Activated Carbons Derived from Different Parts of Corn Plant and Their Ability to Remove Phenoxyacetic Herbicides from Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation and Characterization of the Activated Carbons
2.3. Bath Adsorption Experiments
3. Results
3.1. Characteristics of the Activated Carbons
3.2. Adsorption Studies
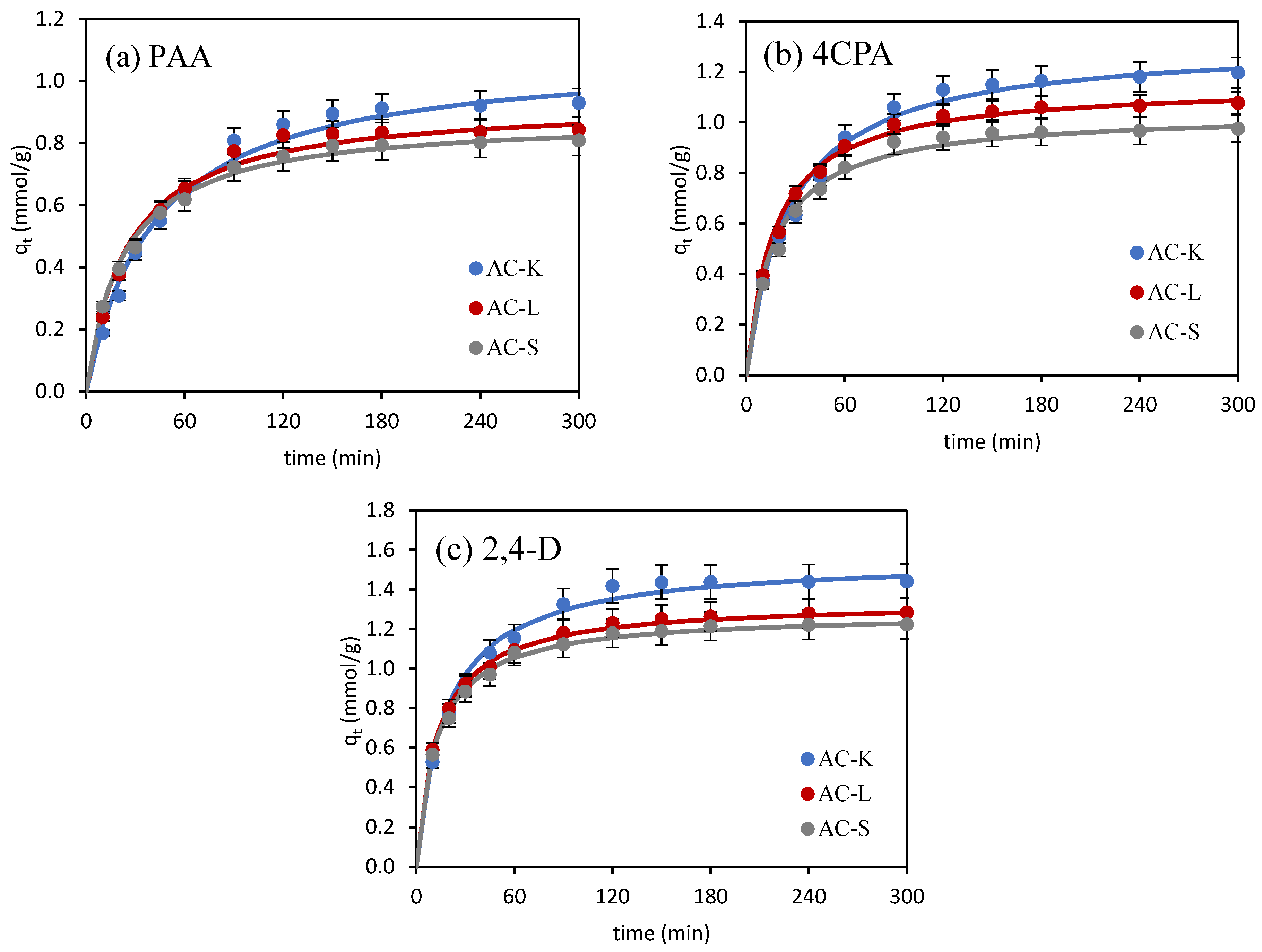
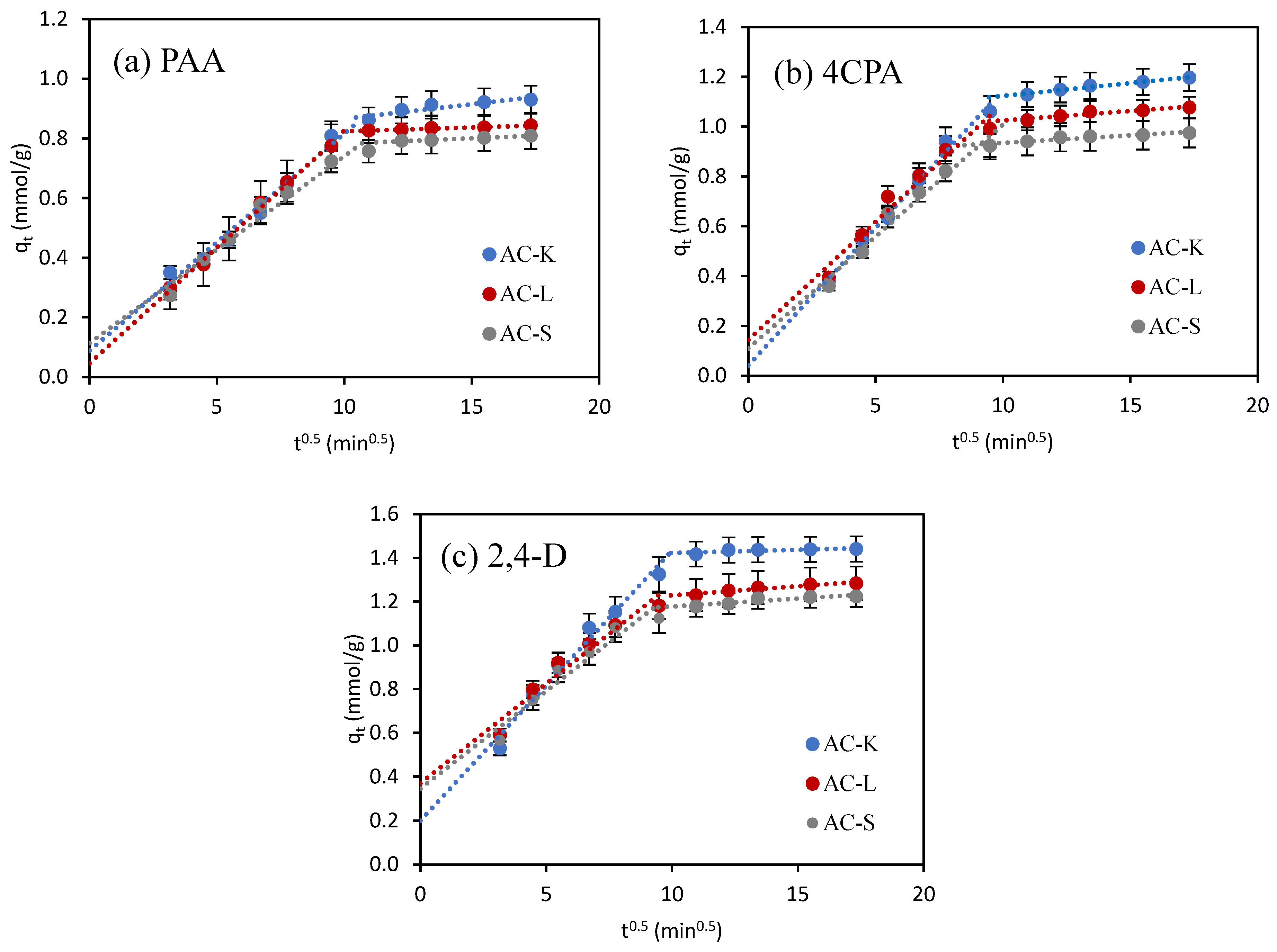
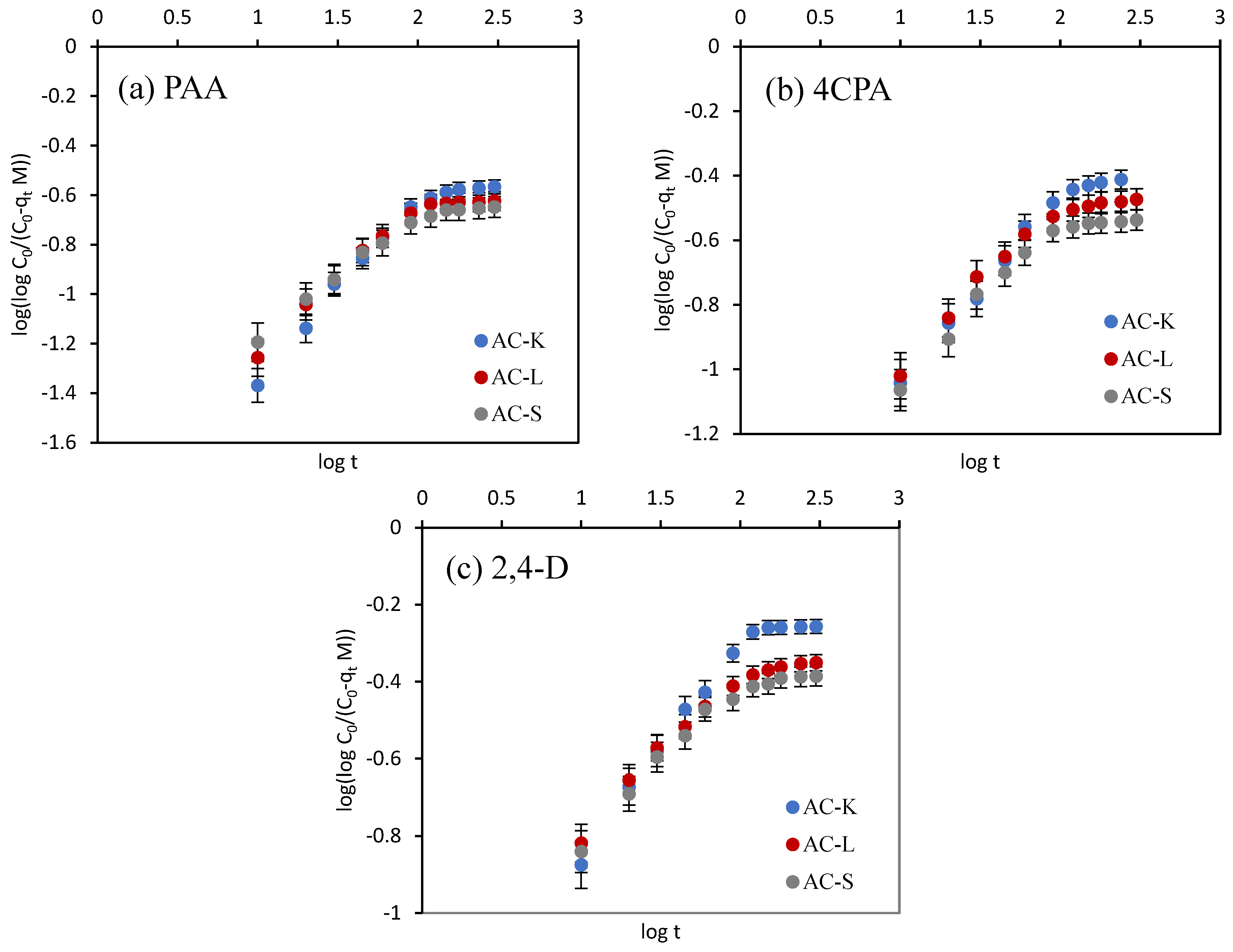
3.2.1. Adsorption Kinetics
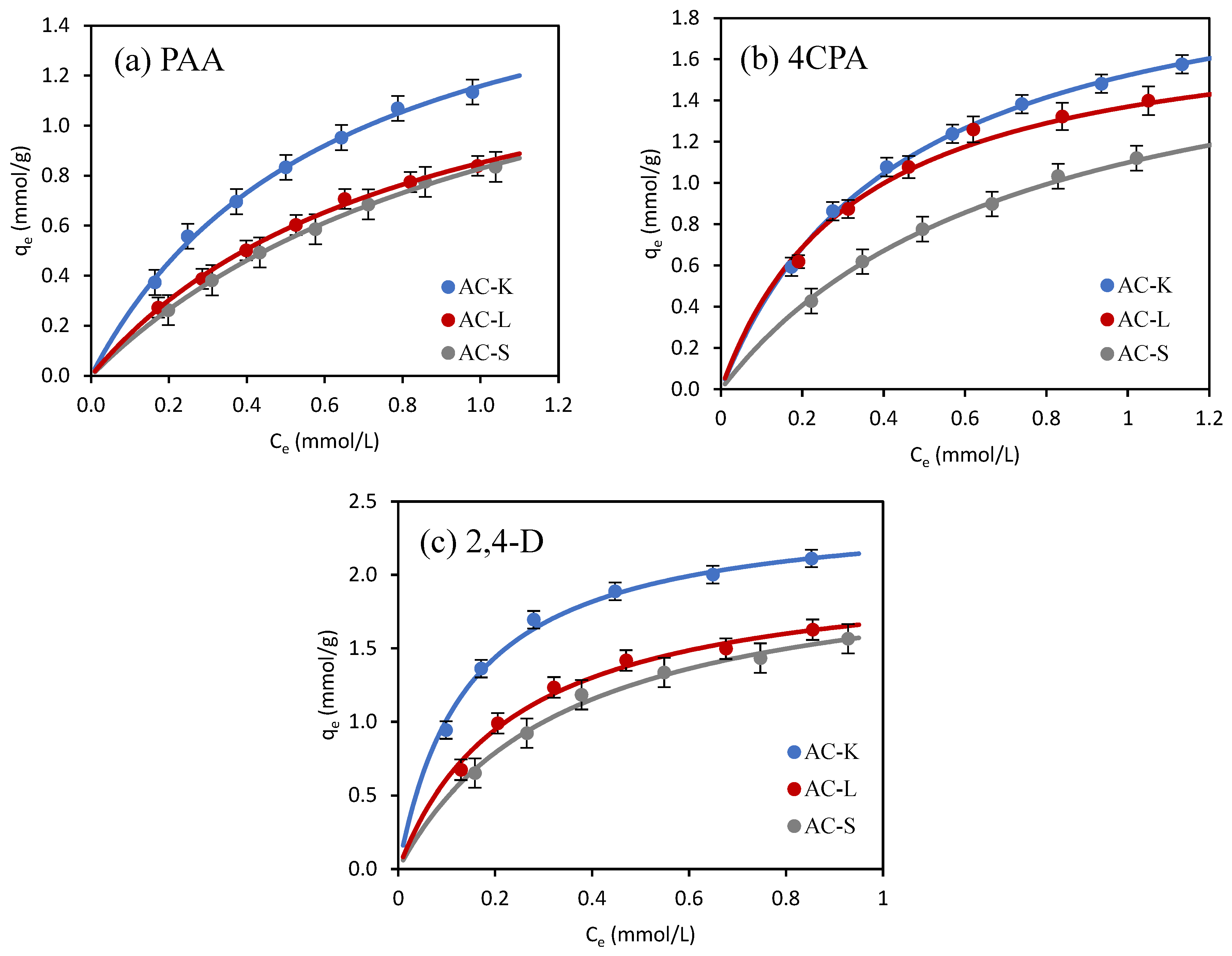
3.2.2. Adsorption Isotherms
3.2.3. Effect of Solution pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwiatkowski, J.F. Activated Carbon: Classifications, Properties and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2012. [Google Scholar]
- Verma, C.; Quraishi, M.A. Activated Carbon: Progress and Applications; The Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Chew, T.W.; H’ng, P.S.; Abdullah, B.C.T.G.L.C.; Chin, K.L.; Lee, C.L.; Hafizuddin, B.M.S.M.N.; TaungMai, L. A Review of Bio-Based Activated Carbon Properties Produced from Different Activating Chemicals during Chemicals Activation Process on Biomass and its Potential for Malaysia. Materials 2023, 16, 7365. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon: Review. RSC Adv. 2023, 13, 4275–4302. [Google Scholar] [CrossRef]
- Blachnio, M.; Kusmierek, K.; Swiatkowski, A.; Derylo-Marczewska, A. Waste-Based Adsorbents for the Removal of Phenoxyacetic Herbicides from Water: A Comprehensive Review. Sustainability 2023, 15, 16516. [Google Scholar] [CrossRef]
- Cuervo, O.H.P.; Gonzalez, C.F.; Rojas, H.A.; Martínez, J.J.; Romanelli, G.P.; Peixoto, A.F. Increasing furfural production from xylose and directly obtaining it from corn residues using Preyssler heteropolyacid. Biomass Convers. Biorefinery 2023, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Zhao, X.; Yin, F.; Yang, H.; Wu, K.; Liang, C.; Yang, B.; Zhang, W. Bioethanol production from corn straw pretreated with deep eutectic solvents. Electron. J. Biotechnol. 2023, 62, 27–35. [Google Scholar] [CrossRef]
