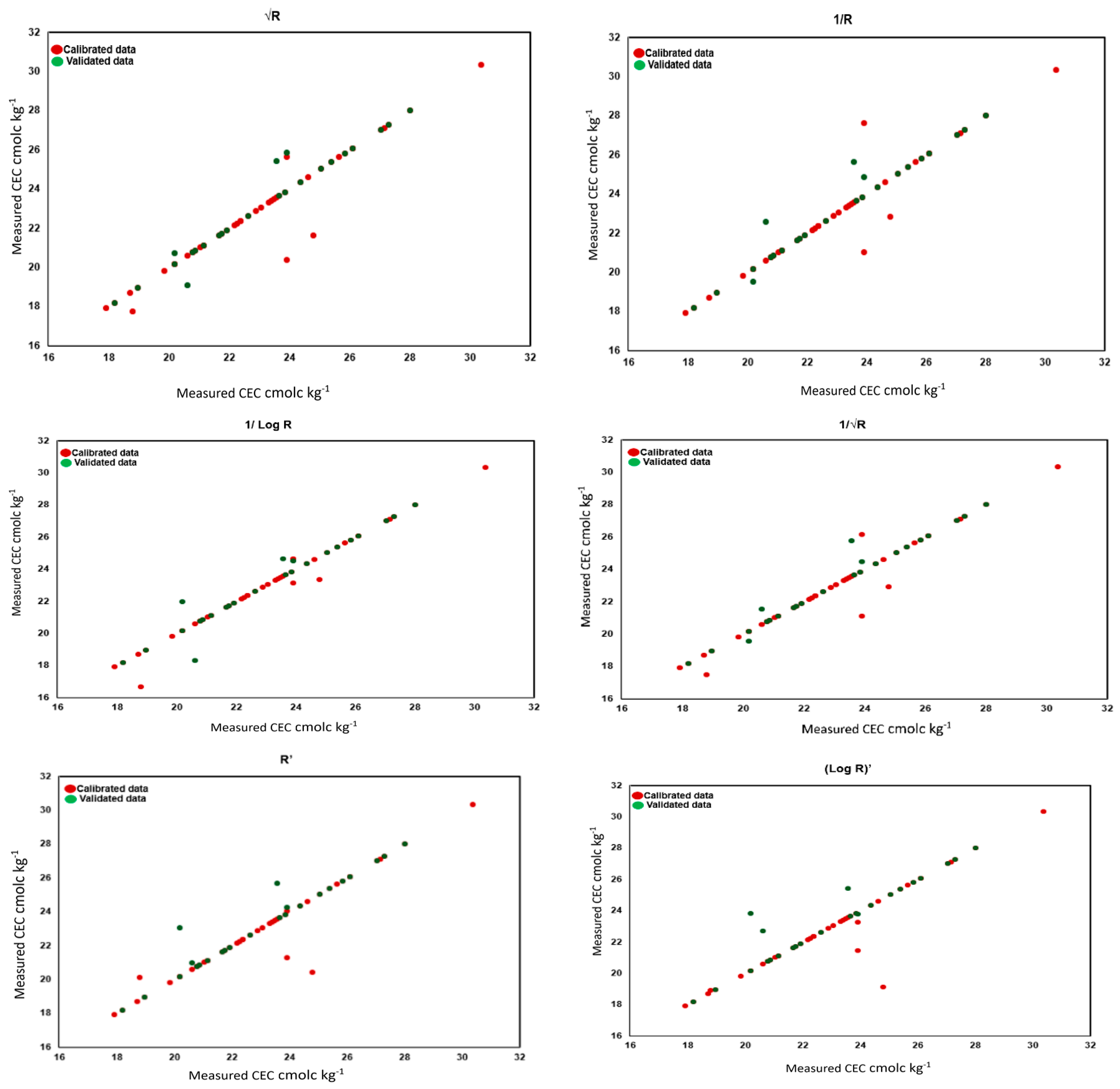
3.3. Prediction of Soil Properties Using PLSR
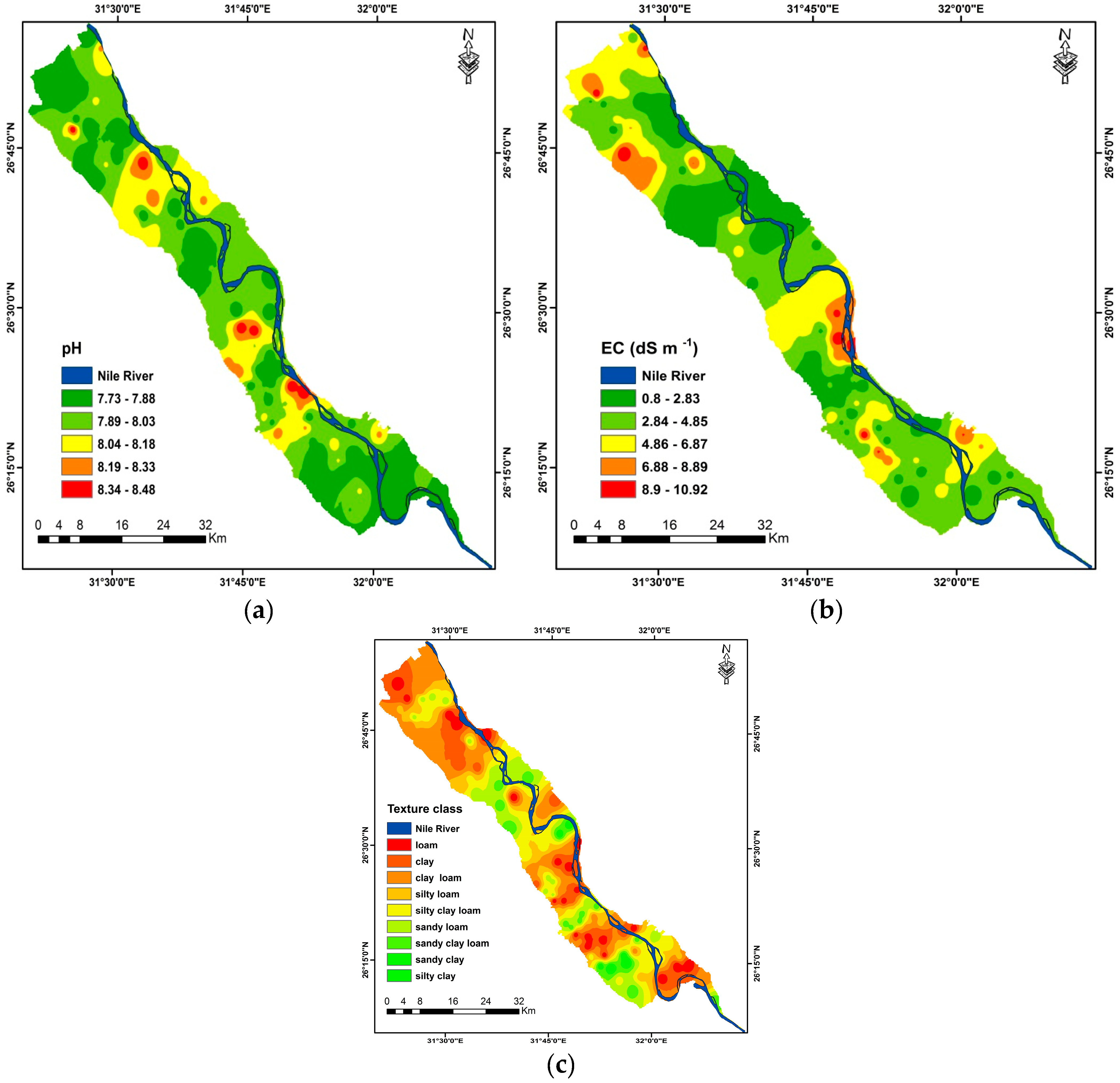
3.4. CEC Mapping Based on Semivariogram Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demattê, J.A.; Ramirez-Lopez, L.; Marques, K.; Rodella, A. Chemometric soil analysis on the determination of specific bands for the detection of magnesium and potassium by spectroscopy. Geoderma 2017, 288, 8–22. [Google Scholar] [CrossRef]
- Ulusoy, Y.; Tekin, Y.; Tümsavaş, Z.; Mouazen, A.M. Prediction of soil cation exchange capacity using visible and near infrared spectroscopy. Biosyst. Eng. 2016, 152, 79–93. [Google Scholar] [CrossRef]
- Fung, E.; Wang, J.; Zhao, X.; Farzamian, M.; Allred, B.; Clevenger, W.B.; Levison, P.; Triantafilis, J.J.S.; Research, T. Mapping cation exchange capacity and exchangeable potassium using proximal soil sensing data at the multiple-field scale. Soil Tillage Res. 2023, 232, 105735. [Google Scholar] [CrossRef]
- Emamgolizadeh, S.; Bateni, S.; Shahsavani, D.; Ashrafi, T.; Ghorbani, H. Estimation of soil cation exchange capacity using Genetic Expression Programming (GEP) and Multivariate Adaptive Regression Splines (MARS). J. Hydrol. 2015, 529, 1590–1600. [Google Scholar] [CrossRef]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Wiley Online Library: Hoboken, NJ, USA, 1982; Volume 9, pp. 149–157. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Polemio, M.; Rhoades, J.D. Determining Cation Exchange Capacity: A New Procedure for Calcareous and Gypsiferous Soils. Soil Sci. Soc. Am. J. 1977, 41, 524–528. [Google Scholar] [CrossRef]
- Takele, C.; Iticha, B. Use of infrared spectroscopy and geospatial techniques for measurement and spatial prediction of soil properties. Heliyon 2020, 6, e05269. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, A.; Sarmadian, F.; Shiri, J.; Iqbal, M.; Tirado-Corbalá, R.; Omran, E.-S.E. Application of ANFIS-based subtractive clustering algorithm in soil Cation Exchange Capacity estimation using soil and remotely sensed data. Measurement 2017, 95, 173–180. [Google Scholar] [CrossRef]
- Mishra, G.; Sulieman, M.M.; Kaya, F.; Francaviglia, R.; Keshavarzi, A.; Bakhshandeh, E.; Loum, M.; Jangir, A.; Ahmed, I.; Elmobarak, A.; et al. Machine learning for cation exchange capacity prediction in different land uses. CATENA 2022, 216, 106404. [Google Scholar] [CrossRef]
