Abstract
The cross-regional spread of epidemics, such as COVID-19, poses significant challenges due to the spillover of false-negative individuals resulting from incubation periods, detection errors, and individual irrationality. This study develops a stylized model to address the trade-offs faced by the planner in designing optimal lockdown policies: curbing the cross-regional spread of epidemics while balancing economic costs and ensuring long-term sustainability. The model integrates a queuing network to calculate the influx of false-negative cases, which more accurately reflects real-world scenarios and captures the complexity of regional interactions during an outbreak. Subsequently, a SIR network is used to estimate the spread of infections. Unlike similar studies, our approach focuses specifically on the cross-regional dynamics of epidemic spread and the formulation of optimal lockdown policies that consider both public health and economic impacts. By optimizing the lockdown threshold, the model aims to minimize the total costs associated with lockdown implementation and infection spread. Our theoretical and numerical results underscore the crucial role of timely nucleic acid testing in reducing infection rates and highlight the delicate balance between public health benefits and economic sustainability. These findings provide valuable insights for developing sustainable epidemic management strategies.
1. Introduction
The emergence and rapid spread of infectious diseases pose significant challenges to public health systems worldwide. In recent years, the world has witnessed the devastating impact of pandemics, such as the COVID-19 outbreak, which highlighted the critical need for effective and timely interventions. Among the various strategies employed to curb the spread of infectious diseases, lockdowns have emerged as a crucial tool. By April 2020, about half of the world’s population was under some form of lockdown, with more than 3.9 billion people in more than 90 countries or territories having been asked or ordered to stay at home by their governments [1].
However, implementing lockdowns is not without its challenges. Policymakers must navigate the significant downsides associated with such measures, including their negative impact on the workforce and the broader economy. For instance, lockdown policies affected 93% of the global workforce, with the severity of impact varying across regions depending on the level of restrictions and enforcement [2]. Moreover, the uncertainty surrounding lockdown policies further complicates decision-making. Policymakers often face difficult questions: When should a lockdown be implemented? Under what circumstances should it be lifted? A case in point is the statewide stay-at-home order imposed by New York Governor Andrew Cuomo on 22 March 2020, initially set to end on 19 April but subsequently extended to 29 April and then to 15 May [3].
The implementation of lockdown policies, especially across regions with varying epidemiological profiles, requires a careful balance between the immediate public health benefits and the long-term socio-economic costs. Policymakers must consider not only the effectiveness of lockdowns in reducing transmission but also the broader implications of these measures on society.
In this paper, we develop a fundamental framework to guide policymakers in designing and implementing optimal lockdown policies tailored to the cross-regional spread of epidemics. Specifically, we use a queuing network to model the spillover process from the source region to the destination region, accounting for the presence of false-negative individuals due to incubation periods, detection errors, and individual irrationality. We consider a two-server queuing network where false-negative individuals arrive at queue 1. An individual then joins queue 2 if found to be positive. The capacity of server 2 is finite, representing the intensity of the prevention policy. Once queue 2 is full, indicating that the spillover of false-negative cases has reached this threshold, the planner will lock down the destination region. We then use the SIR model to estimate the infected cases in the destination region. Finally, the planner’s optimization problem is to minimize the total costs by considering both lockdown costs and epidemic costs and setting the optimal threshold.
The primary objective of this research is to formulate and analyze the optimal lockdown policy for managing the cross-regional spread of epidemics. The key contributions of this study are as follows: First, we develop a comprehensive model that captures the dynamics of disease transmission across multiple regions, incorporating key variables such as infection rates, mobility patterns, healthcare capacity, and economic factors. Second, we propose an optimization framework that balances the trade-offs between public health benefits and economic costs, facilitating the determination of region-specific lockdown intensities and durations that minimize the overall impact of the epidemic while ensuring the sustainability of both public health and economic systems.
2. Literature Review
There has been an increasing interest in the lockdown policy following the outbreak of COVID-19. One stream of literature showed that the lockdown policy was beneficial to public health or social welfare. For instance, an 81% reduction in average contact numbers during lockdowns, which helped alleviate socio-economic pressures while preventing healthcare systems from being overwhelmed [4]. Similarly, a downward trend in daily COVID-19 cases and the disease’s growth factor 15 days post-lockdown, though the prevalence and mortality rates remained largely unaffected due to the virus’s nature [5]. Additionally, preventative restrictions significantly curbed virus transmission [6]. A macroeconomic model based on SIR to examine how testing, quarantining, social distancing, and mask use affected health and economic outcomes, finding these measures can significantly lower epidemic costs [7]. Without testing and quarantining, social distancing and mask use primarily delay epidemic-related deaths rather than reduce them, also mitigating the severity of epidemic-induced recessions but extending their duration. Furthermore, nationwide lockdowns effectively reduced case numbers and helped contain the virus [8].
Conversely, another research stream argues that lockdowns might not be optimal and could have adverse effects. Limiting government lockdown discretion could enhance social welfare [9]. While early social distancing interventions could significantly limit epidemics in China, early epicenter lockdowns might counteract these benefits by causing localized deterioration [10]. They proposed a stepwise implementation of social distancing starting in the epicenter city, then the province, and finally nationwide without an epicenter lockdown to effectively minimize epidemic size and deaths. People’s efforts to avoid infection by reducing consumption and work exacerbate recession severity despite reducing epidemic impact [11].