- Fua, R.; Zhanga, Y.; Yanga, X.; Chenb, W. Study on biogas production from corn straw pretreated by compound microorganisms. Energy Rep. 2022, 8, 279–285. [Google Scholar] [CrossRef]
- Malacara-Becerra, A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Riquelme-Jiménez, L.M.; Mansouri, S.S.; Iqbal, H.M.N.; Parra-Saldívar, R. Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability 2022, 14, 11799. [Google Scholar] [CrossRef]
- Rahman, M.M.; Roy, A.; Nayeem, J.; Popy, R.S.; Ferdous, T.; Jahan, M.S. Tissue paper from corn stalk pulp in biorefinery concept. Biomass Convers. Biorefinery 2023, 1–8. [Google Scholar] [CrossRef]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and prospects of corn husk as a sustainable material in composites and other technical applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar] [CrossRef]
- Elvira-Hernandez, E.A.; Hernandez-Hernandez, J.; de Leon, A.; Gallardo-Vega, C.; Delgado-Alvarado, E.; Lopez-Huerta, F.; Herrera-May, A.L. Green energy harvesting to power electronic devices using portable triboelectric nanogenerator based on waste corn husk and recycled polystyrene. Energy Rep. 2024, 11, 276–286. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Danish, M.; Anastopoulos, I.; Iwuozor, K.O. Recent progress on corn (Zea mays L.)-based materials as raw, chemically modified, carbonaceous, and composite adsorbents for aquatic pollutants: A review. J. Anal. Appl. Pyrolysis 2023, 172, 106004. [Google Scholar] [CrossRef]
- Chaudhari, V.; Patkar, M. Removal of nickel from aqueous solution by using corncob as adsorbent. Mater. Today Proc. 2022, 61, 307–314. [Google Scholar] [CrossRef]
- Vafakhah, S.; Bahrololoom, M.E.; Bazarganlari, R.; Saeedikhani, M. Removal of copper ions from electroplating effluent solutions with native corn cob and corn stalk and chemically modified corn stalk. J. Environ. Chem. Eng. 2014, 2, 356–361. [Google Scholar] [CrossRef]
