Coniferous Biomass for Energy Valorization: A Thermo-Chemical Properties Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Samples
2.2. Chemical Characterization
2.2.1. Proximate Analysis
2.2.2. Elemental Analysis
2.2.3. Calorimetry
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization
3.1.1. Proximate Analysis
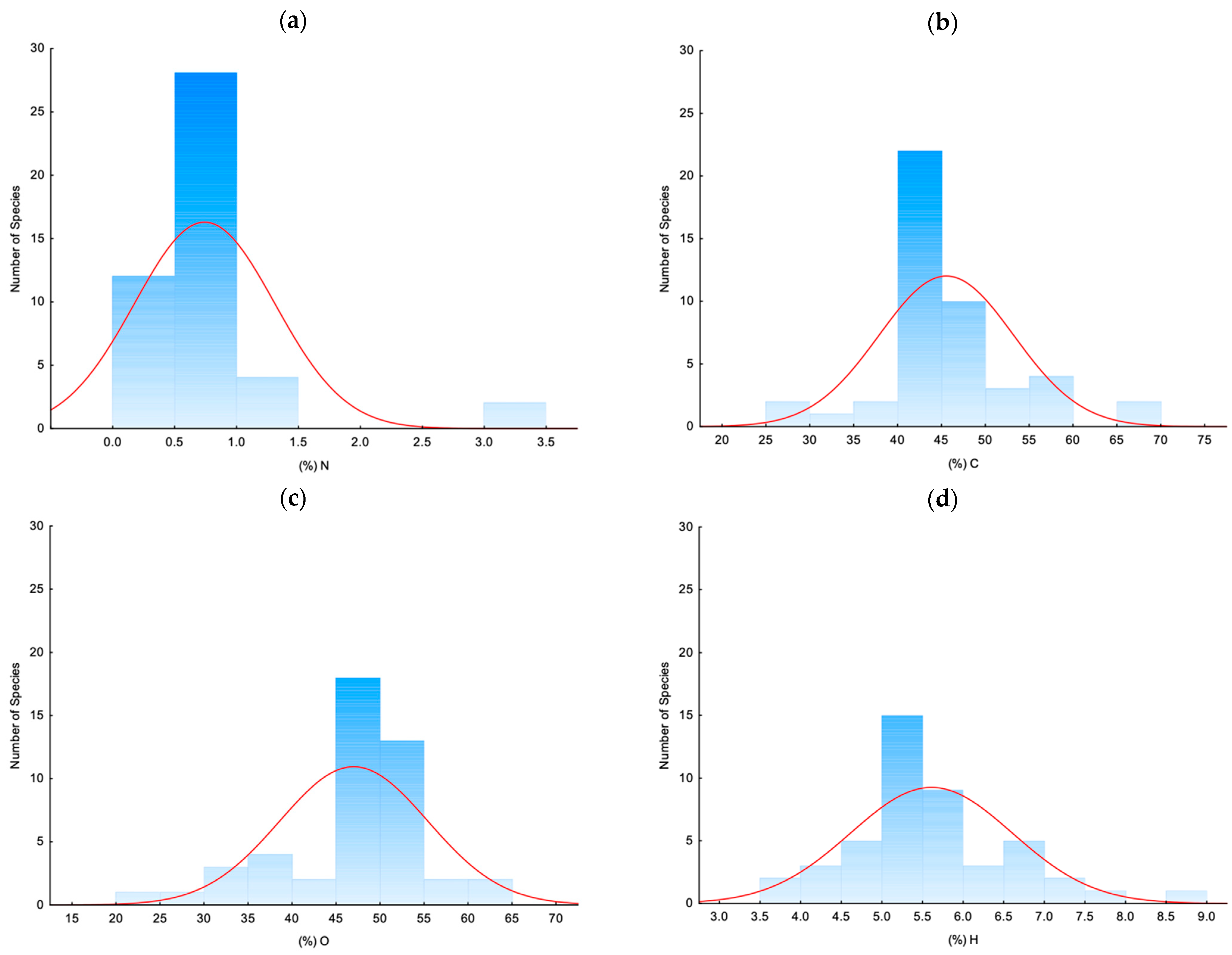
3.1.2. Elemental Chemical Analysis
3.1.3. Calorimetric Analysis
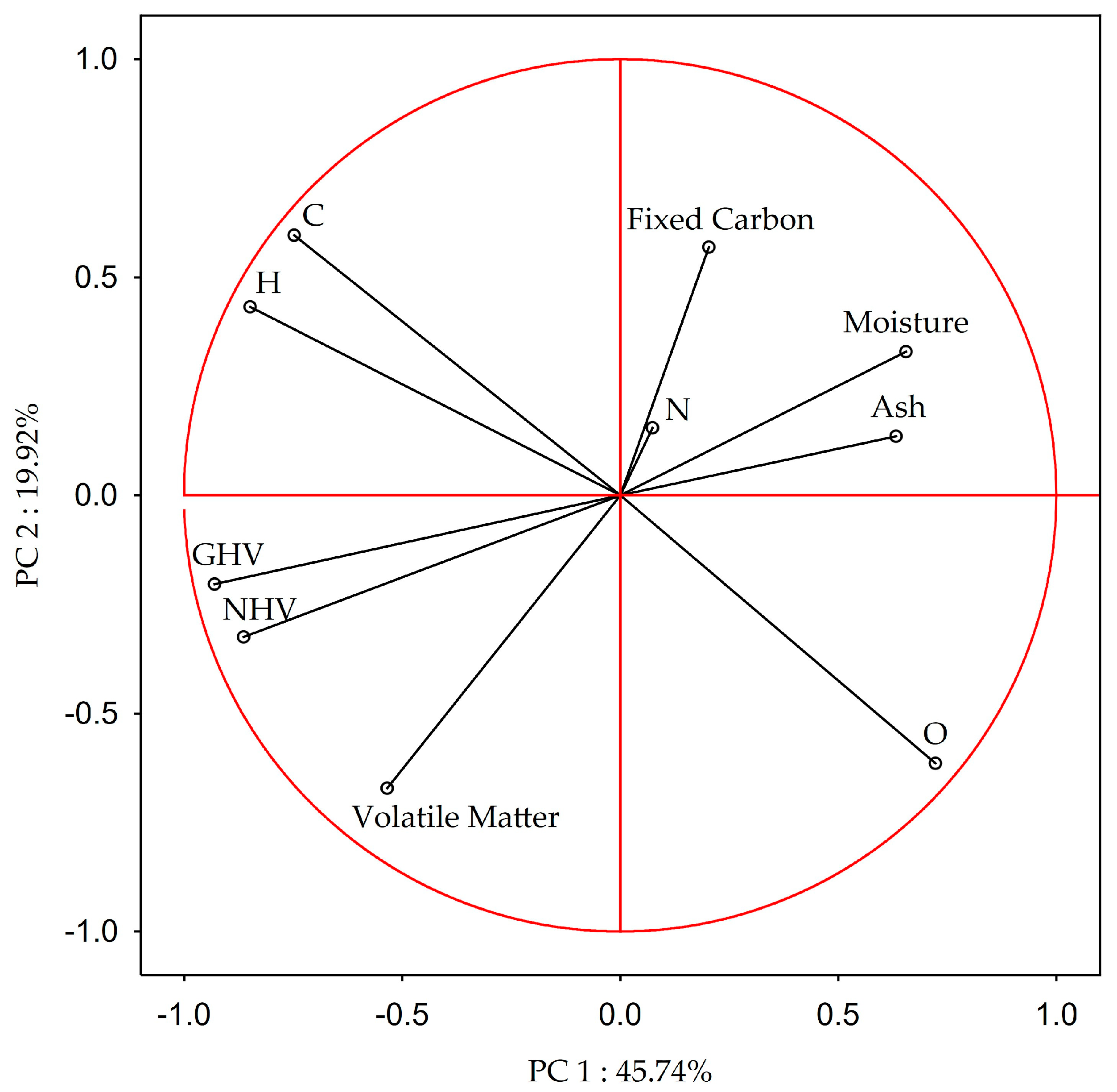
3.2. Relationship between Proximate Analysis, Elemental Composition, and Heating Values of Biomass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adebayo, T.S.; Awosusi, A.A.; Kirikkaleli, D.; Akinsola, G.D.; Mwamba, M.N. Can CO2 emissions and energy consumption determine the economic performance of South Korea? A time series analysis. Environ. Sci. Pollut. Res. 2021, 28, 38969–38984. [Google Scholar] [CrossRef]
- Yuping, L.; Ramzan, M.; Xincheng, L.; Murshed, M.; Awosusi, A.A.; Bahs, I.; Adebayo, T.S. Determinants of carbon emissions in Argentina: The roles of renewable energy consumption and globalization. Energy Rep. 2021, 7, 4747–4760. [Google Scholar] [CrossRef]
- Maluf, C.D. Qual a Importância da Energia Renovável. AmbScience Engenharia. Available online: https://ambscience.com/energia-renovavel/ (accessed on 3 May 2022).
- European Environment Agency. About Energy, EEA 2018. Available online: https://www.eea.europa.eu/pt/themes/energy/about-energy (accessed on 3 May 2022).