Several researchers have explored the optimal design of policies and influential factors. One of them provides a quantitative framework to balance lives and economic costs [12]. A paper conducted a basic calculation of the ‘lives vs economy’ trade-off [13]. Several researchers considered the long-run implications of the virus for the economy [14,15]. The optimal lockdown intensity is influenced by the fatality rate gradient relative to the infected and the availability of antibody testing, offering a welfare gain of 2% of GDP [16]. Higher testing rates coupled with targeted quarantine measures can lessen the economic impact of the virus and reduce peak symptomatic infections, easing hospital capacity issues [17]. Effective tracking technology allows targeted testing and isolation policies to deliver significant welfare benefits compared to optimal policies [18]. Two major factors influencing policy enforcement: national and local government tensions, and the robustness of local territorial institutions [19]. Lastly, optimal solutions vary depending on the epidemic stage, suggesting region-specific differentiated policies or a unified approach as appropriate [20].
There are two key distinctions between our study and the existing literature. First, our research focuses on the cross-regional spread of the epidemic, employing a queuing network model integrated with a SIR network to estimate the number of infected individuals. This approach aligns more closely with real-world scenarios, capturing the complexity of regional interactions during an outbreak. Second, our study incorporates two critical factors highly relevant to lockdown policies: spillover cases and economic costs. Both elements are essential for policymakers when designing optimal lockdown strategies, as they balance the health benefits of reduced transmission against the economic repercussions of restrictive measures.
3. Method
3.1. Model Overview
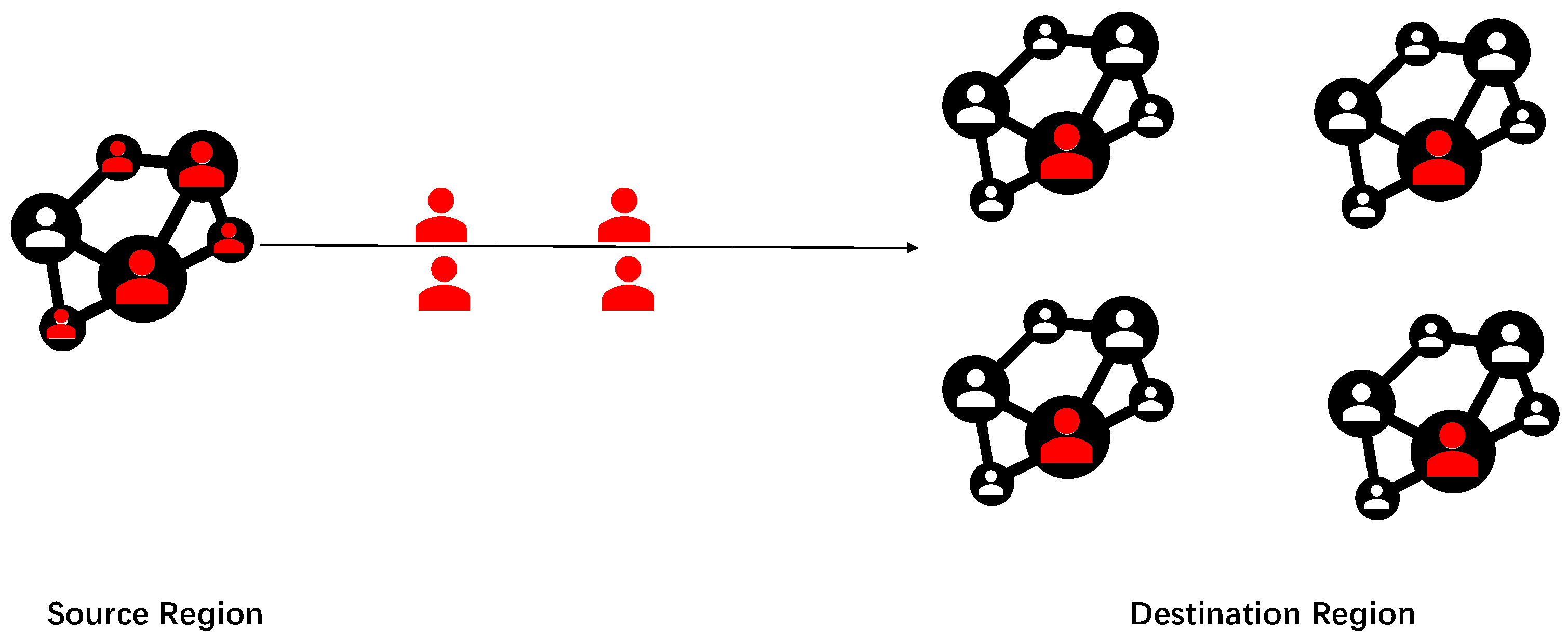
In Figure 1, we present a schematic diagram illustrating the spread of the epidemic across different regions. Test results are typically not entirely accurate. A systematic review of the real-world performance of COVID-19 tests, demonstrating a true negative rate consistently above 98% [21]. However, there may be false negative cases (marked in red), where infected individuals are incorrectly tested as negative. These individuals may travel from the source region to the destination region, leading to the spread of the epidemic. This study employs a queuing network model with two service lines to calculate the expected number of spillover cases and the duration of epidemic transmission in the destination region. Additionally, the SIR model is utilized to estimate the number of infections in the destination region. In the subsequent section, we use data to demonstrate that the arrival rate from the source region to the destination region follows a Poisson process. Then, we can formulate a queue system with a Poisson arrival rate.
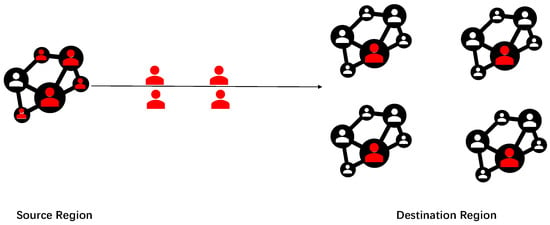
Figure 1.
