The Eliciting Effect of Aqueous Extracts from Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2. Mineral Composition of the Extract
2.3. Qualitative Analysis of the Extract
2.4. Pot Experiment
2.5. Analysis of Antioxidant Activity Assay in Microgreens
2.6. Determination of Enzymatic Activity
2.7. Statistical Analysis
3. Results and Discussion
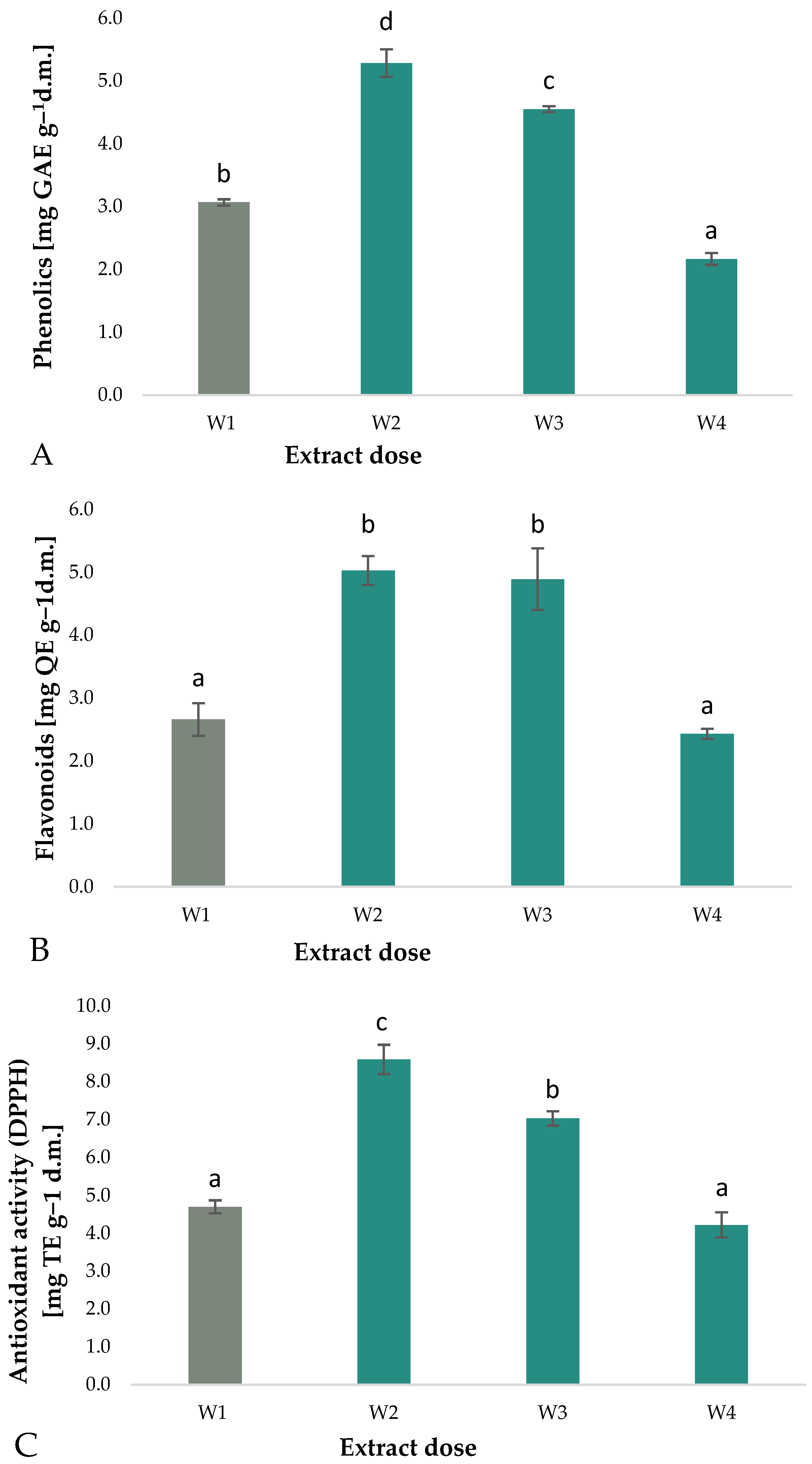
3.1. Total Phenolic Compounds, Flavonoids, and Antioxidant Activity against DPPH
3.2. Phenylalanine Ammonia Lyase and Polyphenol Oxidase Activities
3.3. Catalase and Superoxide Dismutase Activities
4. Conclusions
- The total phenolic content of arugula microgreens was found to increase significantly following the application of aqueous extracts of A. nodosum to the substrate. The greatest increase (72%) was observed at a concentration of 2.5%.