- Assireyand, E.A.; Altamimi, L.R. Chemical analysis of corn cob-based biochar and its role as water decontaminants. J. Taibah Univ. Sci. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Gairola, S.; Naik, T.P.; Sinha, S.; Singh, I. Corncob waste as a potential filler in biocomposites: A decision towards sustainability. Compos. Part C 2022, 9, 100317. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Nowicki, P.; Pietrzak, R. Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 2013, 19, 273–281. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Łopatka, H.; Zborowska, M. Activated carbon prepared from corn biomass by chemical activation with potassium hydroxide. BioResources 2022, 17, 1794–1804. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Qu, B.; Liu, X.; Yi, W.; Zhang, H. Study on Adsorption Properties of Modified Corn Cob Activated Carbon for Mercury Ion. Energies 2021, 14, 4483. [Google Scholar] [CrossRef]
- Agustina, S.; Yulianto, A.; Kamil, A.; Saepudin, A.; Fajriyan; Manurung, S.E.R.; Budiyanto; Majid, A.; Fitrina, A. Corn cob usage as activated carbon using KOH as activator in 3rd International Seminar of Natural Resources and Environmental Management 2023. IOP Conf. Ser. Earth Environ. Sci. 2023, 1266, 012082. [Google Scholar] [CrossRef]
- Yuliusman; Al Farouq, F.; Sipangkar, S.P.; Fatkhurrahman, M.; Putri, S.A. Preparation and characterization of activated carbon from corn stalks by chemical activation with KOH and NaOH. AIP Conf. Proc. 2020, 2255, 060006. [Google Scholar] [CrossRef]
- Afandi, S.A.R.; Bermakai, M.Y. The Effect of Impregnation Ratio on the Yield of Corn Cob Activated Carbon by Chemical Activation. Sci. Res. J. 2022, 19, 55–67. [Google Scholar] [CrossRef]
- Ammar, N.S.; Fathy, N.A.; Ibrahim, H.S.; Mousa, S.M. Micro mesoporous modified activated carbon from corn husks for removal of hexavalent chromium ions. Appl. Water Sci. 2021, 11, 154. [Google Scholar] [CrossRef]
