Improved Straw Decomposition Products Promote Peanut Growth by Changing Soil Chemical Properties and Microbial Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Straw Decomposition Products and Tested Soil
2.2. Pot Experiment
2.3. Soil and Plant Analysis
2.4. Soil DNA Extraction and High-Throughput Sequencing
2.5. Data Analysis
3. Results
3.1. Effect of Ca(OH)2-Treated Straw Decomposition Products on Soil Chemical Properties
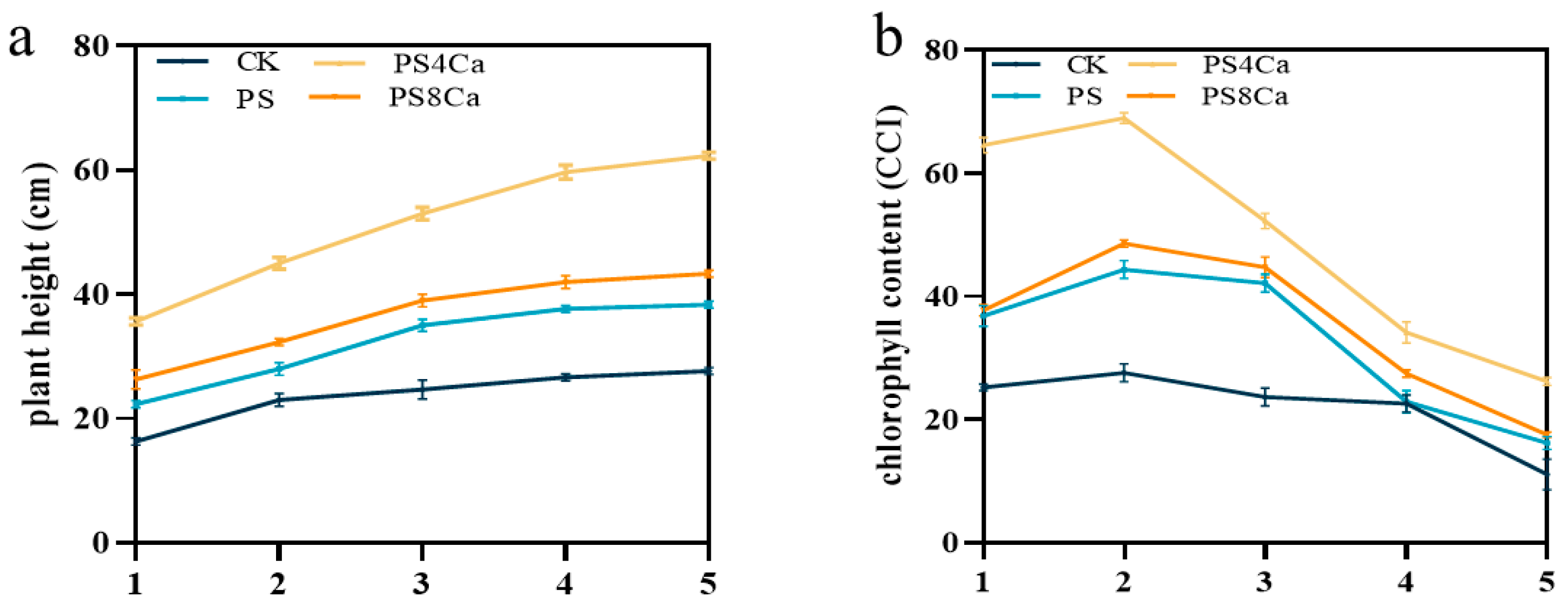
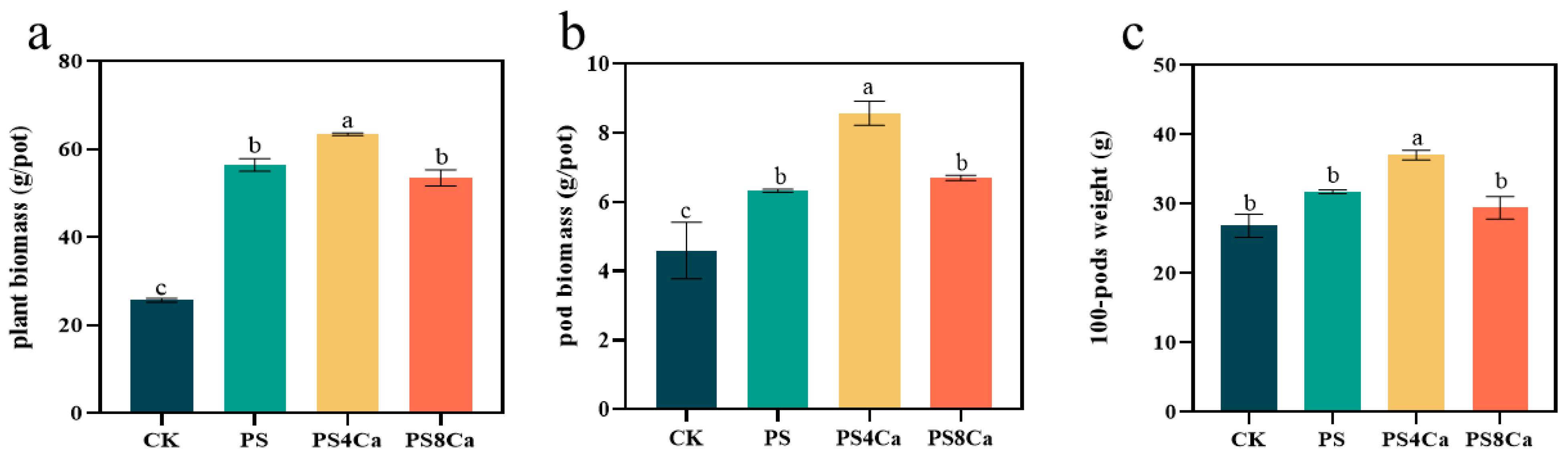
3.2. Effect of Ca(OH)2-Treated Straw Decomposition Products on Peanut Growth
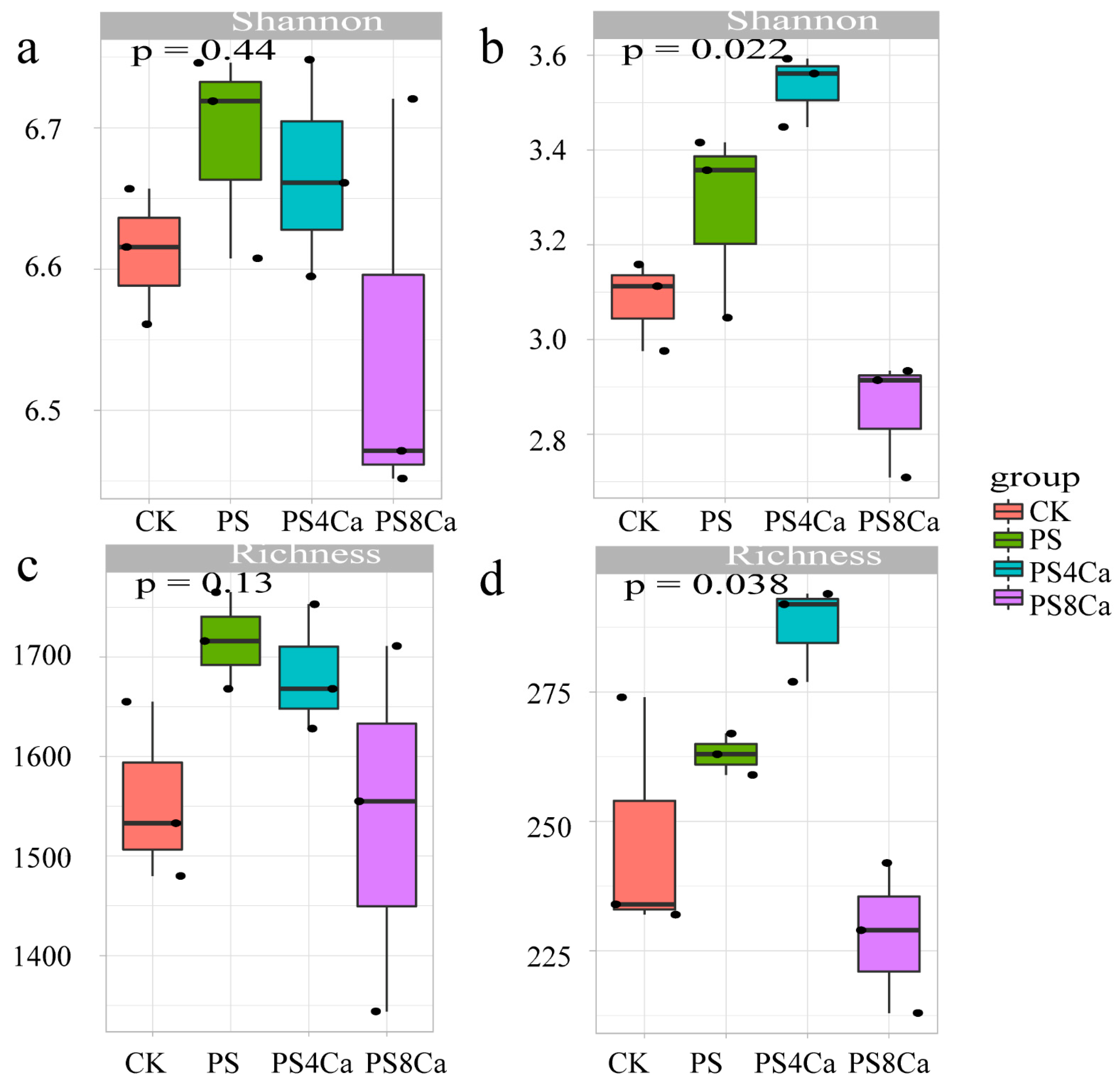
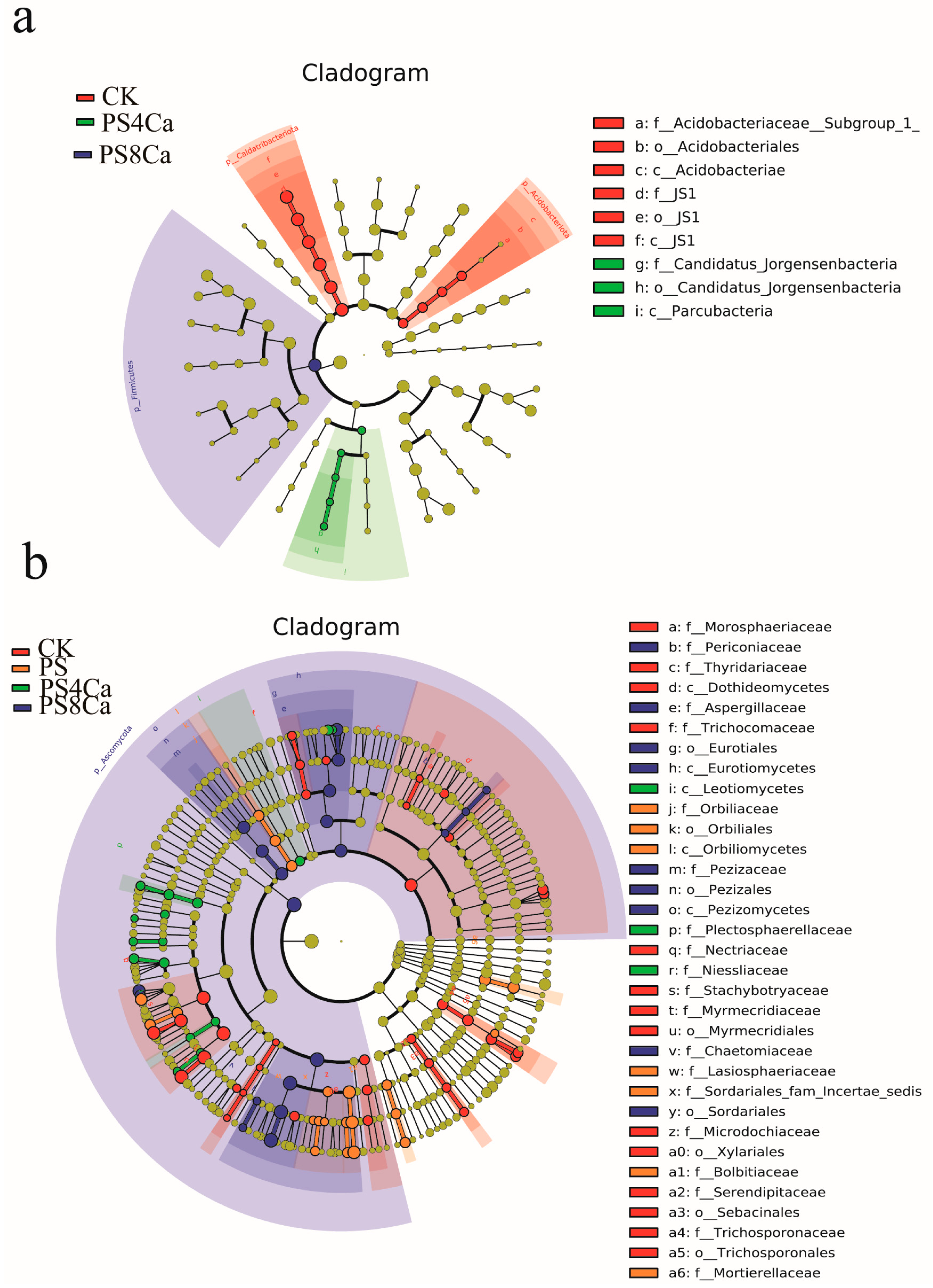
3.3. Effect of Ca(OH)2-Treated Straw Decomposition Products on Soil Microbial Diversity
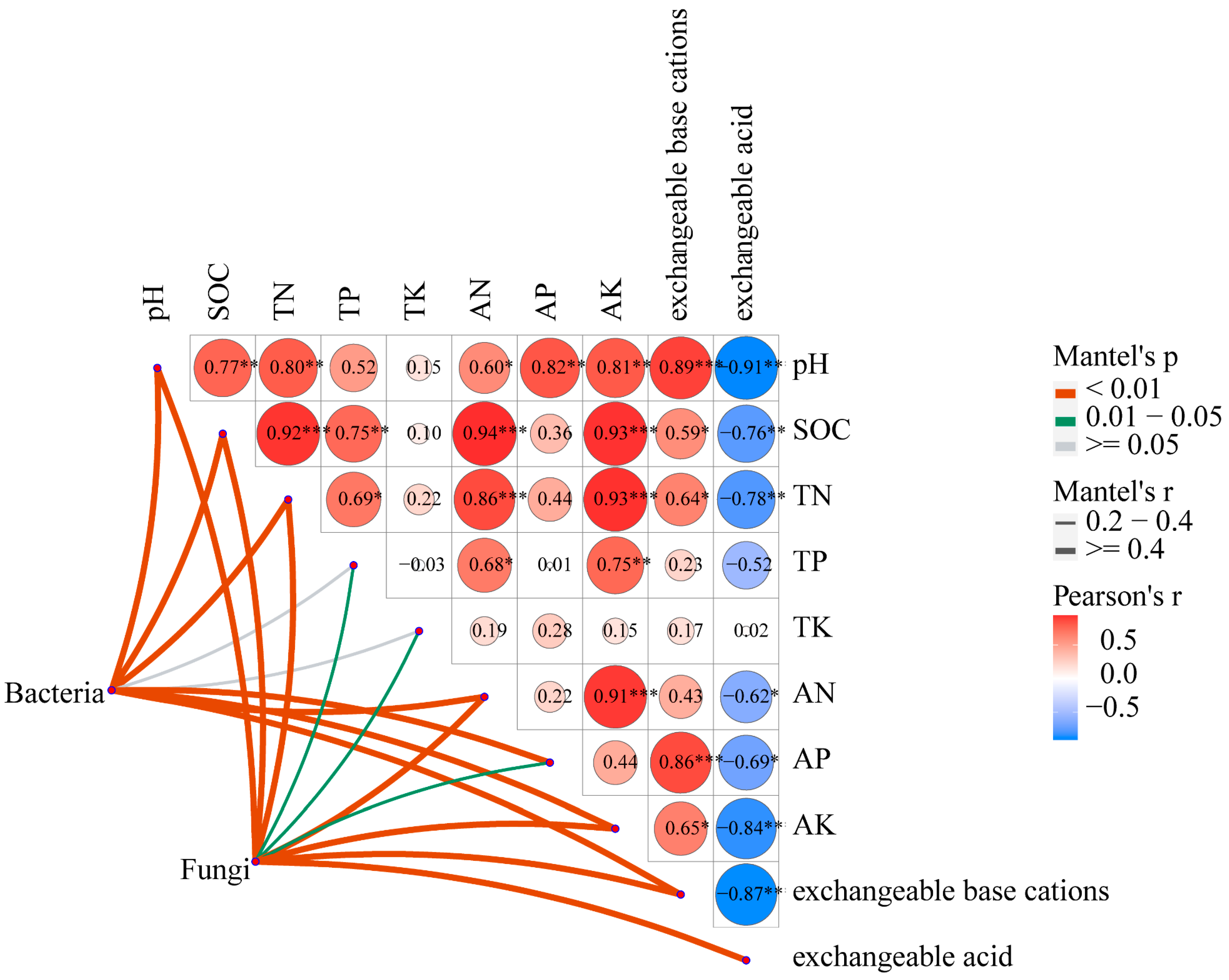
3.4. Relationships between Soil Chemical Properties, Microbial Diversity, and Peanut Growth
4. Discussion
4.1. Beneficial Effects of Ca(OH)2-Treated Straw Decomposition Products on Soil Acidification, Soil Fertility, and Peanut Growth
4.2. Impact of Ca(OH)2-Treated Straw Decomposition Products on Bacterial and Fungal Diversity
4.3. Potential Effects of Ca(OH)2-Treated Straw Decomposition Products on Peanut Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Wang, X.; Yang, Y.; Duan, G.; Zhang, H.; Cheng, W. The effect of straw-returning on antimony and arsenic volatilization from paddy soil and accumulation in rice grains. Environ. Pollut. 2020, 263, 114581. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wang, X.; Guan, Z.; Gu, Y.; Wu, C.; Hu, W. Effects of different patterns of maize-straw application on soil microorganisms, enzyme activities, and grain yield. Bioengineered 2021, 12, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wu, M.; Che, S.; Yuan, S.; Yang, X.; Li, S.; Tian, P.; Wu, L.; Wu, Z. Effects of continuous straw returning on soil functional microorganisms and microbial communities. J. Microbiol. 