Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Analysis
2.2.1. Sample Collection
2.2.2. Sample Analysis
pH (Active Acidity)
Hydrolytic Acidity
Exchangeable Acidity, Exchangeable H+, and Exchangeable Al3+
Cation Exchange Capacity (CEC)
Total Exchangeable Base (TEB) and Base Saturation (BS)
Soil Organic Matter (SOM)
Active Heavy Metals
2.2.3. Data Processing
3. Results
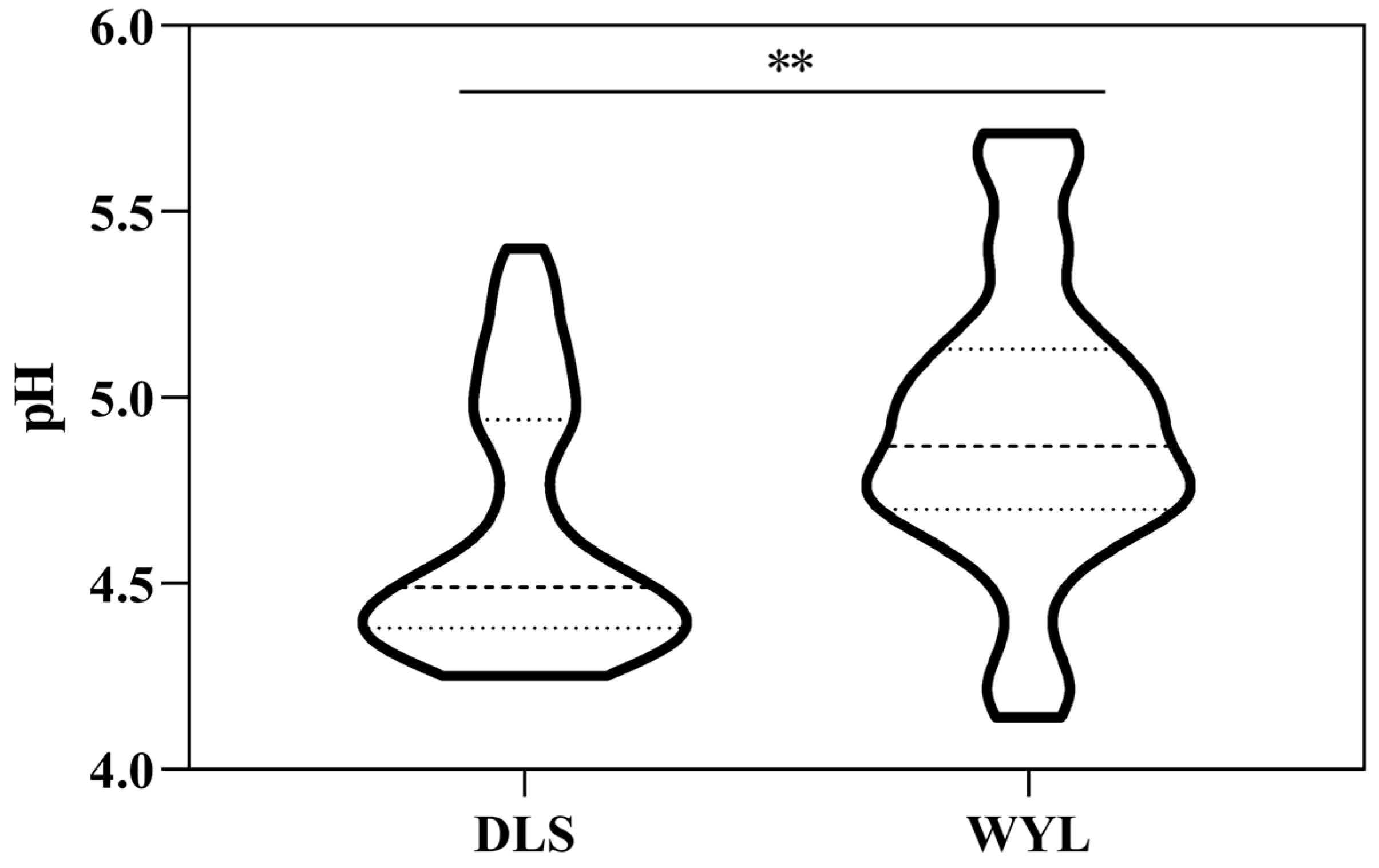
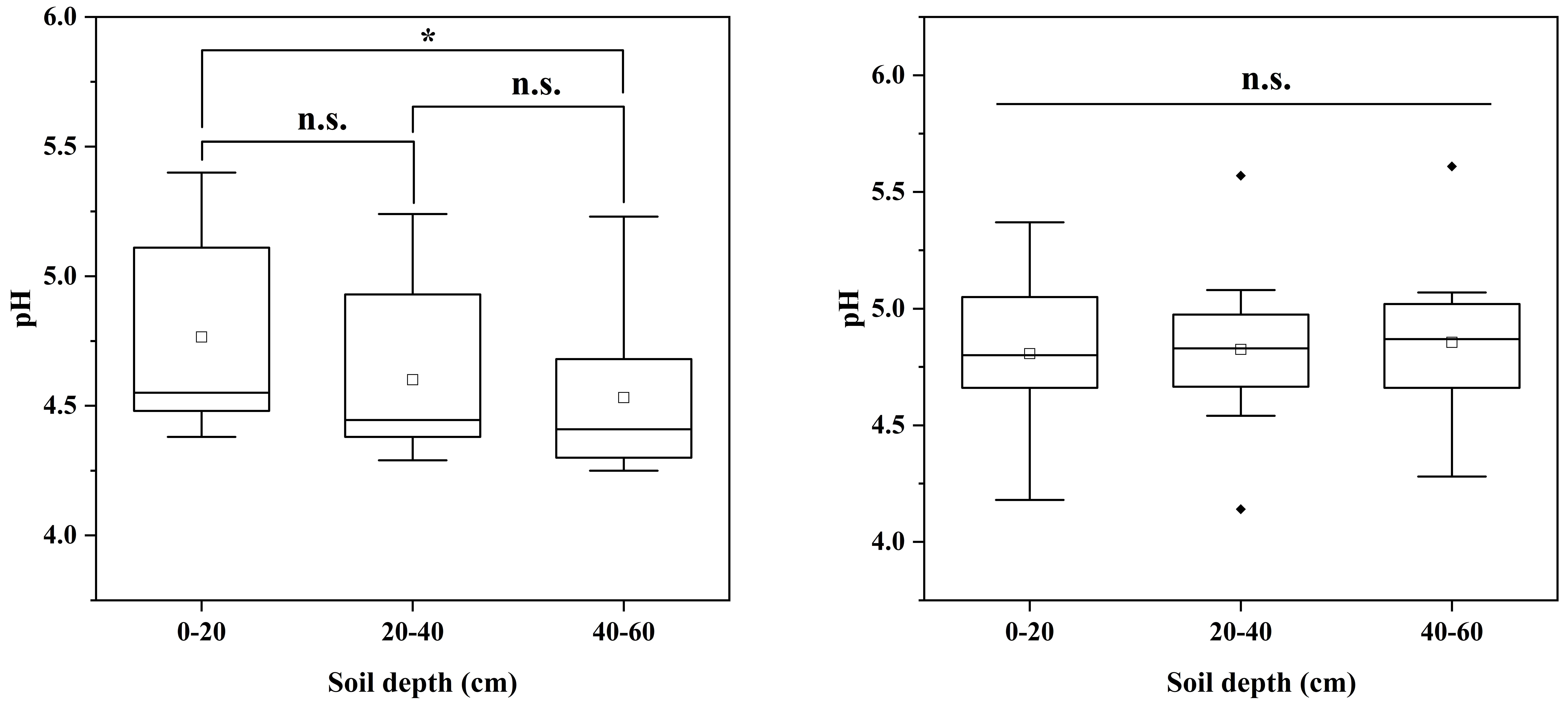
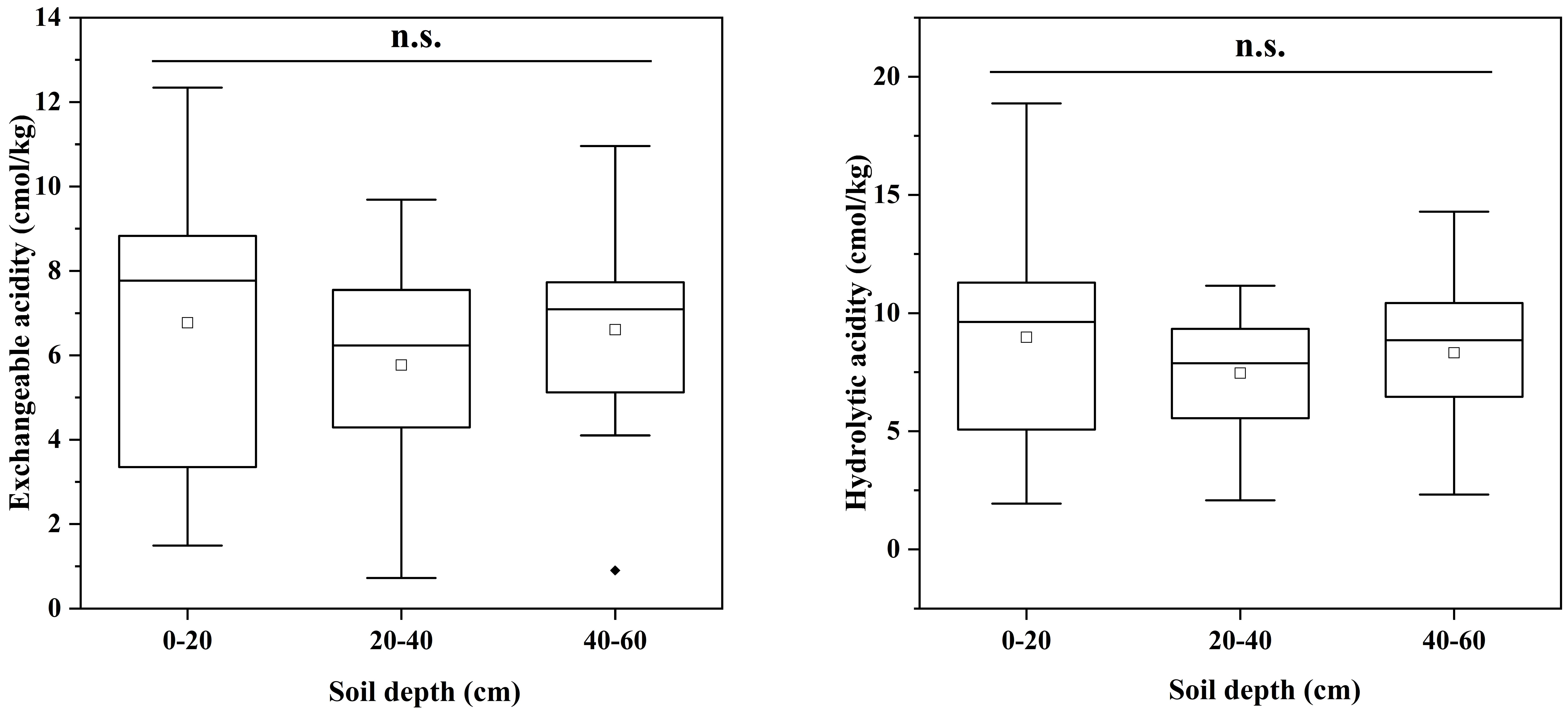
3.1. Acidification of Forest Soils in Different Layers of the Study Area
3.2. Forest Soil Acidification at Different Elevations in the Study Area
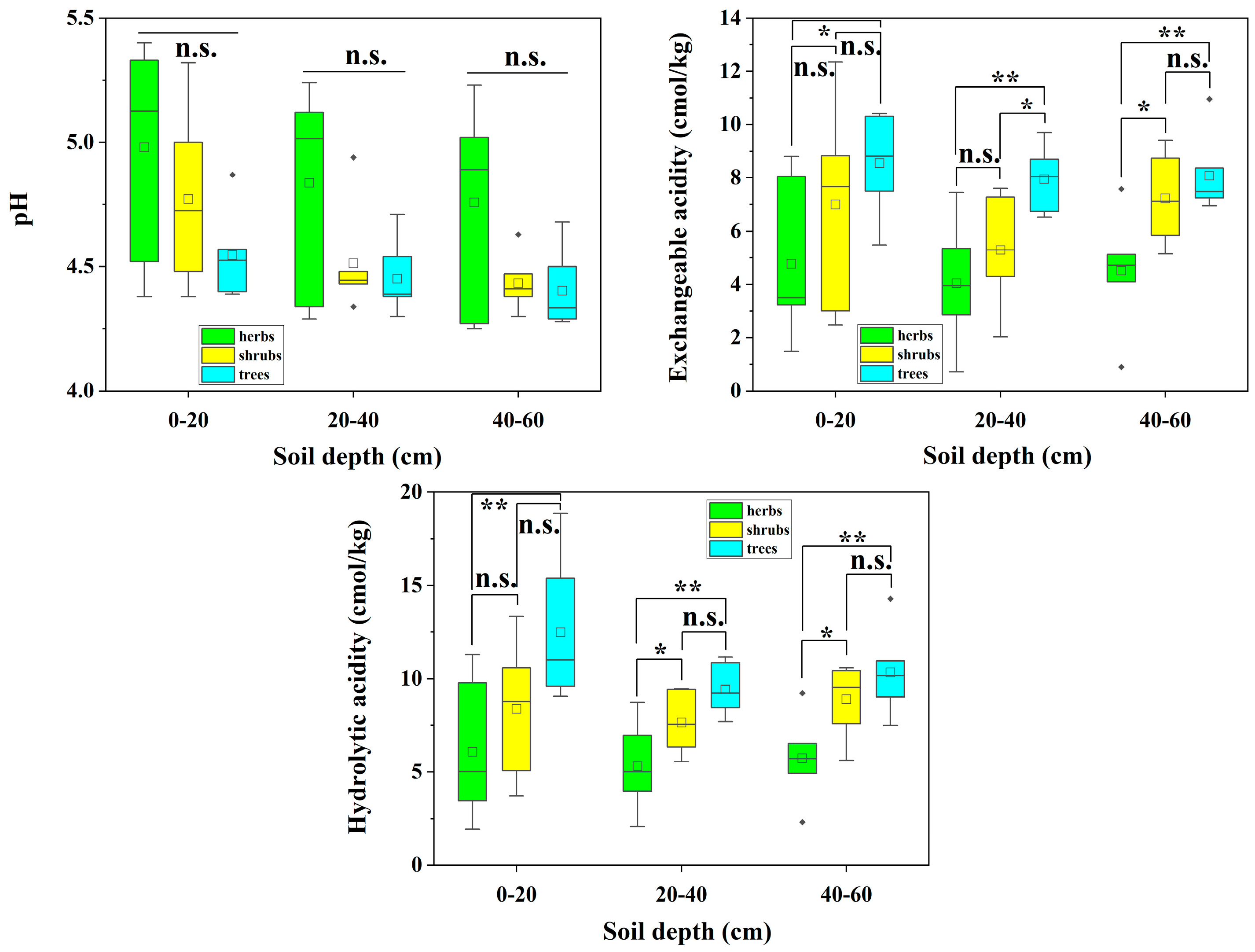
3.3. Soil Acidification in Forest Soils of Different Plant Types in the Study Area
3.4. Soil Acidification in Forest Soils from Different Slope Orientations in the Study Area
3.5. Relationship between Forest Soil Acidity Indexes and Soil Physicochemical Properties
4. Discussion
4.1. Differential Analysis of Soil Acidification in Different Layers of Forest Soil in Two Forest Sites
4.2. Differential Analysis of Forest Soils at Different Elevations and with Different Plant Types and Slope Orientations in Two Forest Sites