- Njoku, V.O.; Hameed, B.H. Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J. 2011, 173, 391–399. [Google Scholar] [CrossRef]
- Christica, I.S.; Julia, R. Activated carbon utilization from corn cob (Zea mays) as a heavy metal adsorbent in industrial waste. Asian J. Pharm. Res. Dev. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Sabri, M.A.; Ibrahim, T.H.; ElSayed, Y.A. Synthesis and characterization of activated carbon fibers derived from corn silk and its application in p-Cresol removal. Desalination Water Treat. 2019, 168, 77–87. [Google Scholar] [CrossRef]
- Lala, M.A.; Olomowewe, Z.O.; Adeniyi, A.T.; Giwa, A. Maize cob-derived activated carbon as an adsorbent for the removal of nickel (II) cation from aqueous solution: Optimization and kinetic studies. Arab. J. Basic Appl. Sci. 2023, 30, 573–582. [Google Scholar] [CrossRef]
- Medhat, A.; El-Maghrabi, H.H.; Abdelghany, A.; Abdel Menem, N.M.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently activated carbons from corn cob for methylene blue adsorption. Appl. Surf. Sci. Adv. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Blachnio, M.; Kusmierek, K.; Swiatkowski, A.; Derylo-Marczewska, A. Adsorption of Phenoxyacetic Herbicides from Water on Carbonaceous and Non-Carbonaceous Adsorbents. Molecules 2023, 28, 5404. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Ojukwu, V.E.; Umeh, C.T.; Aniagor, C.O.; Chinyelu, C.E.; Ajala, O.J.; Dulta, K.; Adeola, A.O.; Rangabhashiyam, S. Recent advances in the adsorptive removal of 2,4-dichlorophenoxyacetic acid from water. J. Water Proc. Eng. 2023, 56, 104514. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. Removal of phenoxy herbicides from aqueous solutions using lignite as a low-cost adsorbent. Desalination Water Treat. 2022, 260, 111–118. [Google Scholar] [CrossRef]
