Abstract
In order to reduce global warming, new energy fuels that use waste biomass to replace traditional coal are rapidly developing. The main purpose of this study is to investigate the feasibility behavior of different biomass materials such as spent coffee grounds (SCGs) and spent tea grounds (STGs) as fuel during combustion and their impact on the environment. This study involves using fuel shaping and co-firing methods to increase the fuel calorific value and reduce the emissions of pollutants, such as NOX and SO2, and greenhouse gas CO2. The produced gas content was analyzed using the HORIBA (PG-250) laboratory combustion apparatus. The results indicate that, among the measured formed particles, SCG:STG = 8:2, 6:4, and 4:6 had the lowest post-combustion pollutant gas emissions. Compared to using only waste coffee grounds as fuel, the NOx emissions were reduced from 166 ppm to 102 ppm, the CO emissions were reduced from 22 ppm to 12 ppm, and the CO2 emissions were reduced from 629 ppm to 323 ppm. In addition, the emission of SO2, the main component of acid rain, was reduced by 20 times compared to the combustion of traditional fuels. The SO2 emission of five different proportions of biomass fuels was 5 ppm, which is much lower than that of traditional coal fuels. Therefore, SCG and STG mixed fuels can replace coal as fuel while reducing harmful gasses.
1. Introduction
The need for alternative and renewable sources of energy has never been more pressing [1]. In response to the global energy crisis, efforts have been devoted to exploring and exploiting a myriad of unconventional, sustainable, and green potential energy sources [2]. One such alternative is waste biomass and, more specifically, spent coffee grounds (SCGs) and spent tea grounds (STGs). The ubiquity of coffee and tea consumption has created a wealth of bio-waste, much of which remains underutilized [3].
SCGs and STGs are both abundant and underutilized sources of biomass [4]. After the process of brewing, these grounds are typically discarded, contributing significantly to food waste [5,6]. However, the spent grounds are still rich in lignocellulosic substances, indicating their potential as an alternative and renewable source of energy. The initiative to repurpose these waste materials into biomass fuel integrates a resource-conservative approach into a green energy solution [7]. It is not only logical but also highly beneficial from an environmental perspective to solve the problem of waste recycling and effectively replace fossil fuels. Furthermore, the biomass mixture showed good grindability, which is an important aspect of the pulverization process in most power generation systems. Traditional biomass sources such as wood, bamboo, etc., need to be pulverized to adjust the particle size for mixing with other biomasses. The waste SCGs and STGs collected in this article are materials with a relatively uniform particle size distribution, which can save labor and machinery costs in the pulverization process, promote uniform combustion, and reduce the possibility of blocking the fuel supply system [8]. The concept of a biomass mixture involves the amalgamation of different types of renewable organic materials, in this case, SCG and STG, to be co-fired as sources of energy [9]. Co-firing is a promising solution for energy generation from renewable sources as it leverages the existing power infrastructure and lays a foundation for a reduced reliance on fossil fuels [10]. Recent research into the energy potential of SCGs and STGs has indicated that they may provide a viable source of renewable energy, making them an area of interest for further investigation [11].
In the process of pellet formation, biomass mixing plays an indispensable role. For producing efficient energy from SCGs and STGs, pelleting has proven to be a decisive step. This section explains the importance of biomass mixing, its influence on pellet quality, and its role in combustion performance. Biomass mixing primarily influences the physical and thermal properties of the pellets [12,13]. For example, the particle size, moisture content, and volatile matter content of the blend can impact pellet density, durability, and calorific value—critical parameters for a fuel’s efficacy [14]. Additionally, the proportion of biomass components used in the blending process significantly affects the pellet’s combustion characteristics and energy potential. It is important to note that the success of biomass mixing is heavily reliant on the uniformity of the mix. Uniform mixing ensures a stable and controlled burning process by promoting homogeneity in the combustion properties [15,16]. A homogeneous mix guarantees consistent heat release, reducing chances for clinker formation and slagging in the combustion unit. The influence of blending extends beyond the level of pellet formation to the performance of the pellet during combustion. The blend composition can also dictate the emission characteristics of the produced pellets. SCG and STG have notably low sulfur and chlorine percentages, thereby reducing the emission of harmful sulfur oxides (SO2) and chlorides when combusted [17]. In summary, biomass mixing in pellet formation not only improves the pellet quality but also maximizes the efficiency and sustainability of the resultant fuel. Further studies could delve into the precise composition and ratio of SCGs and STGs required to optimize pellet performance. Furthermore, there is a largely unexplored area concerning the emissions resulting from SCG and STG co-firing. As with all combustion processes, emissions may contain pollutants that could potentially affect human health and contribute to climate change. The identification and measurement of the possible pollutants and their potential impacts is a vital part of this study [18].
The purpose of this study is to evaluate the chemical and physical preparation of SCGs and STGs as alternative biomass fuels through to the environmental impact of their emissions [19]. Because the temperature of SCGs and STGs during the roasting process is 80–120 °C, they can be regarded as raw materials after low-temperature carbonization. In the research, it was found that more SCG biochar was used and mixed with coal or other biomass [20]. There was a lack of research on SCG as a raw material, especially when the mixed materials were all waste biomass from beverages. This fills the research gap in this area. It further provides a comprehensive understanding that will inform future research and the development of this promising renewable energy source [2].
2. Materials and Methods
2.1. Collection of Spent Coffee Grounds and Spent Tea Grounds
Spent coffee grounds (SCGs) were collected from various local restaurants within Tokyo, Japan. The collection process was designed to conveniently match the daily operation schedule of the restaurants, and it was structured to not interfere or impede their operations under any circumstances [21]. For SCGs, upon collection, they were stored in separate sealed glass containers to prevent contamination and maintain their physical properties as much as possible. The containers were labeled according to the source and the date of collection. The average quantity collected daily was 1 kg of SCGs. The collected waste coffee grounds were wet waste coffee grounds. Following collection, they were oven-dried (Kosumosu SSN-113S, Isuzu, Yokohama, Japan) at 105 °C for 72 h until an acceptable moisture level approximately under 10% was achieved. This material was used as a raw material and stored at room temperature. Since coffee grounds have a strong water absorption capacity [22], the moisture content is bound to increase during storage.
Because the particle size of the hot coffee powder (HSCG, UCC Company Blended Coffee Granze Strong, UCC Ueshima Coffee Co., Ltd., Tokyo, Japan), iced coffee powder (CSCG, UCC Company Iced Coffee Granze Strong), and origin of the used STG ( tea powder residue (originating local tea plantations in Hangzhou, China, but material already existing in the laboratory) was less than 1 mm in size, the grinding step was omitted, saving the manual energy which would have been consumed during grinding, with increased cycling possibilities for fuel formation.
2.2. Pelleting Process for Spent Coffee and Tea Grounds
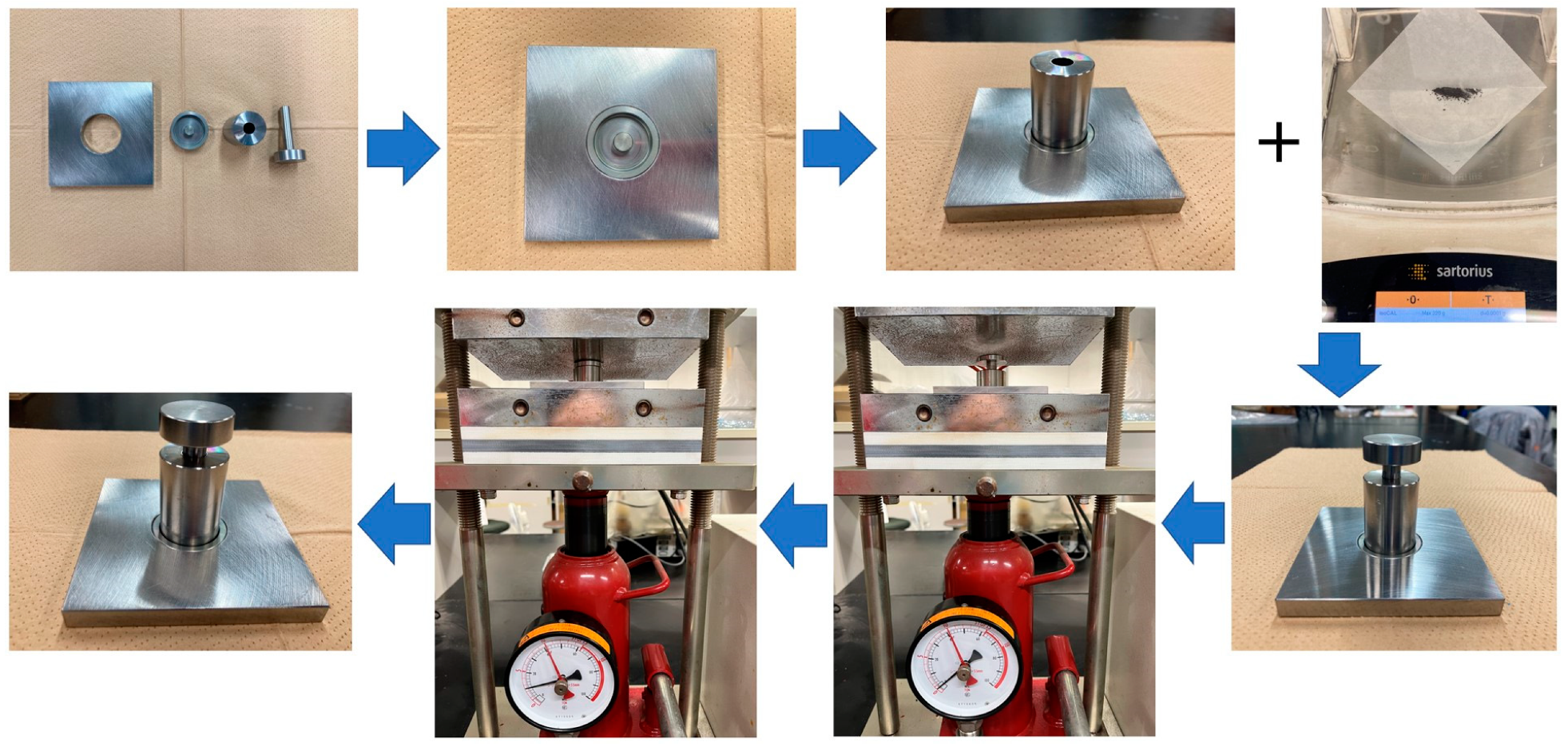
In the pelleting process, as shown in Figure 1, both SCGs and STGs were initially dried to a moisture content of less than 10% and a particle diameter of less than 1 mm.
