Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO)
Abstract
:1. Introduction
2. Materials and Methods
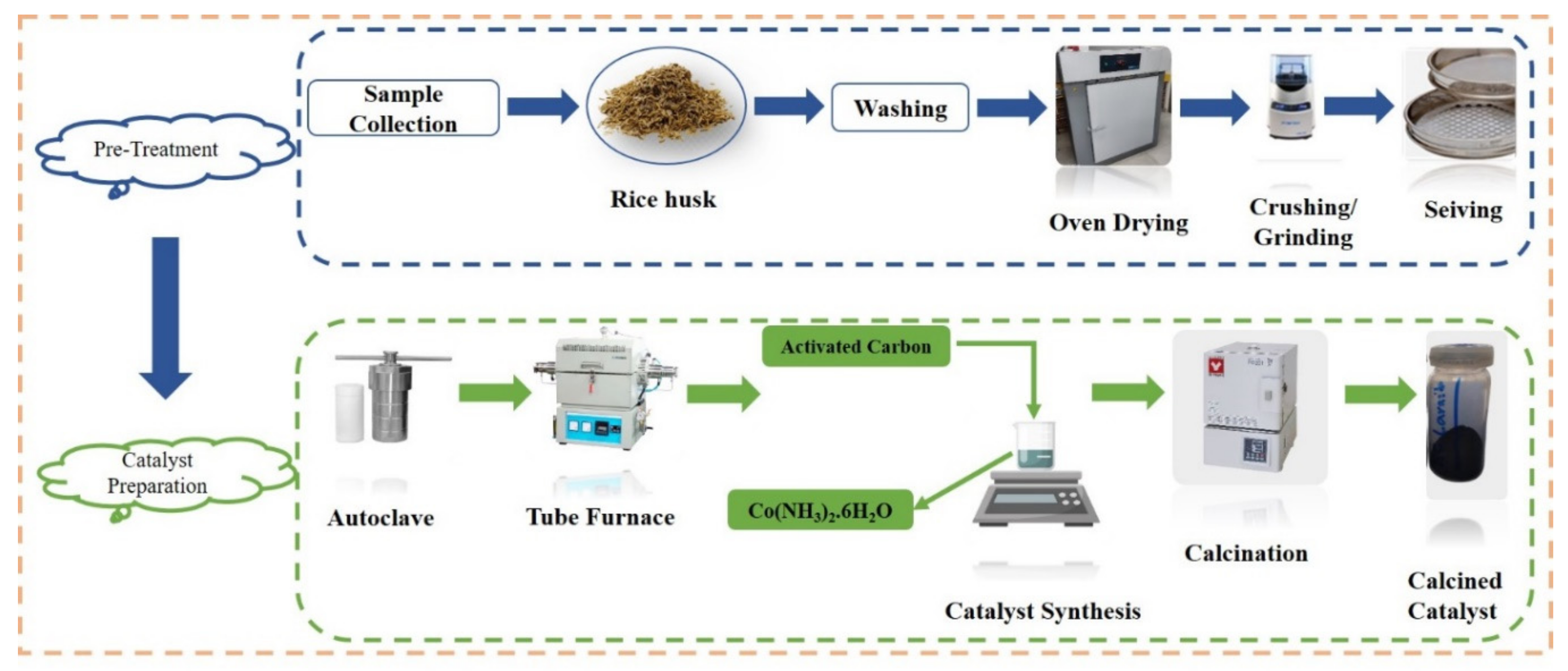
2.1. Material Collection
2.2. Catalyst Synthesis
2.2.1. Mechanical Pre-Treatment of Rice Husk
2.2.2. Hydrochar Production by Hydrothermal Carbonization
2.2.3. Chemical Activation
2.2.4. Preparation of Metal-Loaded Activated Carbon
2.3. Catalyst Characterization
2.4. Biodiesel Production through Transesterification
2.4.1. Esterification
2.4.2. Transesterification
2.5. Characterization of Biodiesel
2.6. Optimization Studies for Biodiesel Yield by Response Surface Methodology (RSM)
Experimental Design
3. Results and Discussion
3.1. Catalyst Characterizations
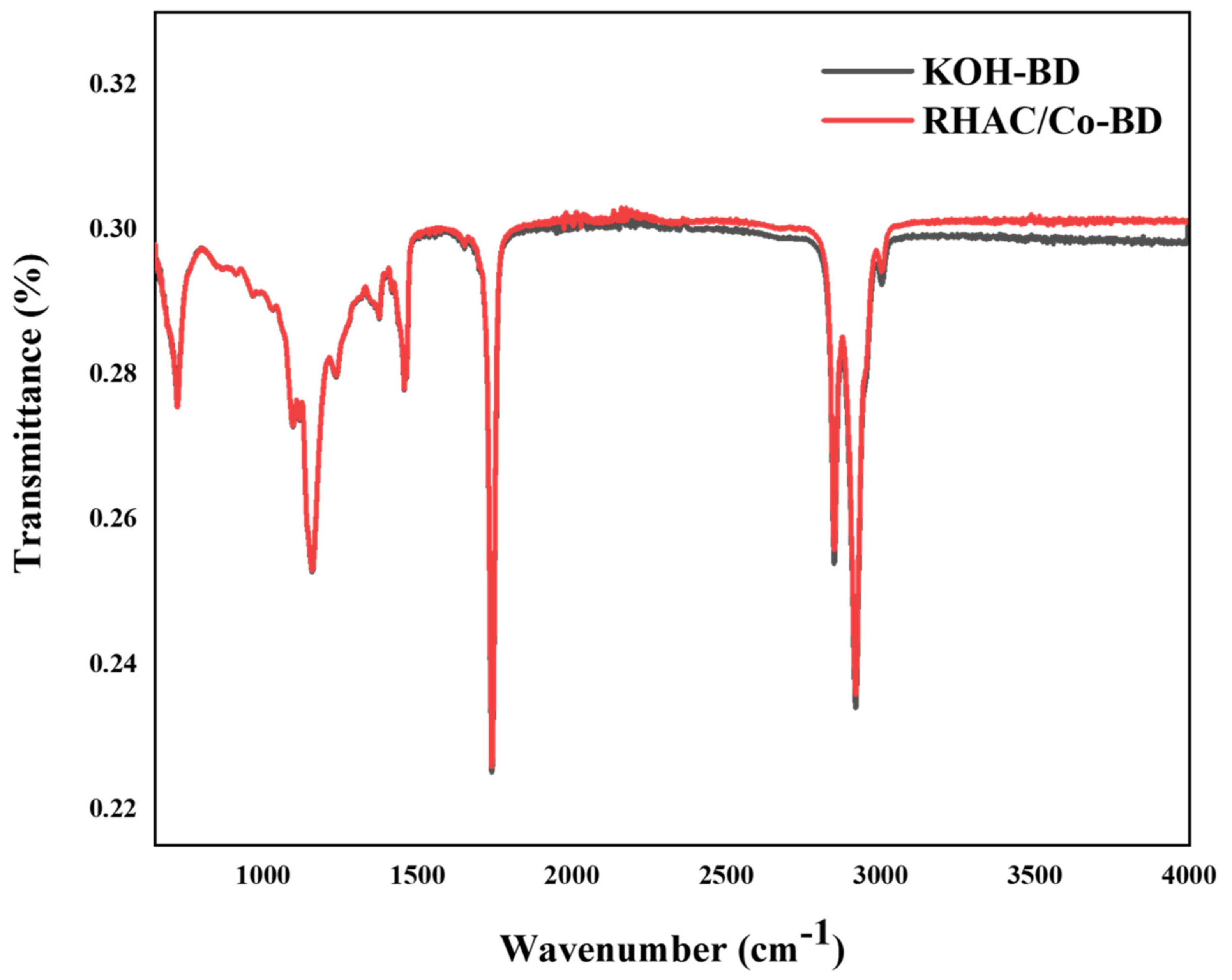
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
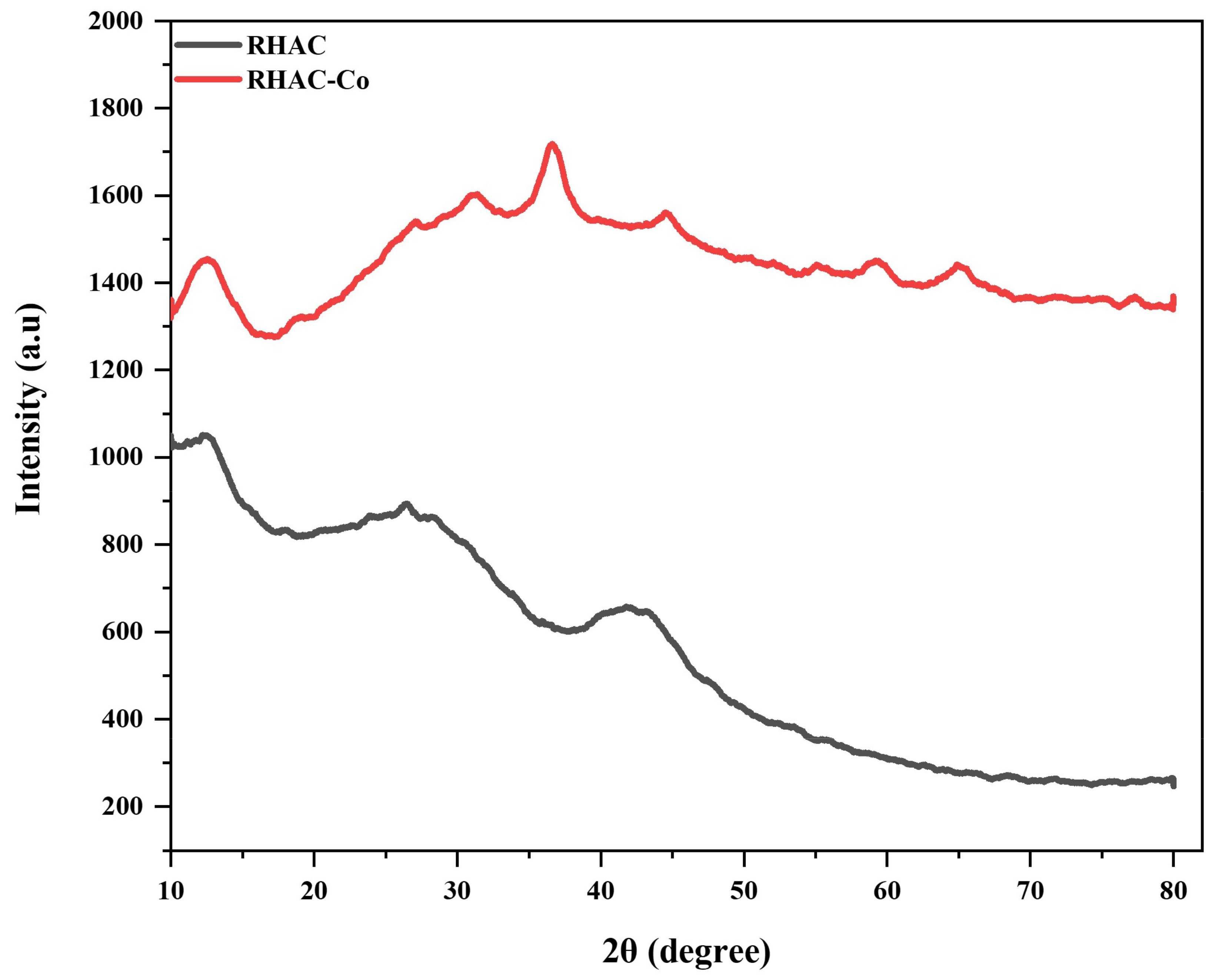
3.1.2. X-ray Diffraction (XRD)
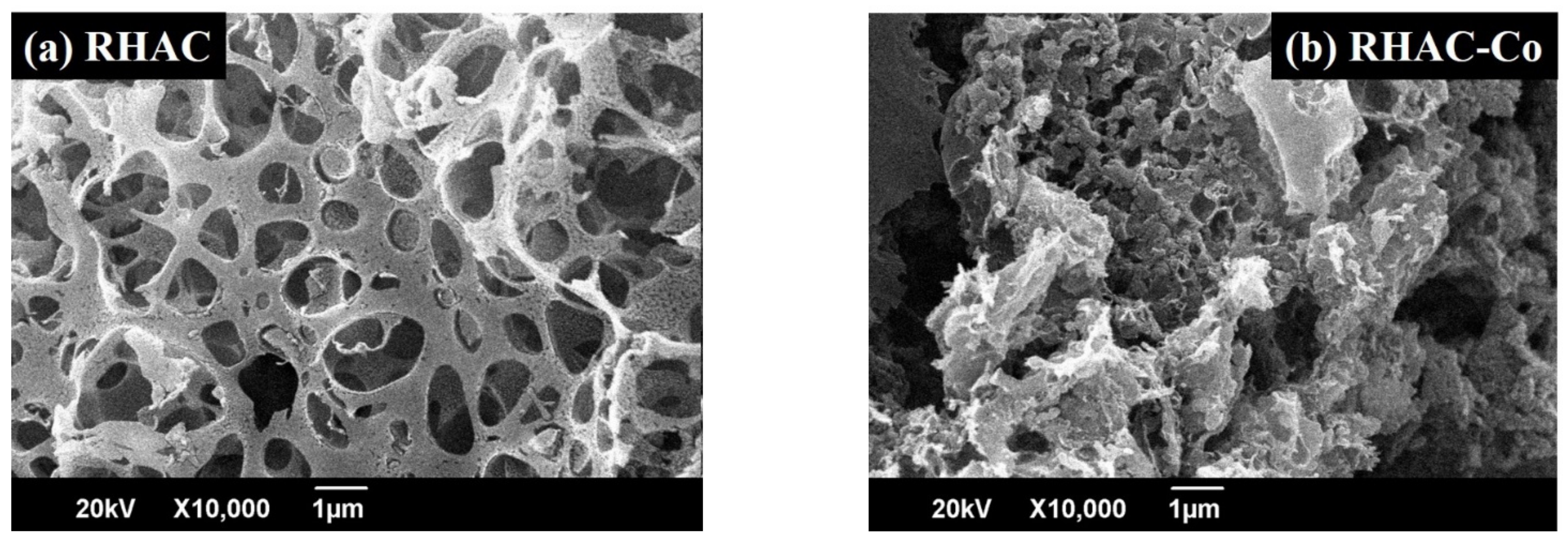
3.1.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
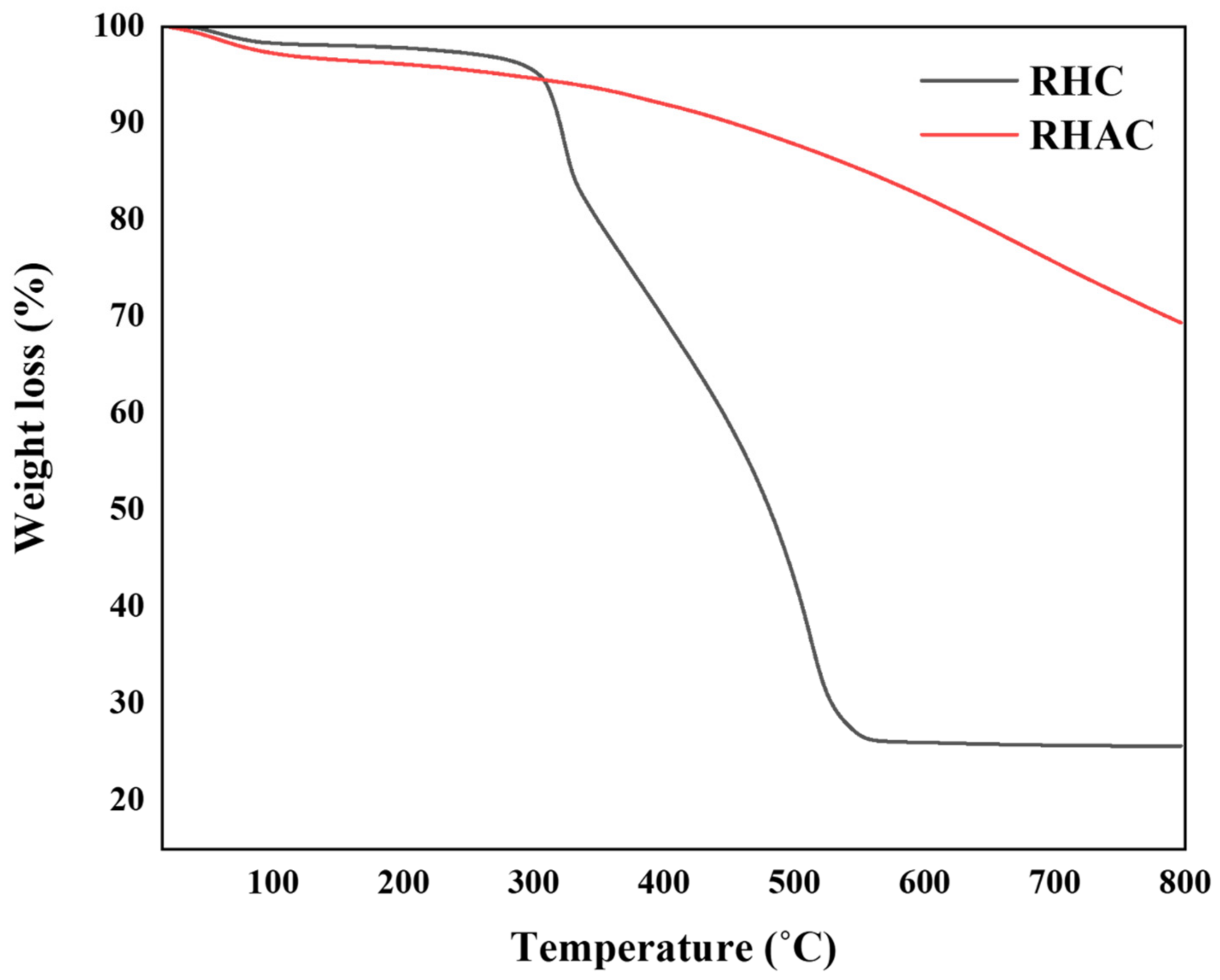
3.1.4. Thermo-Gravimetric Analysis (TGA)
3.1.5. BET Analysis
3.2. Biodiesel Yield Characterization
3.2.1. Esterification
3.2.2. Physio-Chemical Properties of Produced Biodiesel through Transesterification
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
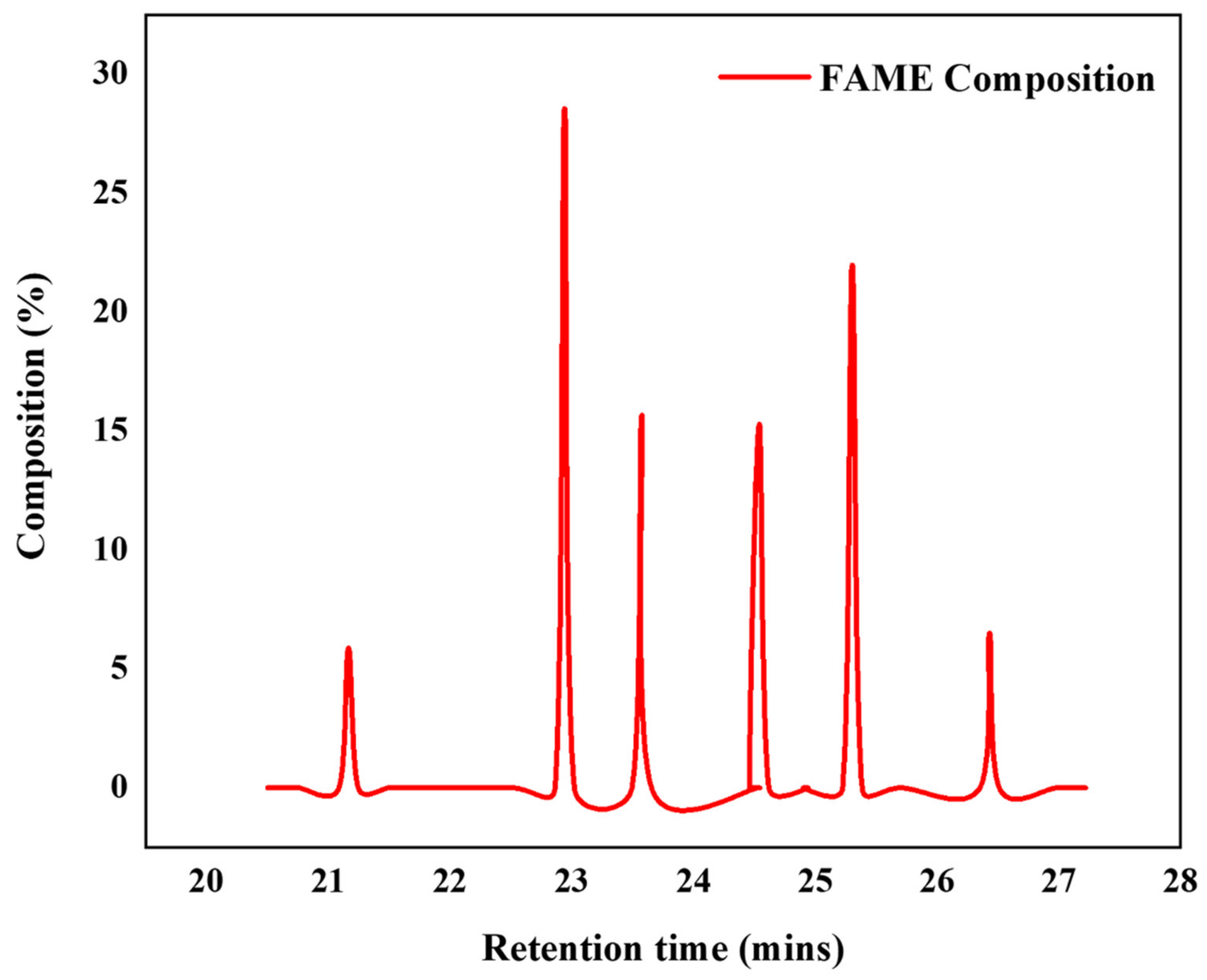
3.2.4. Gas Chromatography-Mass Spectroscopy (GC-MS)
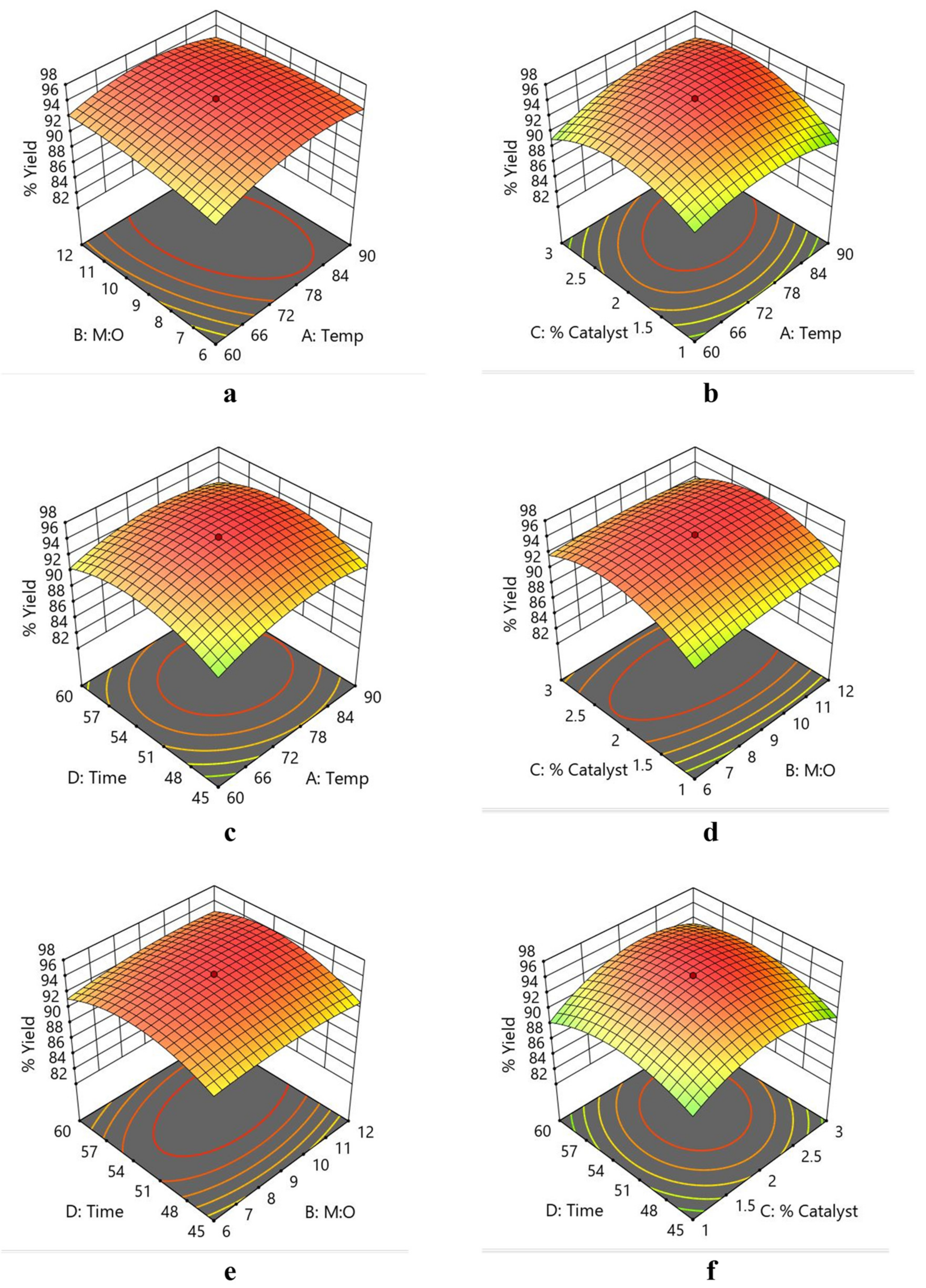
3.3. Optimization Studies for Biodiesel Yield by RSM
Interaction Effect of Independent Variables for Yield Studies
3.4. Significance of Using RHAC-Co Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging technologies for biodiesel production: Processes, challenges, and opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Trivedi, J.; Bangwal, D.P.; Singh, L.P.; Atray, N. Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew. Energy 2021, 170, 302–314. [Google Scholar] [CrossRef]
- Vargas, E.M.; Ospina, L.; Neves, M.C.; Tarelho, L.A.C.; Nunes, M.I. Optimization of FAME production from blends of waste cooking oil and refined palm oil using biomass fly ash as a catalyst. Renew. Energy 2021, 163, 1637–1647. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. An overview of microdiesel—A sustainable future source of renewable energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar] [CrossRef]