- Nasser, S.M.; Abbas, M.; Trari, M. Understanding the rate-limiting step adsorption kinetics onto biomaterials for mechanism adsorption control. Prog. React. Kinet. Mech. 2024, 49, 1–26. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Kavanagh, B.V.; Posner, A.M.; Quirk, J.P. The adsorption of phenoxyacetic acid herbicides on goethite. J. Colloid Interface Sci. 1977, 61, 545–553. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Pakuła, M.; Biniak, S.; Świątkowski, A.; Dąbek, L. Adsorption and electrodegradation of phenoxyacetic acids on various activated carbons. Int. J. Electrochem. Sci. 2020, 15, 5770–5781. [Google Scholar] [CrossRef]
- Binh, Q.A.; Nguyen, H.-H. Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y.; Li, G. Characterization and evaluation of surface modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 2018, 210, 734–744. [Google Scholar] [CrossRef]
- Brito, G.M.; Roldi, L.L.; Schetino, M.A.; Freitas, J.C.C.; Coelho, E.R.C. High-performance of activated biocarbon based on agricultural biomass waste applied for 2,4-D herbicide removing from water: Adsorption, kinetic and thermodynamic assessments. J. Environ. Sci. Health Part B Pestic. 2020, 55, 767–782. [Google Scholar] [CrossRef]
- Doczekalska, B.; Kuśmierek, K.; Świątkowski, A.; Bartkowiak, M. Adsorption of 2,4-dichlorophenoxyacetic acid and 4-chloro-2-metylphenoxyacetic acid onto activated carbons derived from various lignocellulosic materials. J. Environ. Sci. Health Part B Pestic. 2018, 53, 290–297. [Google Scholar] [CrossRef]
- Legocka, I.; Kuśmierek, K.; Świątkowski, A.; Wierzbicka, E. Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane. Materials 2022, 15, 8433. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Pérez, R.; Daiem, M.M.A.; Rivera-Utrilla, J.; Méndez-Díaz, J.D.; Sánchez-Polo, M. Modeling adsorption rate of organic micropollutants present in landfill leachates onto granular activated carbon. J. Colloid Interface Sci. 2012, 385, 174–182. [Google Scholar] [CrossRef] [PubMed]



| Herbicide | CAS No. | Molecular Formula | Molar Mass (g/mol) | Water Solubility * (g/L) | log P | pKa |
