Threshold Response Identification to Multi-Stressors Using Fish- and Macroinvertebrate-Based Diagnostic Tools in the Large River with Weir-Regulated Flow
Abstract
1. Introduction
2. Materials and Methods
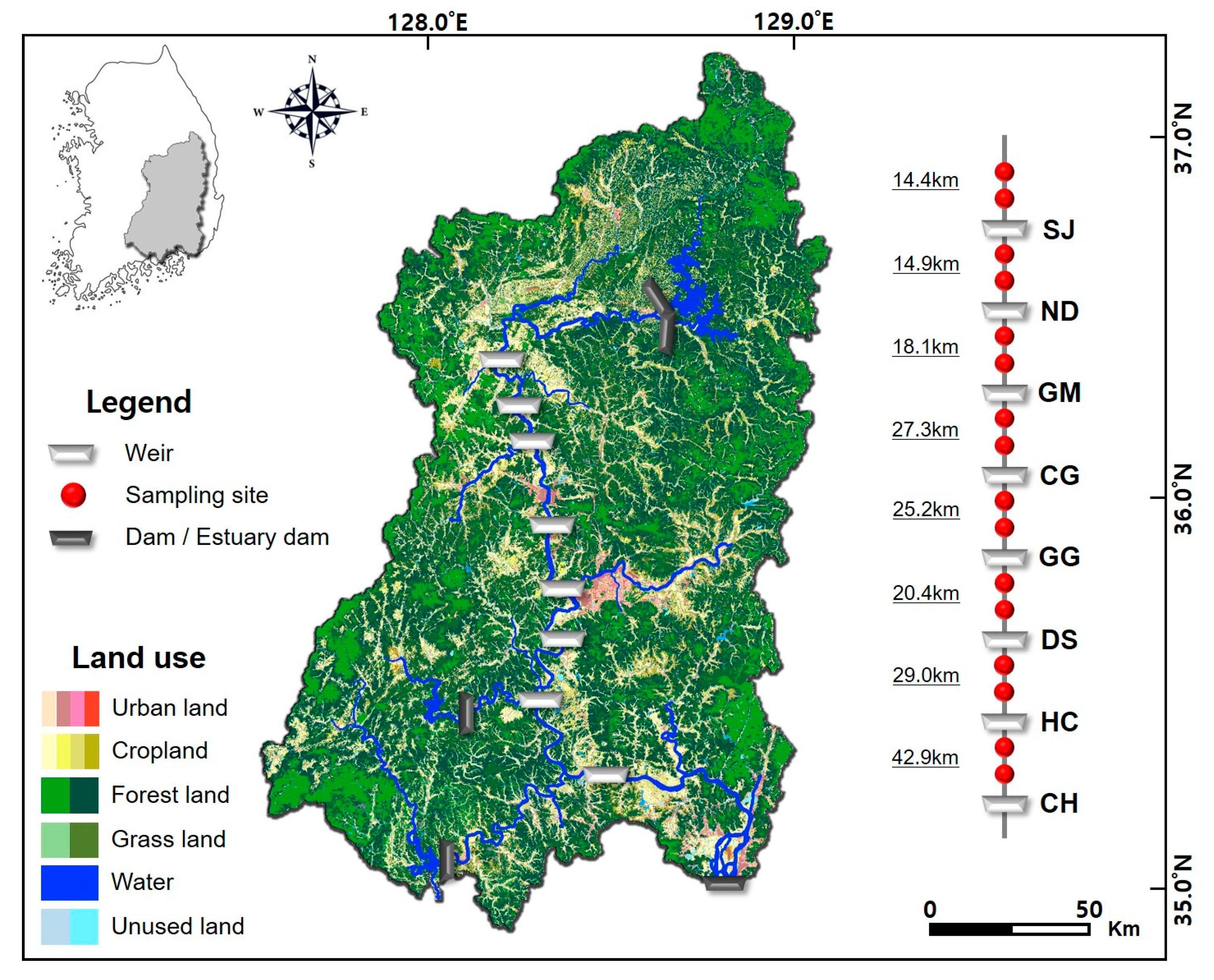
2.1. Study Area Description
2.2. Data Collection Design
2.3. Data-Driven Model Quantifying Biological Assembalge Response to Stressors
3. Results
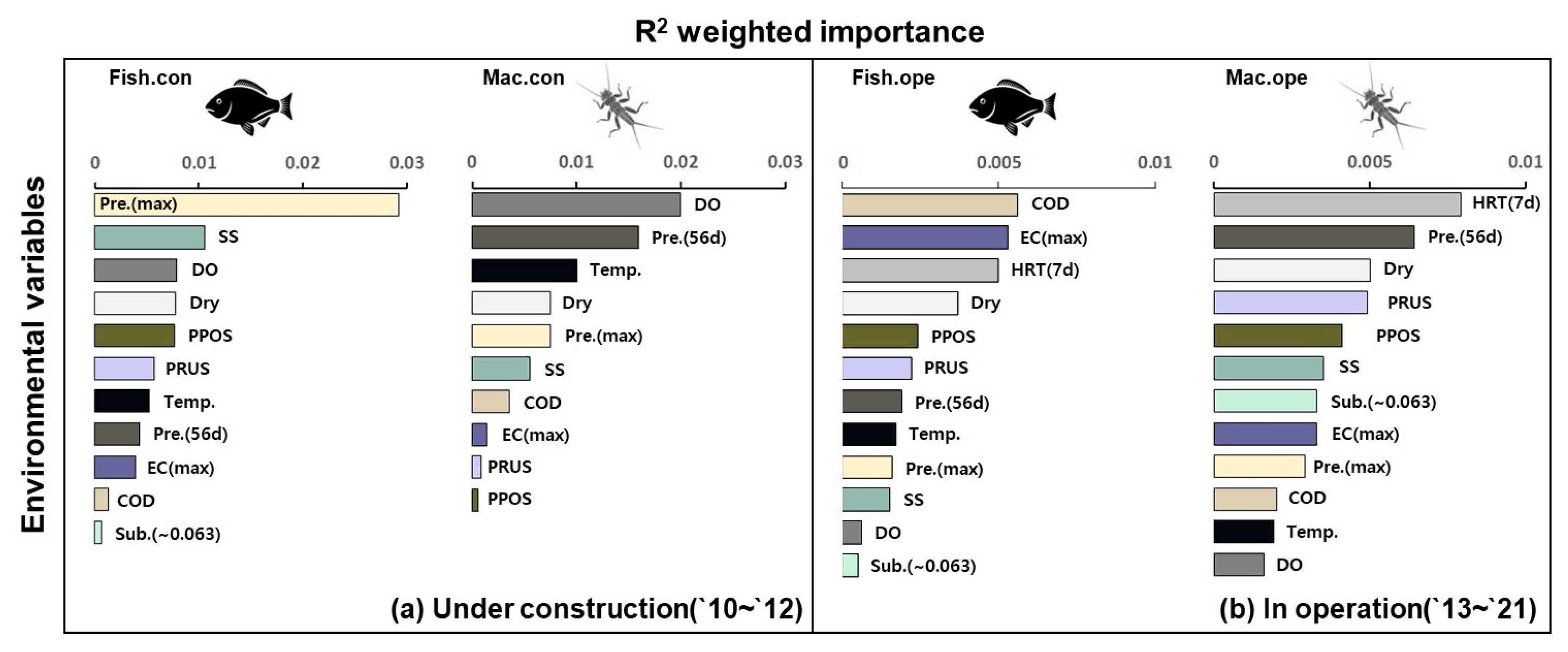
3.1. Overall Important Predictor of Biological Assemblage
3.2. Specific-Taxon Response to Environmental Thresholds
3.2.1. Fish
3.2.2. Macroinvertebrate
3.3. Comparison of Two Methods (The GF Models and the NMDS Plot)
4. Discussion
4.1. Specific-Taxon Response to Multi-Stressors
4.2. Effors to Identify ‘True’ Thresholds
4.3. Preservation Implicatrion Using Environmental Thresholds
5. Conclusions
- i.