2023, 61, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Z.; Zhai, X. Peanut straw decomposition products promoted by chemical additives and their effect on enzymatic activity and microbial functional diversity in red soil. Compost Sci. Util. 2013, 21, 76–86. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.Y.; Shi, R.Y.; Xu, R.K.; Shen, R.F. Contribution of soil diazotrophs to crop nitrogen utilization in an acidic soil as affected by organic and inorganic amendments. Plant Soil 2024, 1–15. [Google Scholar] [CrossRef]
- Montecchia, M.S.; Correa, O.S.; Soria, M.A.; Frey, S.D.; García, A.F.; Garland, J.L. Multivariate approach to characterizing soil microbial communities in pristine and agricultural sites in Northwest Argentina. Appl. Soil Ecol. 2011, 47, 176–183. [Google Scholar] [CrossRef]
- Ma, X.W.; Ren, B.H.; Yu, J.X.; Wang, J.Y.; Bai, L.; Li, J.H.; Li, D.Y.; Meng, M. Changes in grassland soil types lead to different characteristics of bacterial and fungal communities in Northwest Liaoning, China. Front. Microbiol. 2023, 14, 1205574. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.Y.; Zhang, P.P.; He, L.L.; Gao, X.X.; Li, W.J.; Zhou, Y.Y.; Li, Z.X.; Li, H.; Yang, L. Effects of tillage managements and maize straw returning on soil microbiome using 16S rDNA sequencing. J. Integr. Plant Biol. 2019, 61, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.Q.; Chen, F.S.; Duncan, D.S.; Fang, X.M.; Wang, H. Reforestation and slope-position effects on nitrogen, phosphorus pools, and carbon stability of various soil aggregates in a red soil hilly land of subtropical China. Can. J. For. Res. 2015, 45, 26–35. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, F.B.; Liu, C.S.; Huang, W.; Liu, T.X.; Yu, W.M. Iron redox cycling coupled to transformation and immobilization of heavy metals: Implications for paddy rice safety in the red soil of south China. Adv. Agron. 2016, 137, 279–317. [Google Scholar]
- Wang, H.X.; Xu, J.L.; Liu, X.J.; Zhang, D.; Li, L.W.; Li, W.; Sheng, L.X. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Till. Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Li, P.; Dai, C.; Wang, X.; Zhang, T.; Chen, Y. Variation of soil enzyme activities and microbial community structure in peanut monocropping system in subtropical China. Afr. J. Agric. Res. 2012, 7, 1870–1879. [Google Scholar]
- Dell’Agnola, G.; Ferrari, G. Characteristics of laboratory-prepared humified organic matter as affected by the composition of starting materials. Soil Sci. 1979, 128, 105–109. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic And Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Biddle, J.F.; Fitz-Gibbon, S.; Schuster, S.C.; Brenchley, J.E.; House, C.H. Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. Proc. Natl. Acad. Sci. USA 2008, 105, 10583–10588. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Shi, R.Y.; Hong, Z.N.; Jiang, J.; He, X.; Xu, R.K.; Qian, W. Characteristics of crop straw decayed products and their ameliorating effects on an acidic Ultisol. Arch. Agron. Soil Sci. 2020, 67, 1708–1721. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, N.; Xu, R.K.; Tiwari, D. Potential of industrial byproducts in ameliorating acidity and aluminum toxicity of soils under tea plantation. Pedosphere 2010, 20, 645–654. [Google Scholar] [CrossRef]