- Musthafa, M.M.; Kumar, T.A.; Mohanraj, T.; Chandramouli, R. A comparative study on performance, combustion and emission characteristics of diesel engine fuelled by biodiesel blends with and without an additive. Fuel 2018, 225, 343–348. [Google Scholar] [CrossRef]
- Niju, S.; Janaranjani, A.; Nanthini, R.; Sindhu, P.A.; Balajii, M. Valorization of banana pseudostem as a catalyst for transesterification process and its optimization studies. Biomass Convers. Biorefin. 2023, 13, 1805–1818. [Google Scholar] [CrossRef]
- Yameen, M.Z.; Naqvi, S.R.; AlMohamadi, H.; Wang, S. Process optimization, kinetic, and thermodynamic studies of biodiesel production using KOH-modified bio-carbon catalyst derived from marine macroalgae. Carbon Lett. 2023, 33, 1571–1590. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Akram, F.; Haq, I.U.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current trends in biodiesel production technologies and future progressions: A possible displacement of the petro-diesel. J. Clean. Prod. 2022, 370, 133479. [Google Scholar] [CrossRef]
- Muratori, M.; Kheshgi, H.; Mignone, B.; Clarke, L.; McJeon, H.; Edmonds, J. Carbon capture and storage across fuels and sectors in energy system transformation pathways. Int. J. Greenh. Gas Control 2017, 57, 34–41. [Google Scholar] [CrossRef]
- Leblanc, F.; Bibas, R.; Mima, S.; Muratori, M.; Sakamoto, S.; Sano, F.; Bauer, N.; Daioglou, V.; Fujimori, S.; Gidden, M.J.; et al. The contribution of bioenergy to the decarbonization of transport: A multi-model assessment. Clim. Chang. 2022, 170, 21. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Abro, R.; Harijan, K.; Zhao, Z.; Bazmi, A.A.; Abbas, T.; Yu, G. Progress in the production of biomass-to-liquid biofuels to decarbonize the transport sector-prospects and challenges. RSC Adv. 2016, 6, 32140–32170. [Google Scholar] [CrossRef]
- Hannula, I.; Reiner, D.M. Near-Term Potential of Biofuels, Electrofuels, and Battery Electric Vehicles in Decarbonizing Road Transport. Joule 2019, 3, 2390–2402. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Hassan Shah, M.U.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Don, T.; Sinks, D.; Other, O.R. COOKING OIL Used Cooking Oil Recycling. Processes 2013, 8, 1–13. [Google Scholar]
- Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Kalogirou, S.A.; Gupta, V.K.; Park, Y.-K.; Fallahi, A.; Sulaiman, A.; Ranjbari, M.; Rahnama, H.; Aghbashlo, M.; et al. Environmental life cycle assessment of biodiesel production from waste cooking oil: A systematic review. Renew. Sustain. Energy Rev. 2022, 161, 112411. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Oduwale, A.I.; Owootomo, Y.; Obisesan, O.R.; Elugoke, S.E.; Durodola, S.S.; Akintunde, S.B.; Oluwafemi, O.S. Biodiesel potential of used vegetable oils transesterified with biological catalysts. Energy Rep. 2020, 6, 2861–2871. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, P.H.; Lan, N.V.; Hou, S.S. Enhancement of biodiesel production from high-acid-value waste cooking oil via a microwave reactor using a homogeneous alkaline catalyst. Energies 2021, 14, 437. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.M.; Khan, H.M.; Jamil, A.; Yaqoob, H.; Soudagar, M.E.M.; Imran, M.; Ahmad, M.; Munir, M. Review waste animal bones as catalysts for biodiesel production; a mini review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Su, C.H.; Ong, H.C.; Juan, H.Y.; Wu, S.J. Bio-derived catalysts: A current trend of catalysts used in biodiesel production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- Lee, H.; Wu, W.H.; Chen, B.H.; Liao, J.D. Heterogeneous catalysts using strontium oxide agglomerates depositing upon titanium plate for enhancing biodiesel production. Catalysts 2021, 11, 30. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production process using solid acid catalyst: Influence of market variables on the process’s economic feasibility. Biofuels Bioprod. Biorefin. 2021, 15, 815–824. [Google Scholar] [CrossRef]
- Banurea, I.R.; Setyawan, N.; Yuliani, S.; Herawati, H.; Hoerudin. Utilization of rice husk silica as solid catalyst in the transesterification process for biodiesel production. IOP Conf. Ser. Earth Environ. Sci. 2021, 739, 012083. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Mohammed, E.A.; Shaban, M.; Abou Kana, M.T.H.; Negm, N.A. Synthesis, characterization and catalytic performances of activated carbon-doped transition metals during biofuel production from waste cooking oils. J. Mol. Liq. 2020, 306, 112749. [Google Scholar] [CrossRef]