4.3. Analysis of the Relationship between Forest Soil pH and Soil Physicochemical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Mo, J.; Zhang, W.; Lu, X. Research on acidification in forest soil driven by atmospheric nitrogen deposition. Acta Ecol. Sin. 2014, 34, 302–310. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; De Vries, W.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Fujii, K.; Kanetani, S.; Tetsuka, K. Effects of volcanic parent materials on the acid buffering capacity of forest soils on Yakushima Island, Japan. Soil Sci. Plant Nutr. 2020, 66, 680–692. [Google Scholar] [CrossRef]
- Cecchini, G.; Andreetta, A.; Marchetto, A.; Carnicelli, S. Soil solution fluxes and composition trends reveal risks of nitrate leaching from forest soils of Italy. CATENA 2021, 200, 105175. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, J.; Wang, Q.; Xu, L.; Li, M.; Dai, G.; Mulder, J.; Xi, Y.; He, N.; Dai, H. Soil acidification in China’s forests due to atmospheric acid deposition from 1980 to 2050. Sci. Bull. 2022, 67, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Zarfos, M.R.; Dovciak, M.; Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J. Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States. Plant Soil 2019, 438, 461–477. [Google Scholar] [CrossRef]
- De Schrijver, A.; Mertens, J.; Geudens, G.; Staelens, J.; Campforts, E.; Luyssaert, S.; De Temmerman, L.; De Keersmaeker, L.; De Neve, S.; Verheyen, K. Acidification of forested podzols in North Belgium during the period 1950–2000. Sci. Total Environ. 2006, 361, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.E.; Lee, W.Y.; Ok, Y.S.; Skousen, J. Soil nutrient bioavailability and nutrient content of pine trees (Pinus thunbergii) in areas impacted by acid deposition in Korea. Environ. Monit. Assess. 2009, 157, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hopf, S.-E.; Tresch, S.; Belyazid, S.; Sverdrup, H.; Augustin, S.; Kurz, D.; Rihm, B.; Braun, S. Dendrochemical indicators of tree rings reveal historical soil acidification in Swiss forest stands. Dendrochronologia 2023, 81, 126099. [Google Scholar] [CrossRef]
- Fujii, K.; Funakawa, S.; Kosaki, T. Effects of forest management on soil acidification in cedar plantation. Geoderma 2022, 424, 115967. [Google Scholar] [CrossRef]