- Kania, M.; Gruba, P. Estimation of selected properties of forest soils using near-infrared spectroscopy (NIR). Soil Sci. Annu. 2016, 67, 32–36. [Google Scholar] [CrossRef]
- Rehman, H.U.; Knadel, M.; de Jonge, L.W.; Moldrup, P.; Greve, M.H.; Arthur, E. Comparison of Cation Exchange Capacity Estimated from Vis–NIR Spectral Reflectance Data and a Pedotransfer Function. Vadose Zone J. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Waruru, B.K.; Shepherd, K.D.; Ndegwa, G.M.; Kamoni, P.T.; Sila, A.M. Rapid estimation of soil engineering properties using diffuse reflectance near infrared spectroscopy. Biosyst. Eng. 2014, 121, 177–185. [Google Scholar] [CrossRef]
- Ben-Dor, E. Quantitative remote sensing of soil properties. Adv. Agron. 2002, 75, 173–243. [Google Scholar]
- Demattê, J.A.M. Characterization and discrimination of soils by their reflected electromagnetic energy. Pesqui. Agropecuária Bras. 2002, 37, 1445–1458. [Google Scholar] [CrossRef]
- Abdul Munnaf, M.; Nawar, S.; Mouazen, A.M. Estimation of Secondary Soil Properties by Fusion of Laboratory and On-Line Measured Vis–NIR Spectra. Remote Sens. 2019, 11, 2819. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra-Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Noon, C.; van Wesemael, B. Prediction of soil organic carbon for different levels of soil moisture using Vis-NIR spectroscopy. Geoderma 2013, 199, 37–42. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, Y.; Wang, M.; Shi, X. Comparison of multivariate methods for estimating selected soil properties from intact soil cores of paddy fields by Vis–NIR spectroscopy. Geoderma 2018, 310, 29–43. [Google Scholar] [CrossRef]
- Fan, S.; Wang, X.; Lei, J.; Ran, Q.; Ren, Y.; Zhou, J. Spatial distribution and source identification of heavy metals in a typical Pb/Zn smelter in an arid area of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1661–1687. [Google Scholar] [CrossRef]
- Mondal, B.P.; Sekhon, B.S.; Sahoo, R.N.; Paul, P. Vis-nir reflectance spectroscopy for assessment of soil organic carbon in a rice-wheat field of ludhiana district of punjab. ISPRS-Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. W6. [CrossRef]
- Conforti, M.; Castrignanò, A.; Robustelli, G.; Scarciglia, F.; Stelluti, M.; Buttafuoco, G. Laboratory-based Vis–NIR spectroscopy and partial least square regression with spatially correlated errors for predicting spatial variation of soil organic matter content. CATENA 2015, 124, 60–67. [Google Scholar] [CrossRef]