- Pan, X.; Xu, R.; Nkoh, J.N.; Lu, H.; Hua, H.; Guan, P. Effects of straw decayed products of four crops on the amelioration of soil acidity and maize growth in two acidic Ultisols. Environ. Sci. Pollut. Res. 2021, 28, 5092–5100. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Soil pH buffering capacity and nitrogen availability following compost application in a tropical acid soil. Compost Sci. Util. 2017, 26, 1–15. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.T.; Wang, X.C.; Chen, C.; Hu, Y.; Cheng, L.J.; Gu, S.Y.; Xu, X.H. Temporal variations of soil microbial community under compost addition in black soil of northeast China. Appl. Soil Ecol. 2017, 121, 214–222. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Moreno, J.L.; Ros, M.; Garcia, C.; Hernandez, T. Application of fresh and composted organic wastes modifies structure: Size and activity of soil microbial community under semiarid climate. Appl. Soil Ecol. 2008, 40, 318–329. [Google Scholar] [CrossRef]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: A useful tool to improve soil quality under intensive farming systems. Appl. Soil Ecol. 2016, 107, 13–23. [Google Scholar] [CrossRef]
- Manirakiza, N.; Seker, C. Effects of compost and biochar amendments on soil fertility and crop growth in a calcareous soil. J. Plant Nutr. 2020, 20, 3002–3019. [Google Scholar] [CrossRef]
- Zhao, W.R.; Li, J.Y.; Jiang, J.; Lu, H.L.; Hong, Z.N.; Qian, W.; Xu, R.K.; Deng, K.Y.; Guan, P. The mechanisms underlying the reduction in aluminum toxicity and improvements in the yield of sweet potato (Ipomoea batatas L.) after organic and inorganic amendment of an acidic ultisol. Agric. Ecosyst. Environ. 