|---|---|---|---|---|---|---|
| PAA | 122-59-8 |  | 152.15 | 10 | 1.48 | 3.70 |
| 4CPA | 122-88-3 |  | 186.59 | 0.96 | 1.85 | 3.10 |
| 2,4-D | 94-75-7 | 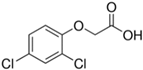 | 221.04 | 0.89 | 2.37 | 2.98 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmi (cm3/g) | Vme (cm3/g) | Vmi/Vt | D = 4 Vt/SBET (nm) |
|---|---|---|---|---|---|---|
| AC-K | 1965 | 1.112 | 0.932 | 0.180 | 0.838 | 2.264 |
| AC-L | 1720 | 1.286 | 0.869 | 0.417 | 0.676 | 2.989 |
| AC-S | 1600 | 1.144 | 0.798 | 0.346 | 0.697 | 2.860 |
| Sample | Σ of Acidic Groups (mmol/g) | Σ of Basic Groups (mmol/g) | pHPZC |
|---|---|---|---|
| AC-K | 2.15 | 0.77 | 6.85 |
| AC-L | 1.93 | 1.83 | 7.10 |
| AC-S | 2.25 | 0.48 | 6.60 |
| Sample | Mass Loss (%) in Temperature Range (°C) | |||
|---|---|---|---|---|
| 200–400 | 400–600 | 600–750 | 750–950 | |
| AC-K | 3.2 | 5.5 | 5.7 | 5.6 |
| AC-L | 3.0 | 2.3 | 4.8 | 5.5 |
| AC-S | 3.6 | 5.6 | 5.2 | 5.4 |
| Kinetic Model | Adsorbate | ||
|---|---|---|---|
| PAA | 4CPA | 2,4-D | |
| AC-K | |||
| qe (exp) (mmol/g) | 0.930 | 1.197 | 1.441 |
| pseudo-first-order | |||
| k1 (1/min) | 0.0207 | 0.0182 | 0.0318 |
| qe1 (cal) (mmol/g) | 0.894 | 0.895 | 0.813 |
| R2 | 0.991 | 0.974 | 0.976 |
| χ2 | 0.188 | 0.163 | 0.417 |
| pseudo-second-order | |||
| k2 (g/mmol∙min) | 0.0219 | 0.0292 | 0.0353 |
| qe2 (cal) (mmol/g) | 1.093 | 1.318 | 1.556 |
| R2 | 0.996 | 0.998 | 0.998 |
| χ2 | 0.021 | 0.016 | 0.015 |
| AC-L | |||
| qe (exp) (mmol/g) | 0.844 | 1.077 | 1.284 |
| pseudo-first-order | |||
| k1 (1/min) | 0.2140 | 0.0193 | 0.0246 |
| qe1 (cal) (mmol/g) | 0.634 | 0.683 | 0.834 |
| R2 | 0.936 | 0.961 | 0.978 |
| χ2 | 0.388 | 0.596 | 0.487 |
| pseudo-second-order | |||
| k2 (g/mmol∙min) | 0.0424 | 0.0496 | 0.0554 |
| qe2 (cal) (mmol/g) | 0.933 | 1.151 | 1.350 |
| R2 | 0.997 | 0.999 | 0.999 |
| χ2 | 0.017 | 0.008 | 0.003 |
| AC-S | |||
| qe (exp) (mmol/g) | 0.809 | 0.975 | 1.224 |
| pseudo-first-order | |||