- Curcio, D.; Ciraolo, G.; D’asaro, F.; Minacapilli, M. Prediction of Soil Texture Distributions Using VNIR-SWIR Reflectance Spectroscopy. Procedia Environ. Sci. 2013, 19, 494–503. [Google Scholar] [CrossRef]
- Mousavi, F.; Abdi, E.; Ghalandarzadeh, A.; Bahrami, H.A.; Majnounian, B.; Ziadi, N. Diffuse reflectance spectroscopy for rapid estimation of soil Atterberg limits. Geoderma 2019, 361, 114083. [Google Scholar] [CrossRef]
- Yitagesu, F.A.; Van der Meer, F.; Van der Werff, H.; Hecker, C. Spectral characteristics of clay minerals in the 2.5–14 μm wavelength region. Appl. Clay Sci. 2011, 53, 581–591. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Modeling of soil organic carbon fractions using visible–near-infrared spectroscopy. Appl. Clay Sci. 2009, 73, 176–184. [Google Scholar] [CrossRef]
- Kaya, F.; Mishra, G.; Francaviglia, R.; Keshavarzi, A. Combining Digital Covariates and Machine Learning Models to Predict the Spatial Variation of Soil Cation Exchange Capacity. Land 2023, 12, 819. [Google Scholar] [CrossRef]
- Saidi, S.; Ayoubi, S.; Shirvani, M.; Azizi, K.; Zeraatpisheh, M. Comparison of Different Machine Learning Methods for Predicting Cation Exchange Capacity Using Environmental and Remote Sensing Data. Sensors 2022, 22, 6890. [Google Scholar] [CrossRef]
- Mishra, G.; Das, J.; Sulieman, M. Modelling Soil Cation Exchange Capacity in Different Land-Use Systems using Artificial Neural Networks and Multiple Regression Analysis. Curr. Sci. 2019, 116, 2020–2027. [Google Scholar] [CrossRef]
- Fang, Q.; Hong, H.; Zhao, L.; Kukolich, S.; Yin, K.; Wang, C. Visible and Near-Infrared Reflectance Spectroscopy for Investigating Soil Mineralogy: A Review. J. Spectrosc. 2018, 2018, 3168974. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Janik, L.J.; Rossel, R.A.V.; Macdonald, L.M.; McLaughlin, M.J. The Performance of Visible, Near-, and Mid-Infrared Reflectance Spectroscopy for Prediction of Soil Physical, Chemical, and Biological Properties. Appl. Spectrosc. Rev. 2013, 49, 139–186. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Engineering, A. Incorporate the Fertility Capability Classification and Geo-informatics for Assessing Soil: A Case Study on Some Soils of Sohag Governorate, Egypt. J. Soil Sci. Agric. Eng. 2023, 14, 187–193. [Google Scholar] [CrossRef]