2020, 288, 106716. [Google Scholar] [CrossRef]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive effects of crop diversity on productivity driven by changes in soil microbial composition. Front. Microbiol. 2021, 12, 660749. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, K.; Tang, L.; Jia, Z.; Li, Y. Change in deep soil microbial communities due to long-term fertilization. Soil Biol. Biochem. 2014, 75, 264–272. [Google Scholar] [CrossRef]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Zhao, S.C.; Qiu, S.J.; Xu, X.P.; Ciampitti, I.A.; Zhang, S.Q.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Song, K.; Sun, Y.F.; Qin, Q.; Sun, L.J.; Zheng, X.Q.; Terzaghi, W.; Lv, W.G.; Xue, Y. The effects of earthworms on fungal diversity and community structure in farmland soil with returned straw. Front. Microbiol. 2020, 11, 594265. [Google Scholar] [CrossRef]
- He, H.B.; Li, W.X.; Zhang, Y.W.; Cheng, J.K.; Jia, X.Y.; Li, S.; Yang, H.R.; Chen, B.M.; Xin, G.R. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass (Lolium multiflorum L.)-rice (Oryza sativa L.) rotation. Soil Till. Res. 2020, 196, 104487. [Google Scholar] [CrossRef]
- Zhang, C.W.; Fang, Y.X.; Yin, X.R.; Lai, H.F.; Kuang, Z.G.; Zhang, T.X.Y.; Xu, X.P.; Wegener, G.; Wang, J.H.; Dong, X.Y. The majority of microorganisms in gas hydrate-bearing subseafloor sediments ferment macromolecules. Microbiome 2023, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of continuous nitrogen fertilizer application on the diversity and composition of rhizosphere soil bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Weber, R.W.S. Introduction to