- Awosusi, A.A.; Adebayo, T.S.; Altuntaş, M.; Agyekum, E.B.; Zawbaa, H.M.; Kamel, S. The dynamic impact of biomass and natural resources on ecological footprint in BRICS economies: A quantile regression evidence. Energy Rep. 2022, 8, 1979–1994. [Google Scholar] [CrossRef]
- Júnior, S.V.; Soares, I.P. Chemical analysis of biomass—A review of techniques and applications. Quím. Nova 2014, 37, 709–715. [Google Scholar] [CrossRef]
- DGEG. Fontes de Energia Renovável: Biomassa Assegura 45% da Produção. Energia 2021. Available online: https://florestas.pt/valorizar/energia-renovavel-biomassa-assegura-56-da-producao/#:~:text=A%20produ%C3%A7%C3%A3o%20anual%20de%20energia,Energia%20e%20Geologia%20(DGEG) (accessed on 31 May 2022).
- Baker, J.S.; Wade, C.M.; Sohngen, B.L.; Ohrel, S.; Fawcett, A.A. Potential complementarity between forest carbon sequestration incentives and biomass energy expansion. Energy Policy 2019, 126, 391–401. [Google Scholar] [CrossRef]
- ICNF. IFN6—6° Inventário Florestal Nacional—Relatório Final; Instituto da Conservação da Natureza e das Florestas: Lisbon, Portugal, 2015. [Google Scholar]
- Röser, D.; Asikainen, A.; Stupak, I.; Pasanen, K. Forest Energy Resources and Potentials. In Sustainable Use of Forest Biomass for Energy; Röser, D., Asikainen, A., Raulund-Rasmussen, K., Stupak, I., Eds.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; Volume 12. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística Statistics Portugal. Contas Económicas da Silvicultura. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=473108475&DESTAQUESmodo=2 (accessed on 31 May 2022).
