Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China
Abstract
1. Introduction
2. Materials and Methods
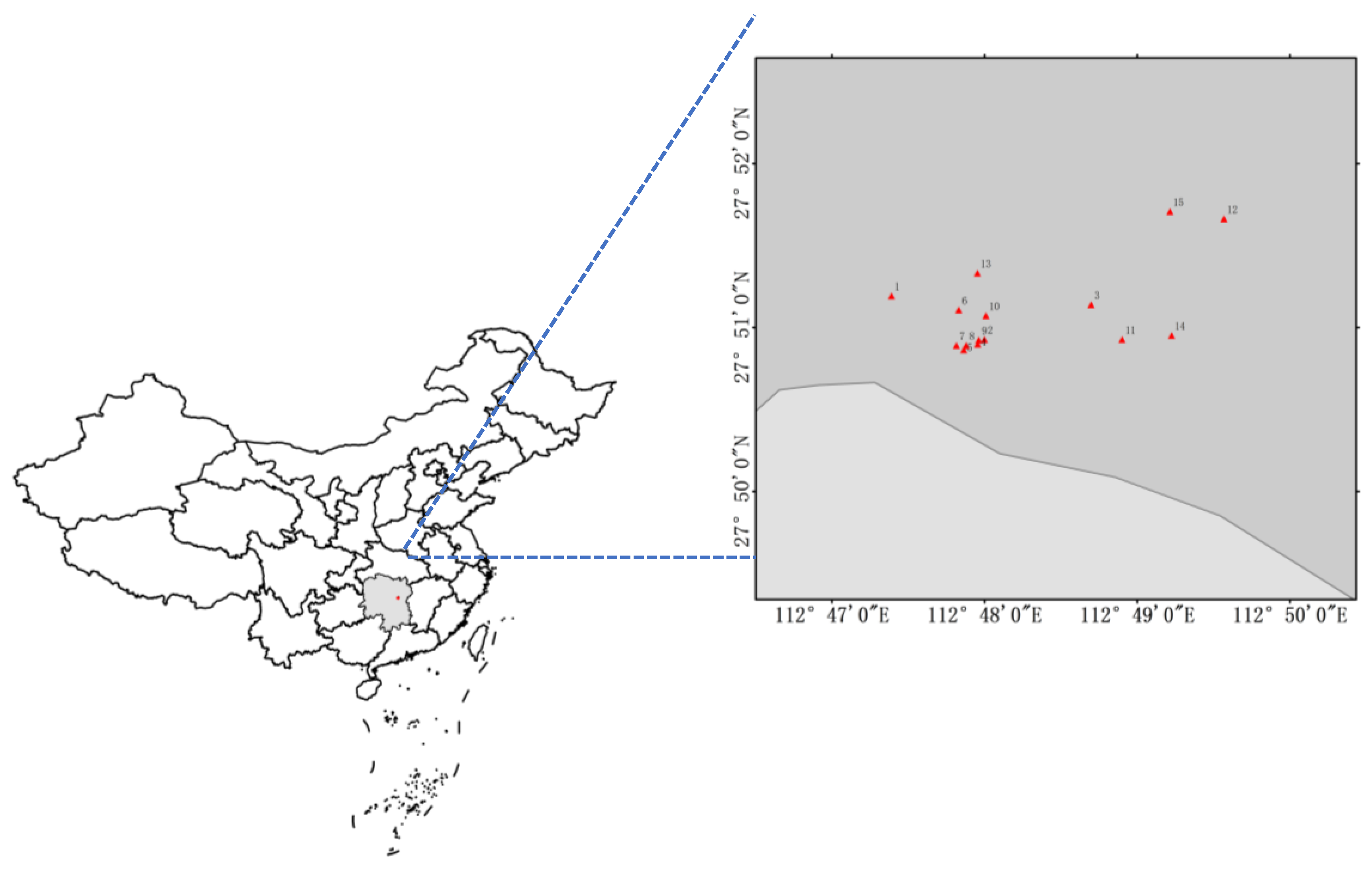
2.1. Overview of the Study Area
2.2. Experimental Reagents
2.3. Experimental Instruments
2.4. Soil Sample Collection and Pretreatment
2.5. Separation and Digestion of Soil Microplastics