- El-Rawy, M.; Abdalla, F.; El Alfy, M.; El-Rawy, M.; Abdalla, F.; El Alfy, M.; Abdalla, F. Water resources in Egypt. In The Geology of Egypt; Springer: Berlin/Heidelberg, Germany, 2019; pp. 687–711. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Thomas, G.W. Soil pH and Soil Acidity. In Method of Soil Analysis, Part 3: Chemical Methods; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Bingham, J.M., Ed.; ASA-SSSA: Madison, WI, USA, 1996; Volume 5, pp. 1201–1229. [Google Scholar]
- Lavkulich, L.M. Methods Manual: Pedology Laboratory; University of British Columbia, Department of Soil Science: Vancouver, BC, Canada, 1981. [Google Scholar]
- Asgari, N.; Ayoubi, S.; Demattê, J.A.M.; Dotto, A.C. Carbonates and organic matter in soils characterized by reflected energy from 350–25000 nm wavelength. J. Mt. Sci. 2020, 17, 1636–1651. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Borůvka, L.; Saberioon, M.; Vašát, R. Visible, Near-Infrared, and Mid-Infrared Spectroscopy Applications for Soil Assessment with Emphasis on Soil Organic Matter Content and Quality: State-of-the-Art and Key Issues. Appl. Spectrosc. 2013, 67, 1349–1362. [Google Scholar] [CrossRef]
- Jinliang, W.; Qiming, Q.; Heng, D.; Chao, C.; Qingye, M. Study on quantitative retrieval of soil organic matter based on bare soil spectrum. In Proceedings of the 3rd International Conference on Multimedia Technology (ICMT-13); Atlantis Press: Amsterdam, The Netherlands, 2013; pp. 382–389. [Google Scholar]
- Li, J.; Deng, X.; Wang, Y.; Fu, Y.; Li, E.; Peng, M. Quantitative prediction of soil organic matter of mined-land by hyperspectral remote sensing and mathematical statistics. Sens. Transducers 2014, 162, 227. [Google Scholar]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification, 2nd ed.; NIR Publiscations: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Aslam, M.W.; Wajid, M.; Waheed, A.; Ahmad, S.; Jafar, K.; Akmal, H.; Khan, T.; Maqsud, M.S.; Khan, M.S. Revision of some mensural measurements, food preference, and haematological parameters in breeding pairs of blue rock pigeon, Columba livia sampled from punjab Pakistan. Braz. J. Biol. 2023, 83, e252059. [Google Scholar] [CrossRef]
- Maurel, V.B.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.; McBratney, A. Prediction of soil attributes by NIR spectroscopy. A critical review of chemometric indicators commonly used for assessing the quality of the prediction. Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Zhang, W.; Zhu, J.; Sun, J. Prediction of Soil-Available Potassium Content with Visible Near-Infrared Ray Spectroscopy of Different Pretreatment Transformations by the Boosting Algorithms. Appl. Sci. 2020, 10, 1520. [Google Scholar] [CrossRef]