- Girón, R.P.; Ruiz, B.; Fuente, E.; Gil, R.R.; Suárez-Ruiz, I. Properties of fly ash from forest biomass combustion. Fuel 2013, 114, 71–77. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Hall, D.O. Biomass, Energy, and Environment: A Developing Country Perspective from India; Oxford Academic: Oxford, UK, 1995. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Hiloidhari, M.; Baruah, D.C.; Kumaria, M.; Kumari, S.; Thakur, I.S. Prospect and potential of biomass power to mitigate climate change: A case study in India. J. Clean. Prod. 2019, 220, 931–944. [Google Scholar] [CrossRef]
- Sulaiman, C.; Abdul-Rahim, A.S.; Ofozor, C.A. Does wood biomass energy use reduce CO2 emissions in European Union member countries? Evidence from 27 members. J. Clean. Prod. 2020, 253, 119996. [Google Scholar] [CrossRef]
- IRENA. Global Energy Transformation: A Roadmap to 2050 (2019 Edition); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- Observatório da Energia; DGEG—Direção Geral de Energia e Geologia; Direção de Serviços de Planeamento Energético e Estatística; ADENE—Agência para a Energia; Unidade de Informação. Energia em Números; ADENE: Lisbon, Portugal, 2020. [Google Scholar]
- Hall, D.O.; Rosillo-Calle, F.; de Groot, P. Biomass energy: Lessons from case studies in developing countries. Energy Policy 1992, 20, 62–73. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- ISO 18135:2017 (E); Solid Biofuels—Sampling. ISO: Geneva, Switzerland, 2017.
- Makepa, D.C.; Chihobo, C.H. Sustainable pathways for biomass production and utilization in carbon capture and storage—A review. Biomass Convert. Biorefinery 2024. [Google Scholar] [CrossRef]
- Sorek, N.; Yeats, T.H.; Szemenyei, H.; Youngs, H.; Somerville, C.R. The Implications of Lignocellulosic Biomass Chemical Composition for the Production of Advanced Biofuels. BioScience 2014, 64, 192–201. [Google Scholar] [CrossRef]
- Michael, T.; Dananjali, G.; Naoki, H.; Anke, M.; Saman, S. Effects of Elevated Carbon Dioxide on Photosynthesis and Carbon Partitioning: A Perspective on Root Sugar Sensing and Hormonal Crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef]
- ISO 14780:2017 (E); Solid Biofuels—Solid Biofuels—Sample Preparation. ISO: Geneva, Switzerland, 2017.
- ISO 18134-3:2015 (E); Solid Biofuels—Determination of Moisture Content Oven Dry Method. ISO: Geneva, Switzerland, 2015.
- ISO 18122:2015 (E); Solid Biofuels—Determination of Ash Content, Geneva. ISO: Geneva, Switzerland, 2015.
- ISO 18123:2015 (E); Solid Biofuels—Determination of the Content of Volatile Matter. ISO: Geneva, Switzerland, 2015.
- ISO 16948:2015 (E); Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. ISO: Geneva, Switzerland, 2015.
- ISO 18125:2017 (E); Solid Biofuels—Determination of Calorific Value. ISO: Geneva, Switzerland, 2017.