2.6. Identification and Analysis of Microplastics
2.7. Data Processing
2.8. Quality Control
3. Results and Analysis
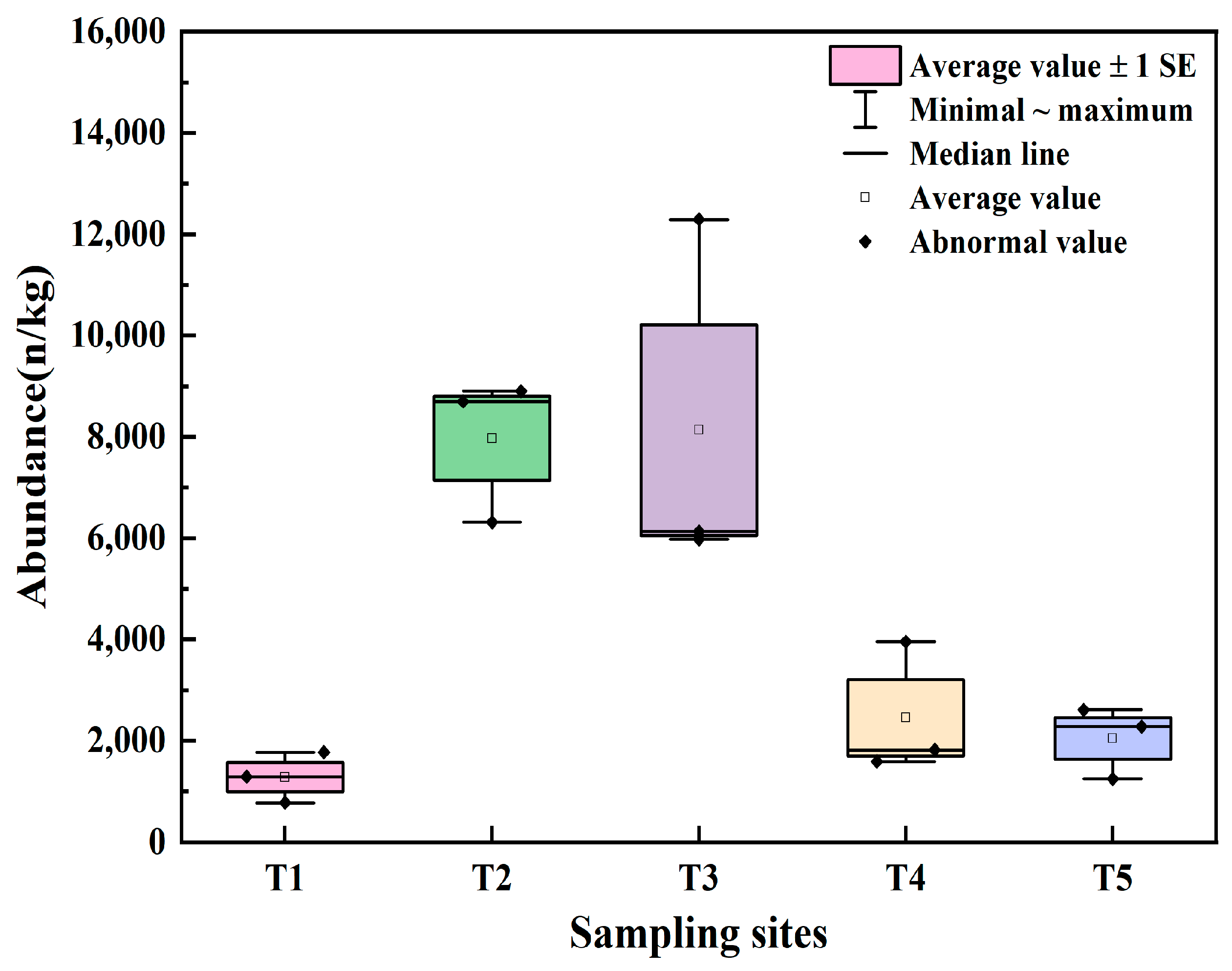
3.1. Abundance of Microplastics
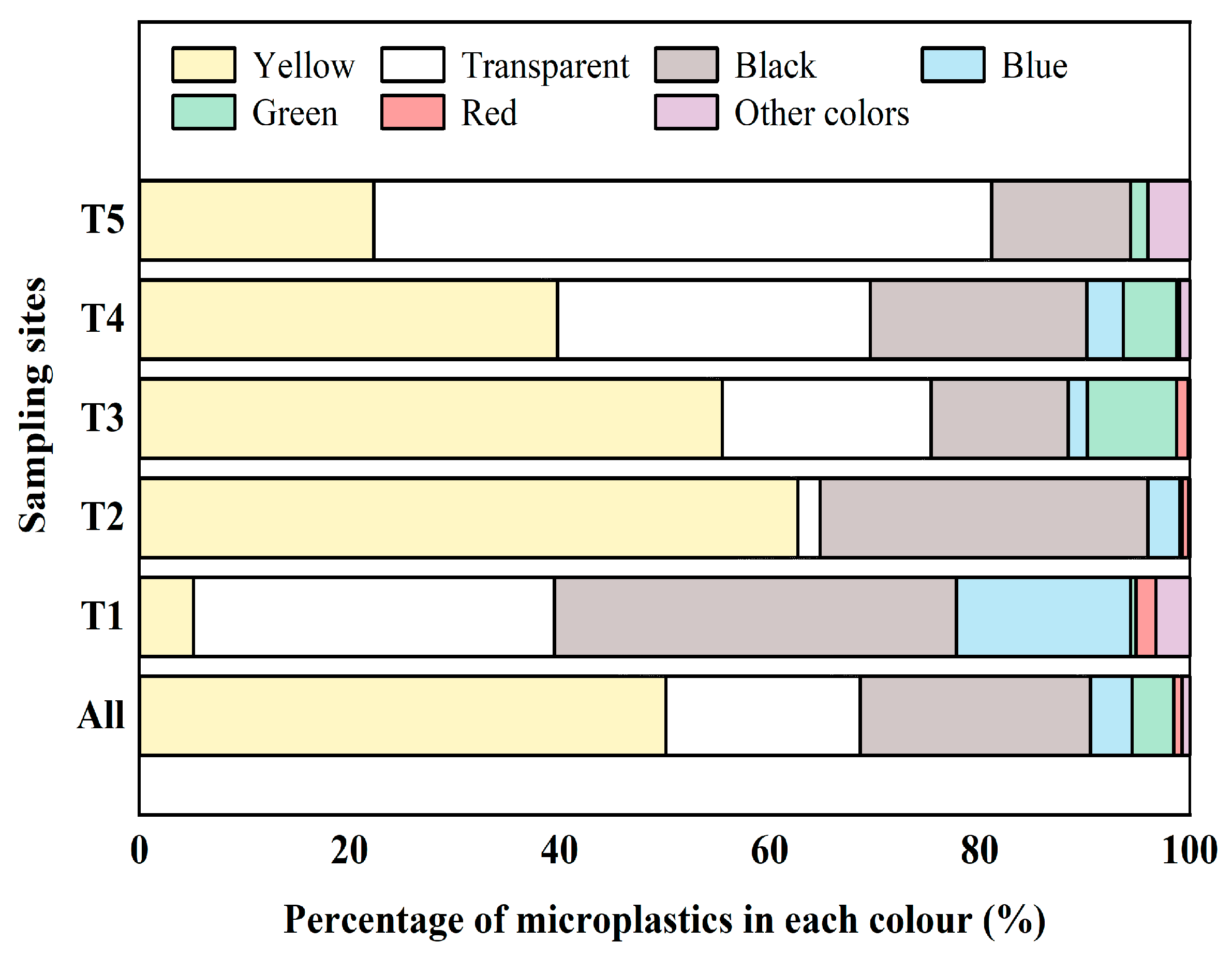
3.2. Color of Microplastics
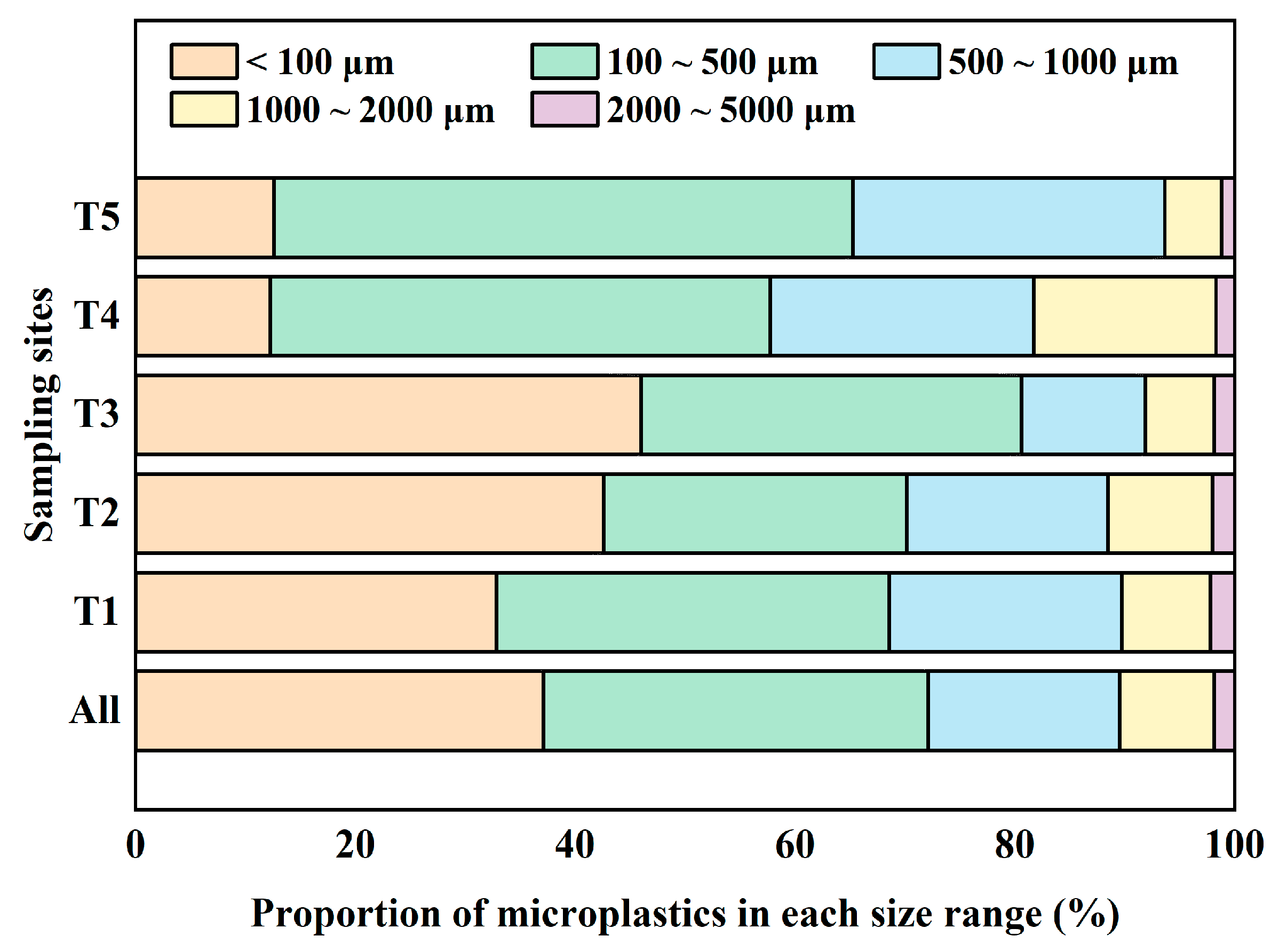
3.3. Particle Size of Microplastics