- The community turnover and thresholds that responded multi-stressors differed depending on the biological assemblage, even under the same environmental conditions. In particular, operation weirs have increased the importance of certain species (e.g., non-native species). Therefore, it was confirmed that diagnostic tools that use multiple biological assemblages can be useful for identifying multiple environmental stressors caused by large artificial structures such as weirs.
- ii.
- Thresholds for each type of major taxon were identified in a large river with weirs, which are the first reference points presented in similar ecological environments. In the long term, this approach can be used as a guideline for major taxa that must be managed from a biodiversity perspective.
- iii.
- The limitations of the Gradient Forest (GF) technique (machine learning method), which is dependent on field data due to its characteristics, were found while identifying ‘true’ thresholds. Therefore, reliable results were obtained by applying several processes (comparison with field data, interpretation through ecological information, and application of similar statistical techniques).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atique, U.; Kwon, S.; An, K. Linking weir imprints with riverine water chemistry, microhabitat alterations, fish assemblages, chlorophyll-nutrient dynamics, and ecological health assessments. Ecol. Indic. 2020, 117, 106652. [Google Scholar] [CrossRef]
- Tóth, R.; Czeglédi, I.; Kern, B.; Erős, T. Land use effects in riverscapes: Diversity and environmental drivers of stream fish communities in protected, agricultural and urban landscapes. Ecol. Ind. 2019, 101, 742–748. [Google Scholar] [CrossRef]
- Miao, Y.; Li, J.; Feng, P.; Dong, L.; Zhang, T.; Wu, J.; Katwal, R. Effects of land use changes on the ecological operation of the Panjiakou-Daheiting Reservoir system, China. Ecol. Eng. 2020, 152, 105851. [Google Scholar] [CrossRef]
- Salant, N.L.; Schmidt, J.C.; Budy, P.; Wilcock, P.R. Unintended consequences of restoration: Loss of riffles and gravel substrates following weir installation. J. Environ. Manag. 2012, 109, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Merg, M.L.; Dézerald, O.; Kreutzenberger, K.; Demski, S.; Reyjol, Y.; Usseglio-Polatera, P.; Belliard, J. Modeling diadromous fish loss from historical data: Identification of anthropogenic drivers and testing of mitigation scenarios. PLoS ONE 2020, 15, e0236575. [Google Scholar] [CrossRef]
- Petts, G.E.; Gurnell, A.M. Dams and geomorphology: Research progress and future directions. Geomorphology 2005, 71, 27–47. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Mac Nally, R.; de Sostoa, A. The presence of non-native species is not associated with native fish sensitivity to water pollution in greatly hydrologically altered rivers. Sci. Total Environ. 2017, 607–608, 549–557. [Google Scholar] [CrossRef]
- Jo, H.; Jeppesen, E.; Ventura, M.; Buchaca, T.; Gim, J.S.; Yoon, J.D.; Kim, D.H.; Joo, G.J. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total Environ. 2019, 657, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- van Oorschot, M.; Kleinhans, M.; Buijse, T.; Geerling, G.; Middelkoop, H. Combined effects of climate change and dam construction on riverine ecosystems. Ecol. Eng. 2018, 120, 329–344. [Google Scholar] [CrossRef]
- Waite, I.R.; Pan, Y.; Edwards, P.M. Assessment of multi-stressors on compositional turnover of diatom, invertebrate and fish assemblages along an urban gradient in Pacific Northwest streams (USA). Ecol. Indic. 2020, 112, 106047. [Google Scholar] [CrossRef]