- Hartmann, H. Solid Biofuels, Fuels, and Their Characteristics. In Encyclopedia of Sustainability Science and Technology; Meyers, R., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Bajwa, S.G. Properties of densified solid biofuels in relation to chemical composition, moisture content, and bulk density of the biomass. BioResources 2019, 14, 4996–5015. [Google Scholar] [CrossRef]
- Ferreira, A.C.O. Caracterização de Vários Tipos de Biomassa para Valorização Energética. Master’s Thesis, Universidade de Aveiro, Departamento de Engenharia Mecânica, Aveiro, Portugal, 2013. [Google Scholar]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Oliveira, H.S.M.; Silva, F.G.C.S.; Pio, D.T.; Matos, M.A.A. Valorisation of residual biomass by pyrolysis: Influence of process conditions on products. Sustain. Energy Fuels 2024, 8, 379–396. [Google Scholar] [CrossRef]
- Chen, L.; Lin, S.; Liang, J.; Evrendilek, F.; He, Y.; Liu, J. Combustion behaviors of Lycium barbarum L.: Kinetics, thermodynamics, gas emissions, and optimization. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Vassilev, S.; Baxter, D.; Andersen, L.; Vassileva, C. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Chang, B.; Ma, K.; Lu, Z.; Lu, J.; Cui, J.; Wang, L.; Jin, B. Physiological, Transcriptomic, and Metabolic Responses of Ginkgo biloba L. to Drought, Salt, and Heat Stresses. Biomolecules 2020, 10, 1635. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Ali, F.; Dawood, A.; Hussain, A.; Alnasir, M.H.; Khan, M.A.; Butt, T.M.; Janjua, N.K.; Hamid, A. Fueling the future: Biomass applications for green and sustainable energy. Discov. Sustain. 2024, 5, 156. [Google Scholar] [CrossRef]
- Varghese, R.T.; Antony, T.; Chirayil, C.J. Biomass: Abundance, Classification, Energy Potential. In Handbook of Advanced Biomass Materials for Environmental Remediation; Thomas, S., Chirayil, C.J., Varghese, R.T., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Matin, B.; Leto, J.; Antonović, A.; Brandić, I.; Jurišić, V.; Matin, A.; Krička, T.; Grubor, M.; Kontek, M.; Bilandžija, N. Energetic Properties and Biomass Productivity of Switchgrass (Panicum virgatum L.) under Agroecological Conditions in Northwestern Croatia. Agronomy 2023, 13, 1161. [Google Scholar] [CrossRef]
- Petráš, R.; Mecko, J.; Kukla, J.; Kuklová, M.; Krupová, D.; Pástor, M.; Raček, M.; Pivková, I. Energy Stored in Above-Ground Biomass Fractions and Model Trees of the Main Coniferous Woody Plants. Sustainability 2021, 13, 12686. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Nathans, L.; Oswald, F.; Nimon, K. Interpreting multiple linear regression: A guidebook of variable importance. Practical Assessment. Res. Eval. 2012, 17, 1–19. [Google Scholar] [CrossRef]
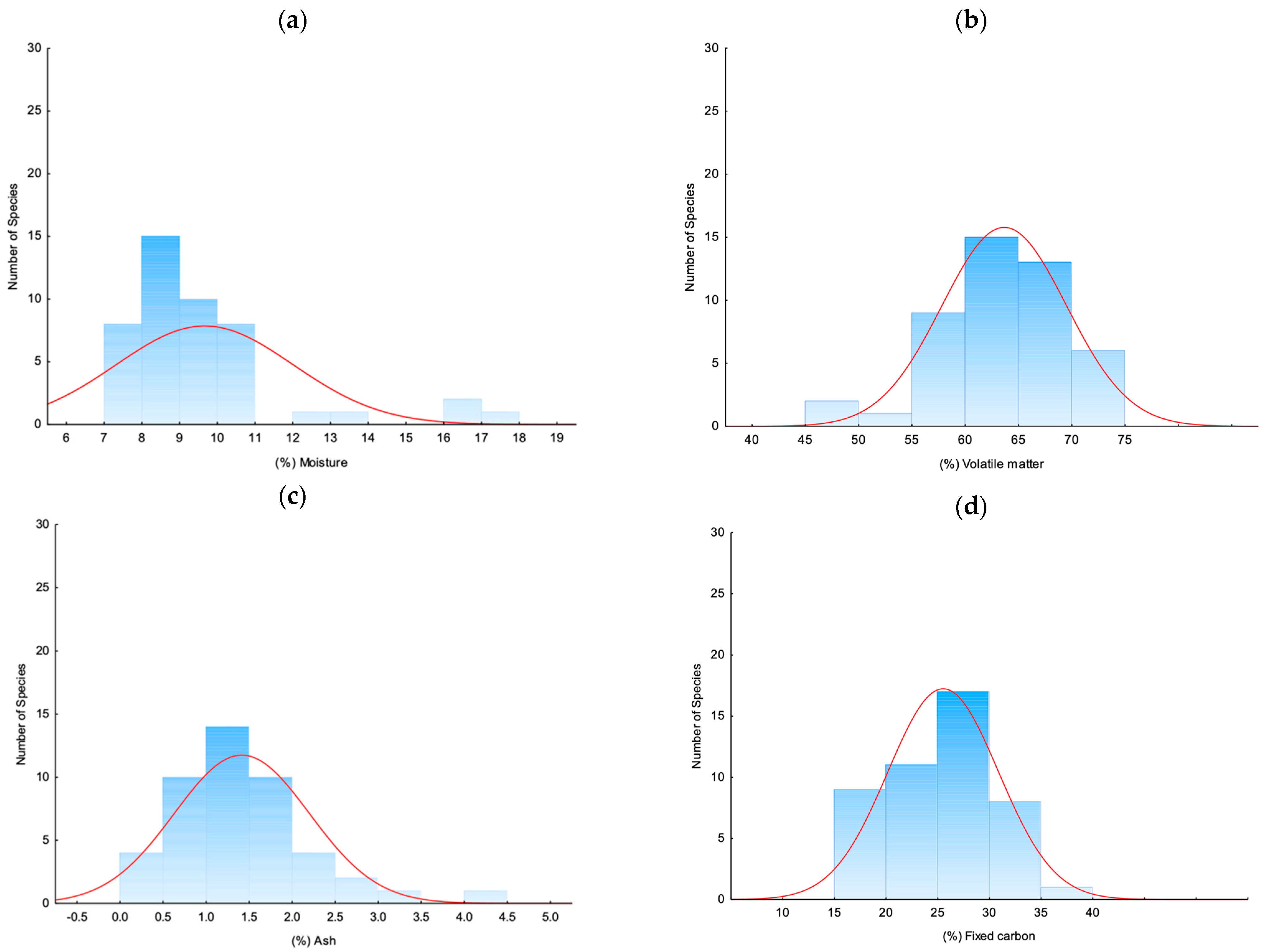







| ID | Sample | Moisture ad ± σ (%) | Volatile Matter d ± σ (%) | Ash d ± σ (%) | Fixed Carbon d ± σ (%) |
|---|---|---|---|---|---|
| 1 | Abies alba | 9.00 ± 0.300 | 61.50 ± 1.973 | 1.30 ± 0.027 | 28.20 ± 0.517 |
| 2 | Abies grandis | 8.30 ± 0.299 | 63.90 ± 0.717 | 0.90 ± 0.030 | 26.90 ± 0.534 |