- Jandl, R.; Leitgeb, E.; Englisch, M. Decadal changes of organic carbon, nitrogen, and acidity of Austrian forest soils. Soil Syst. 2022, 6, 28. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Wu, J.; Liang, G.; Hui, D.; Deng, Q.; Xiong, X.; Qiu, Q.; Liu, J.; Chu, G.; Zhou, G.; Zhang, D. Prolonged acid rain facilitates soil organic carbon accumulation in a mature forest in Southern China. Sci. Total Environ. 2016, 544, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, P.; Wang, Y.; Li, T.; Webb, A.A.; Wang, Y.; Li, Z.; Kou, T.; Shi, G.; Zhang, B. Effects of liming on health and growth of young Schima superba trees under canopy of a Pinus massoniana stand damaged by soil acidification in Chongqing, China. New For. 2016, 47, 801–813. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, T.; Ma, Y.; Zhang, K.; Soltis, P.S.; Soltis, D.E.; Gilbert, J.A.; Zhao, Y.; Fu, C.; Chu, H. Soil pH determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Glob. Ecol. Biogeogr. 2021, 30, 2164–2177. [Google Scholar] [CrossRef]
- Liao, B.; Guo, Z.; Probst, A.; Probst, J.-L. Soil heavy metal contamination and acid deposition: Experimental approach on two forest soils in Hunan, Southern China. Geoderma 2005, 127, 91–103. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Chen, R.; Ni, X.; Cao, J. Effects of forest type on nutrient fluxes in throughfall, stemflow, and litter leachate within acid-polluted locations in Southwest China. Int. J. Environ. Res. Public Health 2022, 19, 2810. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Q.; Peng, H.; Zhu, T. Soil acidification in moso bamboo (Phyllostachys edulis) forests and changes of soil metal ions (Cu, Pb) concentration. Arch. Agron. Soil Sci. 2021, 67, 1799–1808. [Google Scholar] [CrossRef]
- Renyi, G.U.I.; Yuyan, H.U.; Qiang, L.I.; Zhuang, S. Effect of cultivation time on soil heavy metal accumulation and bioavailability in Phyllostachys praecox stands. Pedosphere 2020, 30, 810–816. [Google Scholar]
- Lu, R. Methods of Agricultural Chemical Analysis of Soils; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Forestry Research Institute of China Academy of Forestry Sciences. Determination of Hydrolyzable Total Acidity of Forest Soil: LY/T1241-1999; China Standard Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Renault, P.; Cazevieille, P.; Verdier, J.; Lahlah, J.; Clara, C.; Favre, F. Variations in the cation exchange capacity of a ferralsol supplied with vinasse, under changing aeration conditions: Comparison between CEC measuring methods. Geoderma 2009, 154, 101–110. [Google Scholar] [CrossRef]
- Verstraeten, G.; Baeten, L.; De Frenne, P.; Vanhellemont, M.; Thomaes, A.; Boonen, W.; Muys, B.; Verheyen, K. Understorey vegetation shifts following the conversion of temperate deciduous forest to spruce plantation. For. Ecol. Manag. 2013, 289, 363–370. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef]
- Clesse, M.; Legout, A.; Ranger, J.; Zeller, B.; van der Heijden, G. Soil chemical fertility change over four decades in the Morvan Mountains and influence of tree species (Burgundy, France). For. Ecosyst. 2022, 9, 100043. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Peng, S.; Kong, G. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For. Ecol. Manag. 2003, 175, 573–583. [Google Scholar] [CrossRef]
- Fang, K.; Kou, D.; Wang, G.; Chen, L.; Ding, J.; Li, F.; Yang, G.; Qin, S.; Liu, L.; Zhang, Q.; et al. Decreased soil cation exchange capacity across northern China’s grasslands over the last three decades. J. Geophys. Res. Biogeosci. 2017, 122, 3088–3097. [Google Scholar] [CrossRef]
- Křeček, J.; Palán, L.; Stuchlík, E. Impacts of land use policy on the recovery of mountain catchments from acidification. Land Use Policy 2019, 80, 439–448. [Google Scholar] [CrossRef]
