Degradation of Malachite Green Dye by Solar Irradiation Assisted by TiO2 Biogenic Nanoparticles Using Vaccinium corymbosum Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursors
2.2. Preparation of the Blueberry (Vaccinium corymbosum) Extract
2.3. Green Synthesis of TiO2 NPs
2.4. Characterization of TiO2 NPs
2.5. Photocatalytic Malachite Green Dye Degradation
3. Results and Discussion
3.1. Characterization
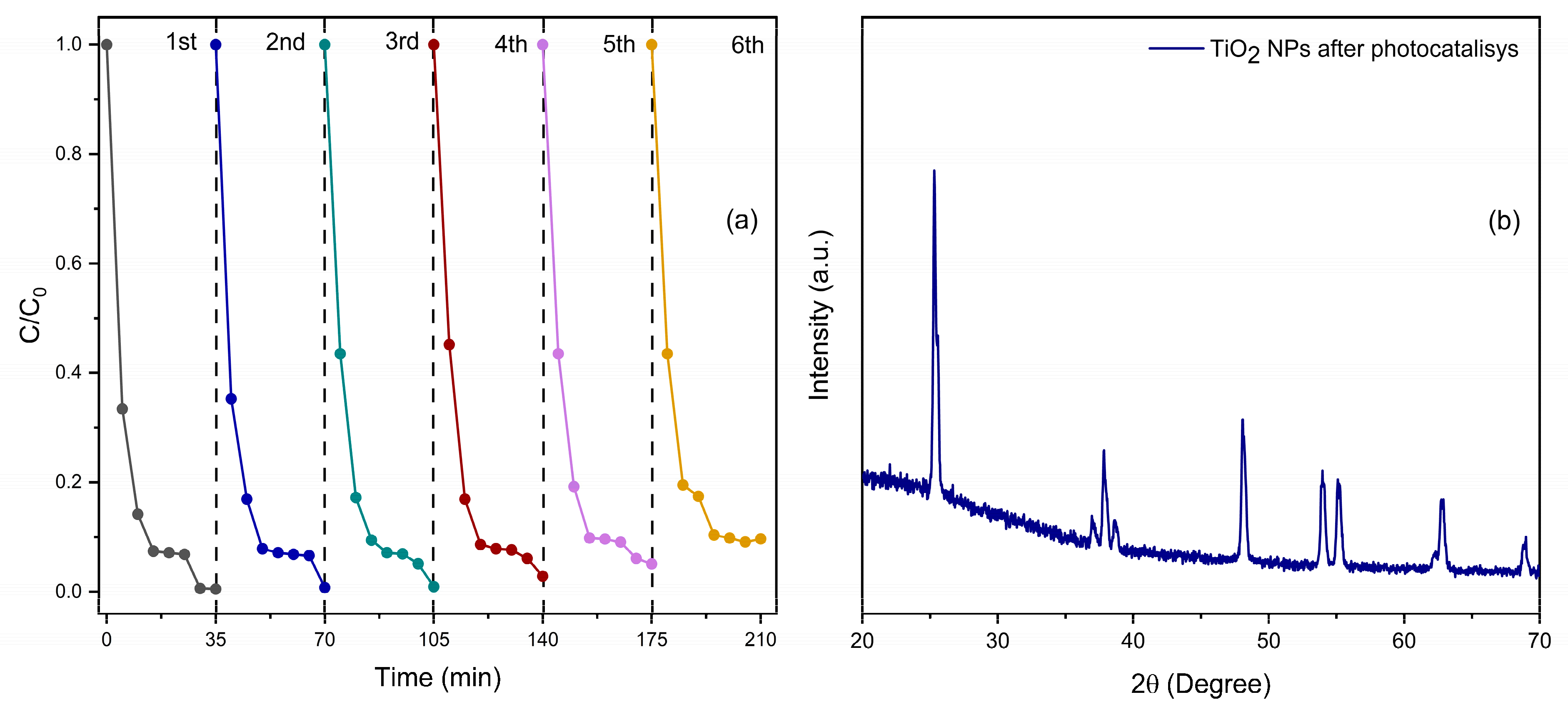
3.1.1. XRD Analysis
3.1.2. UV-Vis Spectroscopy
3.1.3. FTIR Analysis
3.1.4. XPS Analysis
3.1.5. TEM Analysis
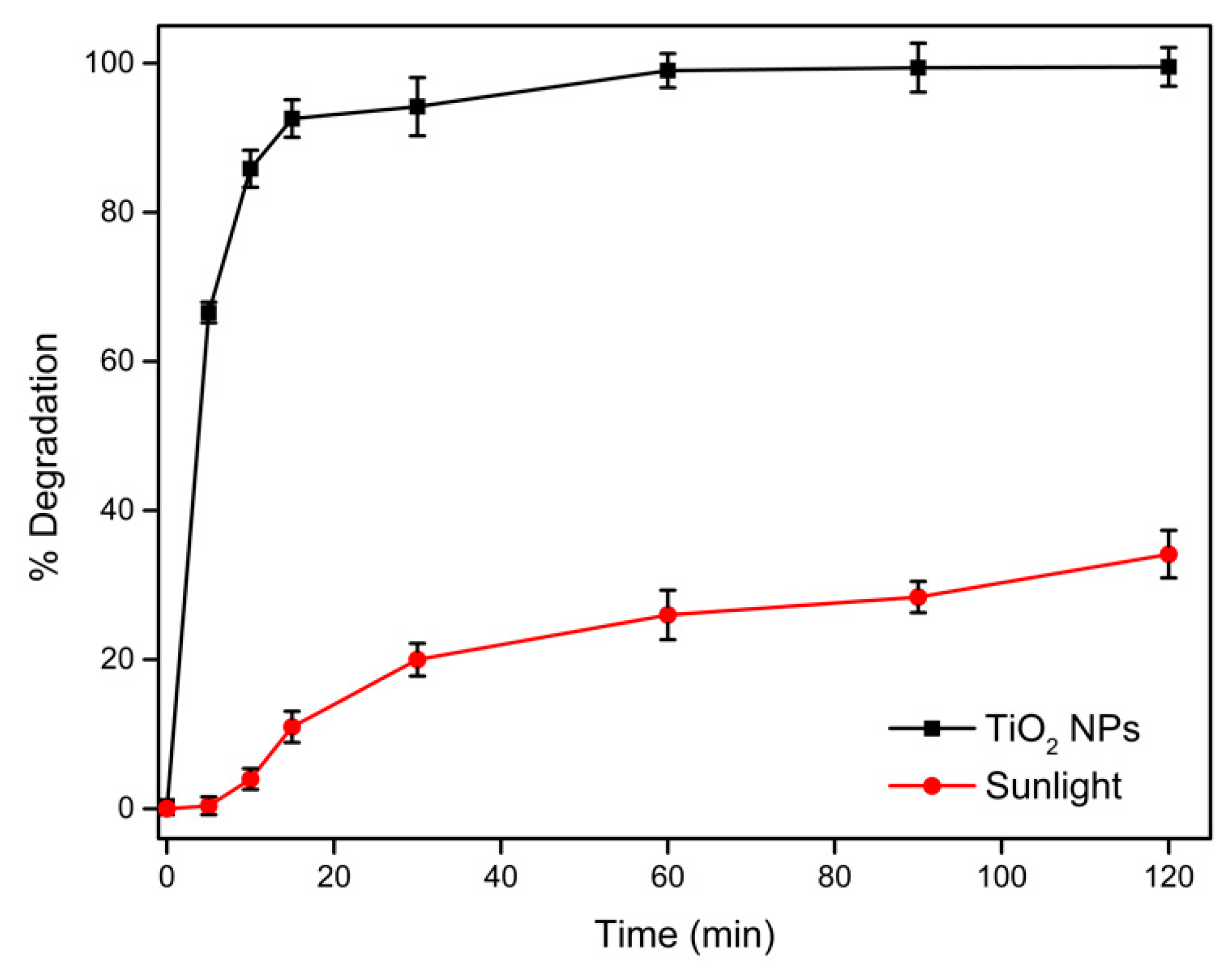
3.2. Evaluation of Photocatalytic Activity of TiO2 NPs in Malachite Green Dye Degradation
3.3. Reusability Test
3.4. Scavenger Test
3.5. Mechanism of Photodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A review on green synthesis of TiO2 NPs: Photocatalysis and antimicrobial applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Ropers, M.-H.; Terrisse, H.; Mercier-Bonin, M.; Humbert, B. Titanium Dioxide as Food Additive. In Application of Titanium Dioxide; Janus, M., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G.; Saravanan, N.; Murugesan, S.; Ramachandran, S.; Ayyasamy, R.; Pugazhendhi, A. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, D.; Prasad, R.; Singh, J. Green synthesis of titanium dioxide nanoparticles: Development and applications. J. Agric. Food Res. 2022, 10, 100361. [Google Scholar] [CrossRef]