| 3 | Abies koreana | 10.90 ± 0.185 | 59.70 ± 1.648 | 1.20 ± 0.033 | 28.20 ± 0.547 |
| 4 | Abies nebrodensis | 7.70 ± 0.367 | 64.70 ± 1.489 | 1.60 ± 0.065 | 26.00 ± 1.010 |
| 5 | Abies nordmanniana | 8.10 ± 0.188 | 70.00 ± 1.818 | 1.30 ± 0.066 | 20.60 ± 0.434 |
| 6 | Abies pinsapo | 9.20 ± 0.159 | 66.10 ± 0.662 | 2.00 ± 0.096 | 22.70 ± 0.448 |
| 7 | Araucaria araucana | 7.80 ± 0.394 | 59.10 ± 0.934 | 0.70 ± 0.023 | 32.40 ± 1.048 |
| 8 | Calocedrus decurrens | 10.90 ± 0.406 | 61.00 ± 0.807 | 3.40 ± 0.165 | 24.70 ± 0.524 |
| 9 | Cedrus atlantica | 10.20 ± 0.289 | 69.80 ± 0.882 | 1.50 ± 0.061 | 18.50 ± 0.328 |
| 10 | Cedrus deodara | 13.30 ± 0.471 | 59.70 ± 1.057 | 1.50 ± 0.064 | 25.50 ± 0.607 |
| 11 | Cedrus libani | 8.90 ± 0.255 | 55.80 ± 1.408 | 1.30 ± 0.039 | 34.00 ± 0.617 |
| 12 | Chamaecyparis lawsoniana | 7.10 ± 0.255 | 64.10 ± 1.089 | 1.60 ± 0.106 | 27.20 ± 0.692 |
| 13 | Chamaecyparis obtusa | 8.70 ± 0.464 | 71.70 ± 1.733 | 1.20 ± 0.031 | 18.40 ± 0.377 |
| 14 | Cryptomeria japonica | 9.20 ± 0.265 | 68.60 ± 2.156 | 1.80 ± 0.028 | 20.40 ± 0.306 |
| 15 | Cupressus arizonica | 8.60 ± 0.560 | 58.80 ± 1.033 | 1.20 ± 0.049 | 31.40 ± 1.126 |
| 16 | Cupressus lusitanica | 16.70 ± 0.460 | 66.90 ± 0.547 | 1.00 ± 0.052 | 15.40 ± 0.146 |
| 17 | Cupressus macrocarpa | 8.50 ± 0.253 | 71.00 ± 1.745 | 1.60 ± 0.058 | 18.90 ± 0.381 |
| 18 | Cupressus sempervirens | 8.40 ± 0.093 | 72.20 ± 1.430 | 1.40 ± 0.045 | 18.00 ± 0.508 |
| 19 | Ginkgo biloba | 17.00 ± 0.440 | 45.50 ± 1.469 | 4.10 ± 0.148 | 33.40 ± 0.990 |
| 20 | Juniperus horizontalis | 9.10 ± 0.177 | 70.10 ± 1.896 | 2.00 ± 0.078 | 18.80 ± 0.370 |
| 21 | Juniperus oxycedrus | 8.10 ± 0.201 | 68.60 ± 1.051 | 2.30 ± 0.121 | 21.00 ± 0.357 |
| 22 | Juniperus sabina | 7.70 ± 0.263 | 64.60 ± 0.881 | 2.60 ± 0.142 | 25.10 ± 0.395 |
| 23 | Juniperus sabina var. tamariscifolia | 9.50 ± 0.275 | 60.00 ± 1.473 | 2.30 ± 0.058 | 28.20 ± 0.606 |
| 24 | Juniperus squamata | 8.00 ± 0.184 | 66.20 ± 2.252 | 1.50 ± 0.043 | 24.30 ± 0.520 |
| 25 | Larix decidua | 10.60 ± 0.176 | 64.80 ± 2.081 | 1.60 ± 0.123 | 23.00 ± 0.500 |
| 26 | Metasequoia glyptostroboides | 10.20 ± 0.138 | 63.20 ± 1.989 | 1.40 ± 0.033 | 25.20 ± 1.042 |
| 27 | Picea abies | 9.20 ± 0.271 | 60.20 ± 0.790 | 2.50 ± 0.078 | 28.10 ± 0.627 |
| 28 | Picea glauca | 9.50 ± 0.108 | 68.60 ± 1.249 | 1.50 ± 0.053 | 20.40 ± 0.521 |
| 29 | Picea pungens | 8.30 ± 0.246 | 60.60 ± 2.057 | 1.90 ± 0.057 | 29.20 ± 0.655 |
| 30 | Pinus heldreichii | 10.70 ± 0.215 | 66.20 ± 1.733 | 1.30 ± 0.057 | 21.80 ± 0.661 |
| 31 | Pinus mugo | 7.50 ± 0.194 | 53.80 ± 0.612 | 0.80 ± 0.026 | 37.90 ± 0.683 |
| 32 | Pinus nigra | 9.00 ± 0.200 | 58.10 ± 1.255 | 0.10 ± 0.003 | 32.80 ± 0.526 |
| 33 | Pinus pinaster | 9.30 ± 0.677 | 58.00 ± 0.694 | 0.80 ± 0.031 | 31.90 ± 0.776 |
| 34 | Pinus pinea | 8.90 ± 0.306 | 65.10 ± 1.276 | 0.50 ± 0.023 | 25.50 ± 0.671 |
| 35 | Pinus radiata | 8.90 ± 0.433 | 63.00 ± 1.823 | 0.60 ± 0.041 | 27.50 ± 0.672 |