Figure 1.
Procedure for making pellets of spent coffee and tea grounds.
The next step involved the mixing of the spent coffee and tea grounds in specific ratios (10:0, 8:2, 6:4, 4:6, 2:8, and 0:10), as shown in Figure 2, to achieve optimal calorific properties. One consideration was to balance the high-energy coffee grounds with the lower-energy tea grounds to produce pellets with a good calorific value while remaining cost-efficient [23]. In the actual pelleting process, the pressure of the manual hydraulic press was 10 Mpa, and the holding time was 30 s. This high pressure resulted in heat generation, reaching temperatures of around 60–80 °C [24]. This caused the inherent lignin content within the materials to soften and act as a binding agent, facilitating the formation of solid pellets. The pellets were released from the pelleting machine, and a cooling process occurred. Once cooled, the pellets hardened, with the natural lignin inside the biomass solidifying [25].
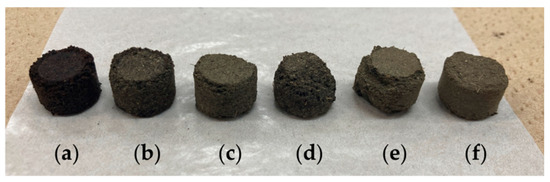
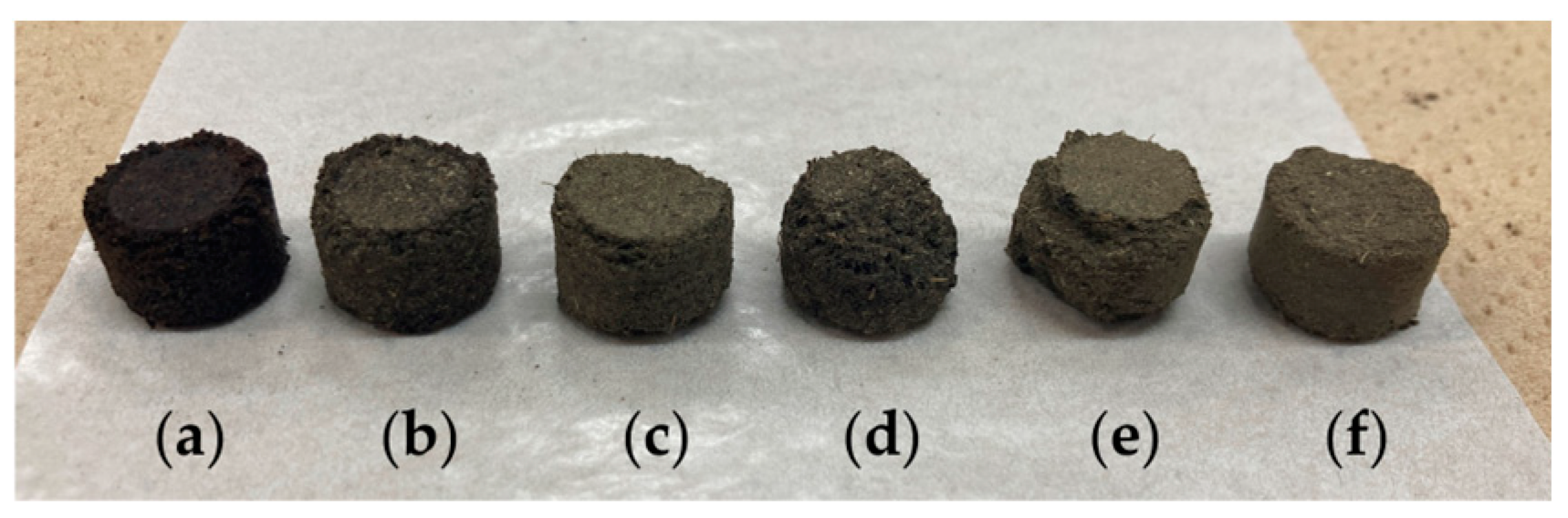
Figure 2.
The produced pellets of spent coffee and tea grounds (from the left): CSCG:STG = (a) 10:0, (b) 8:2, (c) 6:4, (d) 4:6, (e) 2:8, and (f) 0:10.
The pelleting process’s efficiency was measured not only by the throughput, but also in terms of the pellets’ mechanical durability and hardness, as well as the process’s energy consumption [26,27]. Therefore, optimization of the pelleting parameters was crucial in ensuring an efficient and sustainable production of fuel pellets from SCGs and STGs [28].
2.3. Analyzing the Properties of the Waste Biomass Mixture
The SCGs and STGs were dried and ground into a fine powder. The moisture content, volatile matter, ash content, and fixed carbon needed to be independently analyzed for both the SCGs and the STGs before testing, following the ASTM standard D3173 [29].
In the proximate analysis using the Ceramic Muffle furnace (CM-150, Sibata Scientific Technology Ltd., Tokyo, Japan), the percentage of volatile matter was substantial, a characteristic which typically corresponds to enhanced combustion properties. As part of the analytical procedure, the results of the ultimate analysis of the SCGs and STGs were examined using CHN-coder (MT-5, Yanaco Ltd., Kyoto, Japan) and the Microcomputer Han Display Sulfur Analyzer (HYDL-9, Minsheng Ltd., Hebi, China). We then determined the metal content of the SCGs and STGs with an X-ray analytical microscope (XGT-5000, Horiba Ltd., Tokyo, Japan). We measured the cellulose, hemicellulose, and lignin contents of the SCGs and STGs in the same way as [30], using an aspirator (A-3S, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and water baths (Yazawa scientific Ltd., Tokyo, Japan) to determine the content of cellulose and lignin. ATR-FTIR (attenuated total reflectance; ATR PRO ONE Base Kit, JASCO FTIR-6100, JASCO Co., Ltd., Tokyo, Japan) equipment was used to determine the functional groups of the materials [27]. A variable-pressure scanning electron microscope (VP-SEM, Hitachi Ltd., Tokyo, Japan ) SU-1510 with an accelerating voltage of 15 kV (Hitachi Ltd., Tokyo, Japan) was used to observe the surface lignin and cellulose and the surface morphology of the SCGs and STGs. Finally, a portable gas analyzer (PG-250, Horiba Ltd., Tokyo, Japan) was used to evaluate the exhaust gas.
2.4. Assessment of Calorific Value
We calculated the calorific value using the results of the ultimate analysis obtained using CHN-coder. The heating value including both the higher heating value (HHV) and lower heating value (LHV) was determined using Equations (1) and (2), given by the Japan Environmental Sanitation Center. We entered the carbon (C), hydrogen (H), oxygen (O), and sulfur (S) amounts obtained from the ultimate analysis and the moisture content (W) from the proximate analysis results in the below equations.
HHV (kcal/kg) = 81C + 345H − 33.3 × O + 25S
LHV (kcal/kg) = 81C + 345H − 33.3 × O + 25S − 6(9H + W)
2.5. Analysis of Surface Functional Groups
The surface functional groups on the surface of the produced pellet from spent coffee and tea grounds as waste biomass fuel with ratios of CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10 were identified using ATR-FTIR (attenuated total reflectance; ATR PRO ONE Base Kit, JASCO FTIR-6100) equipment. Before employing the device to evaluate the mixed biomass, it was emptied. The samples with ratios of CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10 were then placed directly inside the FTIR equipment for analysis [31].
2.6. Test Setup and Equipment
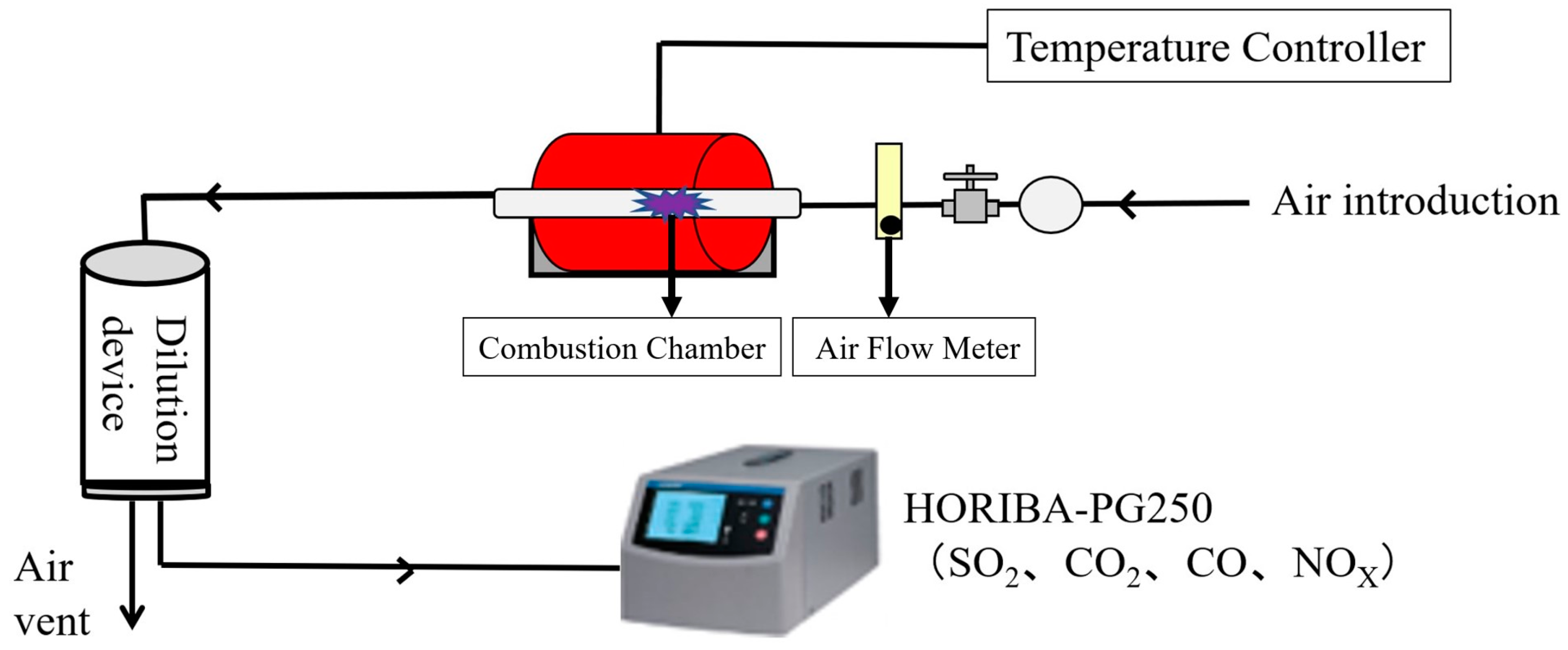
The test setup and equipment employed to assess the feasibility of co-firing SCGs and STGs as potential fuel options are presented in Figure 3. To conduct the experiment, a lab-scale combustor system specifically designed to evaluate the emissions of alternative fuels was utilized. The system, equipped with appropriate fuel feeding and air supply mechanisms, was also connected to a gas-analyzing equipment (HORIBA-PG250, HORIBA, Ltd., Kyoto, Japan) for real-time emission monitoring [32].