- The flavonoid content was found to increase significantly following the application of aqueous extracts of seaweed. The greatest increase was observed at a concentration of 2.5% with a value of 89%.
- The antioxidant activity (DPPH) demonstrated a notable increase following the application of aqueous extracts of A. nodosum. The greatest increase was observed at a concentration of 2.5% (82%).
- The application of aqueous extracts of algae to Eruca sativa microgreens resulted in a notable elevation in CAT activity. The greatest increase was observed at a concentration of 5% with a value of 68.2%.
- The application of A. nodosum extract resulted in an increase in SOD activity. The greatest increase was observed in dose W3 which equates to a concentration of 5% (25% increase).
- PAL activity increased to a considerable extent following the application of aqueous extracts of Ascophyllum nodosum. The greatest increase was observed at dose W3 (1213.5% relative to the control).
- PPO activity increased significantly when the substrate in which the plants were grown was treated with algal extracts. The greatest increase (84.2%) was observed at a concentration of 5%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2016, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens. A novel and promissory source of sustainable bioactive compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, E.; Ugolini, L.; Matteo, R.; Righetti, L. Bioactive Compounds from Eruca sativa Seeds. Encyclopedia 2022, 2, 1866–1879. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.E.O.; Adnan, M.; Siddiqui, A.J.; Snoussi, M.; Khan, M.I.; Azad, Z.R.A.A.; Patel, M.; Ashraf, S.A. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules 2022, 27, 1409. [Google Scholar] [CrossRef]
- Ramazzina, I.; Lolli, V.; Lacey, K.; Tappi, S.; Rocculi, P.; Rinaldi, M. Fresh-Cut Eruca Sativa Treated with Plasma Activated Water (PAW): Evaluation of Antioxidant Capacity, Polyphenolic Profile and Redox Status in CaCO2 Cells. Nutrients 2022, 14, 5337. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Srivastava, A. Green Chemistry: A Promising Route to Sustainable Agriculture. IJIRT 2023, 9, 631–640. [Google Scholar]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Rathod, S.G.; Bhushan, S.; Mantri, V.A. Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry. Phycology 2024, 4, 1–36. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Tuvikene, R.; Fernando, P.H.P.; Adhikari, R.; Perera, M.C.N.; Ranahewa, T.H.; Howlader, M.; Wangchuk, P.; Jayasooriya, A.P.; Rajapakse, R.P.V.J. Comparative analysis of proximate compositions, mineral and functional chemical groups of 15 different seaweed species. Sci. Rep. 2022, 12, 19610. [Google Scholar] [CrossRef] [PubMed]
- Samuels, L.J.; Setati, M.E.; Blancquaert, E.H. Towards a Better Understanding of the Potential Benefits of Seaweed Based Biostimulants in Vitis vinifera L. Cultivars. Plants 2022, 11, 348. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Blunden, G.; Wildgoose, P.B. The effects of aqueous seaweed extract and kinetin on potato yields. J. Sci. Food Agric. 1977, 28, 121–125. [Google Scholar] [CrossRef]