- Haase, P.; Pilotto, F.; Li, F.; Sundermann, A.; Lorenz, A.W.; Tonkin, J.D.; Stoll, S. Moderate warming over the past 25 years has already reorganized stream invertebrate communities. Sci. Total Environ. 2019, 658, 1531–1538. [Google Scholar] [CrossRef]
- Alric, B.; Dézerald, O.; Meyer, A.; Billoir, E.; Coulaud, R.; Larras, F.; Mondy, C.P.; Usseglio-Polatera, P. How diatom-, invertebrate- and fish-based diagnostic tools can support the ecological assessment of rivers in a multi-pressure context: Temporal trends over the past two decades in France. Sci. Total Environ. 2021, 762, 143915. [Google Scholar] [CrossRef] [PubMed]
- Ozolinš, D.; Skuja, A.; Jēkabsone, J.; Kokorite, I.; Avotins, A.; Poikane, S. How to assess the ecological status of highly humic lakes? Development of now method based on benthic invertebrates. Water 2021, 13, 223. [Google Scholar] [CrossRef]
- Poikane, S.; Várbíró, G.; Kelly, M.G.; Birk, S.; Phillips, G. Estimating river nutrient concentration consistent with good ecological condition: More stringent nutrient thresholds needed. Ecol. Ind. 2021, 121, 107017. [Google Scholar] [CrossRef]
- Vitecek, S.; Johnson, R.K.; Poikane, S. Assessing the ecological status of European rivers and lakes using benthic invertebrate communities: A practical catalogue of metrics and methods. Water 2021, 13, 346. [Google Scholar] [CrossRef]
- Kelly, M.G.; Whitton, B.A. The trophic diatom index: A new index for monitoring eutrophication in rivers. J. Appl. Phycol. 1995, 7, 433–444. [Google Scholar] [CrossRef]
- Mondy, C.P.; Usseglio-Polatera, P. Using conditional tree forests and life history traits to assess specific risks of stream degradation under multiple pressure scenario. Sci. Total Environ. 2013, 461 462, 750–760. [Google Scholar] [CrossRef]
- Larras, F.; Billoir, E.; Gautreau, E.; Coulaud, R.; Rosebery, J.; Usseglio-Polatera, P. Assessing anthropogenic pressures on streams: A random forest approach based on benthic diatom communities. Sci. Total Environ. 2017, 586, 1101–1112. [Google Scholar] [CrossRef]
- Dézerald, O.; Mondy, C.P.; Dembski, S.; Kreutzenberger, K.; Reyjol, Y.; Chandesris, A.; Valette, L.; Brosse, S.; Toussaint, A.; Belliard, J.; et al. A diagnosis-based approach to assess specific risks of river degradation in a multiple pressure context: Insights from fish communities. Sci. Total Environ. 2020, 734, 139467. [Google Scholar] [CrossRef]
- Sultana, J.; Tibby, J.; Recknagel, F.; Maxwell, S.; Goonan, P. Comparison of two commonly used methods for identifying water quality thresholds in freshwater ecosystems using field and synthetic data. Sci. Total Environ. 2020, 724, 137999. [Google Scholar] [CrossRef]
- Wagenhoff, A.; Clapcott, J.E.; Lau, K.E.M.; Lewis, G.D.; Young, R.G. Identifying congruence in stream assemblage thresholds in response to nutrient and sediment gradients for limit setting. Ecol. Appl. 2017, 27, 469–484. [Google Scholar] [CrossRef]
- Waite, I.R.; Munn, M.D.; Moran, P.W.; Konrad, C.P.; Nowell, L.H.; Meador, M.R.; Van Metre, P.C.; Carlisle, D.M. Effects of urban multi-stressors on three stream biotic assemblages. Sci. Total Environ. 2019, 660, 1472–1485. [Google Scholar] [CrossRef]
- Anunciação, P.R.; Barros, F.M.; Ribeiro, M.C.; Carvalho, L.M.; Ernst, R. Taxonomic and functional threshold responses of vertebrate communities in the Atlantic Forest Hotspot. Biol. Conserv. 2021, 257, 109137. [Google Scholar] [CrossRef]