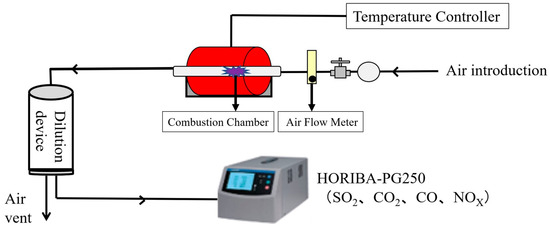
Figure 3.
Schematic diagram of the pellet combustion system.
As shown in Figure 3, the thermal efficiency and emission characteristics of SCG and STG co-firing were studied using a bench-scale combustion-testing facility, equipped with a fuel hopper, a feeder, a combustion chamber, an air flow meter, an exhaust fan, and a chimney. The combustion chamber was a tubular updraft combustor, specifically designed to simulate the typical conditions of a grate-fired burner system.
The system was connected to a gas analyzer (HORIBA-PG250) that consisted of carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOX), and sulfur dioxide (SO2) analyzers for the real-time analysis of flue gas composition. The pellet combustion experiment was carried out at 800 °C and an air flow rate of 30 L/min. Flue gas samples were continuously collected during the combustion tests.
3. Results and Discussion
3.1. Chemical Properties of Spent Coffee and Tea Grounds
3.1.1. Proximate and Ultimate Analyses of Spent Coffee and Tea Grounds
SCGs and STGs are agro-industrial by-products, saturated with organic matter such as carbon, oxygen, nitrogen, sulfur, and ash content. These elements primarily define the fuel characteristics of these biomass fuels.
For instance, the carbon content of SCGs and STGs directly correlates with the heating value of the fuel. The oxygen content, meanwhile, influences both the heating value and the fuel characteristics [33]. A higher oxygen content often results in a lower heating value and higher combustion rates. As shown in Table 1, the nitrogen and sulfur contents affect the emission of nitrogen oxide (NOX) and sulfur oxide (SO2) gasses.

Table 1.
Proximate and ultimate analyses of HSCGs, CSCGs, and STGs.
The chemical composition of the SCGs was measured using elemental analysis, and Table 1 showcases the results of nine experiments, showing that CSCGs consisted of 57.61% carbon, 7.74% hydrogen, 2.44% nitrogen, 0.09% sulfur, and 32.14% oxygen by weight, while HSCGs consisted of 53.65% carbon, 6.77% hydrogen, 2.38% nitrogen, 0.1% sulfur, and 36.61% oxygen. The ash content was determined to be 1.45%.
The STGs were found to have 45.44% carbon, 5.92% hydrogen, 3.26% nitrogen, 0.17% sulfur, and about 45.24% oxygen by weight. The ash content of the STGs was slightly higher than in the SCGs, with a value of 3.66%.
As revealed by JIS M8813 [34], a higher carbon content contributes directly towards a high calorific value. Additionally, as shown in Table 1, the nitrogen and sulfur contents were reasonably low, suggesting reduced emissions of harmful gasses such as NOX and SOX during combustion.
It is worth noting that both SCGs and STGs showcased high carbon and oxygen contents in this study, hinting at their potential as viable renewable biomass fuels. However, differences in the carbon, oxygen, hydrogen, nitrogen, sulfur, and ash contents in both SCGs and STGs might result in variations in their fuel properties, combustion behaviors, and emission characteristics. It should be noted that there are certain limitations associated with the individual use of these materials. For instance, tea grounds have a relatively high nitrogen content, which could lead to elevated nitrogen oxide emissions. Comparatively, coffee grounds have a lower nitrogen content. However, co-firing these materials in appropriate proportions can potentially moderate these limitations.
Moreover, the ash content of both SCGs and STGs is appreciably significant, which may cause slagging and fouling issues during combustion. Slagging and fouling are common problems in biomass combustion, where ash components form deposits on combustion chamber surfaces, leading to a reduced heat transfer efficiency and, eventually, equipment damage [35]. Future studies might focus on developing effective solutions for ash-related issues in SCG and STG co-firing. However, the measured ash content in the biomass mixture in this study fell within the acceptable range for typical biomass fuels.
The proximate and ultimate analyses demonstrate the potential of this biomass mixture to be an effective co-firing fuel possessing combustion characteristics that are comparable to traditional biomass fuels such as wood pellets.
3.1.2. Calculation of the Calorific Value
As shown in Table 2, the calorific value of the HSCGs ranged between 21.16 and 22.84 MJ/kg, whereas, for CSCG, the energy potential ranged from 24.29 to 26.2 MJ/kg, which is comparable to the calorific value of some lignite varieties. This was even higher than the LHV of 16.53 MJ/kg found in Fushun peat and was similar to the low calorific value of 21.31 MJ/kg observed in ordinary clean coal, such as Nantong coal, from China. This clearly demonstrates that these waste products have the potential to serve as an effective alternative to traditional fuels, also promoting recycling and reducing the environmental burden.

Table 2.
Calorific value of HSCGs, CSCGs, STGs, FuShun peat, and NanTong coal.
The high heating value of SCGs and STGs was largely due to their high carbon and hydrogen contents (Table 1) and the presence of several organic compounds such as cellulose, hemicellulose, and lignin (Table 3). Also, the moisture content, which negatively affected the heating value, was relatively low, further enhancing their potential as a fuel source.

Table 3.
Chemical composition analysis of lignin and cellulose contents in SCGs and STGs.
3.1.3. Biomass Composition of Spent Coffee and Tea Grounds
The SCGs consisted mainly of cellulose, hemicellulose, and lignin, as shown in Table 3 and Figure 4. In Table 3, the holocellulose in the SCGs accounts for 47.29% of the total weight, while the lignin is 22.05%. The holocellulose content in STGs is 55.73%, and the lignin is 29.33%. The same results are presented in Figure 4.
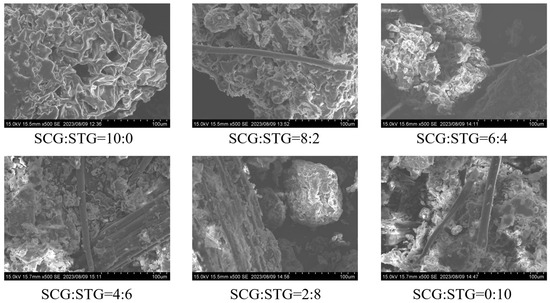
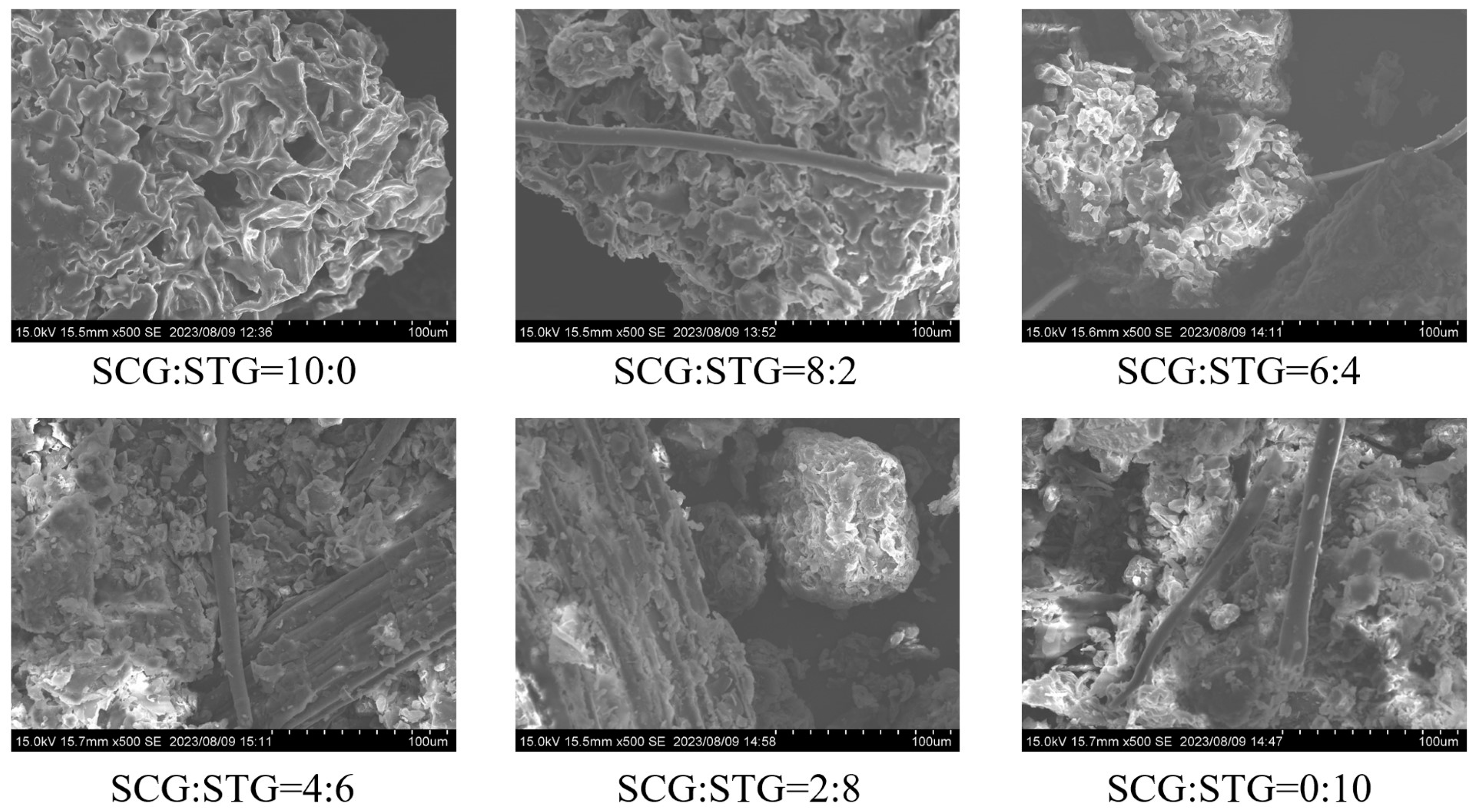
Figure 4.