- Fujii, K.; Hayakawa, C.; Panitkasate, T.; Maskhao, I.; Funakawa, S.; Kosaki, T.; Nawata, E. Acidification and buffering mechanisms of tropical sandy soil in northeast Thailand. Soil Tillage Res. 2017, 165, 80–87. [Google Scholar] [CrossRef]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Yamada, T.; Takenaka, C.; Yoshinaga, S.; Hirai, K. Long-term changes in the chemical properties of Japanese cedar (Cryptomeria japonica) forest soils under high precipitation in southwest Japan. J. For. Res. 2013, 18, 466–474. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, Z.; Shao, S.; Li, F.; Yang, S.; Song, W.; Li, W.; Li, S. Effects of aluminum toxicity induced by acid deposition on pine forest ecosystem in Longli of Guizhou Province, Southwestern China. Chin. Geogr. Sci. 2016, 26, 495–507. [Google Scholar] [CrossRef]



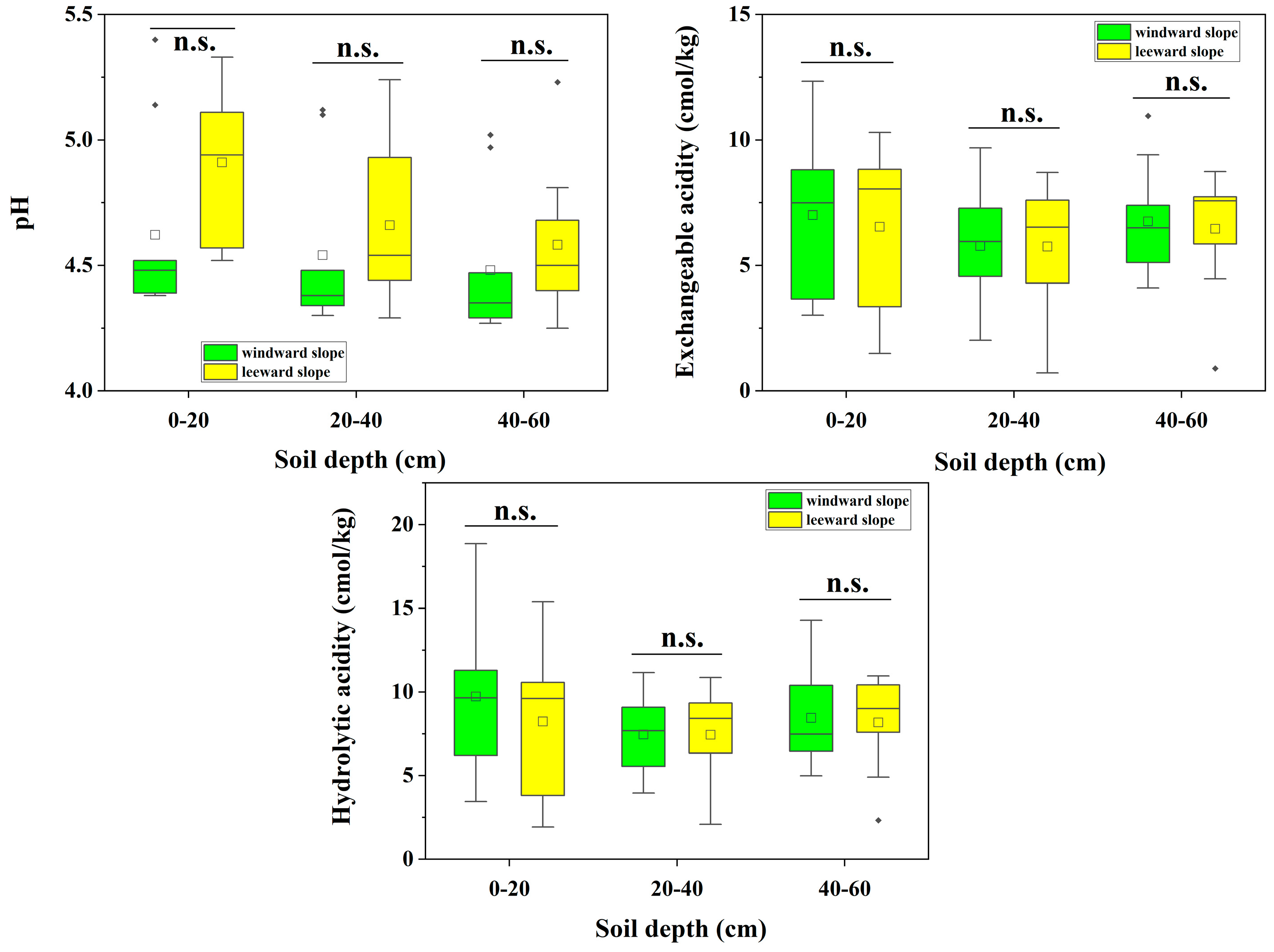


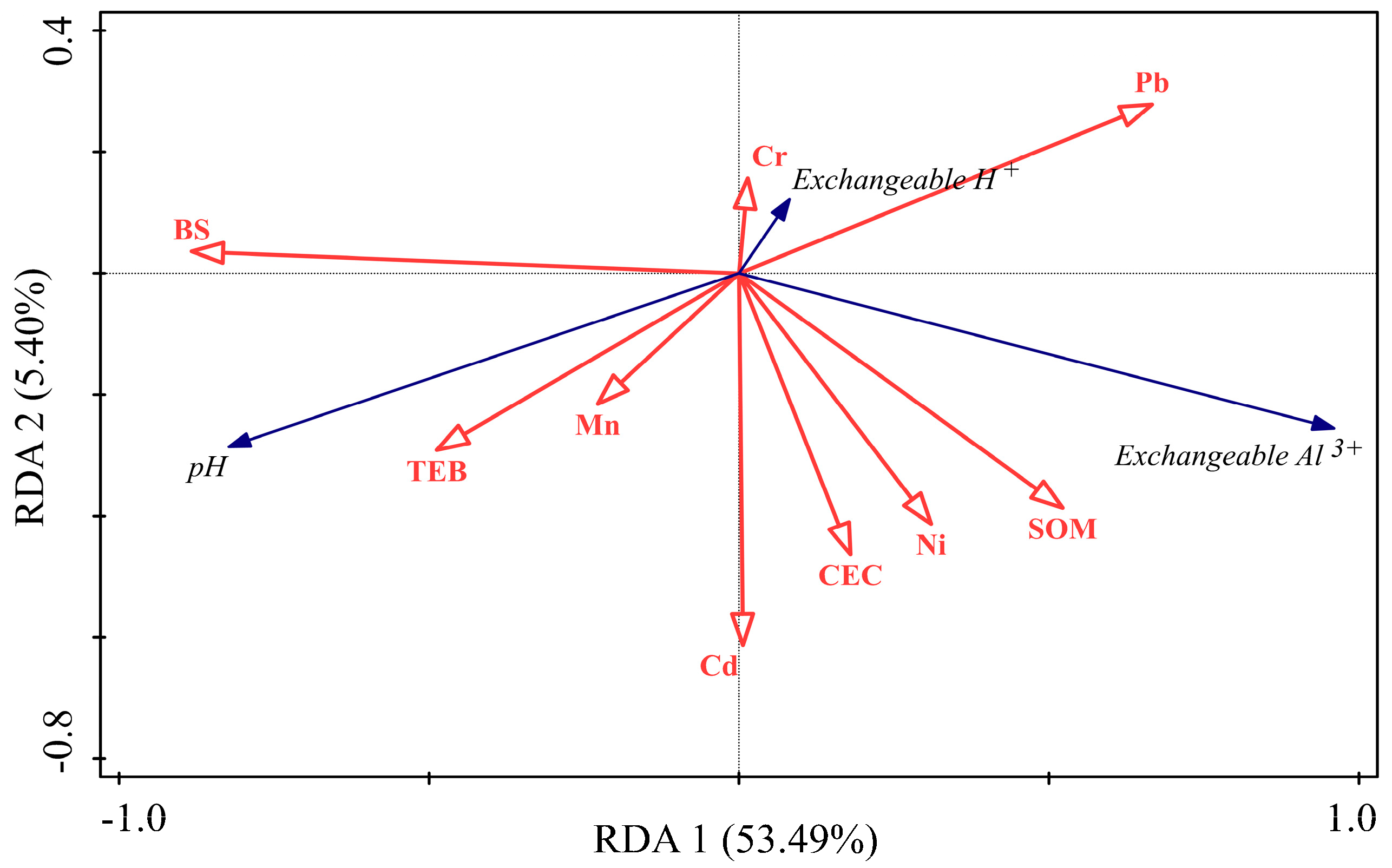
| pH | Grade | Number of Soil pH Distributions in DLS (Individual) | Number of Soil pH Distributions in WYL (Individual) |
|---|---|---|---|
| <4.5 | Very acidic soil | 28 | 5 |
| 4.5~5.5 | Strongly acidic soil | 26 | 39 |
| 5.5~6.5 | Acid soil | 0 | 5 |
| 6.5~7.5 | Neutral soil | 0 | 0 |