- Moon, M.Y.; Ji, C.W.; Lee, D.-S.; Lee, D.-Y.; Hwang, S.-J.; Noh, S.-Y.; Kwak, I.-S.; Park, Y.-S. Characterizing responses of biological trait and functional diversity of benthic macroinvertebrates to environmental variables to develop aquatic ecosystem health assessment index. Korean J. Ecol. Environ. 2020, 53, 31–45. [Google Scholar] [CrossRef]
- Schinegger, R.; Palt, M.; Segurado, P.; Schmutz, S. Untangling the effects of multiple human stressors and their impacts on fish assemblages in European running waters. Sci. Total Environ. 2016, 573, 1079–1088. [Google Scholar] [CrossRef]
- Feng, C.; Li, H.; Yan, Z.; Wang, Y.; Wang, C.; Fu, Z.; Liao, W.; Giesy, J.P.; Bai, Y. Technical study on national mandatory guideline for deriving water quality criteria for the protection of freshwater aquatic organisms in China. J. Environ. Manag. 2019, 250, 109539. [Google Scholar] [CrossRef]
- Nichols, J.D.; Eaton, M.J.; Martin, J. Thresholds for conservation and management: Structured decision making as a conceptual framework. In Application of Threshold Concepts in Natural Resource Decision Making; Guntenspergen, G., Ed.; Springer: New York, NY, USA, 2014; pp. 9–28. [Google Scholar]
- Baker, M.E.; King, R.S. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 2010, 1, 25–37. [Google Scholar] [CrossRef]
- Bennett, E.M.; Balvanera, P. The future of production systems in a globalized world. Front. Ecol. Environ. 2007, 5, 191–198. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. [Google Scholar]
- NIER (National Institute of Environmental Research). Guidelines on Investigating the Current Status of Aquatic Ecosystems and Assessment Method of Integrity; The Ministry of Environment (ME): Incheon, Republic of Korea, 2019. [Google Scholar]
- WEIS (Water Environment Information System). Water Quality Monitoring System by Ministry of Environment. Available online: https://water.nier.go.kr/web (accessed on 15 May 2021).
- WIP (Water Information Portal). Real-Time Weir Operation Information by K-Water of the Republic of Korea: My Water Database. Available online: https://www.water.or.kr/kor/menu/sub.do?menuId=13_91_93 (accessed on 11 May 2021).
- Craig, D.A. Some of what you should know about water of K.I.S.S. for hydrodynamics (*Keeping It Stupidly Simple). Bull. North Am. Benthol. Soc. 1987, 4, 178–182. [Google Scholar]
- Cummins, K.W. An Evaluation of some techniques for the collection and analysis of benthic samples with special emphasis on lotic waters. Am. Midl. Nat. 1962, 67, 477–504. [Google Scholar] [CrossRef]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef]
- Ferrier, S.; Guisan, A. Spatial modelling of biodiversity at the community level. J. Appl. Ecol. 2006, 43, 393–404. [Google Scholar] [CrossRef]
- D’Amen, M.; Rahbek, C.; Zimmermann, N.E.; Guisan, A. Spatial predictions at the community level: From current approaches to future frameworks. Biol. Rev. Camb. Philos. Soc. 2017, 92, 169–187. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 10 January 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. R Package. Version 2.5-3. 2018. Available online: https://www.researchgate.net/publication/275524120_Multivariate_analysis_of_ecological_communities_in_R_vegan_tutorial_R_package_version_17 (accessed on 10 January 2023).
- EFE (Enforcement Decree of the Framework act on Environmental policy) Ministry of Government Legislation, Korean Law Information Center. Available online: https://www.law.go.kr/LSW/eng/engLsSc.do?menuId=2§ion=lawNm&query=%ED%99%98%EA%B2%BD%EC%A0%95%EC%B1%85&x=0&y=0#liBgcolor1/ (accessed on 10 January 2023).