SEM photographs of SCG and STG pellet fuels (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10).
3.1.4. Metal Contents of Spent Coffee and Tea Grounds
The metal analysis results are shown in Table 4. SCGs and STGs contained trace metals, and the metal content of STGs was higher than that of SCGs. Since the root system of tea trees is more developed, tea leaves may absorb more metal elements from the soil during growth. On the other hand, this content is also related to the brewing method. Although coffee grounds and tea grounds contain some metal elements, the content of these metal elements is very low and does not pose a threat to human health. In addition, these trace metals form ash after combustion. The metal content in STGs is higher, so more ash is produced. The experimental results are the same as those in Table 1.

Table 4.
Metal analysis of Na, K, Ca, and Mg contents in SCGs and STGs.
3.1.5. Functional Group Analysis of SCG and STG Pellet Fuels
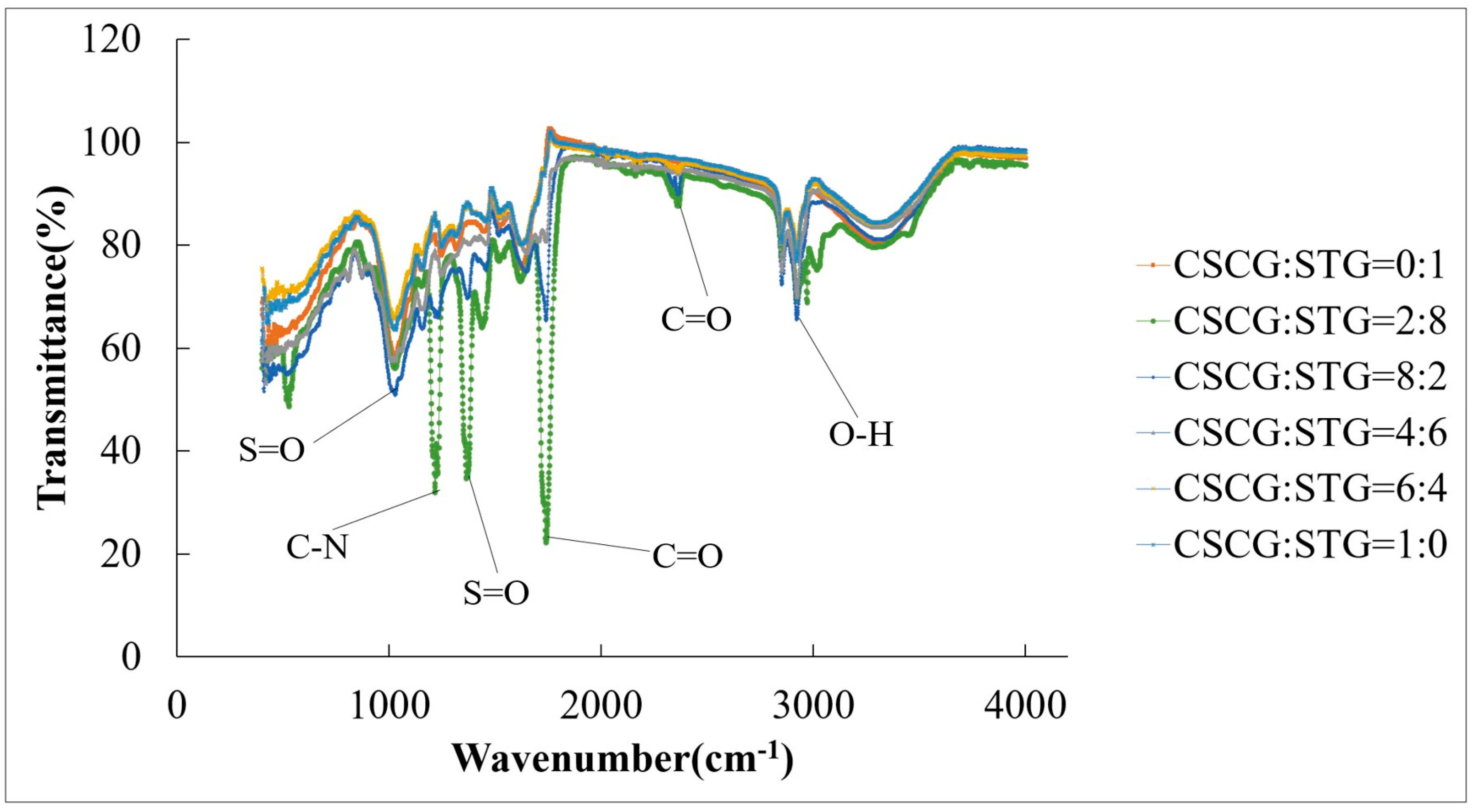
The FTIR graph in Figure 4 shows the infrared spectral transmission data of the CSCG and STG samples with different ratios, namely 0:1, 2:8, 8:2, 4:6, 6:4, and 1:0. Several characteristic absorption peaks are marked in the spectrum, indicating the main chemical groups in the samples. The main absorption peaks include the following: S=O bond vibration peak at 1365 cm−1, indicating the presence of thioester or thioketone groups; C-N bond vibration peak at 1217 cm−1, which may be attributed to nitrile or amine bonds; C=O bond vibration peak at 1738 cm−1, suggesting the presence of carboxyl or carbonyl groups, indicating carbon dioxide emissions; and a broad peak at about 2850 cm−1, representing the stretching vibration of O-H. Samples with different CSCG and STG ratios show obvious differences in the intensity and position of these absorption peaks, indicating that their chemical composition and structure change with the ratio. For example, the intensities of the O-H and C=O peaks change with the change in the CSCG and STG ratio. In the following combustion experiments, the emission gas output of the mixed pellet fuels with different ratios was different due to the strength of the functional groups. The most noticeable was CSCG:STG = 2:8. There were noticeable differences in the strength of the S-N, S=O, and C=O functional groups compared to the other five mixed pellet fuels. Indeed, they were all stronger than their respective values in the other five mixed pellet fuels. Therefore, the emission of SO2, NOX, and CO2 after CSCG:STG = 2:8 combustion was also higher than that measured with the other five mixed pellet fuels. These data reveal the diversity in the chemical composition of samples with different CSCG and STG ratios, which is of great significance for material science and chemical analysis research [23,36].
3.2. Physical Properties of SCG and STG Pellet Fuels
3.2.1. SEM Analysis of SCG and STG Pellet Fuels
The set of scanning electron microscope (SEM) images showed the microstructure of the material under different mixing ratios of SCGs and STGs. The images corresponded to samples with SCG:STG ratios of 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10, respectively. It could be observed that, when the SCG content was high (such as in SCG:STG = 10:0), the surface of the material presented a denser, wrinkle-like rough structure with relatively few pores. As the STG content increased (such as in SCG:STG = 8:2, 6:4), more linear structures could be seen, the surface became relatively looser, and the pores became more recognizable. When STGs completely replaced SCGs (such as in SCG:STG = 0:10), the surface of the material showed a large number of fibrous structures, the pores were more significant, and the structure was more porous overall. These microstructural differences can have significant effects on the physical and chemical properties of a material. For instance, regarding chemical properties such as the cellulose content when the STG ratio was high, the fibrous materials were intertwined with the bulk materials to form a tightly aggregated structure (such as in SCG:STG = 4:6). Meanwhile, for physical properties such as the calorific value, a higher SCG content corresponded to higher calorific values, both in terms of the high calorific value and the low calorific value. Hence, the calorific value of SCG:STG = 10:0 was the highest among the six pellets and comparable to that of conventional coal fuel [36].
3.2.2. Physical Characterization of SCG and STG Pellet Fuels
The length, width, height, volume, and bulk density of the six mixed pellet fuel particles are shown in Table 5. Under the same extrusion pressure of 10 MPa and holding time of 30 s, the bulk density of CSCG:STG = 2:8 and CSCG:STG = 0:10 was significantly lower than that of the other four mixed pellet fuels. Although Table 3 and Figure 5 mention that the STGs had a higher cellulose content, this was easier to form after extrusion. The reason for the opposite result is that coffee grounds are a special material that contains a large amount of biomass oil [37], which just acts as an adhesive. Therefore, the higher the CSCG ratio, the greater the bulk density after forming. At the same time, this material is not easy to break.

Table 5.
Physical characterization of SCG and STG pellet fuels.
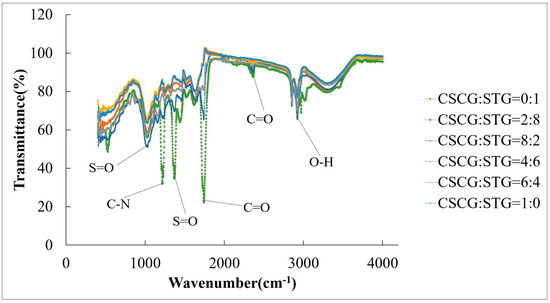
Figure 5.