- Crouch, I.J.; van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Drygaś, B.; Depciuch, J.; Puchalski, C. Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L. Agronomy 2021, 11, 1446. [Google Scholar] [CrossRef]
- Michalak, J.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production—A review. Vet Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Heida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1553–1907. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, M.; Barcala, M.; Rosell, C.M.; Sineiro, J.; Moreira, R. Aqueous extracts characteristics obtained by ultrasound-assisted extraction from Ascophyllum nodosum seaweeds: Effect of operation conditions. J. Appl. Phycol. 2021, 33, 3297–3308. [Google Scholar] [CrossRef]
- Mroczek, K.; Saletnik, B.; Bajcar, M.; Saletnik, A.; Puchalski, C.; Zaguła, G. Effect on Ionic Composition and Tonic Parameters of Sweeteners Used in the Production of Functional Beverages. Beverages 2023, 9, 98. [Google Scholar] [CrossRef]
- Aziz, M.A. Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J. Integr. Med. 2015, 13, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Nhon, H.T.N.; Phan, T.T.; Lien, P.T.K.; Van, N.N.H.; Dao, D.T.A.; Anh, L.T.H. Phytochemical screening, extraction, and determination of the bioactivities of the extract-enriched polyphenols and saponins from Musa balbisiana Fruit. J. Food Process Preserv. 2023, e2581641. [Google Scholar] [CrossRef]
- Jabeen, S.; Ali, M.F.; Mohiud, D.A.; Javed, T.; Mohammed, N.S.; Chaudhari, S.K.; Javed, M.A.; Ali, B.; Zhang, L.; Rahimi, M. Phytochemical screening and allelopathic potential of phytoextracts of three invasive grass species. Sci. Rep. 2023, 13, 8080. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Szumny, A.; Szymczycha-Madeja, A.; Wełna, M.; Michalak, I. Methods for Rapid Screening of Biologically Active Compounds Present in Plant-Based Extracts. Molecules 2022, 27, 7094. [Google Scholar] [CrossRef]
- Alemu, M.; Lulekal, E.; Asfaw, Z.; Warkineh, B.; Debella, A.; Abebe, A.; Degu, S.; Debebe, E. Antibacterial activity and phytochemical screening of traditional medicinal plants most preferred for treating infectious diseases in Habru District, North Wollo Zone, Amhara Region, Ethiopia. PLoS ONE 2024, 19, e0300060. [Google Scholar] [CrossRef]
- Zeleke, B.; Mekonnen, Z.; Bireda, M.; Yitbarek, M.; Dendir, A. Phytochemical screening and antimicrobial activity of Polygala sadebeckiana Gürke extracts on bacterial isolates from Wound samples of patients with “Shimetere”. BMC Complement Med. Ther. 2024, 24, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar Singh, P.; Singh, J.; Medhi, T.; Kumar, A. Phytochemical Screening, Quantification, FT-IR Analysis, and In Silico Characterization of Potential Bio-active Compounds Identified in HR-LC/MS Analysis of the Polyherbal Formulation from Northeast India. ACS Omega 2022, 37, 33067–33078. [Google Scholar] [CrossRef] [PubMed]
- Bakir, Ç.N.; Yalçin, E.; Çavuşoğlu, K.; Sipahi Kuloğlu, S. Qualitative and quantitative phytochemical screening of Nerium oleander L. extracts associated with toxicity profile. Sci. Rep. 2022, 12, 21421. [Google Scholar] [CrossRef]
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.E.; Balakrishanan, M. Phytochemical Profile and Anti-ulcer Activities of Extracts of Unripen Fruits of Psidium Guajava Linn by Ethanol Induced Ulcer Method. Int. J. Pharm. Drug Anal. 2016, 4, 310–317. [Google Scholar]