- Amigun, A.T.; Adekola, F.A.; Tijani, J.O.; Mustapha, S. Photocatalytic degradation of malachite green dye using nitrogen/sodium/iron-TiO2 nanocatalysts. Results Chem. 2022, 4, 100480. [Google Scholar] [CrossRef]
- Kamble, R.J.; Gaikwad, P.V.; Garadkar, K.M.; Sabale, S.R.; Puri, V.R.; Mahajan, S.S. Photocatalytic degradation of malachite green using hydrothermally synthesized cobalt-doped TiO2 nanoparticles. J. Iran. Chem. Soc. 2022, 19, 303–312. [Google Scholar] [CrossRef]
- Ma, Y.; Ni, M.; Li, S. Optimization of malachite green removal from water by TiO2 nanoparticles under UV irradiation. Nanomaterials 2018, 8, 428. [Google Scholar] [CrossRef]
- Tiwari, N.; Chakrabortty, S.; Samal, K.; Moulick, S.; Mohapatra, B.G.; Samanta, S.; Mohapatra, P.K.; Sanjay, K.; Nayak, J.; Banerjee, S.; et al. Photocatalytic degradation of malachite green using TiO2 and ZnO impregnated on fecal sludge derived biochar. J. Taiwan Inst. Chem. Eng. 2023, 145, 104800. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Srujana, S.; Anjamma, M.; Alimuddin; Singh, B.; Dhakar, R.C.; Natarajan, S.; Hechhu, R.; Lakshmipathy, R. A Comprehensive Study on the Synthesis and Characterization of TiO2 Nanoparticles Using Aloe vera Plant Extract and Their Photocatalytic Activity against MB Dye. Adsorpt. Sci. Technol. 2022, 2022, 7244006. [Google Scholar] [CrossRef]
- Pavithra, S.; Bessy, T.C.; Bindhu, M.R.; Venkatesan, R.; Parimaladevi, R.; Alam, M.M.; Mayandi, J.; Umadevi, M. Photocatalytic and photovoltaic applications of green synthesized titanium oxide (TiO2) nanoparticles by Calotropis gigantea extract. J. Alloys Compd. 2023, 960, 170638. [Google Scholar] [CrossRef]