FTIR spectra of SCG and STG pellet fuels.
3.3. Results of the Combustion Test
The combustion tests of the SCG and STG pellets were conducted in a laboratory-scale combustion facility to assess their emission characteristics.
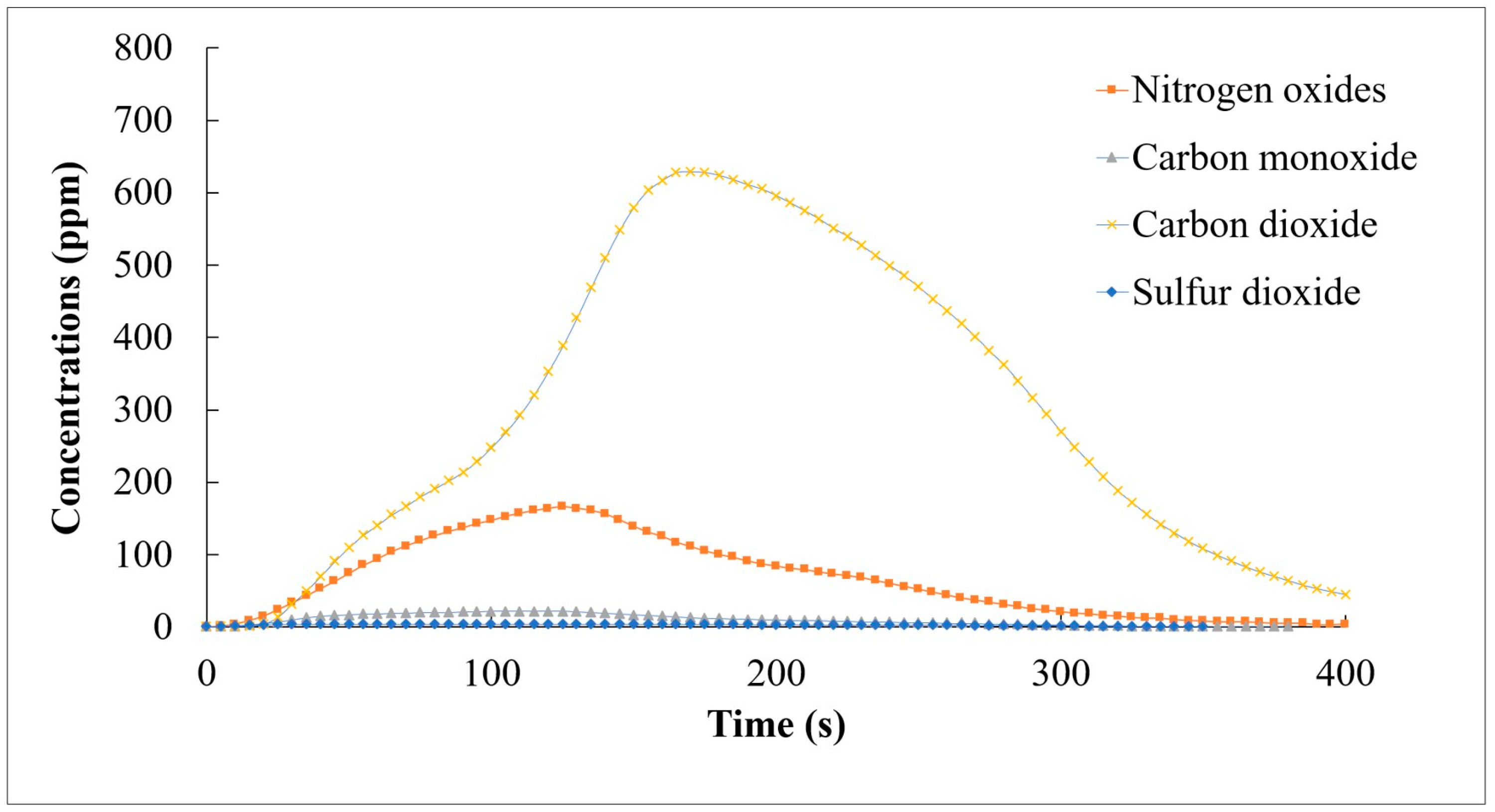
The initial combustion test focused on evaluating the combustion performance of the pellets individually. The SCG and STG pellets exhibited a comparable calorific value to traditional fuels. During the combustion process, both types of pellets burned efficiently, with a relatively high heat value and a low ash content. The specific combustion characteristics of the SCG pellets were as follows: The SCG pellets showed a high level of thermal efficiency [11]. The temperature distribution within the combustion chamber was uniform, indicating that the burning of the coffee ground pellets was stable. The emission analysis (Figure 6) showed a low level of carbon monoxide (CO), implying a high level of oxidization. The sulfur dioxide (SO2) emissions were also negligible.
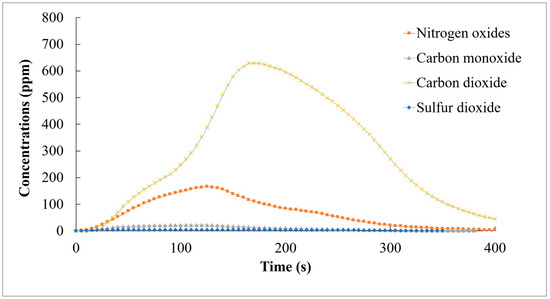
Figure 6.
CO, NOX, CO2, and SO2 emissions during the combustion of SCG-based pellets.
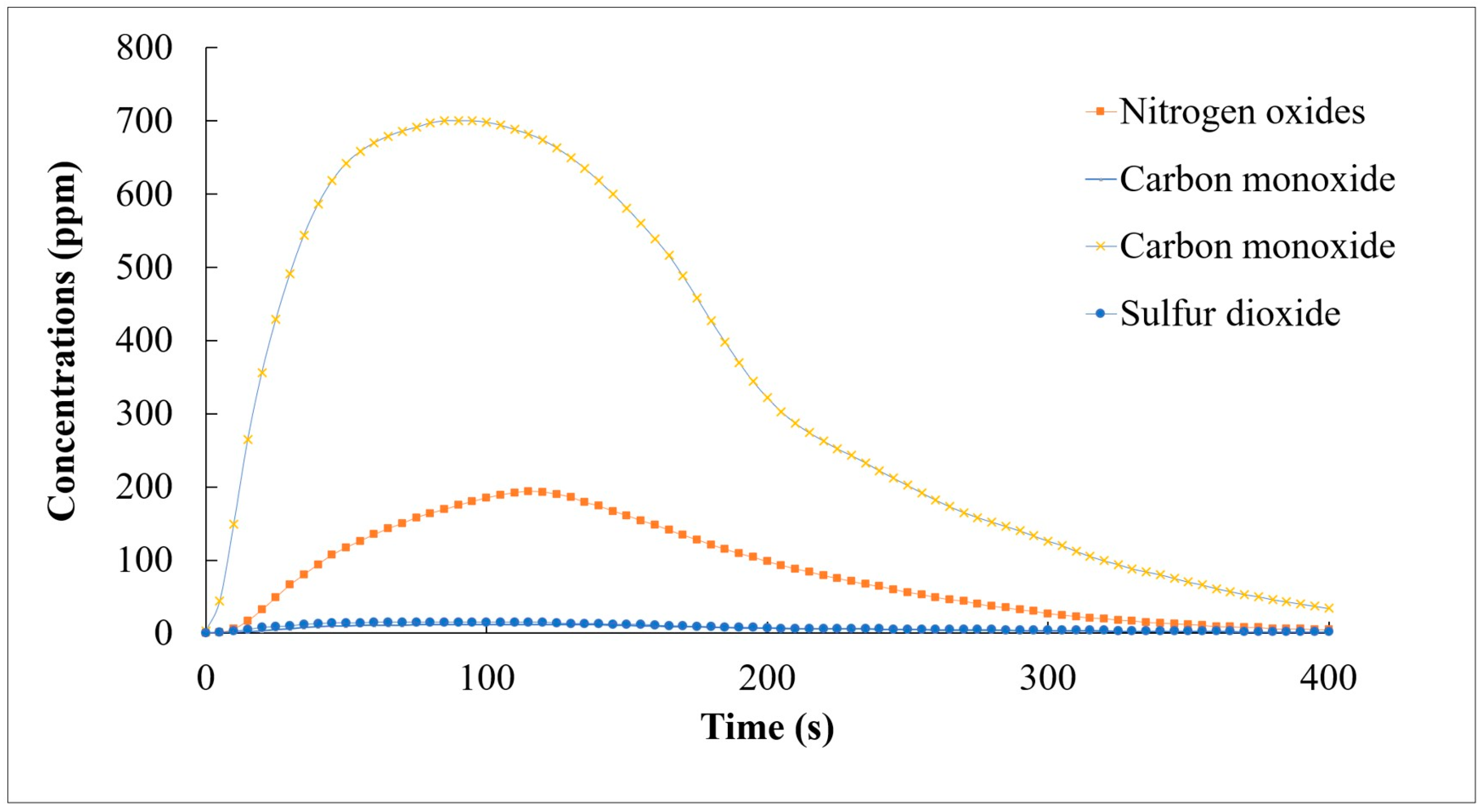
Similar to the coffee grounds, the tea ground pellets demonstrated a consistent temperature distribution within the combustion chamber. As shown in Figure 7, the pellets produced less heat compared to the coffee ground pellets but had a lower emission of pollutants. Both CO and SO2 emissions were low.
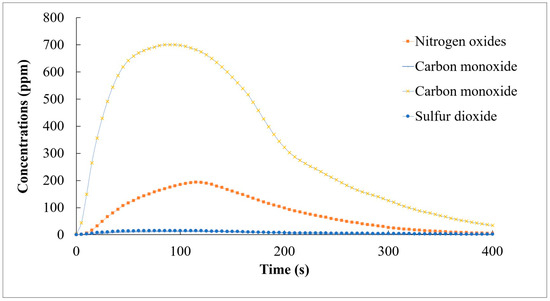
Figure 7.