- Khan, W.U.H.; Khoja, A.H.; Gohar, H.; Naqvi, S.R.; Din, I.U.; Lumbers, B.; Salem, M.A.; Alzahrani, A.Y. In depth thermokinetic investigation on Co-pyrolysis of low-rank coal and algae consortium blends over CeO2 loaded hydrotalcite (MgNiAl) catalyst. J. Environ. Chem. Eng. 2022, 10, 108293. [Google Scholar] [CrossRef]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review. J. Clean. Prod. 2020, 255, 120261. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef]
- Macdermid-Watts, K.; Adewakun, E.; Norouzi, O.; Abhi, T.D.; Pradhan, R.; Dutta, A. Effects of FeCl3 Catalytic Hydrothermal Carbonization on Chemical Activation of Corn Wet Distillers’ Fiber. ACS Omega 2021, 6, 14875–14886. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Omar, R.; Robinson, J.P. Conventional and microwave-assisted pyrolysis of rapeseed oil for bio-fuel production. J. Anal. Appl. Pyrolysis 2014, 105, 131–142. [Google Scholar] [CrossRef]
- Yigezu, Z.D.; Muthukumar, K. Catalytic cracking of vegetable oil with metal oxides for biofuel production. Energy Convers. Manag. 2014, 84, 326–333. [Google Scholar] [CrossRef]
- Koide, T.; Aritome, I.; Saeki, T.; Morita, Y.; Shiota, Y.; Yoshizawa, K.; Shimakoshi, H.; Hisaeda, Y. Cobalt-Carbon Bond Formation Reaction via Ligand Reduction of Porphycene-Cobalt(II) Complex and Its Noninnocent Reactivity. ACS Omega 2018, 3, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, T.; Tye, C.T. Performance of activated carbon supported cobalt oxides and iron oxide catalysts in catalytic cracking of waste cooking oil. Period. Polytech. Chem. Eng. 2021, 65, 350–360. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Mustafa, A.; Ramadan, R.; Niikura, F.; Inayat, A.; Hafez, H. Highly selective synthesis of glyceryl monostearate via lipase catalyzed esterification of triple pressed stearic acid and glycerin. Sustain. Energy Technol. Assess. 2023, 57, 103200. [Google Scholar] [CrossRef]
- Selvaraj, R.; Moorthy, I.G.; Kumar, R.V.; Sivasubramanian, V. Microwave mediated production of FAME from waste cooking oil: Modelling and optimization of process parameters by RSM and ANN approach. Fuel 2019, 237, 40–49. [Google Scholar] [CrossRef]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J. King Saud Univ.—Eng. Sci. 2022, 34, 198–208. [Google Scholar] [CrossRef]