Diagram of epidemic spread. The red and white colors indicate individuals who have been infected and those who have not been infected, respectively.
3.2. Data and Hypothesis Testing
We collected data on 121 spillover cases from the official websites of provincial health committees in China, spanning from 27 March 2022, to 18 April 2022. The health committees provided detailed information about each spillover case, including the origin, arrival time, and diagnosis time. Statistical tests on these 121 cases over the 23-day period indicate that the number of false negative cases spilling over from a given region follows a Poisson process. The specific statistical results are shown in Table 1.

Table 1.
Statistical test results. The statistical test was conducted to determine if the number of spillover cases follows a Poisson distribution. The sample size is 23, with a mean of 5.260. The Chi-Square statistic is 2.820 with 2 degrees of freedom, resulting in a p-value of 0.244. Based on this p-value, we accept the null hypothesis.
Based on the hypothesis testing, this paper assumes that the number of false negative cases spilling over from a given region follows a Poisson process. Consequently, we can formulate a and queue system to conduct a theoretical analysis and obtain closed-form results. For more general distributions, numerical solutions can also be applied, as referenced in related research [22,23].
3.3. Queuing Network
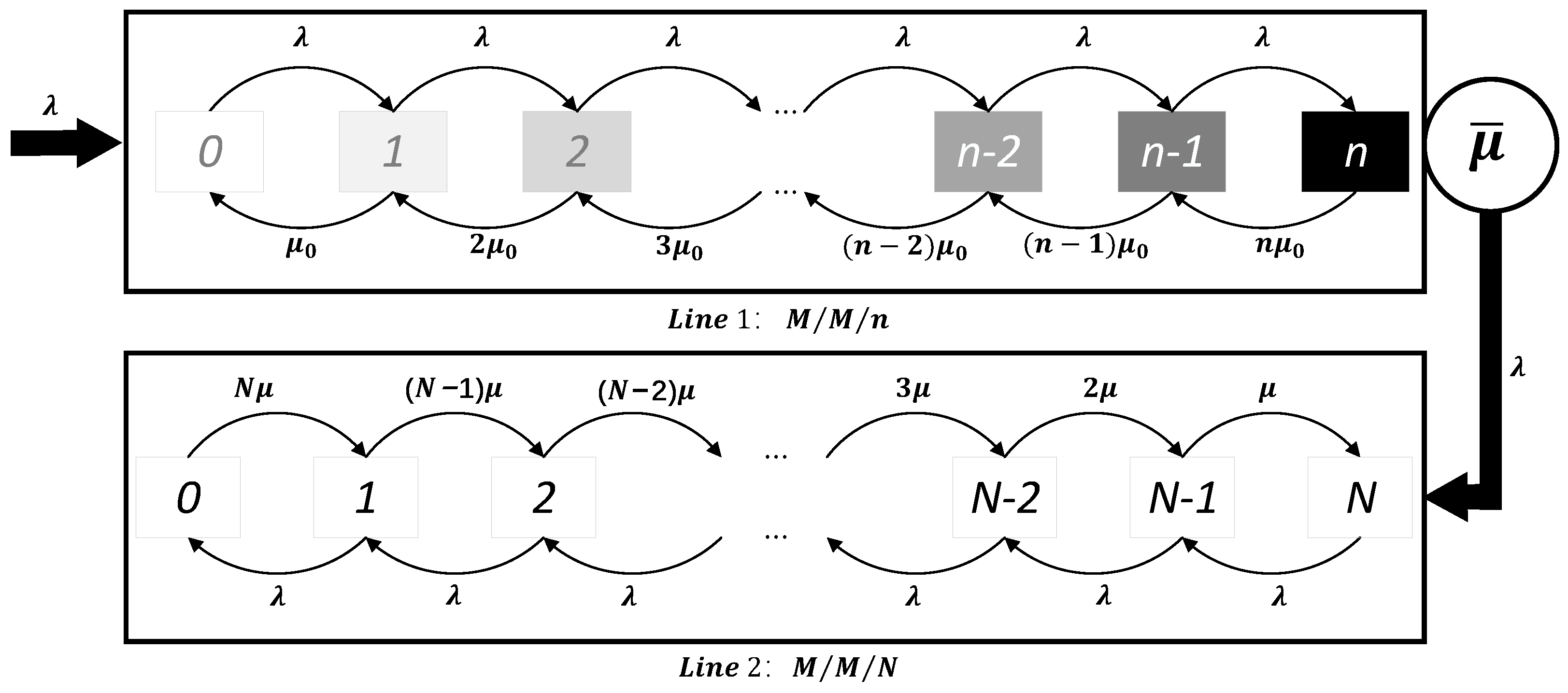
Consider a queuing system with two service lines, as illustrated in Figure 2. Specifically, Service Line 1 represents the destination region, while Service Line 2 is used for isolating negative cases. There are four types of individuals at the source region: positive, false positive, negative, and false negative. It is reasonable to assume that positive or false positive cases are not allowed to travel to other regions, while only negative and false negative cases can travel freely.
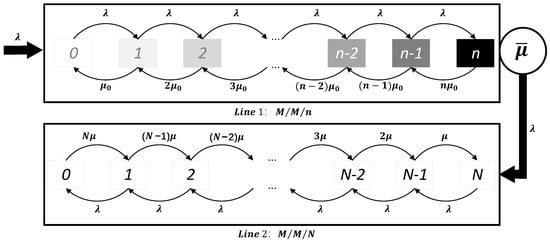
Figure 2.
Queuing network system.