CO, NOX, CO2, and SO2 emissions during the combustion of STG-based pellets (CSCG:STG = 2:8).
The co-firing test was then conducted using a blend of SCGs and STGs. The blend provided a balanced combustion, where the coffee grounds’ higher heat compensated for the less heat generated by the tea grounds. Thus, the combination worked successfully in providing a stable temperature and a clean burn with reduced emissions.
In terms of gaseous emissions, a significant reduction in carbon emissions was observed in the co-firing test compared to traditional biomass fuels. The nitrogen oxide (NOX) and SO2 emissions were within permissible limits, affirming the eco-friendliness of using SCGs and STGs as biofuel.
In conclusion, the results of the combustion test illustrate that SCGs and STGs possess good combustion properties and can serve as viable sources of clean energy. However, more large-scale tests must be conducted to assess the practicality of applying this technology in real-world conditions.
3.4. Concentrations of Different Gasses from the Emissions
One of this study’s primary objectives was measuring the levels of sulfur dioxide (SO2), nitrogen oxide (NOX), carbon dioxide (CO2), and carbon monoxide (CO) generated during the co-firing process.
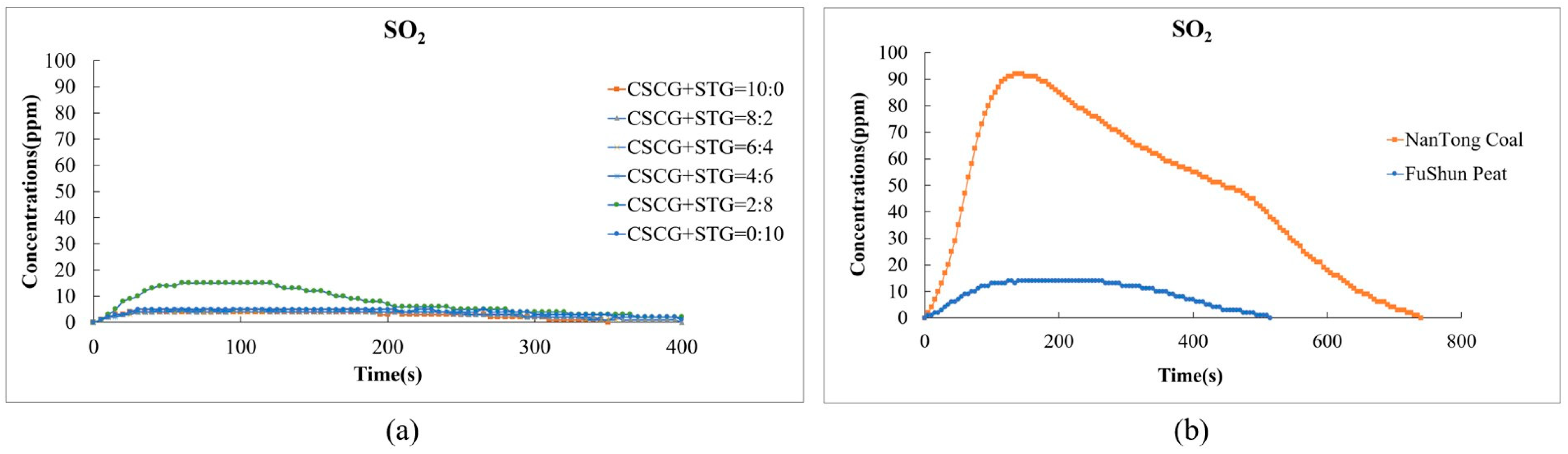
In Figure 8, the results from the laboratory experiment show a significant reduction in the emission of sulfur dioxide (SO2) in the co-firing process compared to traditional fossil fuels. Figure 8a shows the SO2 emissions versus time for six different CSCG and STG mix ratios (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10). The SO2 concentration of each fuel proportion is relatively low, the peak concentration is below 10 ppm, and the fluctuation is small. With the exception of CSCG:STG = 8:2, the peak value of the combined pellet fuels (CSCG:STG = 10:0, 6:4, 4:6, 2:8, and 0:10) is lower compared to that of traditional fuels, averaging below 5 ppm. However, Figure 8b shows that the SO2 concentration of NanTong coal quickly peaks at around 90 ppm after about 100 s and gradually decreases after 400 s. The SO2 concentration of FuShun peat is significantly lower than that of NanTong coal, with a peak value of about 15 ppm. The sulfur dioxide emissions from traditional fuel coal are about 20 times higher than those of mixed biomass fuels such as coffee and tea residues. The low sulfur content of the SCGs and STGs used contributed to this significant reduction. This low-emission feature of SCGs and STGs renders them an environmentally friendly alternative to other fuel sources.
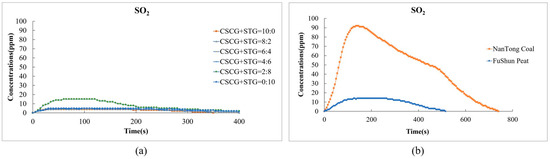
Figure 8.
Sulfur dioxide emissions during the combustion of (a) the produced pellets (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10) and (b) NanTong coal and FuShun peat from China.
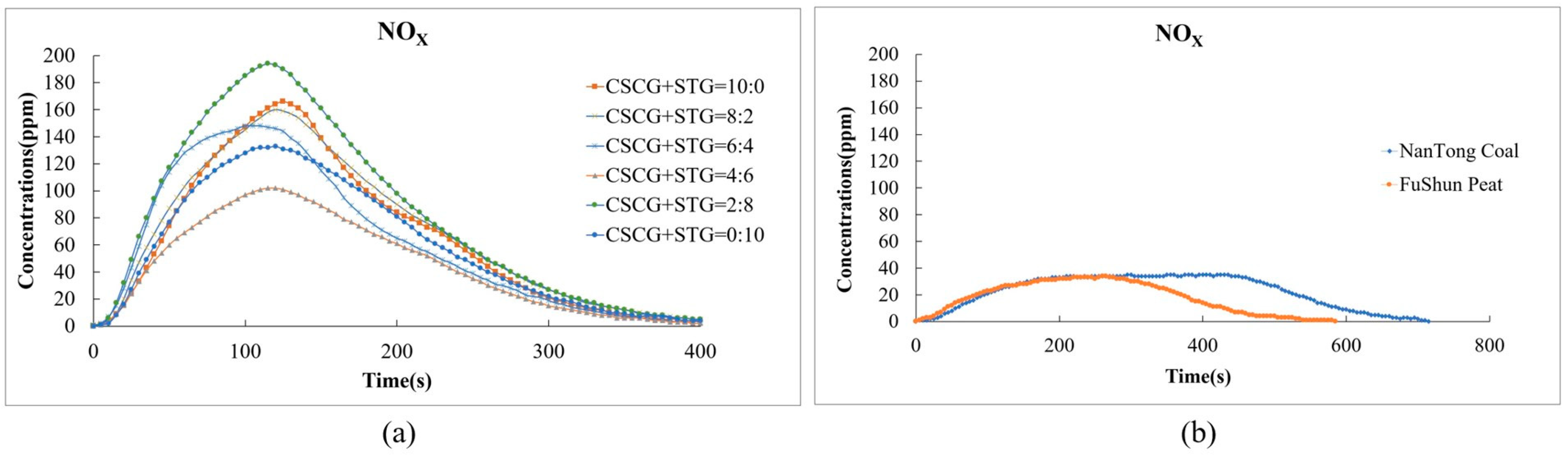
Conversely, the nitrogen oxide (NOX) emissions from our pellets were slightly higher in comparison to other fuels. This can be attributed to the higher nitrogen content within the biomass of SCGs and STGs. Figure 9a shows that the NOx emissions of each fuel ratio reach their peak at about 150 s, and the peak concentration is between around 60 ppm and 180 ppm. As the proportion of STGs in the mixture increases, the NOx concentration gradually increases.
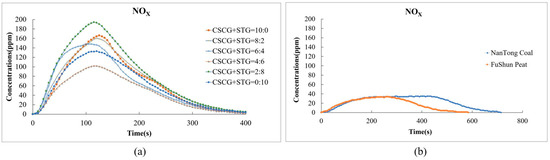
Figure 9.
Nitric oxide and nitrogen dioxide emissions during the combustion of (a) the produced pellets (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10) and (b) NanTong coal and FuShun peat from China.
In Figure 9b, we can see that the NOx concentration of NanTong coal is higher than that of FuShun peat as a whole and that both gradually drop to 0 ppm after around 600 s. Some strategies to mitigate this increase could be the optimization of the combustion temperature and the oxygen supply during co-firing, which, in future studies, could significantly reduce the levels of NOX.