- Kang, Y.-J.; Lee, S.-J.; An, K.-G. Physical habitat and chemical water quality characteristics on the distribution patterns of ecologically disturbing fish (Largemouth bass and Blugill) in Dongjin-River Watershed. Korean J. Environ. Biol. 2019, 37, 177–188. [Google Scholar] [CrossRef]
- Wang, J.H.; Park, H.J.; Park, J.H.; Song, H.S.; Kim, H.J.; Park, Y.J.; Choi, J.K.; Lee, H.G. Comparison of the habitat distribution characteristics of aquatic Oligochaeta according to the construction of weirs in four major rivers in South Korea. Korean J Environ. Biol. 2019, 37, 607–617. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Hong, C.; Choi, B.; Kim, H.; Park, Y.; Park, J.; Song, H.; Kwak, I. Changes in Community Structure of Chironomidae Caused by Variability of Environmental Factor among Weir Sections in Korean Rivers. Korean J. Ecol. Environ. 2020, 53, 46–54. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, Y.-H.; Lee, J.-H.; Kim, D.-G.; Kim, M.-K.; Kim, H.-M. Biodiversity changes and community characteristics of benthic macroinvertebrates in weir section of the Nakdong River, South Korea. Korean J. Environ. Ecol. 2022, 36, 150–164. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human health risk assessment of potentially toxic elements and microplastics accumulation in products from the Danube River Basin fish market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Topa, C.; Timofti, M.; Pintilie, V.; Arseni, M. Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water 2019, 11, 1305. [Google Scholar] [CrossRef]
- Daily, J.P.; Hitt, N.P.; Smith, D.R.; Snyder, C.D. Experimental and environmental factors affect spurious detection of ecological thresholds. Ecology 2012, 93, 17–23. [Google Scholar] [CrossRef]
- Khoshgoftaar, T.M.; Golawala, M.; Van Hulse, J.V. An empirical study of learning from imbalanced data using random forest. In Proceedings of the 19th IEEE International Conference on Tools with Artificial Intelligence (ICTAI 2007), Patras, Greece, 29–31 October 2007; IEEE Publications: Piscataway, NJ, USA; pp. 310–317. [Google Scholar] [CrossRef]
- García-Callejas, D.; Araújo, M.B. The effects of model and data complexity on predictions from species distributions models. Ecol. Modell. 2016, 326, 4–12. [Google Scholar] [CrossRef]
- Balounová, Z.; Pechoušková, E.; Rajchard, J.; Jota, V.; Šinko, J. World-wide distribution of the Bryozoan Pectinatella magnifica (Leidy 1851). Eur. J. Environ. Sci. 2013, 3, 96–100. [Google Scholar] [CrossRef]
- Cha, Y.; Shin, J.; Go, B.; Lee, D.S.; Kim, Y.; Kim, T.; Park, Y.S. An interpretable machine learning method for supporting ecosystem management: Application to species distribution models of freshwater macroinvertebrates. J. Environ. Manag. 2021, 291, 112719. [Google Scholar] [CrossRef]
- Metcalf, J.D.; Karns, G.R.; Gade, M.R.; Gould, P.R.; Bruskotter, J.T. Agency mission statements provide insight into the purpose and practice of conservation. Hum. Dimens. Wildl. 2021, 26, 262–274. [Google Scholar] [CrossRef]
- Rostami, M.A.; Frontalini, F.; Giordano, P.; Francescangeli, F.; Alves Martins, M.V.A.; Dyer, L.; Spagnoli, F. Testing the applicability of random forest modeling to examine benthic foraminiferal responses to multiple environmental parameters. Mar. Environ. Res. 2021, 172, 105502. [Google Scholar] [CrossRef]