When travelers arrive in the destination region, they enter Service Line 1. These travelers can be classified into two categories based on their potential health status: healthy individuals and false negative individuals, which are unknown to the planner. Assume that the process of individuals traveling from the source region to the destination region follows a Poisson process with parameter . The probability that each spillover case arriving in the destination region is not detected is P. This P can also be treated as the test’s false negative rate. Therefore, the rate at which false negative cases from outside the region arrive at Service Line 1 follows a Poisson process with a rate . Assume that the time until each false negative case is discovered follows an exponential distribution with parameter . Denote the capacity of Line 1 by n. This n can be treated as the capacity of a region. Additionally, assume that is positively correlated with the number of infected cases in the source region, I (i.e., ), and with the level of contact between the two regions, r (i.e., ). This implies that the more infected cases in the source region, the more people spillover; the closer the two regions are, the more people spillover.
Once a false negative case is detected as positive, the individual leaves Service Line 1 and enters Service Line 2 for isolation and treatment. Service Line 2 has a capacity of N. When the number of cases in Service Line 2 is less than or equal to N, individuals with a negative test result can pass freely through Service Line 1. If the number of cases exceeds N, the planner will lock down the destination region. The capacity N represents the stringency of the epidemic prevention policies in the destination region: the smaller the N, the stricter the policies. The following lemma shows that the arrival process of Line 2 also follows a Poisson process.
Lemma 1.
In a queuing system, if , the departure process follows a Poisson process with rate λ [23].
According to Lemma 1, individuals who test positive arrive at Service Line 2 following a Poisson process with rate . In the SIR model, it is typically assumed that the recovery time of cases follows an exponential distribution. Here, we also assume that the recovery time of imported cases follows an exponential distribution with parameter [24]. Therefore, Service Line 2 operates as a queuing system with parameters and , where i represents the state i. More specifically, when the system is in state N, entering state N can only be realized by leaving one case from state , since state has only one case, so the arrival rate is . For state i, , such as , leaving the state can be realized by reaching or leaving one case, since state has one case, so the departure rate is . State can enter a case or reach state at a rate of and leave one case at a rate of . When the system is in state 0, the process of leaving can only be realized by leaving one case, since state 0 has N cases, the departure rate is , and the arrival state 0 can only be realized by reaching state 1. Thus, the entire process of arrivals and departures in Service Line 2 follows the dynamics described, with states changing based on these arrival and departure rates.
Using this queuing network, we calculate the probability of each state of Service Lines 1 and 2. Further, we determine the average time a false negative case stays in Service Line 1, as shown in Theorem 1. From these results, we infer the average number of infected cases caused by the spillover cases through the SIR model. Additionally, Theorem 2 provides the probability that the destination region will need to implement the lockdown policy. The detailed proof of Theorem 1 and Theorem 2 is provided in Appendix A.
Theorem 1.
Let represent the probability that the number of existing false negative cases in the destination region is k. Then, , where . The average time a false negative case stays in Service Line 1 is .
Theorem 1 shows that the average stay time of a spillover case in Service Line 1 is . In other words, the expected time from when a spillover case enters the destination region to when it is detected is .
Theorem 2.
Let represent the probability that the number of existing confirmed imported cases in the destination region is . Then, , where .
Theorem 2 shows the steady-state probability of Service Line 2. Recall that is defined as the probability that the number of existing confirmed imported cases in the destination region is . Thus, represents the probability that the number of existing spillover cases is N, indicating that the destination region needs to adopt the lockdown policy.
Proposition 1.
For any given λ, and μ, we have , , and .
Proof of Proposition 1.
By Theorem 2, we have
It is evident that increases with . Based on the assumption that and , we can infer the following results:
and
To determine whether the derivative of with respect to N is positive or negative, we first need to compute this derivative. Starting with the expression for , we denote the denominator by D:
Thus, can be written as . Now, we need to calculate the derivative of with respect to N. Using the chain rule, we obtain
Next, we need to compute . Notice that D consists of polynomials in N. Specifically,
Calculating the derivative of each term and simplifying, we obtain
Since these derivatives are all positive, it follows that . Thus, we have shown that
□
Proposition 1 shows that the probability of the destination region implementing a lockdown policy is positively related to the number of infected cases in the source region and the level of contact between the two regions, while it is negatively related to the threshold for the lockdown policy in the destination region.
First, as stated in the setting, it is reasonable to assume that if the source region has more infected cases, the effective arrival rate of spillover cases is higher. This means that a higher number of false negative cases increases the probability of spillover and, consequently, the likelihood of a lockdown in the destination region. Second, if the level of contact between the two regions is high, more individuals will travel to the destination region, leading to a larger and a higher . Third, if the destination region implements a loose epidemic prevention policy (i.e., a larger N), the probability of a lockdown will decrease. Intuitively, the probability that the destination region reaches its maximum capacity for isolating cases from the source region has an inverse relationship with N.
3.4. SIR Network
In this section, we first introduce the SIR model [25] and combine the results from the queuing network to estimate the number of infected cases.
The SIR model divides the population into three categories: Susceptible (S), Infected (I), and Removed (R). Let denote the probability that susceptible individuals become infected upon contact with infected individuals. Let denote the rate at which infected individuals either die or recover, which depends on the average duration of the infection. The dynamics of the population can be described by the following differential equations:
where Q is the total population in the destination region. Assume that , meaning there are no initial infected cases and everyone is at risk of infection in the destination region. The general solution to these differential equations is . Given , we can deduce that , hence .
Let denote the average number of imported cases per unit time, i.e., per day. If a spillover case is not detected in time, it becomes a new source of infection. According to data collected from the Web of Health Committees, spillover false negative cases are detected within 0 to 9 days, with an average detection time of 2.25 days. By , we know that in a short period, the number of infections does not increase rapidly. Thus, it is reasonable to assume that the sources of infection are independent, meaning there are new sources of infection.
