Screening and Physiological Responses of Maize Inbred Lines to Drought Stress in South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
2.2. The First Experiment
2.3. The Second Experiment
2.4. Sampling and Measurements
2.4.1. Chlorophyll Contents
2.4.2. Morphological Parameters
2.4.3. Relative Water Content
2.4.4. Photosynthetic Pigment
2.4.5. Antioxidant Activity
2.4.6. Malondialdehyde Content
2.4.7. Proline Content
2.5. Tolerance Analysis
2.6. Statistical Analysis
3. Results
3.1. Response of Maize Seedling Traits to Drought Stress
3.2. Comprehensive Evaluation of D Value
3.3. Survival Rate Analysis
3.4. Principal Component Analysis
3.5. Correlation Analysis
3.6. Cluster Analysis
3.7. Phenotypic Differences in Drought Response between Two Maize Inbred Lines
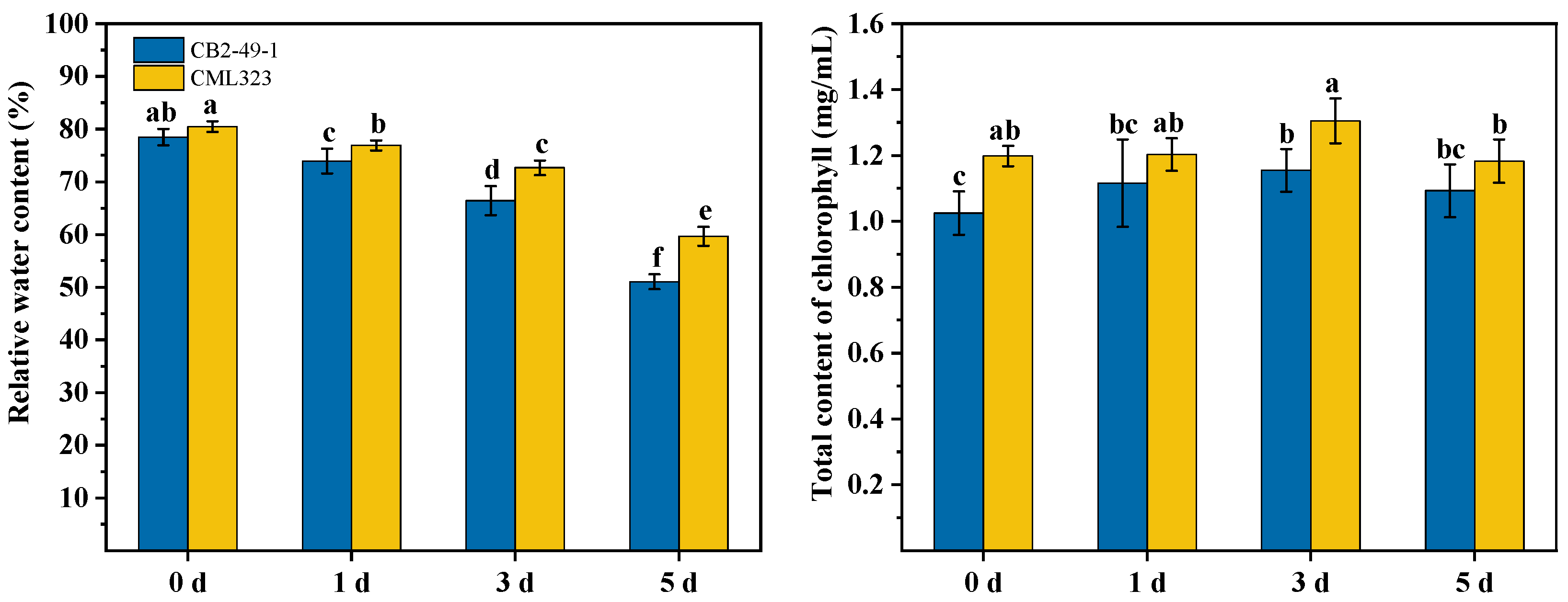
3.8. Differences in Physiological Indices between Two Maize Inbred Lines under Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yao, Z.; Zhang, W.; Wang, X.; Lu, M.; Chadwick, D.; Zhang, Z.; Chen, X. Carbon footprint of maize production in tropical/subtropical region: A case study of Southwest China. Environ. Sci. Pollut. Res. 2021, 28, 28680–28691. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Ding, R.; Du, S.; Kang, S.; Tong, L.; Li, S. Stomatal conductance drives variations of yield and water use of maize under water and nitrogen stress. Agric. Water Manag. 2022, 268, 107651. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Hu, Y.X.; Tung, S.A.; Yang, L.; Wang, Y.; Zhou, X.B. Evaluating the effects of water-nitrogen interactions on carbon and nitrogen accumulation as well as related metabolic enzymes activity in autumn maize. J. Soil Sci. Plant Nutr. 2023, 23, 5245–5256. [Google Scholar] [CrossRef]
- Ahmadi, A.; Emam, Y.; Pessarakli, M. Biochemical changes in maize seedlings exposed to drought stress conditions at different nitrogen levels. J. Plant Nutr. 2010, 33, 541–556. [Google Scholar] [CrossRef]
- Wajhat-Un-Nisa; Sandhu, S.; Ranjan, R.; Sharda, R. Root plasticity: An effective selection technique for identification of drought tolerant maize (Zea mays L.) inbred lines. Sci. Rep. 2023, 13, 5501. [Google Scholar] [CrossRef]
- Dar, I.A.; Sofi, P.A.; Dar, Z.A.; Kamaluddin, X.; Lone, A.A. Screening of maize genotypes for drought tolerance related trait variability. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 668–682. [Google Scholar] [CrossRef]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Dar Shi, C.Q. Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef]
- Zeng, W.; Peng, Y.; Zhao, X.; Wu, B.; Chen, F.; Ren, B.; Zhuang, Z.; Gao, Q.; Ding, Y. Comparative proteomics analysis of the seedling root response of drought-sensitive and drought-tolerant maize varieties to drought stress. Int. J. Mol. Sci. 2019, 20, 2793. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2014, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Gheysari, M.; Mirlatifi, S.M.; Bannayan, M.; Homaee, M.; Hoogenboom, G. Interaction of water and nitrogen on maize grown for silage. Agric. Water Manag. 2009, 96, 809–821. [Google Scholar] [CrossRef]