| k1 (1/min) | 0.0205 | 0.0209 | 0.0222 |
| qe1 (cal) (mmol/g) | 0.624 | 0.6094 | 0.831 |
| R2 | 0.976 | 0.944 | 0.954 |
| χ2 | 0.205 | 0.406 | 0.398 |
| pseudo-second-order | |||
| k2 (g/mmol∙min) | 0.0493 | 0.0563 | 0.0605 |
| qe2 (cal) (mmol/g) | 0.883 | 1.043 | 1.286 |
| R2 | 0.998 | 0.998 | 0.999 |
| χ2 | 0.006 | 0.014 | 0.004 |
| Isotherm Model | Adsorbate | ||
|---|---|---|---|
| PAA | 4CPA | 2,4-D | |
| AC-K | |||
| Langmuir | |||
| qm (mmol/g) | 1.891 | 2.188 | 2.467 |
| KL (L/mmol) | 1.580 | 2.288 | 7.001 |
| R2 | 0.993 | 0.998 | 0.992 |
| χ2 | 0.009 | 0.011 | 0.007 |
| ΔG0 (kJ/mol) | −28.0 | −28.9 | −31.7 |
| RL | 0.209–0.514 | 0.186–0.477 | 0.188–0.447 |
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 1.230 | 1.576 | 2.403 |
| 1/n | 0.613 | 0.504 | 0.356 |
| R2 | 0.981 | 0.968 | 0.922 |
| χ2 | 0.123 | 0.235 | 0.152 |
| AC-L | |||
| Langmuir | |||
| qm (mmol/g) | 1.763 | 1.911 | 2.135 |
| KL (L/mmol) | 0.886 | 1.355 | 2.937 |
| R2 | 0.995 | 0.995 | 0.994 |
| χ2 | 0.008 | 0.017 | 0.055 |
| ΔG0 (kJ/mol) | −29.2 | −27.6 | −29.6 |
| RL | 0.221–0.531 | 0.207–0.511 | 0.211–0.484 |
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 0.886 | 1.114 | 1.860 |
| 1/n | 0.706 | 0.589 | 0.483 |
| R2 | 0.993 | 0.980 | 0.958 |
| χ2 | 0.381 | 0.133 | 0.251 |
| AC-S | |||
| Langmuir | |||
| qm (mmol/g) | 1.559 | 1.822 | 2.078 |
| KL (L/mmol) | 1.203 | 3.033 | 4.187 |
| R2 | 0.993 | 0.993 | 0.995 |
| χ2 | 0.029 | 0.030 | 0.031 |
| ΔG0 (kJ/mol) | −30.0 | −29.6 | −30.4 |
| RL | 0.243–0.562 | 0.215–0.523 | 0.216–0.490 |
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 0.821 | 1.020 | 1.713 |
| 1/n | 0.658 | 0.548 | 0.411 |
| R2 | 0.991 | 0.934 | 0.935 |
| χ2 | 0.157 | 0.815 | 0.711 |
| Adsorbent | SBET (m2/g) | Adsorption Capacity (mmol/g) | Ref. | ||
|---|---|---|---|---|---|
| 2,4-D | 4CPA | PAA | |||
| AC from corn kernels (AC-K) | 1965 | 2.467 | 2.188 | 1.891 | this paper |
| AC from corn leaves (AC-L) | 1720 | 2.135 | 1.911 | 1.763 | this paper |