Without any preventive measures, by Theorem 1, the average time a false negative case is detected is . Thus, the expected number of infections caused by the false negative cases from arrival to detection is .
Next, we consider the problem that the planner may face. When the destination region implements the lockdown policy, it will negatively impact the economy. Thus, the planner faces a trade-off: preventing the spread of the epidemic or ensuring economic stability. Recall that the destination region locks down when the number of existing spillover cases reaches N, leading to economic losses. Let c denote the cost of implementing these policies, and h represents the unit cost of the infected cases, including costs from regional nucleic acid tests to prevent the spread of the epidemic or expenses for treatment. Referring to the research by [26], we assume that the cost caused by new cases is linearly related to the number of new cases. Let be the number of existing confirmed imported cases at time t. Therefore, for the destination region, if economic factors are considered when formulating the intensity of epidemic prevention policies, N, the problem faced is minimizing the expected cost:
where is an indicator function with if hold.
Proposition 2.
If is positive, the total cost decreases with N; otherwise, the total cost increases with N.
Proof of Proposition 2.
The terms and represent the times when the number of confirmed spillover cases in the system is greater than or equal to N and less than N, respectively. The expected values correspond to the probabilities that the number of confirmed cases in the system is greater than or equal to N and less than N. Thus, the problem can be simplified as:
By Proposition 1, we have . Therefore, if , the objective function decreases with N. Otherwise, it increases with N. □