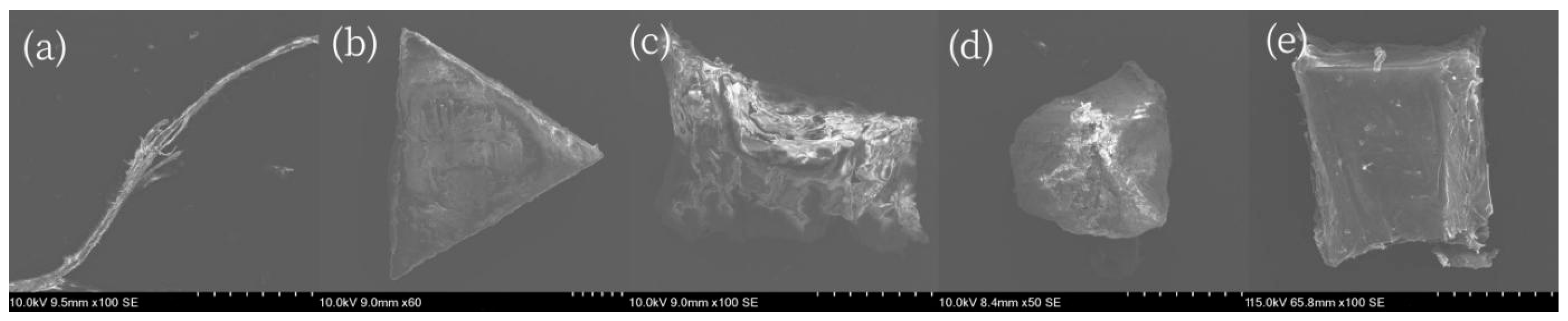
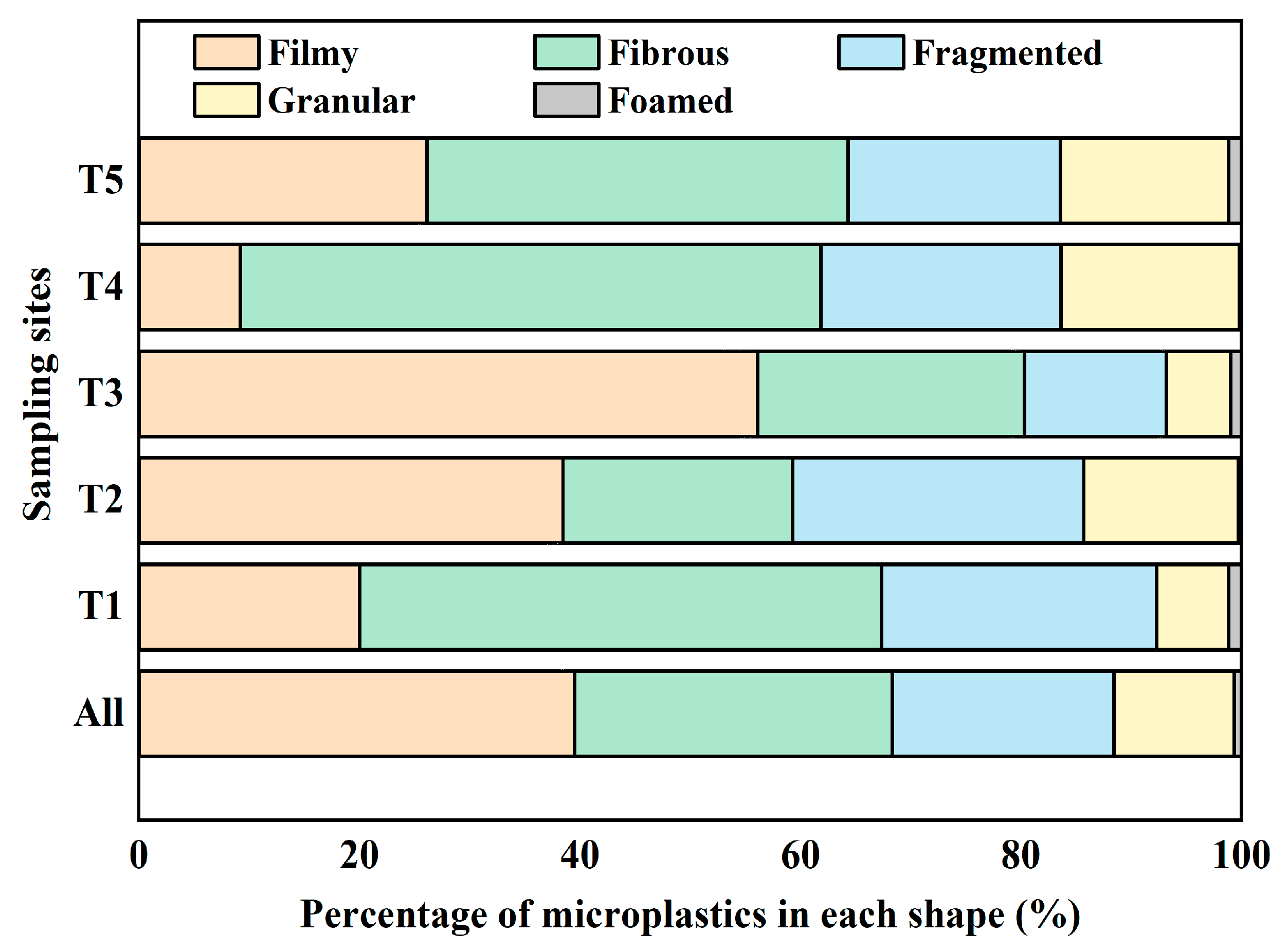
3.4. Shapes of Microplastics
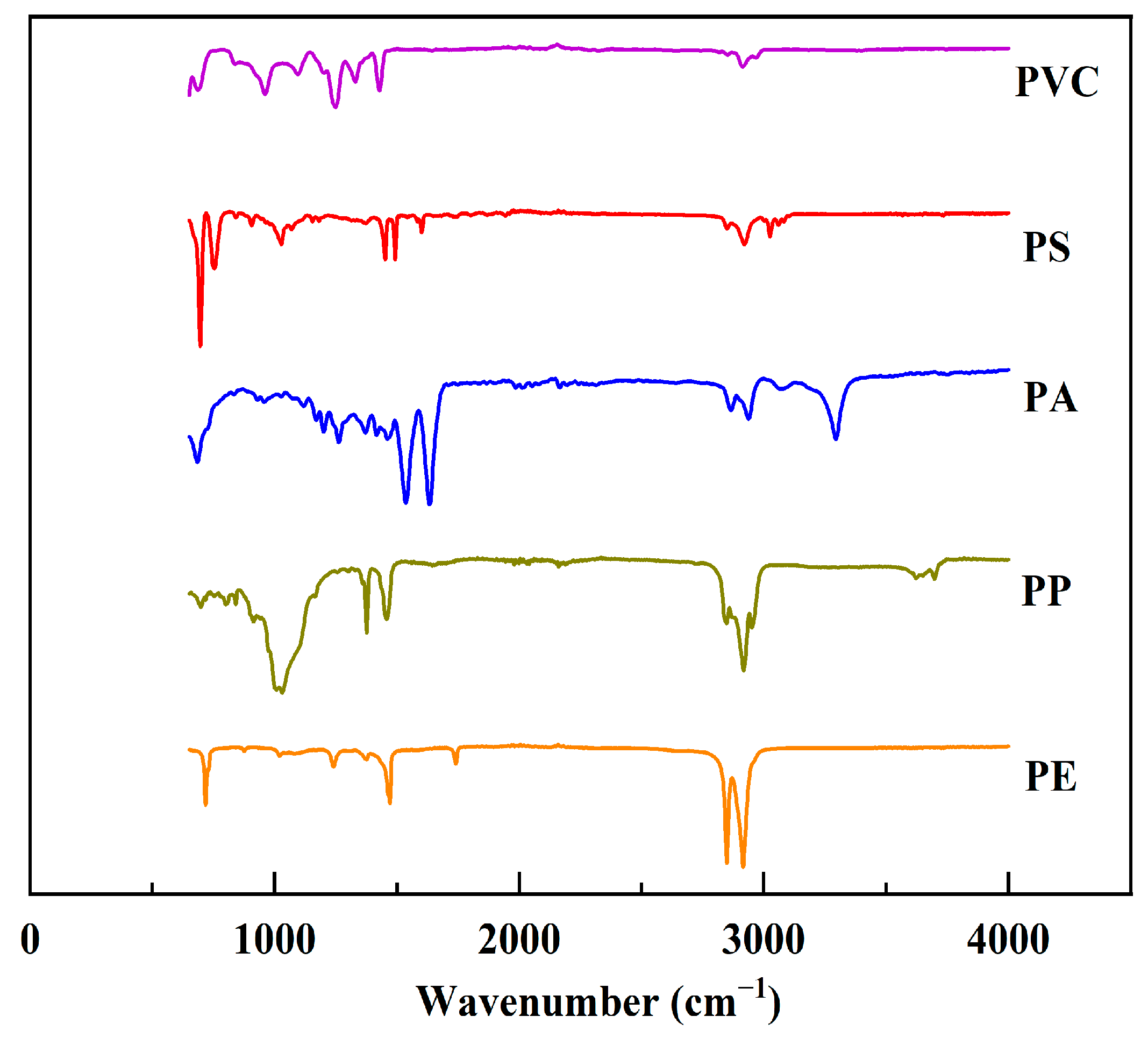
3.5. Types of Microplastics
4. Discussion
4.1. Pollution Status of Microplastics in Agricultural Soils in Xiangtan
4.2. Comparison of Microplastic Characteristics of Agricultural Soils
4.3. Potential Sources of Soil Microplastics
5. Conclusions
- (1)
- Microplastics were found in each of the 15 sampling locations of agricultural soils in Xiangtan, China. The average abundance of microplastics was 4377.44 items/kg, with a maximum abundance of 12,292.33 items/kg. Microplastics of a small and medium size (<500 μm) dominated, and their colors were mainly yellow, transparent, and black; their shapes were mainly filmy and fibrous; and their types mainly included PE and PP.
- (2)
- Variations in the prevalence of microplastics were observed in the soil across different farming techniques. The abundance of microplastics in the vegetable soil with agricultural films was four times higher than that without agricultural films, the number of microplastics in the vegetable soil in greenhouses was slightly higher than that in the open fields, and the average abundance of microplastics in the vegetable soil in the open fields without agricultural films was 1.60 times more than that in the rice soil.