| 36 | Pinus strobus | 9.20 ± 0.329 | 71.10 ± 1.414 | 1.10 ± 0.034 | 18.60 ± 0.394 |
| 37 | Pinus sylvestris | 8.80 ± 0.197 | 60.70 ± 1.921 | 0.80 ± 0.020 | 29.70 ± 0.891 |
| 38 | Podocarpus macrophyllus | 17.40 ± 0.695 | 49.80 ± 1.784 | 2.30 ± 0.054 | 30.50 ± 0.616 |
| 39 | Pseudotsuga menziesii | 7.10 ± 0.125 | 63.90 ± 1.311 | 1.20 ± 0.054 | 27.80 ± 0.300 |
| 40 | Sequoia sempervirens | 9.60 ± 0.219 | 60.40 ± 0.580 | 0.60 ± 0.029 | 29.40 ± 0.783 |
| 41 | Sequoiadendron giganteum | 10.30 ± 0.315 | 56.50 ± 1.319 | 0.40 ± 0.016 | 32.80 ± 0.914 |
| 42 | Taxodium distichum | 8.30 ± 0.173 | 64.30 ± 1.844 | 1.10 ± 0.041 | 26.30 ± 0.831 |
| 43 | Taxus baccata | 12.40 ± 0.335 | 69.30 ± 0.951 | 1.30 ± 0.084 | 17.00 ± 0.345 |
| 44 | Thuja plicata | 10.20 ± 0.378 | 66.00 ± 1.216 | 0.80 ± 0.024 | 23.00 ± 0.854 |
| 45 | Thujopsis dolobrata | 7.90 ± 0.235 | 68.30 ± 1.882 | 0.20 ± 0.005 | 23.60 ± 0.377 |
| 46 | Tsuga heterophylla | 9.40 ± 0.229 | 70.80 ± 0.912 | 0.50 ± 0.021 | 19.30 ± 0.668 |
| ID | Sample | Nd ± σ (%) | Cd ± σ (%) | Od ± σ (%) | Hd ± σ (%) |
|---|---|---|---|---|---|
| 1 | Abies alba | 0.56 ± 0.015 | 47.04 ± 0.997 | 46.45 ± 0.824 | 4.64 ± 0.125 |
| 2 | Abies grandis | 1.45 ± 0.051 | 53.87 ± 0.894 | 38.42 ± 0.815 | 5.36 ± 0.264 |
| 3 | Abies koreana | 0.97 ± 0.037 | 57.94 ± 1.594 | 33.67 ± 0.770 | 6.21 ± 0.105 |
| 4 | Abies nebrodensis | 1.29 ± 0.097 | 46.09 ± 0.906 | 46.39 ± 1.175 | 4.63 ± 0.172 |
| 5 | Abies nordmanniana | 0.62 ± 0.022 | 49.83 ± 0.672 | 42.73 ± 0.926 | 5.51 ± 0.142 |
| 6 | Abies pinsapo | 1.13 ± 0.043 | 59.60 ± 1.802 | 31.11 ± 0.449 | 6.16 ± 0.362 |
| 7 | Araucaria araucana | 0.68 ± 0.024 | 44.77 ± 2.160 | 49.20 ± 0.857 | 4.65 ± 0.287 |
| 8 | Calocedrus decurrens | 0.68 ± 0.044 | 36.88 ± 0.829 | 55.89 ± 1.140 | 3.15 ± 0.138 |
| 9 | Cedrus atlantica | 0.84 ± 0.019 | 50.08 ± 1.210 | 42.51 ± 1.343 | 5.07 ± 0.155 |
| 10 | Cedrus deodara | 1.04 ± 0.031 | 63.84 ± 1.487 | 26.89 ± 0.649 | 6.73 ± 0.218 |
| 11 | Cedrus libani | 3.34 ± 0.107 | 47.72 ± 1.192 | 43.68 ± 0.840 | 3.95 ± 0.157 |
| 12 | Chamaecyparis lawsoniana | 0.62 ± 0.016 | 46.05 ± 0.548 | 47.03 ± 0.779 | 4.71 ± 0.104 |
| 13 | Chamaecyparis obtusa | 0.62 ± 0.028 | 51.12 ± 2.158 | 41.62 ± 0.730 | 5.43 ± 0.153 |
| 14 | Cryptomeria japonica | 0.54 ± 0.013 | 50.04 ± 1.390 | 42.48 ± 1.939 | 5.13 ± 0.118 |
| 15 | Cupressus arizonica | 0.52 ± 0.016 | 47.30 ± 1.281 | 46.03 ± 1.317 | 4.94 ± 0.213 |
| 16 | Cupressus lusitanica | 0.58 ± 0.034 | 50.23 ± 1.601 | 44.10 ± 0.691 | 4.10 ± 0.087 |
| 17 | Cupressus macrocarpa | 0.71 ± 0.029 | 48.37 ± 0.542 | 44.15 ± 1.036 | 5.17 ± 0.308 |
| 18 | Cupressus sempervirens | 0.42 ± 0.022 | 49.76 ± 2.136 | 43.29 ± 0.799 | 5.14 ± 0.138 |
| 19 | Ginkgo biloba | 0.77 ± 0.028 | 34.49 ± 0.758 | 58.29 ± 1.564 | 2.35 ± 0.126 |
| 20 | Juniperus horizontalis | 0.60 ± 0.019 | 49.77 ± 1.073 | 42.65 ± 1.799 | 4.97 ± 0.164 |
| 21 | Juniperus oxycedrus | 3.47 ± 0.067 | 48.21 ± 1.475 | 42.28 ± 0.906 | 3.74 ± 0.138 |