Proposition 2 shows that the optimal value of N depends on the value of , which represents the cost of the epidemic prevention policy and the cost of increased cases. From Proposition 1, we know that as N increases, decreases. When is positive, the total cost decreases as N increases, requiring the adoption of looser epidemic prevention policies to prevent the spread of the epidemic through timely nucleic acid testing. Conversely, when is negative, as N increases, decreases, and the total cost increases, necessitating stricter epidemic prevention policies to reduce the impact of spillover cases on the destination region.
In the following section, we will use numerical experiments to demonstrate the theoretical results more directly.
4. Numerical Results
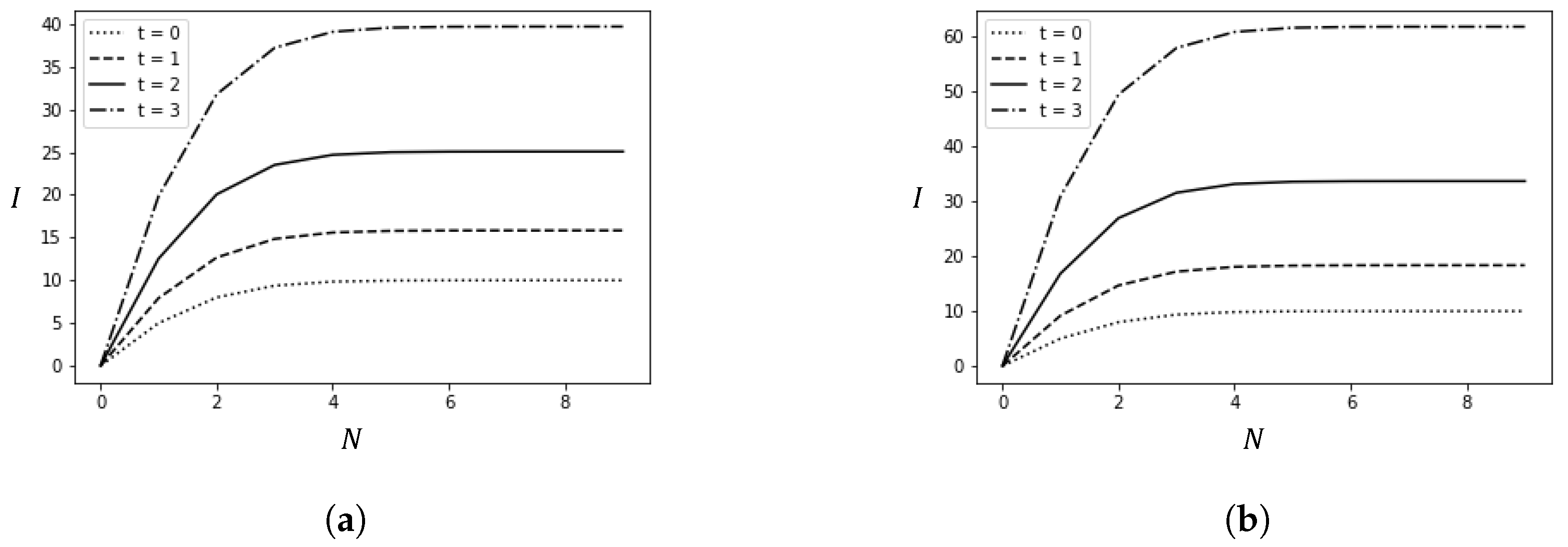
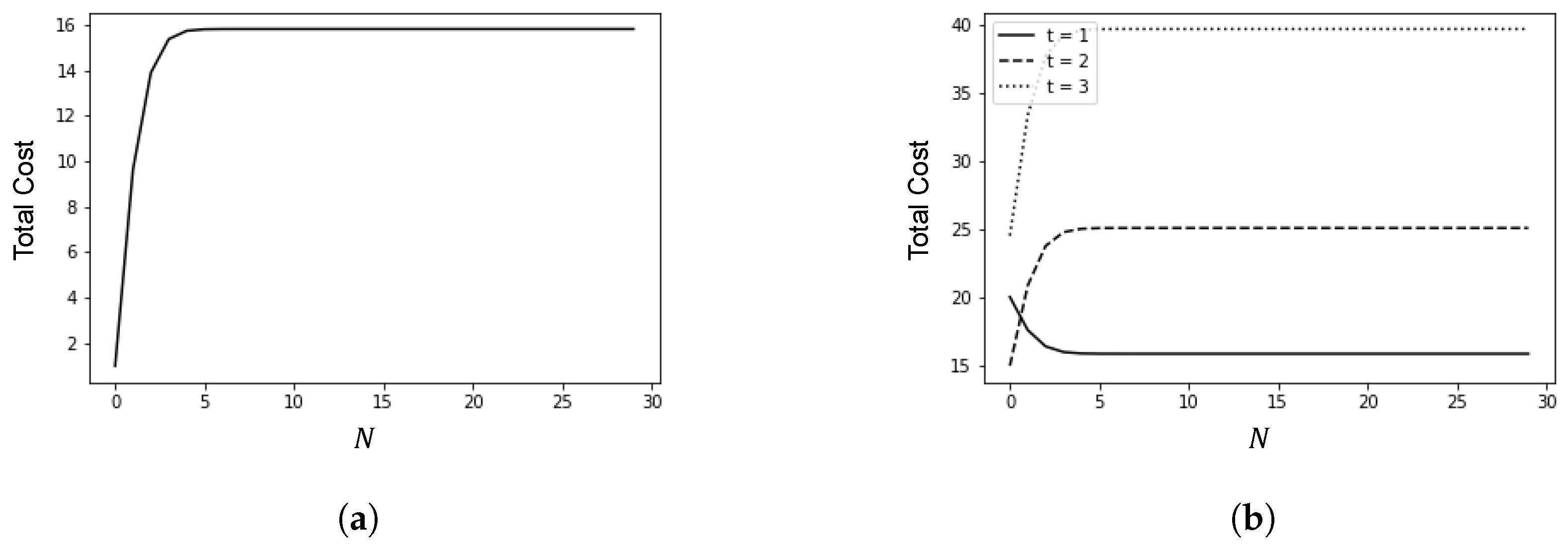
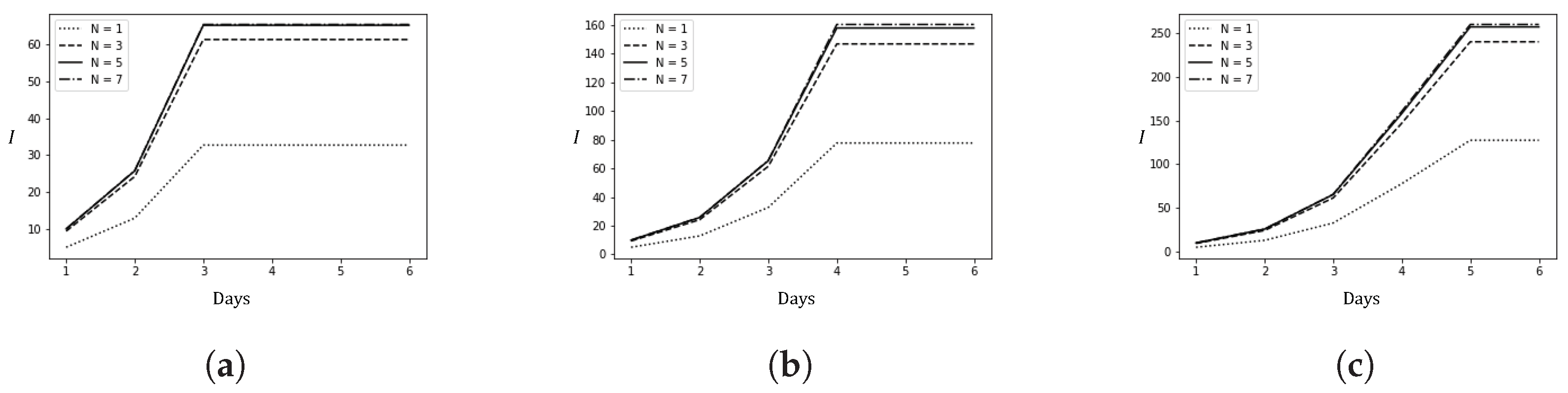
This section presents the results of numerical experiments. Figure 3 shows how the number of infected cases changes with the policy adjustment threshold N and the average time t from arrival to detection. Figure 4 illustrates the relationship between the policy adjustment threshold N and the total cost. Figure 5 depicts the changes in the number of infected cases over time due to spillover cases under different average times t from arrival to detection and policy adjustment thresholds N.
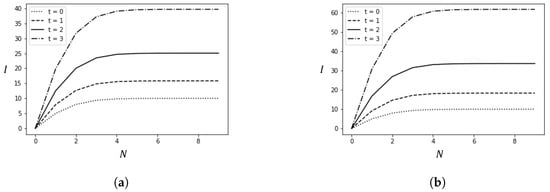
Figure 3.
Changes in the number of infections caused by spillover cases with variations in t and N. (a) ; (b) .
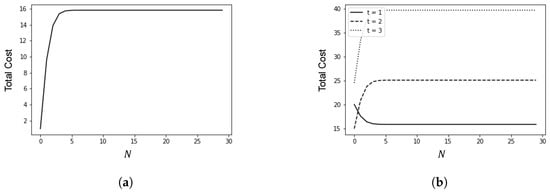
Figure 4.
Relationship between policy adjustment threshold N and total cost. (a) ; (b) .

Figure 5.
Changes in the number of local confirmed cases due to spillover cases under different epidemic prevention policies over time (6 days). (a) ; (b) ; (c) .