- Romdhane, L.; Radhouane, L.; Farooq, M.; Dal Cortivo, C.; Panozzo, A.; Vamerali, T. Morphological and biochemical changes in maize under drought and salinity stresses in a semi-arid environment. Plant Biosyst. 2020, 154, 396–404. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Bai, Y.; Camberato, J.; Xue, J.; Zhang, R. Effects of drought stress on the photosynthesis in maize. Russ. J. Plant Physiol. 2018, 65, 849–856. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, A.; Omrani, S.; Omrani, A.; Shojaei, S.H.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. Response of maize hybrids in drought-stress using drought tolerance indices. Water 2022, 14, 1012. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, Y.; Zhang, J. Identification of QTLs and Meta-QTLs for seven agronomic traits in multiple maize populations under well-watered and water-stressed conditions. Crop Sci. 2018, 58, 507–520. [Google Scholar] [CrossRef]
- Bao, X.; Hou, X.; Duan, W.; Yin, B.; Ren, J.; Wang, Y.; Liu, X.; Gu, L.; Zhen, W. Screening and evaluation of drought resistance traits of winter wheat in the North China Plain. Front. Plant Sci. 2023, 14, 1194759. [Google Scholar] [CrossRef] [PubMed]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of water use strategies at early growth stages in durum wheat from shoot phenotyping and physiological measurements. Front. Plant Sci. 2016, 7, 1155. [Google Scholar] [CrossRef]
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Michela Giuliani, M.; Salvi, S.; Conti, S. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Physiol. Mol. Biol. Plants 2002, 48, 697–712. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Q.; Jessup, K.E.; Hou, X.; Hao, B.; Marek, T.H.; Xu, W.; Evett, S.R.; O’Shaughnessy, S.A.; Brauer, D.K. Shoot and root traits in drought tolerant maize (Zea mays L.) hybrids. J. Integr. Agric. 2018, 17, 1093–1105. [Google Scholar] [CrossRef]
- Ghassemi, S.; Delangiz, N.; Asgari, L.B.; Saghafi, D.; Maggi, F. Review and future prospects on the mechanisms related to cold stress resistance and tolerance in medicinal plants. Acta Ecol. Sinica 2021, 1, 120–129. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, E.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinar, H.C.; Marur, C.J.; Vieira, L.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Shahana, T.; Rao, P.A.; Ram, S.S.; Sujatha, E. Mitigation of drought stress by 24-epibarassinolide and 28-homobrassinolide in pigeon pea seedlings. Int. J. Multidiscipl. Current. Res. 2015, 3, 904–911. [Google Scholar]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Sayed, M.A.; Hassan, L. Discerning of rice landraces (Oryza sativa L.) for morpho-physiological, antioxidant enzyme activity, and molecular’ markers’ responses to induced salt stress at the seedling stage. J. Plant Growth Regul. 2020, 39, 41–59. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.F.; Fu, W.; Guo, W.Q.; Ren, N.; Zhao, Y.N.; Ye, Y.L. Efficient physiological and nutrient use efficiency responses of maize leaves to drought stress under different field nitrogen conditions. Agronomy 2020, 10, 523. [Google Scholar] [CrossRef]
- Kamanga, R.M.; Mbega, E.; Ndakidemi, P. Drought tolerance mechanisms in plants: Physiological responses associated with water deficit stress in Solanum lycopersicum. Adv. Crop Sci. Technol. 2018, 6, 1000362. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Impa, S.S.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and antioxidants in plants. In Abiotic Stress Responses in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 131–147. [Google Scholar]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Slatyer, R.O.; Markus, D.K. Plant-water relationships. Soil Sci. 1968, 106, 478. [Google Scholar] [CrossRef]