- Shimi, A.K.; Ahmed, H.M.; Wahab, M.; Katheria, S.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rane, K.P. Synthesis and Applications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022, 2022, 7060388. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Velayutham, J.; Ramasamy, S. Green synthesis of TiO2 nanoparticles prepared from Phyllanthus niruri leaf extract for dye adsorption and their isotherm and kinetic studies. IET Nanobiotechnol. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, P. Green synthesis of TiO2 nanoparticles using Tinospora cordifolia plant extract & its potential application for photocatalysis and antibacterial activity. Inorg. Chem. Commun. 2023, 156, 111221. [Google Scholar] [CrossRef]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Arshad, K.M.; Al-Habardi, M. Green synthesis of spherical TiO2 nanoparticles using Citrus limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Kumar, S.; Singh, J.; Rawat, M. Eco-friendly Approach: Synthesis of Novel Green TiO2 Nanoparticles for Degradation of Reactive Green 19 Dye and Replacement of Chemical Synthesized TiO2. J. Clust. Sci. 2021, 32, 1191–1204. [Google Scholar] [CrossRef]
- Sunny, N.E.; Mathew, S.S.; Chandel, N.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Vasseghian, Y.N.; Rajamohan, S.; Kumar, V. Green synthesis of titanium dioxide nanoparticles using plant biomass and their applications—A review. Chemosphere 2022, 300, 134612. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. 2-Green synthesis of TiO2 and its photocatalytic activity. In Handbook of Smart Photocatalytic Materials; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 11–61. [Google Scholar] [CrossRef]
- Casillas, J.E.; Tzompantzi, F.; Carbajal-Arizaga, G.G.; Aguilar-Martinez, J.; Fernández-Escamilla, V.V.A.; Ramos-Ramírez, E.; López-Álvarez, M.A.; Tzompantzi-Flores, C.; Barrera, A. Coupled Al-Ga-xAg composites prepared by the sol–gel method and their efficient photocatalytic performance in the degradation of diclofenac. Surf. Interfaces 2022, 30, 101809. [Google Scholar] [CrossRef]
- Azar, B.E.; Ramazani, A.; Fardood, S.T.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Ashour, A.A.; Raafat, D.; El-Gowelli, H.M.; El-Kamel, A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207–7221. [Google Scholar] [CrossRef]
- Kumar, B.; Vizuete, K.S.; Sharma, V.; Debut, A.; Cumbal, L. Ecofriendly synthesis of monodispersed silver nanoparticles using Andean Mortiño berry as reductant and its photocatalytic activity. Vacuum 2019, 160, 272–278. [Google Scholar] [CrossRef]
- Balaji, S.; Guda, R.; Mandal, B.K.; Kasula, M.; Ubba, E.; Khan, F.-R.N. Green synthesis of nano-titania (TiO2 NPs) utilizing aqueous Eucalyptus globulus leaf extract: Applications in the synthesis of 4H-pyran derivatives. Res. Chem. Intermed. 2021, 47, 3919–3931. [Google Scholar] [CrossRef]