- (3)
- The main factor that led to the presence of microplastics in agricultural soils in Xiangtan was the utilization of agricultural films. Additionally, irrigation water and waste plastic products were also being noted as potential sources of microplastics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Pilapitiya, P.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Huang, B.; Sun, L.; Liu, M.; Huang, H.; He, H.; Han, F.; Wang, X.; Xu, Z.; Li, B.; Pan, X. Abundance and Distribution Characteristics of Microplastic in Plateau Cultivated Land of Yunnan Province, China. Environ. Sci. Pollut. Res. 2021, 28, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Ryan, P.G.; Moore, C.J.; Van Franeker, J.A.; Moloney, C.L. Monitoring the Abundance of Plastic Debris in the Marine Environment. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, C.; Li, J.; Zhang, Y.; Zhang, X.; Zhang, P.; Liu, X. High Salinity Alters the Adsorption Behavior of Microplastics towards Typical Pollutants and the Phytotoxicity of Microplastics to Synechococcus. Sustainability 2024, 16, 1107. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric Microplastics: A Review on Current Status and Perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 2018, 137, 362–374. [Google Scholar]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A Review of Microplastic Distribution in Sediment Profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic Marine System: An Emerging Area of Research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic Sea Ice Is an Important Temporal Sink and Means of Transport for Microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhou, Y.; Huang, Y. A review of population exposure routes of microplastics and their possible health hazards. Asian J. Ecotoxicol. 2021, 16, 221–227. [Google Scholar]
- Xie, J.; Zhang, J.; Wei, H.; Liu, Z.; Chen, X. Microplastic-based compound pollution in soil: An overview. Ecol. Environ. Sci. 2022, 31, 2431–2440. [Google Scholar]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the Soil Environment: Occurrence, Risks, Interactions and Fate—A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Gui, J.; Sun, Y.; Wang, J.; Chen, X.; Zhang, S.; Wu, D. Microplastics in Composting of Rural Domestic Waste: Abundance, Characteristics, and Release from the Surface of Macroplastics. Environ. Pollut. 2021, 274, 116553. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in Soil: Analytical Methods and Possible Sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene Film Incorporation into the Horticultural Soil of Small Periurban Production Units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Soil: A Review on Methods, Occurrence, Sources, and Potential Risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and Quantification of Macro- and Microplastics on an Agricultural Farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and Transport of Microplastics from Biosolids to Agricultural Soils and the Wider Environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Distribution of Microplastics in Shanghai Farmland Soil and Its Adsorption Characteristics of Heavy Metals; Shanghai Second Polytechnic University: Shanghai, China, 2021. [Google Scholar]
- Yu, Q.; Liu, S.; Ma, L.; Men, Z.; Li, T.; Cai, L.; Sun, M. Analysis on the occurrence characteristics and influencing factors of microplastics in Harbin agricultural soils. Chin. Environ. Sci. 2023, 43, 793–799. [Google Scholar]
- Shi, X.; Sun, L.; Li, Z.; Lv, L. Composition and distribution of microplastics in farmland soil around Shenyang. J. Agro-Environ. Sci. 2021, 40, 1498–1508. [Google Scholar]
- Lin, J.; Li, Z.; Yu, G.; Zhang, Y.; Song, Y.; Wang, G.; Jiang, X. Optimization of microplastics extraction from soil by density separation. Chin. Environ. Sci. 2022, 42, 3285–3294. [Google Scholar]
- Mausra, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment. Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015. [Google Scholar]
- Yahyanezhad, N.; Bardi, M.J.; Aminirad, H. An Evaluation of Microplastics Fate in the Wastewater Treatment Plants: Frequency and Removal of Microplastics by Microfiltration Membrane. Water. Pract. Technol. 2021, 16, 782–792. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in Agricultural Soils on the Coastal Plain of Hangzhou Bay, East China: Multiple Sources Other than Plastic Mulching Film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Wang, X.; Yang, X.; Zhang, C.; Qi, Y.; Guo, X. The Occurrence and Distribution Characteristics of Microplastics in the Agricultural Soils of Shaanxi Province, in North-Western China. Sci. Total Environ. 2020, 720, 137525. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of Microplastics and the Association of Heavy Metals with Microplastics in Suburban Soil of Central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The Distribution of Microplastics in Soil Aggregate Fractions in Southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic Pollution in Vegetable Farmlands of Suburb Wuhan, Central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Pei, Y.; Luo, K.; Yang, W.; Wu, J.; Yue, X.; Wen, J.; Luo, Y. Microplastics in Agricultural Soils: A Comprehensive Perspective on Occurrence, Environmental Behaviors and Effects. Chem. Eng. J. 2024, 489, 151328. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.-Y.; Lee, M.; Lee, S. Comparison of Microplastic Characteristics in Mulched and Greenhouse Soils of a Major Agriculture Area, Korea. J. Polym. Environ. 2023, 31, 2216–2229. [Google Scholar] [CrossRef]