| 22 | Juniperus sabina | 0.60 ± 0.033 | 47.88 ± 1.084 | 44.25 ± 1.482 | 4.67 ± 0.126 |
| 23 | Juniperus sabina var. tamariscifolia | 0.69 ± 0.029 | 44.94 ± 0.760 | 47.24 ± 1.222 | 4.83 ± 0.129 |
| 24 | Juniperus squamata | 1.12 ± 0.056 | 48.86 ± 1.316 | 43.88 ± 1.164 | 4.65 ± 0.119 |
| 25 | Larix decidua | 0.22 ± 0.010 | 43.48 ± 0.666 | 50.92 ± 0.440 | 3.78 ± 0.111 |
| 26 | Metasequoia glyptostroboides | 0.32 ± 0.010 | 46.90 ± 0.893 | 46.70 ± 1.014 | 4.68 ± 0.223 |
| 27 | Picea abies | 0.84 ± 0.028 | 61.70 ± 1.590 | 28.81 ± 0.363 | 6.14 ± 0.139 |
| 28 | Picea glauca | 0.76 ± 0.032 | 50.34 ± 0.671 | 41.68 ± 0.793 | 5.71 ± 0.194 |
| 29 | Picea pungens | 0.75 ± 0.031 | 60.76 ± 1.045 | 29.91 ± 0.457 | 6.68 ± 0.194 |
| 30 | Pinus heldreichii | 0.59 ± 0.034 | 44.97 ± 1.201 | 48.67 ± 1.267 | 4.48 ± 0.095 |
| 31 | Pinus mugo | 0.66 ± 0.045 | 44.70 ± 0.869 | 49.65 ± 1.535 | 4.19 ± 0.102 |
| 32 | Pinus nigra | 0.55 ± 0.017 | 50.90 ± 1.179 | 43.32 ± 0.525 | 5.14 ± 0.225 |
| 33 | Pinus pinaster | 0.45 ± 0.011 | 46.25 ± 0.723 | 47.67 ± 0.683 | 4.83 ± 0.152 |
| 34 | Pinus pinea | 0.36 ± 0.013 | 40.53 ± 0.774 | 54.95 ± 1.333 | 3.66 ± 0.131 |
| 35 | Pinus radiata | 0.47 ± 0.009 | 46.92 ± 0.850 | 47.41 ± 0.652 | 4.60 ± 0.118 |
| 36 | Pinus strobus | 0.66 ± 0.056 | 53.08 ± 1.763 | 39.18 ± 1.155 | 5.98 ± 0.146 |
| 37 | Pinus sylvestris | 0.73 ± 0.017 | 60.94 ± 1.347 | 31.10 ± 0.740 | 6.43 ± 0.134 |
| 38 | Podocarpus macrophyllus | 1.01 ± 0.034 | 36.23 ± 0.997 | 58.30 ± 1.243 | 2.16 ± 0.062 |
| 39 | Pseudotsuga menziesii | 0.99 ± 0.030 | 45.50 ± 2.331 | 47.65 ± 0.961 | 4.66 ± 0.164 |
| 40 | Sequoia sempervirens | 0.32 ± 0.009 | 45.85 ± 0.502 | 49.00 ± 1.487 | 4.23 ± 0.124 |
| 41 | Sequoiadendron giganteum | 0.75 ± 0.023 | 77.92 ± 1.314 | 12.74 ± 0.159 | 8.20 ± 0.277 |
| 42 | Taxodium distichum | 0.76 ± 0.050 | 70.92 ± 1.340 | 20.01 ± 0.242 | 7.21 ± 0.230 |
| 43 | Taxus baccata | 0.63 ± 0.034 | 49.99 ± 1.305 | 43.45 ± 1.643 | 4.63 ± 0.199 |
| 44 | Thuja plicata | 0.27 ± 0.011 | 60.65 ± 0.846 | 31.89 ± 1.103 | 6.39 ± 0.214 |
| 45 | Thujopsis dolobrata | 0.81 ± 0.035 | 45.93 ± 1.431 | 49.00 ± 1.608 | 4.06 ± 0.213 |
| 46 | Tsuga heterophylla | 0.64 ± 0.018 | 51.68 ± 1.305 | 41.43 ± 0.785 | 5.75 ± 0.104 |
| ID | Sample | GHVd ± σ (MJ/kg) | NHVd ± σ (MJ/kg) |
|---|---|---|---|
| 1 | Abies alba | 18.39 ± 0.690 | 17.43 ± 0.646 |
| 2 | Abies grandis | 18.48 ± 0.621 | 17.38 ± 0.652 |
| 3 | Abies koreana | 18.17 ± 0.487 | 16.89 ± 0.495 |
| 4 | Abies nebrodensis | 18.34 ± 0.345 | 17.39 ± 0.364 |
| 5 | Abies nordmanniana | 18.76 ± 0.894 | 17.62 ± 0.878 |
| 6 | Abies pinsapo | 18.46 ± 0.539 | 17.19 ± 0.551 |
| 7 | Araucaria araucana | 18.23 ± 0.714 | 17.27 ± 0.714 |
| 8 | Calocedrus decurrens | 14.39 ± 0.618 | 13.74 ± 0.626 |
| 9 | Cedrus atlantica | 18.02 ± 0.546 | 16.98 ± 0.575 |
| 10 | Cedrus deodara | 17.55 ± 0.653 | 16.16 ± 0.637 |
| 11 | Cedrus libani | 17.70 ± 0.419 | 16.89 ± 0.411 |
| 12 | Chamaecyparis lawsoniana | 18.26 ± 1.109 | 17.29 ± 1.116 |