- Kholief, M.G.; Hesham, A.E.-L.; Hashem, F.S.; Mohamed, F.M. Synthesis and utilization of titanium dioxide nano particle (TiO2 NPs) for photocatalytic degradation of organics. Sci. Rep. 2024, 14, 11327. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; Lopez, N.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen Vacancies as Active Sites for Water Dissociation on Rutile TiO2 (110). Phys. Rev. Lett. 2001, 87, 266104. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Hamlyn, R.C.E.; Mahapatra, M.; Grinter, D.C.; Xu, F.; Luo, S.; Palomino, R.M.; Kattel, S.; Waluyo, I.; Liu, P.; Stacchiola, D.J.; et al. Imaging the ordering of a weakly adsorbed two-dimensional condensate: Ambient-pressure microscopy and spectroscopy of CO2 molecules on rutile TiO2(110). Phys. Chem. Chem. Phys. 2018, 20, 13122–13126. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Tu, K.-J.; Deng, J.-P.; Lo, Y.-S.; Wu, C.-H. Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with superior water-dispersibility for photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef]
- Qu, Q.; Geng, H.; Peng, R.; Cui, Q.; Gu, X.; Li, F.; Wang, M. Chemically Binding Carboxylic Acids onto TiO2 Nanoparticles with Adjustable Coverage by Solvothermal Strategy. Langmuir 2010, 26, 9539–9546. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Akinola, P.O.; Lateef, A.; Asafa, T.B.; Beukes, L.S.; Hakeem, A.S.; Irshad, H.M. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: Antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon 2020, 6, e04610. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Yang, S.; Ding, Y.; Sun, C.; Zhang, A.; Wang, L. Microwave-Assisted Rapid Photocatalytic Degradation of Malachite Green in TiO2 Suspensions: Mechanism and Pathways. J. Phys. Chem. A 2008, 112, 11172–11177. [Google Scholar] [CrossRef]
- Chen, C.C.; Lu, C.S.; Chung, Y.C.; Jan, J.L. UV light induced photodegradation of malachite green on TiO2 nanoparticles. J. Hazard. Mater. 2007, 141, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pizzio, L.R.; Blanco, M.N.; Roussel, C.; Pulgarin, C. Photocatalytic discoloration of aqueous malachite green solutions by UV-illuminated TiO2 nanoparticles under air and nitrogen atmospheres: Effects of counter-ions and pH. Photochem. Photobiol. Sci. 2011, 10, 29–34. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Shahmoradi, B.; Khosravi, T.; Karimi, K.; Zandsalimi, Y. Solar degradation of malachite green using nickel-doped TiO2 nanocatalysts. Desalin. Water Treat. 2016, 57, 9881–9888. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sayılkan, F.; Arpaç, E. Effect of Fe3+ ion doping to TiO2 on the photocatalytic degradation of malachite green dye under UV and vis-irradiation. J. Photochem. Photobiol. A 2009, 203, 64–71. [Google Scholar] [CrossRef]
- Yitagesu, G.B.; Leku, D.T.; Workneh, G.A. Green Synthesis of TiO2 Using Impatiens rothii Hook. f. Leaf Extract for Efficient Removal of Methylene Blue Dye. ACS Omega 2023, 8, 43999–44012. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]

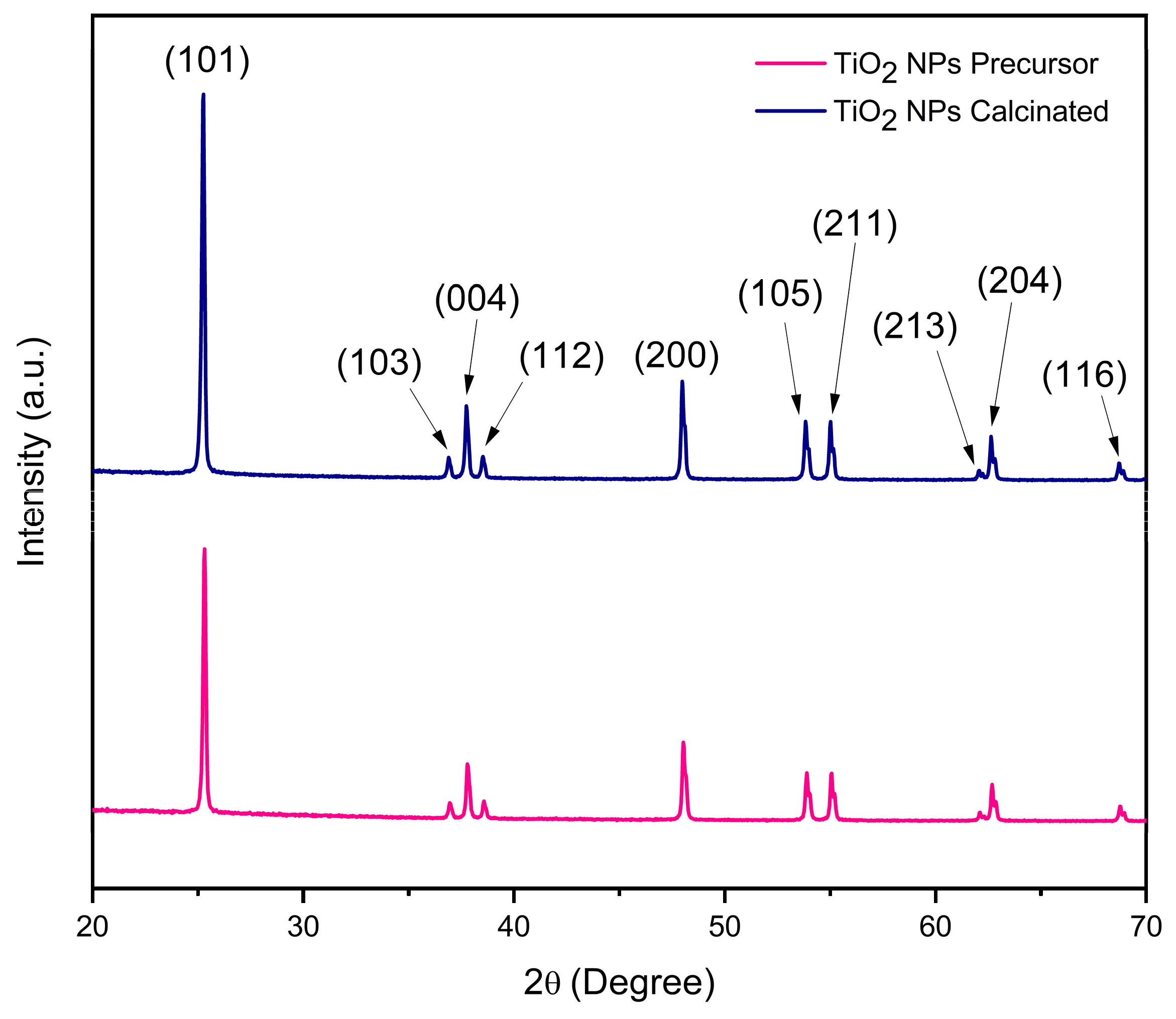