Fungi, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Langarica-Fuentes, A.; Fox, G.; Robson, G.D. Metabarcoding analysis of home composts reveals distinctive fungal communities with a high number of unassigned sequences. Microbiology 2015, 161, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.F.; Ni, M.; Fu, B.; Liu, D.; He, X.; Zhang, Q.; Hu, W.L.; Zhu, H.Y.; Hao, D.C.; Yang, P.W. Microbial community diversity influenced by organic carbon source in rice-rape rotation farmland. Pak. J. Bot. 2022, 54, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, W.W.; Yao, Y.L.; Zhu, F.X.; Wang, W.P.; Chang, X.J. The influence of different concentrations of bio-organic fertilizer on cucumber Fusarium wilt and soil microflora alterations. PLoS ONE 2017, 12, e0171490. [Google Scholar] [CrossRef]
- Chen, L.; Redmile-Gordon, M.; Li, J.W.; Zhang, J.B.; Xin, X.L.; Zhang, C.Z.; Ma, D.H.; Zhou, Y.F. Linking cropland ecosystem services to microbiome taxonomic composition and functional composition in a sandy loam soil with 28-year organic and inorganic fertilizer regimes. Appl. Soil Ecol. 2019, 139, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Kong, L.; Tong, L.; Cao, H.; Zhou, H.; Lv, Y.Z. Long-term application of bio-compost increased soil microbial community diversity and altered its composition and network. Microorganisms 2022, 10, 462. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Qiang, F.F.; Liu, G.Q.; Liu, C.H.; Ai, N. Distribution characteristics of soil microbial communities and their responses to environmental factors in the sea buckthorn forest in the water-wind erosion crisscross region. Front. Microbiol. 2023, 13, 1098952. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Macdonald, L.M.; Rogers, S.L.; Gregg, A.L.; Bolger, T.P.; Baldock, J.A. Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol. Biochem. 2008, 40, 803–813. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Chen, M.; Lou, Y.; Wang, H.; Yang, Q.; Pan, H.; Zhuge, Y. Effects of soil acidification on bacterial and fungal communities in the Jiaodong peninsula, northern China. Agronomy 2022, 12, 927. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, W.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G.X. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610–611, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, C.; Zhang, T.; Wang, X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, M.; Liu, J.; Li, D.; Liu, X.; Chen, L.; Guo, X.; Liu, M. Improved Straw Decomposition Products Promote Peanut Growth by Changing Soil Chemical Properties and Microbial Diversity. Sustainability 2024, 16, 7096. https://doi.org/10.3390/su16167096
Liu Y, Wu M, Liu J, Li D, Liu X, Chen L, Guo X, Liu M. Improved Straw Decomposition Products Promote Peanut Growth by Changing Soil Chemical Properties and Microbial Diversity. Sustainability. 2024; 16(16):7096. https://doi.org/10.3390/su16167096
Chicago/Turabian StyleLiu, Yaxin, Meng Wu, Jia Liu, Daming Li, Xiaoli Liu, Ling Chen, Xi Guo, and Ming Liu. 2024. "Improved Straw Decomposition Products Promote Peanut Growth by Changing Soil Chemical Properties and Microbial Diversity" Sustainability 16, no. 16: 7096. https://doi.org/10.3390/su16167096
APA StyleLiu, Y., Wu, M., Liu, J., Li, D., Liu, X., Chen, L., Guo, X., & Liu, M. (2024). Improved Straw Decomposition Products Promote Peanut Growth by Changing Soil Chemical Properties and Microbial Diversity. Sustainability, 16(16), 7096. https://doi.org/10.3390/su16167096