- Terra, F.S.; Demattê, J.A.M.; Viscarra Rossel, R.A. Spectral libraries for quantitative analyses of tropical Brazilian soils: Comparing vis–NIR and mid-IR reflectance data. Geoderma 2015, 255–256, 81–93. [Google Scholar] [CrossRef]
- Singha, C.; Swain, K.C.; Sahoo, S.; Govind, A. Prediction of soil nutrients through PLSR and SVMR models by VIs-NIR reflectance spectroscopy. Egypt. J. Remote Sens. Space Sci. 2023, 26, 901–918. [Google Scholar] [CrossRef]
- Lark, R.M. A comparison of some robust estimators of the variogram for use in soil survey. Eur. J. Soil Sci. 2000, 51, 137–157. [Google Scholar] [CrossRef]
- Goenster-Jordan, S.; Jannoura, R.; Jordan, G.; Buerkert, A.; Joergensen, R.G. Spatial variability of soil properties in the floodplain of a river oasis in the Mongolian Altay Mountains. Geoderma 2018, 330, 99–106. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Jahin, H.S.; Shokr, M.S.; El Baroudy, A.A.; Mohamed, E.S.; Rebouh, N.Y.; Bassouny, M.A. A Novel Regional-Scale Assessment of Soil Metal Pollution in Arid Agroecosystems. Agronomy 2023, 13, 161. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-Scale Variability of Soil Properties in Central Iowa Soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Dad, J.M.; Shafiq, M.U. Spatial variability and delineation of management zones based on soil micronutrient status in apple orchard soils of Kashmir valley, India. Environ. Monit. Assess. 2021, 193, 797. [Google Scholar] [CrossRef]
- Isaaks, E.H.; Srivastava, R.M. Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Cafarelli, B.; Castrignanò, A.; De Benedetto, D.; Palumbo, A.D.; Buttafuoco, G. A linear mixed effect (LME) model for soil water content estimation based on geophysical sensing: A comparison of an LME model and kriging with external drift. Environ. Earth Sci. 2014, 73, 1951–1960. [Google Scholar] [CrossRef]
- Sebei, A.; Chaabani, A.; Abdelmalek-Babbou, C.; Helali, M.A.; Dhahri, F.; Chaabani, F. Evaluation of pollution by heavy metals of an abandoned Pb-Zn mine in northern Tunisia using sequential fractionation and geostatistical mapping. Environ. Sci. Pollut. Res. 2020, 27, 43942–43957. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.; Mishra, U.; Paul, N. Groundwater suitability analysis for drinking using GIS based fuzzy logic. Ecol. Indic. 2020, 121, 107179. [Google Scholar] [CrossRef]