- Kebede, T.; Gadisa, E. Tufa A Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.A.; Muddathir, A.M.; Osman, M.A. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep. 2020, 10, 17148. [Google Scholar] [CrossRef]
- Subroto, E.; Lemborg, E.; Filianty, F.; Indiarto, R.; Primalia, G.; Zeanal Putri, M.S.K.; Theodora, H.C.; Junar, S. The Analysis Techniques Of Amino Acid And Protein In Food And Agricultural Products. Int. J. Sci. Technol. Res. 2020, 9, 29–36. [Google Scholar]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Ali, S. kadhum. New spectrophotometric assay for assessments of catalase activity in biological samples. Anal. Biochem. 2018, 542, 29–33. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. Changes in phenolic compounds profile and glutathione status in raspberry fruit during storage in ozone-enriched atmosphere. Postharvest Biol. Technol. 2020, 168, 111277. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In Methods in Molecular Biology; Clifton, N.J., Ed.; Springer: New York, NY, USA, 1994; Volume 32, pp. 9–15. [Google Scholar] [CrossRef]
- Nikoogoftar-Sedghi, M.; Rabiei, V.; Razavi, F.; Monalei, S.; Khadivi, A. The effect of foliar application of Ascophyllum nodosum (L.) Le Jol. seaweed extract on biochemical traits related to abiotic stresses in pistachio (Pistacia vera L. cv. Kaleh-Ghoochi). BMC Plant Biol. 2023, 23, 635. Available online: https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-023-04654-5 (accessed on 15 December 2023). [CrossRef] [PubMed]
- Osuna-Ruíz, I.; Ledezma, A.K.D.; Martínez-Montaño, E.; Salazar-Leyva, J.E.; Rodríguez Tirado, V.A.; García, B. Enhancement of in-vitro antioxidant properties and growth of amaranth seed sprouts treated with seaweed extracts. J. Appl. Phycol. 2023, 35, 471–481. [Google Scholar] [CrossRef]
- Hamid, S.; Sahar, A.; Malik, F.; Hussain, S.; Mahmood, R.; Ashfaq, K.; Malik, T.A.; Hassan, A.; Chaudhry, A.H. Physico- chemical investigation and antioxidant activity studies on extracts of Eruca sativa seed. Int. J. Pharm. Chem. 2014, 4, 160–165. [Google Scholar]
- Arbos, K.A.; Freitas, R.J.S.; Stertz, S.C.; Dornas, M.F. Atividade antioxidante e teor de fenólicos totais em hortaliças orgânicas e convencionais (Antioxidant activity and phenolic content in organic and conventional vegetables). Ciência E Tecnol. Aliment. 2010, 30, 501–506. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Effect on yield, total phenolic, total flavonoid and total isothiocyanate content of two broccoli cultivars (Brassica oleraceae var italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). Agric. Food Sci. 2014, 23, 28–37. [Google Scholar] [CrossRef]
- Pacheco, A.C.; Sobral, L.A.; Gorni, P.H.; Carvalho, M.E.A. “Ascophyllum nodosum” extract improves phenolic compound content and antioxidant activity of medicinal and functional food plant “Achillea millefolium” L. Aust. J. Crop Sci. 2019, 13, 418–423. Available online: https://search.informit.org/doi/10.3316/informit.438868076860971 (accessed on 3 December 2023). [CrossRef]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Bioch. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B.A. Biostimulant Preparation of Brown Seaweed Ascophyllum nodosum Suppresses Powdery Mildew of Strawberry. Plant Pathol. J. 2019, 35, 406–416. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Marchica, A.; Cotrozzi, L.; Detti, R.; Lorenzini, G.; Pellegrini, E.; Petersen, M.; Nali, C. The Biosynthesis of Phenolic Compounds Is an Integrated Defence Mechanism to Prevent Ozone Injury in Salvia officinalis. Antioxidants 2020, 9, 1274. [Google Scholar] [CrossRef]