- Yahya, M.D.; Obayomi, K.S.; Abdulkadir, M.B.; Iyaka, Y.A.; Olugbenga, A.G. Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium. Water Sci. Eng. 2020, 13, 202–213. [Google Scholar] [CrossRef]
- Puccini, M.; Ceccarini, L.; Antichi, D.; Seggiani, M.; Tavarini, S.; Hernandez Latorre, M.; Vitolo, S. Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability 2018, 10, 846. [Google Scholar] [CrossRef]
- Khan, M.; Farah, H.; Iqbal, N.; Noor, T.; Amjad, M.Z.B.; Ejaz Bukhari, S.S. A TiO2 composite with graphitic carbon nitride as a photocatalyst for biodiesel production from waste cooking oil. RSC Adv. 2021, 11, 37575–37583. [Google Scholar] [CrossRef] [PubMed]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- ASTM D6751-20a; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Soltani, H.; Karimi, A.; Falahatpisheh, S. The optimization of biodiesel production from transesterification of sesame oil via applying ultrasound-assisted techniques: Comparison of RSM and ANN–PSO hybrid model. Chem. Prod. Process Model. 2022, 17, 55–67. [Google Scholar] [CrossRef]
- Özgür, C. Optimization of biodiesel yield and diesel engine performance from waste cooking oil by response surface method (RSM). Pet. Sci. Technol. 2021, 39, 683–703. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yang, S.; Zhang, S.; Xu, Q.; Chen, P.; Du, Y.; Xing, Y. Cobalt-loaded cherry core biochar composite as an effective heterogeneous persulfate catalyst for bisphenol A degradation. RSC Adv. 2022, 12, 7284–7294. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Y.; Cao, H.; Wei, P.; Chen, C.; Li, X.; Xu, J.; Sheng, G. Activating peroxydisulfate with Co3O4/NiCo2O4 double-shelled nanocages to selectively degrade bisphenol A—A nonradical oxidation process. Appl. Catal. B Environ. 2021, 282, 119585. [Google Scholar] [CrossRef]
- Omri Abdessalem, B.M. Characterization of Activated Carbon Prepared From a New Raw Lignocellulosic Material: Ziziphus Spina-Christi Seeds. J. Soc. Chim. Tunis. 2012, 14, 175–183. [Google Scholar]
- Saad, M.J.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Misran, S.; Rahman, M.H.A.; Chin, S.X. Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malays. 2019, 48, 385–391. [Google Scholar] [CrossRef]