| 13 | Chamaecyparis obtusa | 18.54 ± 0.761 | 17.42 ± 0.750 |
| 14 | Cryptomeria japonica | 18.31 ± 0.419 | 17.25 ± 0.429 |
| 15 | Cupressus arizonica | 18.04 ± 0.677 | 17.02 ± 0.692 |
| 16 | Cupressus lusitanica | 16.75 ± 0.394 | 15.91 ± 0.421 |
| 17 | Cupressus macrocarpa | 18.23 ± 0.970 | 17.16 ± 0.960 |
| 18 | Cupressus sempervirens | 18.24 ± 0.528 | 17.18 ± 0.530 |
| 19 | Ginkgo biloba | 13.45 ± 0.458 | 12.97 ± 0.454 |
| 20 | Juniperus horizontalis | 18.10 ± 0.687 | 17.08 ± 0.676 |
| 21 | Juniperus oxycedrus | 17.61 ± 0.495 | 16.84 ± 0.480 |
| 22 | Juniperus sabina | 18.03 ± 0.641 | 17.07 ± 0.637 |
| 23 | Juniperus sabina var, tamariscifolia | 17.72 ± 0.814 | 16.73 ± 0.813 |
| 24 | Juniperus squamata | 18.10 ± 0.347 | 17.14 ± 0.369 |
| 25 | Larix decidua | 16.53 ± 0.463 | 15.75 ± 0.451 |
| 26 | Metasequoia glyptostroboides | 18.14 ± 0.524 | 17.18 ± 0.539 |
| 27 | Picea abies | 18.33 ± 0.386 | 17.07 ± 0.362 |
| 28 | Picea glauca | 18.63 ± 0.718 | 17.45 ± 0.738 |
| 29 | Picea pungens | 18.24 ± 0.813 | 16.86 ± 0.813 |
| 30 | Pinus heldreichii | 17.26 ± 0.916 | 16.34 ± 0.922 |
| 31 | Pinus mugo | 18.62 ± 0.446 | 17.76 ± 0.469 |
| 32 | Pinus nigra | 18.76 ± 0.636 | 17.70 ± 0.621 |
| 33 | Pinus pinaster | 18.60 ± 0.658 | 17.61 ± 0.607 |
| 34 | Pinus pinea | 18.52 ± 0.732 | 17.77 ± 0.732 |
| 35 | Pinus radiata | 18.39 ± 0.764 | 17.44 ± 0.748 |
| 36 | Pinus strobus | 19.19 ± 0.664 | 17.96 ± 0.635 |
| 37 | Pinus sylvestris | 18.33 ± 0.747 | 17.01 ± 0.750 |
| 38 | Podocarpus macrophyllus | 13.21 ± 0.486 | 12.77 ± 0.481 |
| 39 | Pseudotsuga menziesii | 18.24 ± 0.342 | 17.28 ± 0.360 |
| 40 | Sequoia sempervirens | 18.29 ± 1.386 | 17.42 ± 1.418 |
| 41 | Sequoiadendron giganteum | 18.78 ± 1.413 | 17.09 ± 1.429 |
| 42 | Taxodium distichum | 18.22 ± 0.962 | 16.73 ± 0.938 |
| 43 | Taxus baccata | 17.38 ± 0.444 | 16.43 ± 0.428 |
| 44 | Thuja plicata | 18.36 ± 1.405 | 17.04 ± 1.432 |
| 45 | Thujopsis dolobrata | 18.32 ± 0.822 | 17.48 ± 0.799 |
| 46 | Tsuga heterophylla | 18.48 ± 0.712 | 17.30 ± 0.712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, B.M.M.; Oliveira, M.; da Silva Borges, A.D. Coniferous Biomass for Energy Valorization: A Thermo-Chemical Properties Analysis. Sustainability 2024, 16, 7622. https://doi.org/10.3390/su16177622
Teixeira BMM, Oliveira M, da Silva Borges AD. Coniferous Biomass for Energy Valorization: A Thermo-Chemical Properties Analysis. Sustainability. 2024; 16(17):7622. https://doi.org/10.3390/su16177622
Chicago/Turabian StyleTeixeira, Bruno M. M., Miguel Oliveira, and Amadeu Duarte da Silva Borges. 2024. "Coniferous Biomass for Energy Valorization: A Thermo-Chemical Properties Analysis" Sustainability 16, no. 17: 7622. https://doi.org/10.3390/su16177622
APA StyleTeixeira, B. M. M., Oliveira, M., & da Silva Borges, A. D. (2024). Coniferous Biomass for Energy Valorization: A Thermo-Chemical Properties Analysis. Sustainability, 16(17), 7622. https://doi.org/10.3390/su16177622