| Soil Depth (cm) | 0–20 | 20–40 | 40–60 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant type | Herbs | Shrubs | Trees | Herbs | Shrubs | Trees | Herbs | Shrubs | Trees |
| pH | 4.99 a | 4.90 a | 4.38 a | 5.17 a | 4.91 ab | 4.45 b | 5.09 a | 4.92 a | 4.48 a |
| Exchangeable acidity (cmol/kg) | 3.85 b | 3.44 b | 9.11 c | 4.13 b | 2.37 b | 7.14 c | 3.75 b | 2.35 b | 5.62 c |
| Hydrolytic acidity (cmol/kg) | 9.36 c | 8.06 c | 17.40 c | 8.42 bc | 5.55 b | 13.74 c | 9.25 b | 4.61 b | 10.35 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, J.; Ren, H.; Chen, H. Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve. Sustainability 2024, 16, 7051. https://doi.org/10.3390/su16167051
Zhang Y, Zhou J, Ren H, Chen H. Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve. Sustainability. 2024; 16(16):7051. https://doi.org/10.3390/su16167051
Chicago/Turabian StyleZhang, Yujie, Jiangmin Zhou, Han Ren, and Hualin Chen. 2024. "Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve" Sustainability 16, no. 16: 7051. https://doi.org/10.3390/su16167051
APA StyleZhang, Y., Zhou, J., Ren, H., & Chen, H. (2024). Characterization of Forest Soil Acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve. Sustainability, 16(16), 7051. https://doi.org/10.3390/su16167051