- bin Tang, Y.; Liu, Q.; yan Chen, F. Preparation and characterization of activated carbon from waste ramulus mori. Chem. Eng. J. 2012, 203, 19–24. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kamińska, W.; Michalkiewicz, B.; Koren, Z.C. Production, characterization and methane storage potential of KOH-activated carbon from sugarcane molasses. Ind. Crops Prod. 2013, 47, 153–159. [Google Scholar] [CrossRef]
- Shukla, P.R.; Wang, S.; Sun, H.; Ang, H.M.; Tadé, M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B Environ. 2010, 100, 529–534. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, Y. KOH-activated rice husk char via CO2 pyrolysis for phenol adsorption. Mater. Today Energy 2018, 9, 397–405. [Google Scholar] [CrossRef]
- Scapin, E.; Maciel, G.P.d.S.; Polidoro, A.D.S.; Lazzari, E.; Benvenutti, E.V.; Falcade, T.; Jacques, R.A. Activated carbon from rice husk biochar with high surface area. Biointerface Res. Appl. Chem. 2021, 11, 10265–10277. [Google Scholar] [CrossRef]
- Izhab, I.; Amin, N.A.S.; Asmadi, M. Dry reforming of methane over oil palm shell activated carbon and ZSM-5 supported cobalt catalysts. Int. J. Green Energy 2017, 14, 831–838. [Google Scholar] [CrossRef]
- Das, V.; Tripathi, A.M.; Borah, M.J.; Dunford, N.T.; Deka, D. Cobalt-doped CaO catalyst synthesized and applied for algal biodiesel production. Renew. Energy 2020, 161, 1110–1119. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Zdujić, M.V.; Veljović, D.N.; Krstić, J.B.; Banković-Ilić, I.B.; Veljković, V.B.; Stamenković, O.S. Valorization of walnut shell ash as a catalyst for biodiesel production. Renew. Energy 2020, 147, 1033–1043. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2020, 151, 295–310. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Guo, F.; Peng, Z.G.; Dai, J.Y.; Xiu, Z.L. Calcined sodium silicate as solid base catalyst for biodiesel production. Fuel Process. Technol. 2010, 91, 322–328. [Google Scholar] [CrossRef]
- Ismail, S.; Abu, S.A.; Rezaur, R.; Sinin, H. Biodiesel Production from Castor Oil and Its Application in Diesel Engine. ASEAN J. Sci. Technol. Dev. 2014, 31, 90–100. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Ayetor, G.K.; Sunnu, A.; Parbey, J. Effect of biodiesel production parameters on viscosity and yield of methyl esters: Jatropha curcas, Elaeis guineensis and Cocos nucifera. Alex. Eng. J. 2015, 54, 1285–1290. [Google Scholar] [CrossRef]
- ASTM D6751-02-2002; Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2002.