- Quevedo, Y.M.; Moreno, L.P.; Barragan, E. Predictive models of drought tolerance indices based on physiological morphological and biochemical markers for the selection of cotton (Gossypium hirsutum L.) varieties. J. Integr. Agric. 2022, 21, 1310–1320. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Khorasani, S.K.; Ebrahimi, A. Evaluation of drought tolerance indices for screening some of super sweet maize (Zea mays L. var. saccharata) inbred lines. Agrivita J. Agric. Sci. 2020, 42, 435–448. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Yu, X.; Li, L.; Li, C.; Song, Y.; Xu, Z.; Zhang, J.; Guan, C. Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 2021, 23, 664–667. [Google Scholar] [CrossRef]
- Sakariyahu, S.; Indabo, S.S.; Aliyu, A.; Muhammad, H.U.; Ahmed, H.O.; Mohammed, S.B.; Adamu, A.K.; Aliyu, R.E. Cowpea landraces in northern Nigeria: Overview of seedling drought tolerance. Biologia 2024, 79, 381–392. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Rajarajan, K.; Ganesamurthy, K.; Raveendran, M. Differential responses of sorghum genotypes to drought stress revealed by physio-chemical and transcriptional analysis. Mol. Biol. Rep. 2021, 48, 2453–2462. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Ozgur, R.; Uzilday, B.; Turkan, I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014, 99, 141–149. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, P.M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Wu, S.; Tian, J.; Ren, Y.; Wang, Y. Osmotic adjustment and antioxidant system regulated by nitrogen deposition improve photosynthetic and growth performance and alleviate oxidative damage in dwarf bamboo under drought stress. Front. Plant Sci. 2022, 13, 819071. [Google Scholar] [CrossRef]
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of key drought tolerance indices for cotton at the flowering and boll setting sage using the dimension reduction method. Front. Plant Sci. 2021, 12, 619926. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Laskari, M.; Menexes, G.; Kalfas, I.; Gatzolis, I.; Dordas, C. Water stress effects on the morphological, physiological characteristics of maize (Zea mays L.), and on environmental cost. Agronomy 2022, 12, 2386. [Google Scholar] [CrossRef]
- Shahzad, A.; Gul, H.; Ahsan, M.; Wang, D.; Fahad, S. Comparative genetic evaluation of maize inbred lines at seedling and maturity stages snder drought stress. J. Plant Growth Regul. 2022, 42, 989–1005. [Google Scholar] [CrossRef]
- Jing, L.; Weng, B.; Yan, D.; Yuan, F.; Zhang, S.; Bi, W. Assessment of resilience in maize suitable planting areas under drought stress. Agric. Water Manag. 2023, 277, 108096. [Google Scholar] [CrossRef]
- Li, J.; Abbas, K.; Wang, L.; Gong, B.; Hou, S.; Wang, W.; Dai, B.; Xia, H.; Wu, X.; Lü, G.; et al. Drought resistance index screening and evaluation of lettuce under water deficit conditions on the basis of morphological and physiological differences. Front. Plant Sci. 2023, 14, 1228084. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Fu, B.; Qin, G.; Xing, G.; Wang, Y. Evaluation of drought resistance in Iris germanica L. based on subordination function and principal component analysis. Emir. J. Food Agric. 2017, 29, 770–778. [Google Scholar] [CrossRef]