- Jun, S.Y.; Sattler, S.A.; Cortez, G.S.; Vermerris, W.; Sattler, S.E.; Kang, C.H. Biochemical and Structural Analysis of Substrate Specificity of a Phenylalanine Ammonia-Lyase. Plant Physiol. 2018, 176, 1452–1468. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive Roles of Polyphenol Oxidase in Plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: New York, NY, USA, 2008; pp. 253–270. [Google Scholar] [CrossRef]
- Ali, N.; Ramkissoon, A.; Ramsubhag, A.; Jayaraj, J. Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop Protect. 2016, 83, 67–75. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Lizzi, Y.; Coulomb, C.; Polian, C.; Coulomb, P.J.; Coulomb, P.O. Seaweed and mildew: What does the future hold? Encouraging laboratory results. Phytoma 1998, 508, 29–30. [Google Scholar]
- Abkhoo, J.; Sabbagh, S.K. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 2016, 28, 1333–1342. [Google Scholar] [CrossRef]
- Rinaldi, L.K.; Miamoto, A.; Calandrelli, A.; Rodrigues e Silva, M.T.; Silva Chidichima, L.P.; Pereira, C.B.; Dias-Arieira, C.B. Control of Meloidogyne javanica and induction of resistance-associated enzymes in soybean by extracts of Ascophyllum nodosum. J. Appl. Phycol. 2021, 33, 2655–2666. [Google Scholar] [CrossRef]
- Patel, J.S.; Selvaraj, V.; Gunupuru, L.R.; Rathor, P.K.; Prithiviraj, B. Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant Biol. 2020, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, J.; Wan, A.; Rahma, M.; Punja, Z.K. Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 2008, 10, 1360–1366. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Kapusta, I.; Józefczyk, R.; Balawejder, M. Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water. Molecules 2023, 28, 5038. [Google Scholar] [CrossRef]
- Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Physiological Response of Maize Plants (Zea mays L.) to the Use of the Potassium Quercetin Derivative. Int. J. Mol. Sci. 2021, 22, 7384. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S. and Mittler, Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2019, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Yildiztekin, M.; Tuna, A.L.; Kaya, C. Physiological Effects of the Brown Seaweed (Ascophyllum nodosum) and Humic Substances on Plant Growth, Enzyme Activities of Certain Pepper Plants Grown under Salt Stress. Biol. Futur. 2018, 69, 325–335. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.A.; De Camargo, E.; Castro, P.R.; Gaziola, S.A.; Azevedo, R.A. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comun. Sci. 2018, 9, 292–297. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Fike, J.H.; Allen, V.G.; Schmidt, R.E.; Zhang, X.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Coelho, R.W.; Wester, D.B. Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J. Anim. Sci. 2011, 79, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Fan, D.; Sangha, J.S.; Khan, W.; Evans, F.; Critchley, A.T.; Prithiviraj, B. Tasco®, a Product of Ascophyllum nodosum, Imparts Thermal Stress Tolerance in Caenorhabditis elegans. Mar. Drugs 2011, 9, 2256–2282. [Google Scholar] [CrossRef]