- Gao, Z.; Ma, Y.; Ma, X.; Wang, Q.; Liu, Y. A novel variable pH control strategy for enhancing lipid production from food waste: Biodiesel versus docosahexaenoic acid. Energy Convers. Manag. 2019, 189, 60–66. [Google Scholar] [CrossRef]
- Remón, J.; Ruiz, J.; Oliva, M.; García, L.; Arauzo, J. Effect of biodiesel-derived impurities (acetic acid, methanol and potassium hydroxide) on the aqueous phase reforming of glycerol. Chem. Eng. J. 2016, 299, 431–448. [Google Scholar] [CrossRef]
- Drenth, A.C.; Olsen, D.B.; Denef, K. Fuel property quantification of triglyceride blends with an emphasis on industrial oilseeds camelina, carinata, and pennycress. Fuel 2015, 153, 19–30. [Google Scholar] [CrossRef]
- Drenth, A.C.; Olsen, D.B.; Cabot, P.E.; Johnson, J.J. Compression ignition engine performance and emission evaluation of industrial oilseed biofuel feedstocks camelina, carinata, and pennycress across three fuel pathways. Fuel 2014, 136, 143–155. [Google Scholar] [CrossRef]
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Bayat, A.; Baghdadi, M.; Bidhendi, G.N. Tailored magnetic nano-alumina as an efficient catalyst for transesterification of waste cooking oil: Optimization of biodiesel production using response surface methodology. Energy Convers. Manag. 2018, 177, 395–405. [Google Scholar] [CrossRef]
- Nisar, J.; Razaq, R.; Farooq, M.; Iqbal, M.; Khan, R.A.; Sayed, M.; Shah, A.; Rahman, I.u. Enhanced biodiesel production from Jatropha oil using calcined waste animal bones as catalyst. Renew. Energy 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Rutto, H.L. Optimization and modeling of process variables of biodiesel production from Marula oil using response surface methodology. J. Chem. Soc. Pak. 2015, 37, 256–265. [Google Scholar] [CrossRef]
- Moyo, L.B.; Iyuke, S.E.; Muvhiiwa, R.F.; Simate, G.S.; Hlabangana, N. Application of response surface methodology for optimization of biodiesel production parameters from waste cooking oil using a membrane reactor. S. Afr. J. Chem. Eng. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Ngadi, N.; Ma, L.N.; Alias, H.; Johari, A.; Rahman, R.A.; Mohamad, M. Production of Biodiesel from Waste Cooking Oil via Ultrasonic-Assisted Catalytic System. Appl. Mech. Mater. 2014, 699, 552–557. [Google Scholar] [CrossRef]
- Razzaq, L.; Abbas, M.M.; Miran, S.; Asghar, S.; Nawaz, S.; Soudagar, M.E.M.; Shaukat, N.; Veza, I.; Khalil, S.; Abdelrahman, A.; et al. Response Surface Methodology and Artificial Neural Networks-Based Yield Optimization of Biodiesel Sourced from Mixture of Palm and Cotton Seed Oil. Sustainability 2022, 14, 6130. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Kadry, G.A.; Abdel-Samad, H.A.; Awad, M.E. High operative heterogeneous catalyst in biodiesel production from waste cooking oil. Egypt. J. Pet. 2020, 29, 59–65. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and application of Co doped ZnO as heterogeneous nanocatalyst for biodiesel production from non-edible oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, N.; Ramos-Ramírez, E.; Serafín-Muñoz, A.; Zamorategui-Molina, A.; Monjaraz-Vallejo, J. Use of Co/Fe-Mixed oxides as heterogeneous catalysts in obtaining biodiesel. Catalysts 2019, 9, 403. [Google Scholar] [CrossRef]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl-LDH derived heterogeneous catalysts for the ethanol transesterification of canola oil to biodiesel. Appl. Catal. B Environ. 2009, 88, 42–49. [Google Scholar] [CrossRef]
- Singh, S.; Mukherjee, D.; Dinda, S.; Ghosal, S.; Chakrabarty, J. Synthesis of CoO–NiO promoted sulfated ZrO2 super-acid oleophilic catalyst via co-precipitation impregnation route for biodiesel production. Renew. Energy 2020, 158, 656–667. [Google Scholar] [CrossRef]


| Independent Variables | Value | (Lower–Upper) |
|---|---|---|
| Temp. (°C) | A | 60–90 |
| (M:O) | B | 6–12 |
| Catalyst Conc. (%) | C | 1–3 |
| Time (mins) | D | 45–60 |
| Type | Surface Area (m2/g) | Pore Size (Å) |
|---|---|---|
| RHAC | 1219.618 m2/g | 14.9 |
| RHAC-Co | 324.984 m2/g | 11.3 |
| Properties | Unit | BD Standards (ASTM 6751) [73] | KOH-BD | RHAC/CO-BD |
|---|---|---|---|---|
| Flashpoint | °C | >130 | 130 | 140 |
| Specific gravity | - - - - | 0.86–0.90 | 0.9 | 0.88 |
| Viscosity | mm2/s | 1.9–6.0 | 3.74 | 5.72 |
| Density | kg/m3 | 870–900 | 900 | 899 |
| pH | - - - - | 6.5–7.5 | 8–10 | 7.5 |
| Calorific Value | MJ/Kg | >35 | 46.048 | 40.068 |
| Saponification Value | mg KOH/g | <312 | 112.2 | 252.45 |
| Iodine Value | g I2/100 g oil | <120 | 57.5 | 60 |
| Cetane Number | - - - - | 50.5 | 51 | |
| Yield | (%) | 94 | 98.33 | 96.3 |
| Peak(s) | Fatty Acid Methyl Ester (s) (FAME) | Common Name | Formula | Retention Time (min) | Composition (%) |
|---|---|---|---|---|---|