| AC from corn silk (AC-S) | 1600 | 2.078 | 1.822 | 1.559 | this paper |
| raw lignite | 0.91 | 0.035 | 0.027 | 0.020 | [33] |
| SX2 AC (Norit) | 870 | 1.211 | 1.135 | 1.098 | [38] |
| F-400 AC (Chemviron) | 995 | 1.596 | 1.475 | 1.349 | [38] |
| Corncob biochar | 298 | 0.170 | - | - | [39] |
| Corn stalk biochar (BC) | 523 | 0.039 | - | - | [40] |
| AC from corncob | 1274 | 1.358 | - | - | [26] |
| AC from coconut shell | 991 | 1.054 | - | - | [41] |
| AC from coconut endocarp | 1068 | 1.065 | - | - | [41] |
| AC from sugarcane bagasse | 547 | 0.696 | - | - | [41] |
| AC from willow (AC-W) | 1280 | 2.310 | - | - | [42] |
| AC from hemp shives (AC-H) | 1324 | 2.446 | - | - | [42] |
| AC from miscanthus (AC-M) | 1420 | 2.577 | - | - | [42] |
| AC from flax (AC-F) | 1587 | 2.682 | - | - | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doczekalska, B.; Ziemińska, N.; Kuśmierek, K.; Świątkowski, A. Activated Carbons Derived from Different Parts of Corn Plant and Their Ability to Remove Phenoxyacetic Herbicides from Polluted Water. Sustainability 2024, 16, 7341. https://doi.org/10.3390/su16177341
Doczekalska B, Ziemińska N, Kuśmierek K, Świątkowski A. Activated Carbons Derived from Different Parts of Corn Plant and Their Ability to Remove Phenoxyacetic Herbicides from Polluted Water. Sustainability. 2024; 16(17):7341. https://doi.org/10.3390/su16177341
Chicago/Turabian StyleDoczekalska, Beata, Natalia Ziemińska, Krzysztof Kuśmierek, and Andrzej Świątkowski. 2024. "Activated Carbons Derived from Different Parts of Corn Plant and Their Ability to Remove Phenoxyacetic Herbicides from Polluted Water" Sustainability 16, no. 17: 7341. https://doi.org/10.3390/su16177341
APA StyleDoczekalska, B., Ziemińska, N., Kuśmierek, K., & Świątkowski, A. (2024). Activated Carbons Derived from Different Parts of Corn Plant and Their Ability to Remove Phenoxyacetic Herbicides from Polluted Water. Sustainability, 16(17), 7341. https://doi.org/10.3390/su16177341