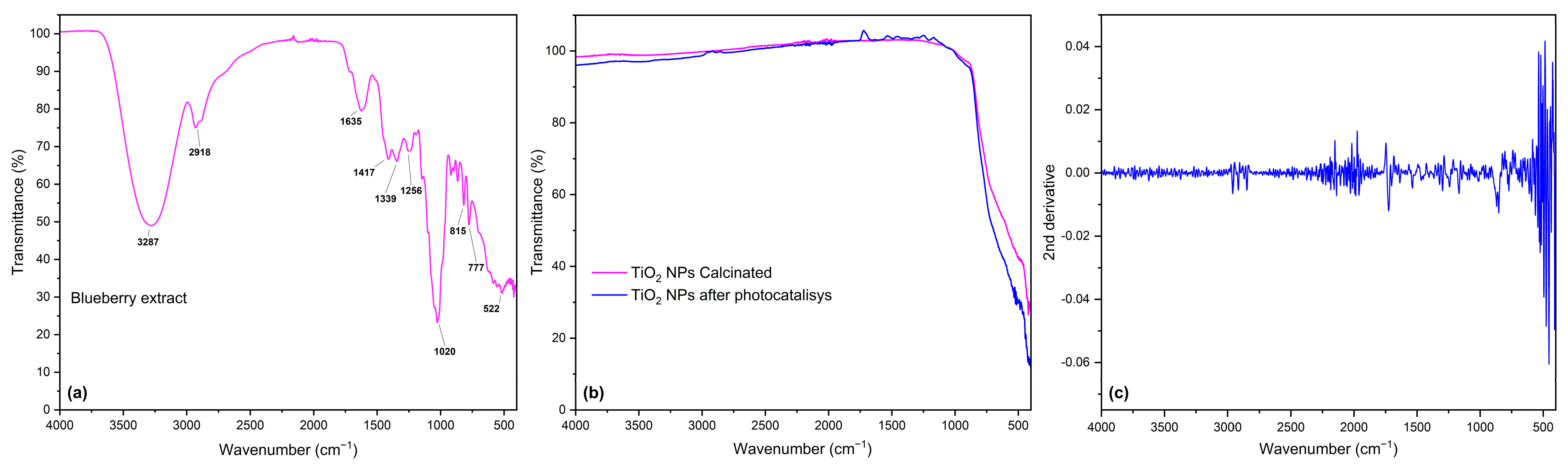

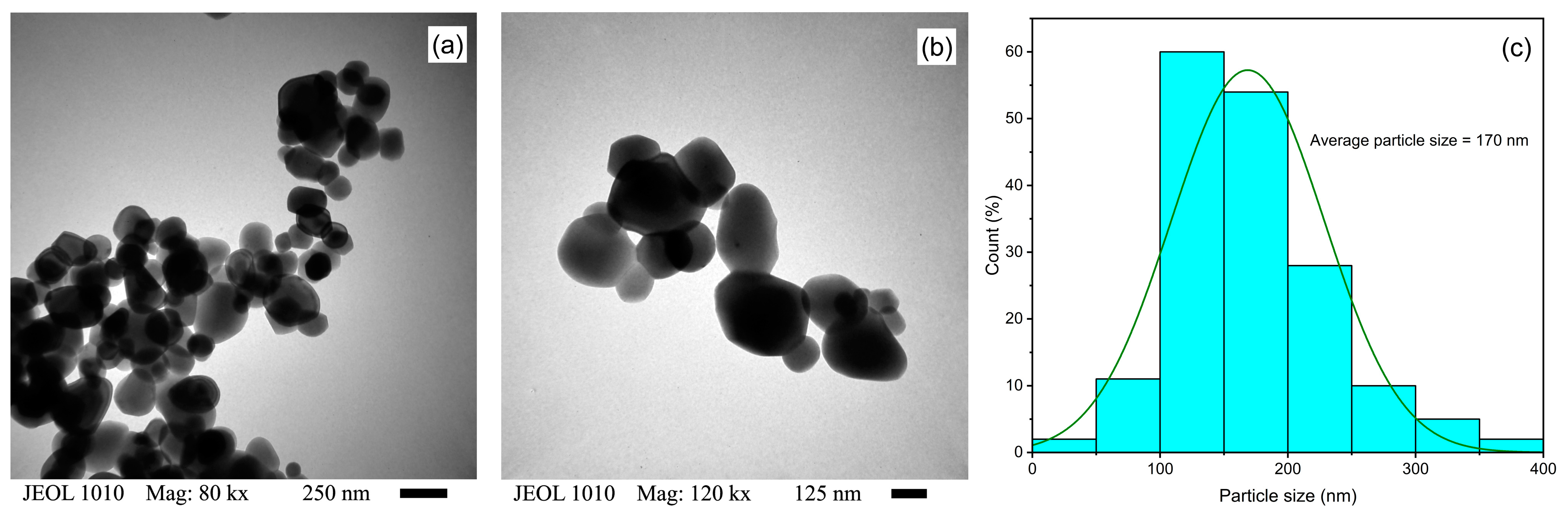
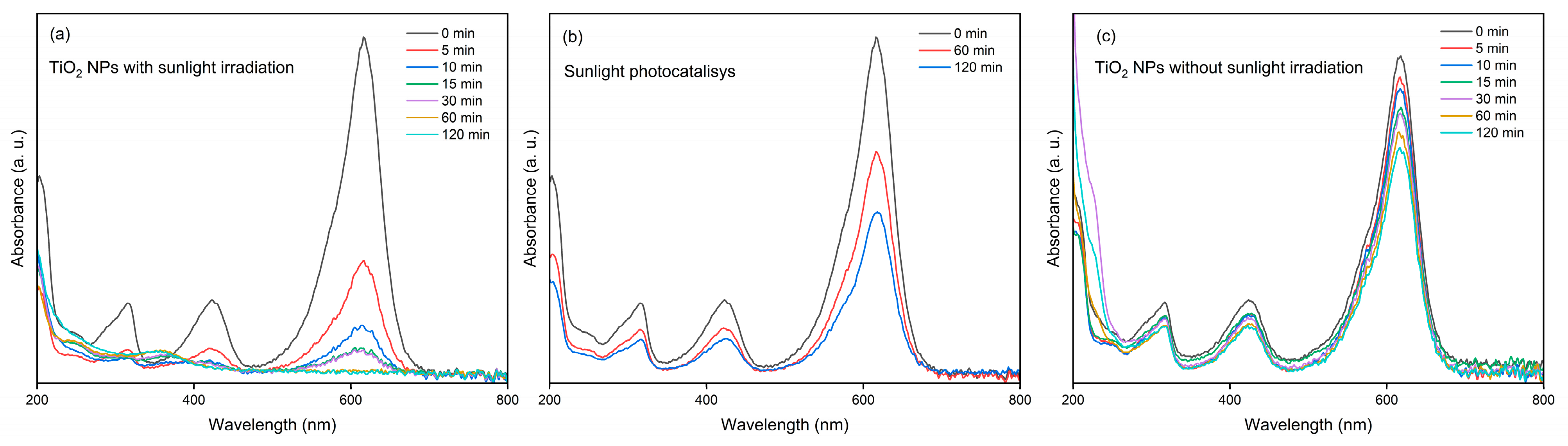



| Powder | d-Spacing(101) (nm) | Cristallite Size (nm) | Crystallinity (%) | Dislocation Density (nm)−2 |
|---|---|---|---|---|
| Precursor | 0.3515 | 54.12 | 77.07 | 3.4141 |
| TiO2 (synthesized) | 0.3522 | 49.98 | 88.01 | 4.0032 |
| Nanoparticle | Precursor | Synthesis Method | Shape/Size | Nanoparticle/Dye Proportion | Conditions | Degradation Efficiency/Time | Reference | |
|---|---|---|---|---|---|---|---|---|
| TiO2 | Titanium Hydroxide | Green synthesis | Spherical/ 25–191 nm | 80 µg/mL/1.098 × 10−4 M | Visible light | 83.48–86.28%/2 h 56.42%/24 h | [36] | |
| TiO2 P-25 | Directly added to the reaction system | Spherical/ 20–30 nm | 0.5 mg/mL/ 1.37 × 10−4 M | 15 W UV-365 nm irradiation | ~99%/4 h | [38] | ||
| Spherical | 1.0 mg/mL/ 5.0 × 10−4 M | 18 W UV (330–400 nm) under oxic conditions | ~99%/1 h | [39] | ||||
| Titanium n-butoxide | Hydrothermal | Spherical/ ~600 nm | 0.8 mg/mL/ 1.0 × 10−5 M | UV irradiation (set at 175 W). | ~99%/2 h | [10] | ||
| Ni-TiO2 | TiO2, and NiO as dopant | Spherical | 1.2 mg/mL/ 6.86 × 10−4 M | Sunlight | [40] | |||
| Co2+-TiO2 | Titanium Isopropoxide and Co(NO3)2, as dopant | Spherical/ 8–11 nm | 50 mg/mL/1.37 × 10−4 M | UV light | 61.16% | 180 min | [9] | |
| Visible light | 74.32% | |||||||
| Sunlight | 82.30% | |||||||
| Fe-TiO2 | Titanium Isopropoxide and Fe(NO3)3·9H2 O, as dopant | Spherical | 0.3 and 7%, iron doping levels in TiO2/ 1.37 × 10−5 M | UV light | ~76%/110 min | [41] | ||