| 1 | Hexadecanoic acid methyl ester | Methyl palmitate | C18H36O2 | 22.942 | 28.56 |
| 2 | Octadecanoic acid methyl ester | Methl Stearate | C18H36O2 | 25.305 | 21.98 |
| 3 | cis-9-Octadecenoic acid methyl ester | Methyl Oleate | C18H34O2 | 24.540 | 15.30 |
| 4 | cis-9-cis-12-Octadecadienoic acid methyl ester | Methyl linoleate | C18H32O2 | 23.575 | 15.67 |
| 5 | cis-9,12,15-octadecatrienoic acid | Methyl Linolenate | C18H30O | 26.432 | 6.50 |
| 6 | Pentadecanoic acid, 14-methyl-, methyl ester | Methyl pentanoate | C17H34O2 | 21.167 | 5.88 |
| Total = 93.89% |
| Runs | Factor 1, A: Temperature (°C) | Factor 2, B: M:O (mol/mol) | Factor 3, C: Catalyst Concentration (wt.%) | Factor 4, D: Time (min) | Response 1 Yield (%) |
|---|---|---|---|---|---|
| 1 | 90 | 6 | 1 | 60 | 87.9 |
| 2 | 75 | 15 | 2 | 52.5 | 94 |
| 3 | 75 | 9 | 2 | 52.5 | 96.3 |
| 4 | 60 | 12 | 3 | 45 | 88 |
| 5 | 90 | 6 | 3 | 45 | 92 |
| 6 | 75 | 9 | 2 | 52.5 | 96.5 |
| 7 | 60 | 12 | 1 | 45 | 89 |
| 8 | 75 | 9 | 2 | 67.5 | 88.5 |
| 9 | 60 | 6 | 1 | 45 | 88.2 |
| 10 | 60 | 12 | 1 | 60 | 91.25 |
| 11 | 75 | 9 | 4 | 52.5 | 86.5 |
| 12 | 90 | 12 | 3 | 60 | 93.5 |
| 13 | 60 | 6 | 1 | 60 | 86.45 |
| 14 | 90 | 12 | 1 | 45 | 88.5 |
| 15 | 75 | 3 | 2 | 52.5 | 92.45 |
| 16 | 90 | 6 | 1 | 45 | 89.5 |
| 17 | 75 | 9 | 2 | 52.5 | 96.3 |
| 18 | 105 | 9 | 2 | 52.5 | 90.5 |
| 19 | 90 | 6 | 3 | 60 | 92.45 |
| 20 | 60 | 12 | 3 | 60 | 89.5 |
| 21 | 75 | 9 | 0 | 52.5 | 83 |
| 22 | 75 | 9 | 2 | 52.5 | 96.3 |
| 23 | 45 | 9 | 2 | 52.5 | 88.15 |
| 24 | 75 | 9 | 2 | 52.5 | 96.3 |
| 25 | 90 | 12 | 1 | 60 | 87.5 |
| 26 | 60 | 6 | 3 | 45 | 87 |
| 27 | 60 | 6 | 3 | 60 | 89.45 |
| 28 | 90 | 12 | 3 | 45 | 90 |
| 29 | 75 | 9 | 2 | 52.5 | 96.3 |
| 30 | 75 | 9 | 2 | 37.5 | 85 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 413.31 | 14 | 29.52 | 37.65 | <0.0001 | significant |
| A-Temp. | 12.33 | 1 | 12.33 | 15.72 | 0.0012 | |
| B-M:O | 2.28 | 1 | 2.28 | 2.91 | 0.1087 | |
| C-Catalyst Conc. | 17.68 | 1 | 17.68 | 22.55 | 0.0003 | |
| D-Time | 6.83 | 1 | 6.83 | 8.71 | 0.0099 | |
| AB | 5.06 | 1 | 5.06 | 6.46 | 0.0226 | |
| AC | 15.02 | 1 | 15.02 | 19.15 | 0.0005 | |
| AD | 0.6006 | 1 | 0.6006 | 0.766 | 0.3953 | |
| BC | 1.05 | 1 | 1.05 | 1.34 | 0.2652 | |
| BD | 2.81 | 1 | 2.81 | 3.58 | 0.078 | |
| CD | 6.25 | 1 | 6.25 | 7.97 | 0.0128 | |
| A2 | 74.49 | 1 | 74.49 | 94.99 | <0.0001 | |
| B2 | 12.42 | 1 | 12.42 | 15.84 | 0.0012 | |
| C2 | 213.76 | 1 | 213.76 | 272.62 | <0.0001 | |
| D2 | 144.05 | 1 | 144.05 | 183.71 | <0.0001 | |
| Residual | 11.76 | 15 | 0.7841 | |||
| Lack of Fit | 11.76 | 10 | 1.18 | |||
| Pure Error | 0 | 5 | 0 | |||
| Cor Total | 425.07 | 29 | ||||
| R2 = 0.9723 | - | R2adj = 0.9465 |
| Item | Temp. °C | M:O mol/mol | Catalyst Conc. wt.% | Time min | Yield % |
|---|---|---|---|---|---|
| Value | 75 | 9 | 2 | 52.5 | 96.3 |
| Catalyst | Oil Used | Synthesis Route | Operating Parameters | Conv. | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Temp. | M:O | Catalyst Conc. | Time | |||||
| Co-CaO | Microalgal biomass | Catalyst Prep.: Co-precipitation impregnation route Transesterification | 65 °C | 3:1 | 0.2% | 120 min | 98% | [58] |
| Co-ZnO | Mesua ferrea oil | Esterification/Transesterification | 60 °C | 9:1 | 2.5% | 180 min | 98.03% | [80] |
| Co/Femixed Oxides | Cooking oil | Transesterification (Catalyst Layered Doubled Hydroxides) | 65 °C | 6:1 | 2% | 20 min | 96% | [81] |
| MgCoAlLa–LDH | Canola oil | Ethanol Transesterification | 100 °C | 16:1 | 1% | 300 min | 95% | [82] |
| CoO-NiO promoted sulphated ZrO2 | Oleophilic oil | Cat. Prep.: Co-precipitation impregnation route Transesterification | 65 °C | 3:1 | 0.2% | 120 min | 98.80% | [83] |
| RHAC-Co | Waste cooking oil | Esterification/Transesterification Catalyst Prep.: Wet impregnation | 75 °C | 9:1 | 2% | 52.5 min | 96.3% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, L.A.; Liaquat, R.; Aman, M.; Kanan, M.; Saleem, M.; khoja, A.H.; Bahadar, A.; Khan, W.U.H. Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO). Sustainability 2024, 16, 7275. https://doi.org/10.3390/su16177275
Khan LA, Liaquat R, Aman M, Kanan M, Saleem M, khoja AH, Bahadar A, Khan WUH. Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO). Sustainability. 2024; 16(17):7275. https://doi.org/10.3390/su16177275
Chicago/Turabian StyleKhan, Laraib Aamir, Rabia Liaquat, Mohammed Aman, Mohammad Kanan, Muhammad Saleem, Asif Hussain khoja, Ali Bahadar, and Waqar Ul Habib Khan. 2024. "Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO)" Sustainability 16, no. 17: 7275. https://doi.org/10.3390/su16177275
APA StyleKhan, L. A., Liaquat, R., Aman, M., Kanan, M., Saleem, M., khoja, A. H., Bahadar, A., & Khan, W. U. H. (2024). Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO). Sustainability, 16(17), 7275. https://doi.org/10.3390/su16177275