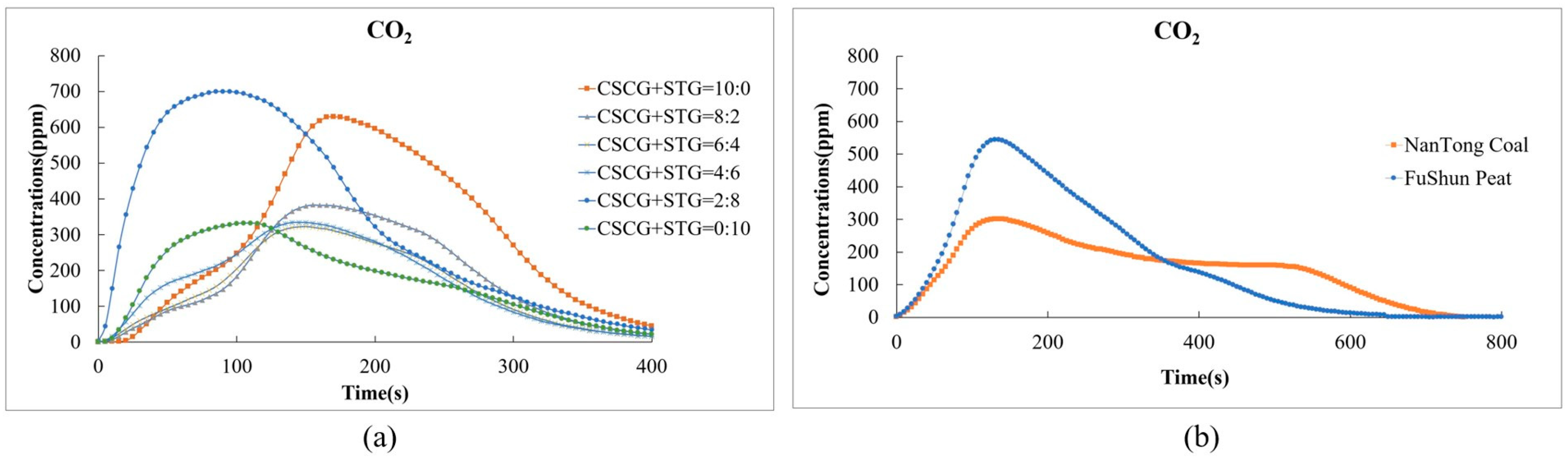
In Figure 10, the carbon dioxide (CO2) emissions of the produced pellets (CSCG:STG = 8:2, 6:4, 4:6, and 0:10) are comparably lower than those of traditional fossil fuels. In particular, traditional fuels burn for a long time, and the total amount of carbon dioxide emitted also increases during this process. In Figure 10a, the CO2 emissions of each fuel ratio reach their peak at about 150 s, and the peak concentration is between about 200 ppm and 700 ppm. In the pellets with ratios of CSCG:STG = 8:2, 6:4, and 4:6, as the STG ratio increases, the peak concentration of CO2 decreases in a relative manner. On the right, Figure 10b shows that the maximum CO2 concentration of NanTong coal is about 300 ppm, while that of FuShun peat is slightly higher, at about 600 ppm, and then gradually decreases.
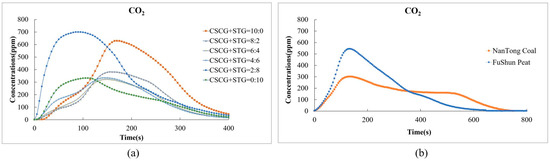
Figure 10.
Carbon dioxide emissions during the combustion of (a) the produced pellets (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10) and (b) NanTong coal and FuShun peat from China.
Secondly, considering the renewability potential of coffee and tea residues as a biomass source, these waste materials absorb CO2 from the atmosphere during their growth process, reducing the CO2 content from the net global emissions to complete the cycle of carbon dioxide in plants, thus achieving zero carbon dioxide emissions.
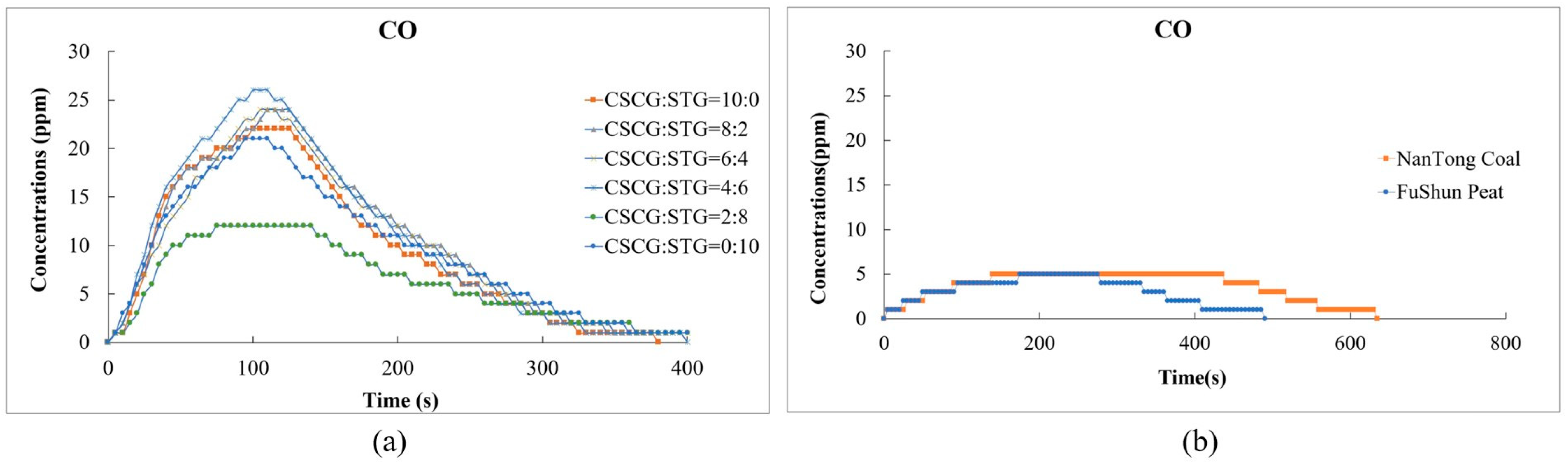
In Figure 11, the levels of carbon monoxide (CO) emissions released during the co-firing of SCGs and STGs were significantly higher than those of conventional fuels. This indicates that incomplete combustion occurs during the combustion of biomass. This is related to the fact that biomass contains a small amount of metal elements, which results in a small amount of ash after combustion.
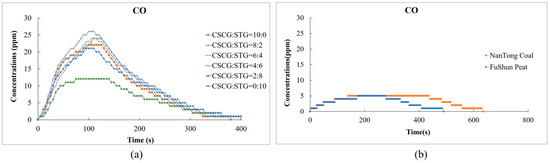
Figure 11.
Carbon monoxide emissions during the combustion of (a) the produced pellets (CSCG:STG = 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10) and (b) NanTong coal and FuShun peat from China.
3.5. Comparing Emissions with Traditional Coal Fuels
The comparison of the emissions derived from the co-firing of SCGs and STGs with those produced by regular fuels presents crucial insights into the potential benefits of adopting sustainable fuel alternatives. It should be noted that the emissions produced during the combustion of both SCGs and STGs vary substantially relative to the characteristic properties of regular fuels such as coal, diesel, and natural gas.
Preliminary studies have revealed lower levels of harmful emissions, particularly SO2 and NOX compounds, which significantly contribute to air pollution, from the combustion of SCGs and STGs compared to regular fuels. This is mainly attributed to the low sulfur and nitrogen content present in tea and coffee grounds, which highly contrasts with the relatively elevated values in conventional fuels [38]. The emission of greenhouse gasses, a critical concern in today’s energy sector, is reportedly diminished in SCG and STG combustion. Specifically, the carbon dioxide emissions are lower compared to coal and gas. With the increasing emphasis on carbon reduction and climate change mitigation, such a reduction gives SCGs and STGs a competitive edge over regular, fossil-derived fuels. Unquestionably, coffee and tea waste possesses a relative moisture content that can have a bearing on the emissions. Preliminary findings suggest that this can amplify the total emissions, given that a portion of the energy is expended on the evaporation of water rather than the generation of heat [3,39]. However, ample drying and appropriate processing of these types of bio-waste can decrease their moisture content, mitigating its impact on the emission levels.
It is important to acknowledge that the variability in the emission outcomes from SCG and STG co-firing could be associated with multiple factors. These include the original characteristics of the feedstock, differences in the processing methods, and the specifics of the combustion technologies utilized [40]. Continued research and development efforts are required to establish consistent emission traits for such biofuels for their widespread industrial application.
In summary, the comparatively lower emission rates of harmful compounds from the combustion of SCGs and STGs highlight their potential as an environmentally friendly alternative to conventional fuels. Further technological and infrastructural advancements shall harness this potential, aligning it with environmental sustainability goals.
3.6. Comparing the Results with Traditional Coal Fuels
In this section, we compare the results obtained from co-firing SCGs and STGs with those from traditional fossil fuels in terms of fuel preparation, properties, combustion tests, and emissions. Firstly, concerning fuel preparation, traditional fuels such as coal and petroleum need extensive mining or drilling, which can result in significant environmental damage [26]. In contrast, spent tea and coffee grounds constitute waste materials that are readily available, requiring only drying and, possibly, pelletizing before their conversion into fuel. This preparation process is a more eco-friendly and effective method, prompting a positive move towards spent tea and coffee ground utilization [3].
The fuel properties of SCGs and STGs are also considered favorable when compared with traditional fuels. Both have high calorific values and low moisture and ash contents, which are desirable properties for fuels. Their energy content is approximately 21.16–26.20 MJ/kg, which is fairly competitive against conventional fuels like coal. In addition, the organic compounds found in SCGs and STGs can lead to a higher efficiency during combustion, enhancing the potential of their position as alternative fuels. Interestingly, in the combustion tests conducted, waste tea and coffee grounds showed a stable and efficient combustion process. The reaction was relatively slow and controlled due to a lower volatility, allowing for a more efficient energy extraction [4,41]. On the other hand, the use of traditional fuels such as coal often leads to unstable and faster burning rates, indicative of a less efficient energy usage.
Finally, emissions from the combustion of SCGs and STGs were found to be within the permissible limits. They produced lower levels of CO2 emissions compared to fossil fuels, making them an environmentally friendlier choice. The significant reduction in SO2 and NOX emissions, major culprits in the formation of acid rain, further underscores their potential as an alternative fuel source.
Overall, the co-firing of SCGs and STGs provides several potential benefits over traditional fossil fuels. These include a more sustainable and cost-effective fuel preparation, favorable fuel properties, an efficient combustion process, and reduced harmful emissions [4]. However, it is crucial to note that more extensive research and pilot tests are needed to optimize the co-firing process and evaluate the long-term implications of this alternative fuel source.