4.1. Parameter Settings
The average daily number of spillover cases, , is set to 10. According to relevant studies, [27] and [28], where is the basic reproduction number. We also estimated the value of using data collected from the Omicron outbreaks in Jilin, Changchun, and Shanghai during the first half of 2022. Table 2 presents an example of the confirmed cases in Jilin. The cumulative number of cases on each day, denoted as , is obtained by summing the confirmed cases from the initial outbreak date up to day t.

Table 2.
Confirmed COVID-19 Cases During the Omicron Outbreak in Jilin (March 2022).
By fitting the collected data to , the objective function is as follows:
Table 3 presents the estimated values of across different regions and varying lengths of time (T), where T generally represents the total duration over which the model is evaluated. As indicated in the table, decreases as T increases, suggesting that provincial health committees have implemented effective epidemic prevention policies. It is reasonable to assume that the estimated during the initial period is closer to the true value. Therefore, the average for is used in the subsequent analysis. In the numerical experiments, is set to 7.43, except in Figure 3b, where it is set to 9.5 to examine the impact of varying on the number of infected cases.

Table 3.
Estimated Basic Reproduction Number () Across Regions and Time Periods.
4.2. Results
Figure 3 depicts how the number of infection cases changes with the policy adjustment threshold N and different average detection times t. Figure 3a,b are set with different values.
The numerical experiment results lead to the following conclusions: First, for any fixed N, as t increases, meaning false negative cases are not detected in time, the number of infection cases increases. Second, as the policy adjustment threshold N increases, the number of infected cases initially increases and then plateaus. The plateau occurs because false negative cases are detected in time, effectively preventing the spread of the epidemic. This highlights the importance of timely nucleic acid testing in preventing the spread of the epidemic. Third, comparing Figure 3a,b, it is evident that as the basic reproduction number of the virus increases, the number of infections also increases, necessitating more timely detection and stricter prevention policies to reduce the number of infections.
Figure 4 illustrates the relationship between the policy adjustment threshold N and the total cost, with h set to 1. In Figure 4a, c is set to 1, and in Figure 4b, c is set to 10.
When , i.e., the cost of epidemic prevention policies is lower than the cost associated with the increase in cases, stricter epidemic prevention policies are required to reduce the number of infections. Conversely, when , i.e., the cost of epidemic prevention policies is higher than the cost associated with the increase in cases, more lenient epidemic prevention policies should be implemented. In such cases, timely nucleic acid testing can reduce the number of infections.
In Figure 4b, as t increases, the total cost initially decreases monotonically with N but then increases monotonically. This indicates that if false negative cases cannot be detected in time, the total cost will increase due to the rise in infection numbers, even if the unit cost associated with the increase in cases is relatively low.
Figure 5 shows the changes in the number of infected cases over a period of 6 days due to spillover cases under different policy adjustment thresholds.
First, as t decreases, meaning the control over the spread of the epidemic from imported cases becomes more timely, the daily number of infected cases decreases, and the epidemic stabilizes earlier. Second, given the high transmissibility of the Omicron variant, effective control of the epidemic’s scale can only be achieved with stricter prevention measures (). Without such measures, the daily number of new confirmed cases will increase significantly. The results show that when , the daily new confirmed cases will multiply. Third, when N is relatively large, further increases in N do not exacerbate the epidemic. This is similar to the results seen in Figure 3: timely detection allows false negative cases to be discovered earlier, effectively preventing the spread of the epidemic. In short, timely detection and stricter prevention measures are crucial for effectively curbing the spread of an epidemic. These findings can also be extended to other diseases with longer incubation periods than COVID-19. For instance, if we consider a disease with a longer incubation period, characterized by a larger t, the dynamics of the epidemic would change. Specifically, as t increases, it is likely that the time required to control the epidemic would also increase, meaning that the trend toward stabilization would occur over a longer period. Consequently, our model suggests that even more stringent and sustained prevention measures might be necessary to achieve the same level of control for such diseases. This would imply that public health policies should be adjusted to account for the longer duration required to detect and manage infections effectively, thereby ensuring that the epidemic does not spread unchecked during the extended incubation period.
5. Discussion
Since the end of 2019, the world has been shrouded in the shadow of the COVID-19 pandemic for nearly three years. From the initial outbreak to the emergence of the Omicron variant, the virus’s transmission capability has rapidly increased, and its stealthiness has significantly enhanced, posing substantial challenges to the detection and management of infection sources. This paper constructs an infectious disease network model incorporating spillover cases based on a queuing network with two service lines, integrating queuing theory with the SIR model. By calculating relevant parameters of the SIR model through the queuing network, we discuss various factors influencing the number of infections. Considering economic factors, we solve for the optimal intensity of epidemic prevention policies in regions experiencing spillover effects. We also conduct numerical experiments to analyze the impact of variables such as detection time and the intensity of prevention policies on the number of infections. The relationship between policy adjustment thresholds and total costs is also examined. The theoretical model and numerical experiment results indicate the following:
First, as the transmissibility of the virus increases, the number of infections rises, necessitating more timely detection and stricter prevention policies to effectively reduce the number of infections. The optimal lockdown intensity is influenced by the availability of antibody testing [16]. Second, if the economic cost associated with an increase in case numbers is high, stricter prevention policies are required to reduce the number of infections. Conversely, if the economic cost of prevention policies is high, more lenient policies should be implemented, with timely nucleic acid testing used to reduce the number of infections. Third, the more timely the control of spillover cases is, the fewer the daily new confirmed cases are, and the earlier the epidemic tends to stabilize. This finding aligns with [18], which show that effective tracking technology enables targeted testing and isolation policies to deliver significant welfare benefits. These findings underscore the importance of sustainable epidemic management practices. Effective lockdown policies must balance public health benefits with economic sustainability, ensuring that regions can maintain both public health and economic stability. The integration of timely detection and strategic prevention measures is essential for minimizing infections while reducing the socio-economic costs of lockdowns.