- Zhang, X. Influence of Plant Growth Regulators on Turfgrass Growth, Antioxidant Status, and Drought Tolerance. Ph.D. Thesis, Virginia Polytech-nic Institute and State University, Blacksburg, VA, USA, 1997; p. 9805556. [Google Scholar]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Sujeeth, N.; Petrov, V.; Guinan, K.J.; Rasul, F.; O’Sullivan, J.T.; Gechev, T.S. Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources. Int. J. Mol. Sci. 2022, 23, 7654. [Google Scholar] [CrossRef]

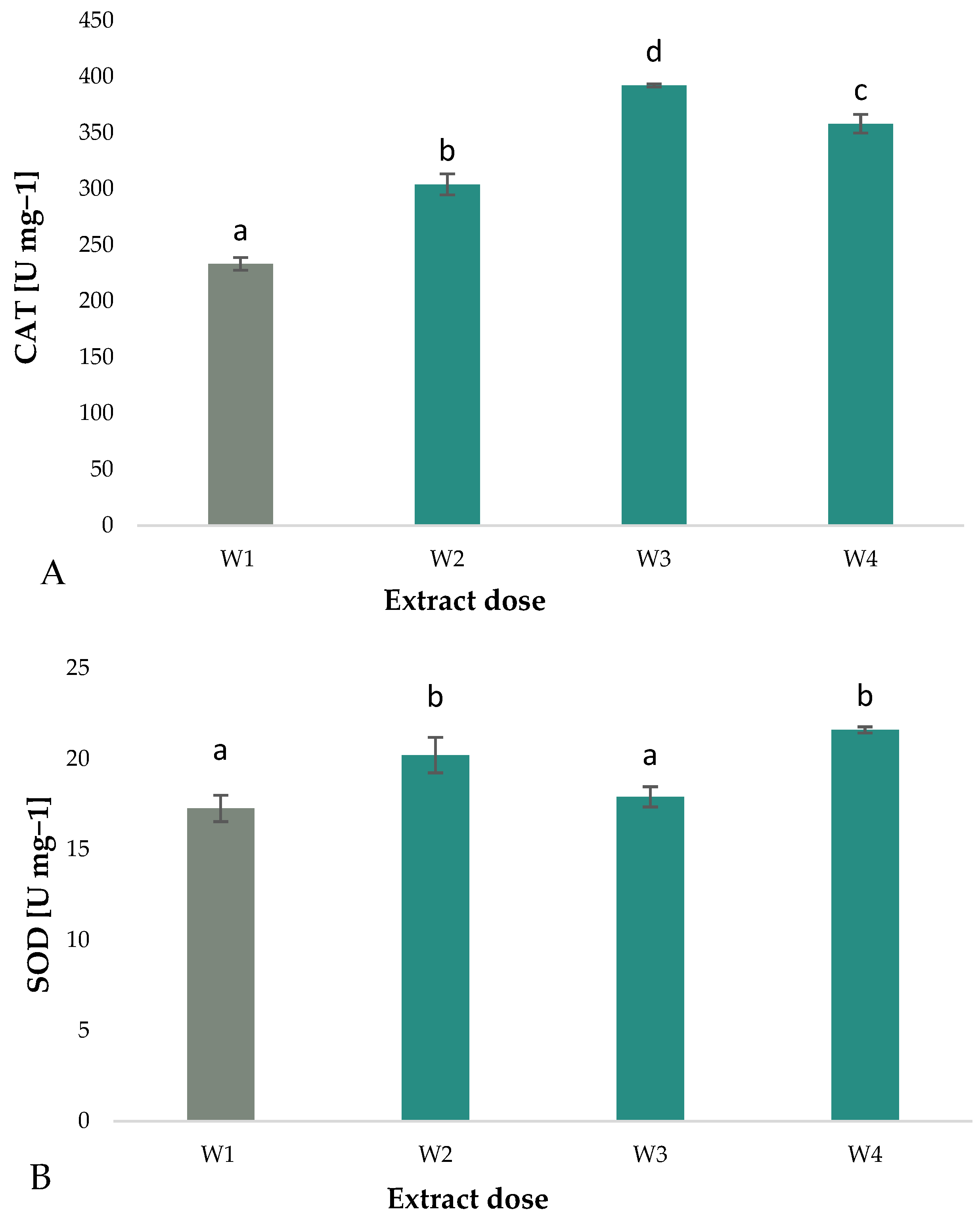
| Macroelements | Dose | Ca | Mg | K | P | S | Na | |
| W1 | 1.33 ± 0.16 | 1.93 ± 0.11 | 5.75 ± 0.44 | Trace | 5.46 ± 0.05 | 6.59 ± 0.13 | ||
| W2 | 1.90 ± 0.10 | 2.76 ± 0.05 | 14.1 ± 0.10 | 0.24 ± 0.03 | 7.624 ± 0.06 | 10.79 ± 0.12 | ||
| W3 | 3.76 ± 0.05 | 5.78 ± 0.09 | 29.01 ± 0.28 | 0.187 ± 0.02 | 16.28 ± 0.13 | 23.913 ± 0.24 | ||
| W4 | 7.96 ± 0.15 | 11.74 ± 0.10 | 57.64 ± 0.36 | 1.833 ± 0.031 | 32.43 ± 0.185 | 48.14 ± 0.337 | ||
| Microelements | Dose | Cu | Zn | Cr | Fe | Mn | Mo | Sr |
| W1 | 0.113 ± 0.015 | 0.063 ± 0.08 | - | 0.018 ± 0.03 | 0.006 ± 0.0 | 0 ± 0.0 | 0.047 ± 0.003 | |
| W2 | 0.149 ± 0.1 | 0.065 ± 0.04 | 0.003 ± 0.01 | 0.022 ± 0.04 | 0.012 ± 0.01 | 0 ± 0.0 | 0.045 ± 0.001 | |
| W3 | 0.110 ± 0.2 | 0.063 ± 0.01 | 0.001 ± 0.0 | 0.074 ± 0.06 | 0.024 ± 0.01 | trace | 0.097 ± 0.001 | |
| W4 | 0.081 ± 0.2 | 0.069 ± 0.01 | 0.004 ± 0.0 | 0.254 ± 0.03 | 0.047 ± 0.005 | 0.001 ± 0.0 | 0.196 ± 0.002 |
| Test Name | Phytoche Mical Group Detected | Sample Name | Positive Test Result—Description | Interpretation | Result Obtained |
|---|---|---|---|---|---|
| foam/emulsion test | saponins | 7A | Persistent foam/formation of an emulsion with oil | foaming, emulsifying, and clouding were found | + |
| FeCl3 test | tannins | 7B | gallic tannins—navy blue catechol tannins—dark green | dark brown gelatinous precipitate | - |
| gelatin test | tannins/phenolic compounds | 7C | precipitation | no chemical reaction | - |
| alkaline test (NaOH) | anthocyanins/flavonoids | 7D | Anthocyanins—blue colour after addition of NaOH, turning yellow or orange after addition of HCl; Flavonoids—intense yellow colour after addition of NaOH, colour fades after addition of HCl | The addition of sodium hydroxide (NaOH) causes the solution to darken, while the addition of hydrochloric acid (HCl) causes it to lighten. | tannins− flavonoids+ |
| lead acetate test | tannins/flavonoids | 7E | white precipitate—tannins; yellow precipitate—flavonoids | yellow precipitate | tannins− flavonoids+ |
| Shinod test | flavonoids | 7F | red colour of the solution | yellowish colour + gelatinous precipitate | - |
| Mayer test | alkaloids | 7G | cream-coloured precipitation/green solution | precipitate after adding HCl | - |
| Wagner test | alkaloids | 7H | brown-red precipitate | no chemical reaction | - |
| Dragendorff test | alkaloids | 7I | orange precipitate | precipitate after adding HCl | - |