- Baruah, T.; Borthakur, H.P. Soil Chemistry. In A Textbook of Soil Analysis; Vikas Publishing House Pvt. Ltd.: New Delhi, India, 1997; pp. 118–132. [Google Scholar]
- Dewangan, S.; Kumari, L.; Minj, P.; Kumari, J.; Sahu, R. The effects of soil ph on soil health and environmental sustainability: A review. JETIR 2023, 10, 611–616. [Google Scholar]
- Curtin, D.; Campbell, C.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. A Provisional Methodology for Soil Degradation Assessment; FAO: Rome, Italy, 1980. [Google Scholar]
- Abdel-Fattah, M.K.; Mohamed, E.S.; Wagdi, E.M.; Shahin, S.A.; Aldosari, A.A.; Lasaponara, R.; Alnaimy, M.A. Quantitative Evaluation of Soil Quality Using Principal Component Analysis: The Case Study of El-Fayoum Depression Egypt. Sustainability 2021, 13, 1824. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; da Silva Terra, F. Spectral pedology: A new perspective on evaluation of soils along pedogenetic alterations. Geoderma 2014, 217–218, 190–200. [Google Scholar] [CrossRef]
- Poppiel, R.R.; Lacerda, M.P.C.; Oliveira, M.P.D.; Demattê, J.A.M.; Romero, D.J.; Sato, M.V.; Almeida, L.R.D.; Cassol, L.F.M. Surface Spectroscopy of Oxisols, Entisols and Inceptisol and Relationships with Selected Soil Properties. Rev. Bras. Cienc. Solo 2018, 42, e0160519. [Google Scholar] [CrossRef]
- Rizzo, R.; Demattê, J.A.M.; Terra, F.d.S. Using numerical classification of profiles based on Vis-NIR spectra to distinguish soils from the Piracicaba Region, Brazil. Rev. Bras. Cienc. Solo 2014, 38, 372–385. [Google Scholar] [CrossRef]
- Adeline, K.; Gomez, C.; Gorretta, N.; Roger, J.-M. Predictive ability of soil properties to spectral degradation from laboratory Vis-NIR spectroscopy data. Geoderma 2016, 288, 143–153. [Google Scholar] [CrossRef]
- Shao, Y.; He, Y. Nitrogen, phosphorus, and potassium prediction in soils, using infrared spectroscopy. Soil Res. 2011, 49, 166–172. [Google Scholar] [CrossRef]
- Wang, W.-J.; Li, Z.; Wang, C.; Zheng, D.; Du, H. Prediction of Available Potassium Content in Cinnamon Soil Using Hyperspectral Imaging Technology. Spectrosc. Spectr. Anal. 2019, 39, 1579–1585. [Google Scholar]