4. Conclusions
In this study, the fuel properties of SCGs and STGs after drying and pelletizing were analyzed. The results showed that the calorific value of the biomass pellets met the requirements of commercial solid biofuels. In our studies, we observed that the biomass mixture displayed a relatively high bulk density, which allowed for easier handling and transportation. The moisture content was found to be minimal, suggesting that the drying process had been effectively carried out prior to co-firing. The SCG and STG biomass mixture possessed several advantageous properties, optimal for co-firing applications. These included a high calorific value, low ash, sulfur, and nitrogen contents, favorable grindability, and stable thermal degradation behaviors. More in-depth studies need to be carried out to fully understand the combustion and emission behaviors of this novel biomass fuel, particularly on a larger, industrial scale. In our study, subsequent combustion tests showed that the SCG and STG pellets could achieve stable combustion in small-scale burners, and their sulfur oxide (SO2) emissions were lower than those of traditional coal fuels. Since SCGs and STGs showed good emission characteristics in thermal power applications, these biomass pellets could be used as economically viable alternative fuels in power plants, schools, hospitals, and private gardens. In addition, the ash obtained after combustion is rich in nutrients and can be used as an organic soil conditioner or fertilizer. However, how to achieve the large-scale collection and processing of SCGs and STGs and ensure their economic feasibility remains a challenge to be solved. At the same time, future research also needs to further evaluate the heavy metal and toxin content in the leftover ash to ensure its environmental friendliness. In summary, SCGs and STGs have great research potential and application prospects as renewable energy sources, but technical and economic barriers need to be overcome to achieve their widespread application.
Author Contributions
Conceptualization, S.W. and Y.W.; methodology, S.W. and D.L.; software, S.W.; validation, S.W.; formal analysis, S.W.; investigation, S.W.; resources, S.W.; data curation, S.W.; writing—original draft preparation, S.W.; writing—review and editing, S.W., Q.W. and W.W.; visualization, S.W.; supervision, Q.W.; project administration, Q.W.; and funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Basic Research (B) (Number. 22H03747, FY2022-FY2024) of the Grant-in-Aid for Scientific Research of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We also thank the Comprehensive Analysis Center for Science, Saitama University for allowing us to conduct some analyses and providing insight and expertise that greatly assisted the research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are provided in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shahzad, U. The Need For Renewable Energy Sources. ITEE J. 2017, 2, 16–18. [Google Scholar]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Haldar, D.; Purkait, M.K. Potential and Sustainable Utilization of Tea Waste: A Review on Present Status and Future Trends. J. Environ. Chem. Eng. 2021, 9, 106179. [Google Scholar] [CrossRef]
- Sermyagina, E.; Mendoza Martinez, C.L.; Nikku, M.; Vakkilainen, E. Spent Coffee Grounds and Tea Leaf Residues: Characterization, Evaluation of Thermal Reactivity and Recovery of High-Value Compounds. Biomass Bioenergy 2021, 150, 106141. [Google Scholar] [CrossRef]
- Agrawal, D.; Gopaliya, D.; Willoughby, N.; Khare, S.K.; Kumar, V. Recycling Potential of Brewer’s Spent Grains for Circular Biorefineries. Curr. Opin. Green Sustain. Chem. 2023, 40, 100748. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Dinesh Kumar, M.; Preethi; Atabani, A.E.; Kumar, G. Biorefinery of Spent Coffee Grounds Waste: Viable Pathway towards Circular Bioeconomy. Bioresour. Technol. 2020, 302, 122821. [Google Scholar] [CrossRef]
- Kalair, A.R.; Seyedmahmoudian, M.; Stojcevski, A.; Abas, N.; Khan, N. Waste to Energy Conversion for a Sustainable Future. Heliyon 2021, 7, e08155. [Google Scholar] [CrossRef]
- Tymoszuk, M.; Mroczek, K.; Kalisz, S.; Kubiczek, H. An Investigation of Biomass Grindability. Energy 2019, 183, 116–126. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.C.; Loha, C.; Akinlabi, E.T. An Overview of Biomass Solid Fuels: Biomass Sources, Processing Methods, and Morphological and Microstructural Properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Roni, M.S.; Chowdhury, S.; Mamun, S.; Marufuzzaman, M.; Lein, W.; Johnson, S. Biomass Co-Firing Technology with Policies, Challenges, and Opportunities: A Global Review. Renew. Sustain. Energy Rev. 2017, 78, 1089–1101. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and Utilization of Fuel Pellets from Biomass: A Review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Pua, F.L.; Subari, M.S.; Ean, L.W.; Krishnan, S.G. Characterization of Biomass Fuel Pellets Made from Malaysia Tea Waste and Oil Palm Empty Fruit Bunch. In Proceedings of the Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 31, pp. 187–190. [Google Scholar]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Kang, S.B.; Oh, H.Y.; Kim, J.J.; Choi, K.S. Characteristics of Spent Coffee Ground as a Fuel and Combustion Test in a Small Boiler (6.5 KW). Renew. Energy 2017, 113, 1208–1214. [Google Scholar] [CrossRef]
- Eisenbies, M.H.; Volk, T.A.; Amidon, T.E.; Shi, S. Influence of Blending and Hot Water Extraction on the Quality of Wood Pellets. Fuel 2019, 241, 1058–1067. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, N.S.; Madhyastha, H. Environmental Pollutants and Their Effects on Human Health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, G.R.; Mahajan, A.; Seth, R.; Mahajan, R. Spent Coffee Ground Briquettes: A Critical Review. In Opportunities and Challenges in Climate-Friendly Clean Water and Energy Technologies; IGI Global: Hershey, PA, USA, 2023; pp. 244–270. [Google Scholar]
- Lee, X.J.; Ong, H.C.; Gao, W.; Ok, Y.S.; Chen, W.H.; Goh, B.H.H.; Chong, C.T. Solid Biofuel Production from Spent Coffee Ground Wastes: Process Optimisation, Characterisation and Kinetic Studies. Fuel 2021, 292, 120309. [Google Scholar] [CrossRef]
- Tatàno, F.; Caramiello, C.; Paolini, T.; Tripolone, L. Generation and Collection of Restaurant Waste: Characterization and Evaluation at a Case Study in Italy. Waste Manag. 2017, 61, 423–442. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Ngo, T.C.Q.; Nguyen, Q.V.; Do, T.S.; Hoang, N.B. Chemistry Potential and Application of Activated Carbon Manufactured from Coffee Grounds in the Treatment of Wastewater: A Review. Mater. Today Proc. 2022, 60, 1914–1919. [Google Scholar] [CrossRef]
- Bejenari, V.; Marcu, A.; Ipate, A.M.; Rusu, D.; Tudorachi, N.; Anghel, I.; Şofran, I.E.; Lisa, G. Physicochemical Characterization and Energy Recovery of Spent Coffee Grounds. J. Mater. Res. Technol. 2021, 15, 4437–4451. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; He, H.; Sun, Y. Analysis of Densification Mechanisms of Feed Pelleting. Biosyst. Eng. 2023, 234, 92–107. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The Role of Lignin and Lignin-Based Materials in Sustainable Construction—A Comprehensive Review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Lachman, J.; Lisý, M.; Baláš, M.; Matúš, M.; Lisá, H.; Milčák, P. Spent Coffee Grounds and Wood Co-Firing: Fuel Preparation, Properties, Thermal Decomposition, and Emissions. Renew. Energy 2022, 193, 464–474. [Google Scholar] [CrossRef]
- de Souza, H.J.P.L.; Arantes, M.D.C.; Vidaurre, G.B.; Andrade, C.R.; Carneiro, A.d.C.O.; de Souza, D.P.L.; Protásio, T.d.P. Pelletization of Eucalyptus Wood and Coffee Growing Wastes: Strategies for Biomass Valorization and Sustainable Bioenergy Production. Renew. Energy 2020, 149, 128–140. [Google Scholar] [CrossRef]
- Thapa, S.; Engelken, R. Optimization of Pelleting Parameters for Producing Composite Pellets Using Agricultural and Agro-Processing Wastes by Taguchi-Grey Relational Analysis. Carbon Resour. Convers. 2020, 3, 104–111. [Google Scholar] [CrossRef]
- ASTM D3173; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017.
- Wang, Q.; Kawamura, S. Decayed Woody Material from Mushroom Cultivation: Characterization of Liquefaction. WIT Trans. Ecol. Environ. 2018, 217, 481–492. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Wang, Q. Combined Experimental and Molecular Dynamics Removal Processes of Contaminant Phenol from Simulated Wastewater by Polyethylene Terephthalate Microplastics. Environ. Technol. 2024, 45, 1183–1202. [Google Scholar] [CrossRef]
- Paulhiac, D.; Cuenot, B.; Riber, E.; Esclapez, L.; Richard, S. Analysis of the Spray Flame Structure in a Lab-Scale Burner Using Large Eddy Simulation and Discrete Particle Simulation. Combust. Flame 2020, 212, 25–38. [Google Scholar] [CrossRef]
- Merckel, R.D.; Labuschagne, F.J.W.J.; Heydenrych, M.D. Oxygen Consumption as the Definitive Factor in Predicting Heat of Combustion. Appl. Energy 2019, 235, 1041–1047. [Google Scholar] [CrossRef]
- JIS M8813 Standard. Available online: https://kikakurui.com/m/M8813-2006-01.html (accessed on 1 July 2019).
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhang, J.; Zhao, J.; Liu, Y.; Sun, L.; Liu, B.; Mao, H.; Lin, Y.; Li, W.; et al. Evaluation of the Potential of Pelletized Biomass from Different Municipal Solid Wastes for Use as Solid Fuel. Waste Manag. 2018, 74, 260–266. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Z.; Yu, T.; Yan, F. Spent Coffee Grounds: Present and Future of Environmentally Friendly Applications on Industries—A Review. Trends Food Sci. Technol. 2024, 143, 104312. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Wang, H.; Tan, M.; Zhang, G.; Huang, Z.; Yuan, X. A Review on Migration and Transformation of Nitrogen during Sewage Sludge Thermochemical Treatment: Focusing on Pyrolysis, Gasification and Combustion. Fuel Process. Technol. 2023, 240, 107562. [Google Scholar] [CrossRef]
- Rijo, B.; Soares Dias, A.P.; Ramos, M.; de Jesus, N.; Puna, J. Catalyzed Pyrolysis of Coffee and Tea Wastes. Energy 2021, 235, 121252. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Tejasvi, R. A Review on Role of Process Parameters on Pyrolysis of Biomass and Plastics: Present Scope and Future Opportunities in Conventional and Microwave-Assisted Pyrolysis Technologies. Process Saf. Environ. Prot. 2022, 162, 435–462. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).