| Keller–Killiani test | cardiac glycosides | 7J | cardiac glycosides—red-brown ring; (steroid aglycone—part of the glycoside) | very subtle darkening at the junction on two phases | inconclusive |
| Liebermann–Burchard test | cardiac glycosides/steroids/terpenoids | 7K | cardiac glycosides—green colour/ring (steroid aglycone—part of the glycoside); steroids—green colour/ring (steroid aglycone—part of the glycoside); terpenoids—red-purple colour | greenish ring | glycosides/steroidal aglycones+ |
| Salkowski test | steroids/terpenoids | 7L | steroids—red-brown ring (steroid aglycone—part of the glycoside);terpenoids—red-brown ring on the interface | brown ring | terpenoids+ |
| Fehling test | reducing sugars/carbohydrates | 7M | brick-red precipitation | blue-green solution | - |
| Biuret reaction | proteins and amino acids | 7N | violet coloration | gelatinous precipitate/green tint | - |
| Xanthoproteic reaction | proteins and amino acids | 7O | After adding HNO3, a white precipitate forms. After dissolving it in ammonia, it turns yellow. | after HNO3—no precipitate; after adding N3—the yellow one | - |
| Borntrager test | anthraquinones | 7P | pink, purple, or violet colouring | brown-yellowish colouration with subtle pinkish tint | inconclusive |
| Molish test | reducing sugars/carbohydrates | 7R | reddish-purple ring at the phase boundary | yellowish colouration with slight darkening at the junction of the phases | inconclusive |
| No. | RT [min] | Peak Share in the Chromatogram [%] | Ordinary Substance Name | Systematic Substance Name | No CAS |
|---|---|---|---|---|---|
| 1 | 4.75 | 1.17 | 1,1-Dimethylsilanediol | 1066-42-8 | |
| 2 | 7.90 | 12.44 | 5-Chloroindole | 17422-32-1 | |
| 3 | 9.80 | 3.75 | Octenal | 25447-69-2 | |
| 4 | 10.91 | 3.27 | Benzene, 1-chloro-3,5-bis(1,1-dimethylethyl)-2-(2-propenyloxy)- | 55955-96-9 | |
| 5 | 13.72 | 19.21 | (E)-2-decenal | 3913-81-3 | |
| 6 | 14.19 | 4.81 | Dodeca-2,4-dienal | 13162-47-5 | |
| 7 | 15.16 | 10.12 | 3-(bromomethyl)cyclohexene | 34825-93-9 | |
| 8 | 15.64 | 4.77 | 2-Cyclohexene-1-methanol, 2-methyl-a-(trichloromethyl)- | 103659-46-7 | |
| 9 | 16.58 | 6.43 | 2,6-di-tert-butylo-p-benzochinon | 719-22-2 | |
| 10 | 16.82 | 20.14 | (E)-beta-ionone | 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one | 79-77-6 |
| 11 | 17.89 | 9.38 | (E,Z,Z)-2,4,7-tridecatrienal | - | |
| 12 | 21.03 | 4.44 | Diisobutyl phthalate | 84-69-5 | |
| 13 | |||||
| 14 | |||||
| TOTAL | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drygaś, B.; Piechowiak, T.; Balawejder, M.; Matłok, N.; Kreczko, J.; Puchalski, C. The Eliciting Effect of Aqueous Extracts from Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens. Sustainability 2024, 16, 7436. https://doi.org/10.3390/su16177436
Drygaś B, Piechowiak T, Balawejder M, Matłok N, Kreczko J, Puchalski C. The Eliciting Effect of Aqueous Extracts from Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens. Sustainability. 2024; 16(17):7436. https://doi.org/10.3390/su16177436
Chicago/Turabian StyleDrygaś, Barbara, Tomasz Piechowiak, Maciej Balawejder, Natalia Matłok, Joanna Kreczko, and Czesław Puchalski. 2024. "The Eliciting Effect of Aqueous Extracts from Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens" Sustainability 16, no. 17: 7436. https://doi.org/10.3390/su16177436