- Füzy, A.; Kovács, R.; Cseresnyés, I.; Parádi, I.; Szili-Kovács, T.; Kelemen, B. Selection of plant physiological parameters to detect stress effects in potexperiments using principal component analysis. Acta Physiol. Plant 2019, 41, 56. [Google Scholar] [CrossRef]




| Indicator | MAX | MIN | Mean ± SD | CV (%) | TC | CV of TC (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WW | DS | WW | DS | WW | DS | WW | DS | Mean | ||
| SPAD | 50.43 | 46.60 | 35.90 | 23.80 | 41.84 ± 2.39 a | 37.70 ± 3.11 b | 5.71 | 8.24 | 0.90 | 6.16 |
| PH (cm) | 154.80 | 117.27 | 81.10 | 64.23 | 122.18 ± 11.12 a | 94.39 ± 8.18 b | 9.03 | 8.67 | 0.77 | 8.47 |
| PFW (g) | 88.69 | 43.93 | 25.10 | 7.83 | 59.95 ± 11.49 a | 23.97 ± 6.30 b | 19.14 | 26.30 | 0.41 | 29.71 |
| PDW (g) | 8.21 | 5.39 | 1.50 | 1.14 | 5.39 ± 1.04 a | 3.68 ± 0.69 b | 19.26 | 18.63 | 0.69 | 18.69 |
| RFW (g) | 25.70 | 7.36 | 2.32 | 1.05 | 5.59 ± 1.86 a | 3.67 ± 0.97 b | 33.37 | 26.43 | 0.69 | 23.43 |
| RDW (g) | 1.22 | 0.89 | 0.24 | 0.08 | 0.59 ± 0.18 a | 0.43 ± 0.16 b | 30.28 | 36.57 | 0.74 | 30.16 |
| RL (cm) | 59.47 | 79.77 | 20.97 | 31.57 | 38.18 ± 15.72 b | 48.72 ± 6.61 a | 15.72 | 13.57 | 1.29 | 13.65 |
| RCR | 0.25 | 0.61 | 0.04 | 0.06 | 0.09 ± 0.03 b | 0.16 ± 0.07 a | 32.18 | 40.32 | 1.79 | 35.10 |
| Indicator | Principal Component | Weight of Principal Component | Total | ||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | F | |
| SPAD | 0.04 | 0.06 | −0.81 | 0.01 | 0.02 | 0.38 | 0.08 |
| PH (cm) | −0.07 | 0.73 | 0.10 | 0.02 | 0.20 | 0.05 | 0.10 |
| PFW (g) | −0.01 | 0.89 | 0.04 | 0.00 | 0.25 | 0.02 | 0.10 |
| PDW (g) | 0.31 | 0.80 | 0.00 | 0.09 | 0.22 | 0.00 | 0.13 |
| RFW (g) | 0.87 | 0.17 | 0.16 | 0.25 | 0.05 | 0.08 | 0.14 |
| RDW (g) | 0.88 | −0.09 | 0.08 | 0.25 | 0.02 | 0.04 | 0.12 |
| RL (cm) | 0.51 | 0.17 | −0.22 | 0.15 | 0.05 | 0.10 | 0.10 |
| RCR | 0.70 | −0.57 | 0.12 | 0.20 | 0.16 | 0.06 | 0.16 |
| SR | 0.09 | 0.14 | 0.58 | 0.03 | 0.04 | 0.28 | 0.08 |
| Eigenvalue | 2.45 | 2.36 | 1.07 | ||||
| CR (%) | 27.20 | 26.24 | 11.88 | ||||
| CCR (%) | 27.20 | 53.44 | 65.32 | ||||
| Inbred Line | Days | Plant Height (cm) | Stem Diameter (mm) | Leaf Area (cm2) |
|---|---|---|---|---|
| CML323 | 0 | 39.35 ± 1.02 b | 3.07 ± 0.08 b | 24.96 ± 6.21 a |
| 1 | 42.85 ± 3.38 b | 3.31 ± 0.10 b | 24.62 ± 5.56 a | |
| 3 | 50.6 ± 4.73 a | 3.74 ± 0.40 a | 23.27 ± 2.27 a | |
| 5 | 50.1 ± 1.95 a | 3.63 ± 0.13 a | 21.29 ± 0.84 a | |
| CB2-49-1 | 0 | 36.78 ± 2.73 c | 3.05 ± 0.22 c | 31.93 ± 5.41 a |
| 1 | 38.98 ± 2.66 ab | 3.19 ± 0.18 bc | 29.01 ± 1.92 ab | |
| 3 | 42.15 ± 2.15 a | 3.47 ± 0.22 a | 29.09 ± 5.26 ab | |
| 5 | 41.83 ± 3.22 a | 3.41 ± 0.13 ab | 26.00 ± 3.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xie, X.; Naseer, M.A.; Zhou, H.; Cheng, W.; Xie, H.; Qin, L.; Yang, X.; Jiang, Y.; Zhou, X. Screening and Physiological Responses of Maize Inbred Lines to Drought Stress in South China. Sustainability 2024, 16, 7366. https://doi.org/10.3390/su16177366
Zhang Z, Xie X, Naseer MA, Zhou H, Cheng W, Xie H, Qin L, Yang X, Jiang Y, Zhou X. Screening and Physiological Responses of Maize Inbred Lines to Drought Stress in South China. Sustainability. 2024; 16(17):7366. https://doi.org/10.3390/su16177366
Chicago/Turabian StyleZhang, Zhiqin, Xiaodong Xie, Muhammad Asad Naseer, Haiyu Zhou, Weidong Cheng, Hexia Xie, Lanqiu Qin, Xiang Yang, Yufeng Jiang, and Xunbo Zhou. 2024. "Screening and Physiological Responses of Maize Inbred Lines to Drought Stress in South China" Sustainability 16, no. 17: 7366. https://doi.org/10.3390/su16177366