- Fu, C.; Xu, Y.; Bundy, A.; Grüss, A.; Coll, M.; Heymans, J.J.; Fulton, E.A.; Shannon, L.; Halouani, G.; Velez, L.; et al. Making ecological indicators management ready: Assessing the specificity, sensitivity, and threshold response of ecological indicators. Ecol. Indic. 2019, 105, 16–28. [Google Scholar] [CrossRef]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; Van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef]






| Category | Predictors (Definition) | Variable Code | Unit |
|---|---|---|---|
| Water quality (W001–W029) | Water temperature (min, medium, max) | Temp | °C |
| Dissolved oxygen (min, medium, max) | DO | mg O2/L | |
| pH (min, medium, max) | pH | ||
| Conductivity (min, medium, max) | EC | μmhos/cm | |
| Suspended solids (medium) | SS | mg/L | |
| Total nitrogen (medium) | TN | mg TN/L | |
| Dissolved total nitrogen (min, medium, max) | DTN | mg DTN/L | |
| Nitrate nitrogen (medium) | NO3-N | mg NO3-N/L | |
| Ammonium nitrogen (medium) | NH3-N | mg NH3-N/L | |
| Total phosphorus (medium) | TP | mg TP/L | |
| Dissolved total phosphorus (min, medium, max) | DTP | mg DTP/L | |
| Phosphates phosphorus (medium) | PO4-P | mg PO4-P/L | |
| Chemical oxygen demand (medium) | COD | mg O2/L | |
| BOD5 (medium) | BOD | mg O2/L | |
| Chlorophyll-a (medium) | Chl-a | mg/m3 | |
| Total coliform (medium) | TC | CFU/100 mL | |
| Fecal coliform (medium) | FC | CFU/100 mL | |
| River flow alternation by weir operation (V001–V019) | Water level (min, medium, max) | EL.m | |
| River flow rate (min, medium, max) | Flow | m3/s | |
| Storage capacity (min, medium, max) | Storage | 106 m3 | |
| Discharge of deep water by water gate | DWG | m3/s | |
| Discharge of deep water by hydroelectric power | DHP | m3/s | |
| Overflow of surface water | Overflow | m3/s | |
| Discharge of surface water by water gate | DSG | m3/s | |
| Number of days corresponding to the intake-water limit level of weir | NIW | day | |
| Number of days corresponding to the lowest water level of weir | NLW | day | |
| Hydraulic retention time (7d, 56d) | HRT | day | |
| Presence of weirs (Presense, Absence) | |||
| Habitat environment (H001–H018) | Precipitation (56d, max) | Pre. (56d, max) | mm |
| Dry day (non-precipiration day during 56d) | Dry | day | |
| Heavy rainfall (day over 80mmy) | HRF | day | |
| Surface water temperature | Sur. T. | °C | |
| Water depth in sampling site | Depth | m | |
| Velocity in sampling site [34] | Vel. | m/s | |
| Substrate size (Eight types) [35] | Substrate | % | |
| Flow schemes (Three types) | PRIS, PRUS, PPOS | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-S.; Heo, J.; Park, K.-J.; Park, H.-K. Threshold Response Identification to Multi-Stressors Using Fish- and Macroinvertebrate-Based Diagnostic Tools in the Large River with Weir-Regulated Flow. Sustainability 2024, 16, 7447. https://doi.org/10.3390/su16177447
Ryu H-S, Heo J, Park K-J, Park H-K. Threshold Response Identification to Multi-Stressors Using Fish- and Macroinvertebrate-Based Diagnostic Tools in the Large River with Weir-Regulated Flow. Sustainability. 2024; 16(17):7447. https://doi.org/10.3390/su16177447
Chicago/Turabian StyleRyu, Hui-Seong, Jun Heo, Kyoung-Jun Park, and Hae-Kyung Park. 2024. "Threshold Response Identification to Multi-Stressors Using Fish- and Macroinvertebrate-Based Diagnostic Tools in the Large River with Weir-Regulated Flow" Sustainability 16, no. 17: 7447. https://doi.org/10.3390/su16177447
APA StyleRyu, H.-S., Heo, J., Park, K.-J., & Park, H.-K. (2024). Threshold Response Identification to Multi-Stressors Using Fish- and Macroinvertebrate-Based Diagnostic Tools in the Large River with Weir-Regulated Flow. Sustainability, 16(17), 7447. https://doi.org/10.3390/su16177447