- Vasques, G.; Grunwald, S.; Sickman, J. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B. On-line visible and near infrared spectroscopy for in-field phosphorous management. Soil Tillage Res. 2016, 155, 471–477. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Baroudy, A.A.E.; El-Beshbeshy, T.; Emam, M.; Belal, A.A.; Elfadaly, A.; Aldosari, A.A.; Ali, A.M.; Lasaponara, R. Vis-NIR Spectroscopy and Satellite Landsat-8 OLI Data to Map Soil Nutrients in Arid Conditions: A Case Study of the Northwest Coast of Egypt. Remote Sens. 2020, 12, 3716. [Google Scholar] [CrossRef]
- Aldabaa, A.; Yousif, I.A.H. Geostatistical approach for land suitability assessment of some desert soils. Egypt. J. Soil Sci. 2020, 60, 195–209. [Google Scholar] [CrossRef]
- Calvete, F.J.S.; Ramírez, J.C. Geoestadística: Aplicaciones a la Hidrogeología Subterránea; Centro Internacional de Métodos Numéricos en Ingeniería: Barcelona, Spain, 1996. [Google Scholar]




| RPD | Interpretation |
|---|---|
| <1.0 | Very poor |
| 1.0–1.4 | Poor |
| 1.4–1.8 | Fair |
| 1.8–2.0 | Good |
| 2.0–2.5 | Very good |
| >2.5 | Excellent |
| RPIQ | Interpretation |
|---|---|
| 2.02–2.7 | Poor |
| 2.7–3.37 | Fair |
| 3.27–4.05 | Good |
| >4.05 | Excellent |
| Properties | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Sand (%) | 40.27 | 25.05 | 74.34 | 9.14 |
| Silt (%) | 33.80 | 22.11 | 60.91 | 6.21 |
| Clay (%) | 25.93 | 13.56 | 49.05 | 8.07 |
| CEC (cmolc kg−1) | 22.72 | 17.91 | 30.35 | 2.66 |
| EC (dSm−1) | 4.01 | 0.80 | 11.00 | 2.75 |
| pH | 7.31 | 7.73 | 8.48 | 0.45 |
| No. | Spectral Transformation | Calibrated (N = 73) | Validated (N = 31) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | RPD | RPIQ | R2 | RMSE | RPD | RPIQ | ||
| 1 | 1/Log R | 0.98 | 0.40 | 6.99 | 9.22 | 0.89 | 0.85 | 3.28 | 4.31 |
| 2 | R | 0.95 | 0.60 | 4.69 | 6.53 | 0.90 | 0.83 | 3.40 | 4.94 |
| 3 | 1/√R | 0.95 | 0.62 | 4.55 | 6.29 | 0.93 | 0.68 | 4.02 | 6.50 |
| 4 | (1/Log R)’ | 0.94 | 0.65 | 4.23 | 6.07 | 0.88 | 0.89 | 2.90 | 4.88 |
| 5 | Log R | 0.94 | 0.69 | 4.09 | 5.83 | 0.93 | 0.69 | 4.04 | 6.50 |
| 6 | √R | 0.93 | 0.76 | 3.79 | 5.39 | 0.89 | 0.86 | 3.34 | 5.52 |
| 7 | R’ | 0.92 | 0.77 | 3.57 | 4.81 | 0.86 | 0.96 | 2.66 | 4.52 |
| 8 | 1/R | 0.90 | 0.85 | 3.47 | 4.61 | 0.90 | 0.83 | 3.30 | 5.31 |
| 9 | (√R)’ | 0.91 | 0.82 | 3.36 | 4.50 | 0.83 | 1.06 | 2.43 | 4.89 |
| 10 | (Log R)’ | 0.89 | 0.90 | 3.09 | 4.08 | 0.77 | 1.23 | 2.01 | 2.99 |
| 11 | (1/√R)’ | 0.82 | 1.16 | 2.40 | 3.19 | 0.51 | 1.21 | 0.99 | 1.17 |