- Yang, J.; Song, K.; Tu, C.; Li, L.; Feng, Y.; Li, R.; Xu, H.; Luo, Y. Distribution and weathering characteristics of microplastics in paddy soils following long-term mulching: A field study in Southwest China. Sci. Total Environ. 2023, 858, 159774. [Google Scholar] [CrossRef]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Gregory, A.S.; Storkey, J.; Stevens, C.J. Agricultural Fertilisers Contribute Substantially to Microplastic Concentrations in UK Soils. Commun. Earth Environ. 2024, 5, 7. [Google Scholar] [CrossRef]
- Tang, K.H.D. Microplastics in Agricultural Soils in China: Sources, Impacts and Solutions. Environ. Pollut. 2023, 322, 121235. [Google Scholar] [CrossRef]
- Liu, M. Study on Characteristics of Microplastic Pollution in Main Types of Soil in Suburb of Shanghai; East China Normal University: Shanghai, China, 2020. [Google Scholar]
- Cao, L.; Wu, D.; Liu, P.; Hu, W.; Xu, L.; Sun, Y.; Wu, Q.; Tian, K.; Huang, B.; Yoon, S.J.; et al. Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Sci. Total Environ. 2021, 794, 148694. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution Characteristics of Microplastics in Agricultural Soils from the Largest Vegetable Production Base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef]
- Sankoda, K.; Yamada, Y. Occurrence, Distribution, and Possible Sources of Microplastics in the Surface River Water in the Arakawa River Watershed. Environ. Sci. Pollut. Res. 2021, 28, 27474–27480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, L.; Li, D. Microplastic in Three Urban Estuaries, China. Environ. Pollut. 2015, 206, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic Abundance, Distribution and Composition in Water, Sediments, and Wild Fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Singh, S.; Chakma, S.; Alawa, B.; Kalyanasundaram, M.; Diwan, V. Assessment of Microplastic Pollution in Agricultural Soil of Bhopal, Central India. J. Mater. Cycles Waste 2024, 26, 708–722. [Google Scholar] [CrossRef]
- Han, L.; Li, Q.; Xu, L.; Lu, A.; Gong, W.; Yu, Q. The pollution characteristics of microplastics in Daliao River sediments. Chin. Environ. Sci. 2020, 40, 1649–1658. [Google Scholar]



| Instrument | Model |
|---|---|
| Vacuum Filtration Pump | SHZ-D (III) (Shanghai Huichuang Chemical Instrument Co., Shanghai, China) |
| Constant Temperature Heating Magnetic Stirrer | 85-2A (Changzhou Yuexin Instrument Manufacture Co., Changzhou, China) |
| Electronic Analytical Balance | TD20002A (Tianjin Tianma Hengji Instrument Co., Tianjin, China) |
| Electric Heating Blast Dryer | DHG-9023A (Shanghai Yiheng Scientific Instrument Co., Shanghai, China) |
| Water Bath Thermostat Oscillator | SHA-B (Changzhou Guowang Instrument Manufacturing Co., Changzhou, China) |
| Stereomicroscopy | SZN71 (Sunny optical technology (group) Co., LTD, Ningbo, China) |
| Fourier Transform Infrared Spectroscopy | NicoletTM iS20 (Thermo Fisher Scientific, Waltham, MA, USA) |
| Sampling Site No. | Soil Type | Form of Cultivation | Use of Agricultural Films | |
|---|---|---|---|---|
| T1 | 1 | Rice soil | Open field | Not used |
| 2 | ||||
| 3 | ||||
| T2 | 4 | Vegetable soil | Greenhouse | Used |
| 5 | ||||
| 6 | ||||
| T3 | 7 | Vegetable soil | Open field | Used |
| 8 | ||||
| 9 | ||||
| T4 | 10 | Vegetable soil | Greenhouse | Not used |
| 11 | ||||
| 12 | ||||
| T5 | 13 | Vegetable soil | Open field | Not used |
| 14 | ||||
| 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Lin, J.; Li, Z.; Wang, G.; Li, Z. Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China. Sustainability 2024, 16, 7254. https://doi.org/10.3390/su16177254
Ye C, Lin J, Li Z, Wang G, Li Z. Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China. Sustainability. 2024; 16(17):7254. https://doi.org/10.3390/su16177254
Chicago/Turabian StyleYe, Cong, Jing Lin, Zhenguo Li, Guanghuai Wang, and Zeling Li. 2024. "Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China" Sustainability 16, no. 17: 7254. https://doi.org/10.3390/su16177254
APA StyleYe, C., Lin, J., Li, Z., Wang, G., & Li, Z. (2024). Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China. Sustainability, 16(17), 7254. https://doi.org/10.3390/su16177254