| Visible light | ~79%/110 min | |||||||
| N/Na/Fe-TiO2 | Titanium Isopropoxide and NaNO3, NH4NO3, and FeCl3·6H2O, as dopants | Hydrothermal-green synthesis | Spherical/ 6–16 nm | 2.0 mg/mL/1.37 × 10−1 M | Visible light | 96.57%/25.83 min | [8] | |
| TiO2 | Food-grade TiO2 | Green synthesis | Spherical/ average 170 nm | 250 µg/mL/ 1.0 × 10−4 M | Sunlight | ~94%/30 min, and 100% within 60 min | This study | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balderas-León, I.; Silva-Jara, J.M.; López-Álvarez, M.Á.; Ortega-Gudiño, P.; Barrera-Rodríguez, A.; Neri-Cortés, C. Degradation of Malachite Green Dye by Solar Irradiation Assisted by TiO2 Biogenic Nanoparticles Using Vaccinium corymbosum Extract. Sustainability 2024, 16, 7638. https://doi.org/10.3390/su16177638
Balderas-León I, Silva-Jara JM, López-Álvarez MÁ, Ortega-Gudiño P, Barrera-Rodríguez A, Neri-Cortés C. Degradation of Malachite Green Dye by Solar Irradiation Assisted by TiO2 Biogenic Nanoparticles Using Vaccinium corymbosum Extract. Sustainability. 2024; 16(17):7638. https://doi.org/10.3390/su16177638
Chicago/Turabian StyleBalderas-León, Iván, Jorge Manuel Silva-Jara, Miguel Ángel López-Álvarez, Pedro Ortega-Gudiño, Arturo Barrera-Rodríguez, and Cristina Neri-Cortés. 2024. "Degradation of Malachite Green Dye by Solar Irradiation Assisted by TiO2 Biogenic Nanoparticles Using Vaccinium corymbosum Extract" Sustainability 16, no. 17: 7638. https://doi.org/10.3390/su16177638