| 12 | (1/R)’ | 0.75 | 1.37 | 2.06 | 2.71 | 0.50 | 1.33 | 1.13 | 1.33 |
| 13 | (1/Log R)” | 0.75 | 1.37 | 2.05 | 2.68 | 0.42 | 1.35 | 1.09 | 1.28 |
| 14 | R” | 0.58 | 1.78 | 1.66 | 2.06 | 0.35 | 1.81 | 0.88 | 1.04 |
| 15 | (√R)” | 0.53 | 1.88 | 1.60 | 1.96 | 0.37 | 2.10 | 1.00 | 1.18 |
| 16 | (Log R)” | 0.52 | 1.90 | 1.62 | 1.95 | 0.41 | 1.88 | 1.28 | 1.50 |
| 17 | (1/√R)” | 0.28 | 2.27 | 1.48 | 1.68 | 0.21 | 2.31 | 1.21 | 1.43 |
| 18 | (1/R)” | 0.42 | 5.25 | 0.74 | 0.73 | 0.33 | 4.86 | 0.99 | 1.16 |
| Spatial Distribution Model | R2 | RMSE | MSE | RMSSE | Range (m) | Nugget | Partial Sill | Sill | Nugget/Sill Ratios | Spatial Dependence |
|---|---|---|---|---|---|---|---|---|---|---|
| Spherical | 0.96 | 2.55 | −0.003 | 1.018 | 14,846.4 | 4.78 | 2.35 | 7.13 | 0.67 | Moderate |
| Exponential | 0.95 | 2.56 | −0.005 | 1.025 | 13,878.2 | 3.68 | 3.53 | 7.21 | 0.51 | Moderate |
| Gaussian | 0.88 | 2.55 | −0.004 | 1.018 | 12,886.0 | 2.21 | 1.93 | 4.14 | 0.53 | Moderate |
| Circular | 0.94 | 2.55 | −0.004 | 1.017 | 13,785.0 | 4.91 | 2.24 | 7.14 | 0.68 | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, A.-r.A.; Abdelsamie, E.A.; Mohamed, E.S.; Rebouh, N.Y.; Shokr, M.S. Modeling of Soil Cation Exchange Capacity Based on Chemometrics, Various Spectral Transformations, and Multivariate Approaches in Some Soils of Arid Zones. Sustainability 2024, 16, 7002. https://doi.org/10.3390/su16167002
Mustafa A-rA, Abdelsamie EA, Mohamed ES, Rebouh NY, Shokr MS. Modeling of Soil Cation Exchange Capacity Based on Chemometrics, Various Spectral Transformations, and Multivariate Approaches in Some Soils of Arid Zones. Sustainability. 2024; 16(16):7002. https://doi.org/10.3390/su16167002
Chicago/Turabian StyleMustafa, Abdel-rahman A., Elsayed A. Abdelsamie, Elsayed Said Mohamed, Nazih Y. Rebouh, and Mohamed S. Shokr. 2024. "Modeling of Soil Cation Exchange Capacity Based on Chemometrics, Various Spectral Transformations, and Multivariate Approaches in Some Soils of Arid Zones" Sustainability 16, no. 16: 7002. https://doi.org/10.3390/su16167002
APA StyleMustafa, A.-r. A., Abdelsamie, E. A., Mohamed, E. S., Rebouh, N. Y., & Shokr, M. S. (2024). Modeling of Soil Cation Exchange Capacity Based on Chemometrics, Various Spectral Transformations, and Multivariate Approaches in Some Soils of Arid Zones. Sustainability, 16(16), 7002. https://doi.org/10.3390/su16167002