Future research should continue to explore sustainable strategies for managing cross-border epidemic spread, focusing on optimizing international travel policies to mitigate global health risks. For example, China implemented the circuit breaker mechanism for airlines: if an airline had a certain number of passengers test positive for COVID-19 after arriving in China, the airline’s route to China would be suspended for a specific period. It is necessary to know how to set the optimal threshold. Moreover, in our model, we assume that the arrival rate follows a Poisson process. In future research, this assumption can be relaxed for a more general distribution. By addressing these challenges through a sustainability lens, policymakers can develop more resilient and adaptive responses to future pandemics, ensuring long-term public health and economic well-being.
6. Conclusions
This study presents a novel approach to modeling the cross-regional spread of epidemics by integrating a queuing network with the SIR model, providing a more realistic and dynamic framework for understanding and managing epidemic outbreaks. The theoretical significance of this work lies in its capacity to model the complexities of cross-regional interactions and the spillover of false-negative cases, which are critical factors in the spread of infectious diseases like COVID-19.
Practically, the model offers valuable insights for policymakers tasked with designing optimal lockdown policies. By emphasizing the balance between public health and economic costs, our findings provide actionable guidance on implementing timely and effective interventions. The results suggest that rapid detection, combined with strategic policy adjustments, is essential for reducing the total costs associated with both infections and economic disruptions.
This research contributes to the broader field of epidemic management by offering a framework that can be adapted to different regional contexts and evolving pandemic scenarios. Future research should explore the application of this model to global epidemic management, particularly in optimizing cross-border travel and trade policies. By advancing our understanding of the interplay between public health and economic factors, this study paves the way for more resilient and adaptive responses to future pandemics.
Author Contributions
Conceptualization, X.G.; methodology, T.Q.; software, Y.L. and C.Q.; validation, Y.L. and C.Q.; formal analysis, T.Q.; investigation, T.Q.; resources, Y.L. and C.Q.; data curation, Y.L. and C.Q.; writing—original draft preparation, T.Q.; writing—review and editing, X.G.; visualization, T.Q.; supervision, X.G.; project administration, X.G.; funding acquisition, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number 7217010132, and supported by Guanghua Talent Project of Southwestern University of Finance and Economics.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Main Proof
Proof of Theorem 1.
The balance equations can be written as
By the balance equations, the steady-state probability is given by
Also, from the normalization condition , we obtain
By the expansion of the Taylor series for , , we can simplify the above equation to
Thus, we have . Therefore, the probability that there are k spillover cases entering the area is
The average stay time of a spillover case on service line 1 is
where L is the average number of customers on service line 1. □
Proof of Theorem 2.
The equilibrium equations for the steady-state of the system can be obtained from the diagram:
Based on the condition , we obtain
□
References
- Sandford, A. Half of Humanity Now on Lockdown as 90 Countries Call for Confinement. 2020. Available online: https://www.euronews.com/2020/04/02/coronavirus-in-europe-spain-s-death-toll-hits-10-000-after-record-950-new-deaths-in-24-hou (accessed on 2 April 2020).
- Al-Jubari, I.; Mosbah, A.; Salem, S.F. Employee well-being during COVID-19 pandemic: The role of adaptability, work-family conflict, and organizational response. Sage Open 2022, 12, 3. [Google Scholar] [CrossRef]
- Joseph, E.; Levenson, E. New York Gov. Cuomo Extends Stay-at-Home Order Until at Least May 15. 2020. Available online: https://www.cnn.com/2020/04/16/us/new-york-coronavirus/index.html (accessed on 16 April 2020).
- Di Domenico, L.; Pullano, G.; Sabbatini, C.E.; Boëlle, P.Y.; Colizza, V. Impact of lockdown on COVID-19 epidemic in Île-de-France and possible exit strategies. BMC Med. 2020, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Abukhalaf, A.A.; Alomar, A.A.; AlMutairi, F.J.; Usmani, A.M.; Klonoff, D.C. Impact of lockdown on COVID-19 prevalence and mortality during 2020 pandemic: Observational analysis of 27 countries. Eur. J. Med. Res. 2020, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- Atalan, A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann. Med. Surg. 2020, 56, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, M.S.; Rebelo, S.; Trabandt, M. The macroeconomics of testing and quarantining. J. Econ. Dyn. Control 2022, 138, 104337. [Google Scholar] [CrossRef]
- Kharroubi, S.; Saleh, F. Are lockdown measures effective against COVID-19? Front. Public Health 2020, 8, 549692. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Yared, P. Pandemic lockdown: The role of government commitment. Rev. Econ. Dyn. 2022, 46, 27–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, B.; Yuan, J.; Tao, Y. The impact of social distancing and epicenter lockdown on the COVID-19 epidemic in mainland China: A data-driven SEIQR model study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Eichenbaum, M.S.; Rebelo, S.; Trabandt, M. The macroeconomics of epidemics. Rev. Financ. Stud. 2021, 34, 5149–5187. [Google Scholar] [CrossRef]
- Kaplan, G.; Moll, B.; Violante, G.L. The Great Lockdown and the Big Stimulus: Tracing the Pandemic Possibility Frontier for the US; NBER: Cambridge, MA, USA, 2020. [Google Scholar]
- Hall, R.E.; Jones, C.I.; Klenow, P.J. Trading off Consumption and COVID-19 Deaths; NBER: Cambridge, MA, USA, 2020. [Google Scholar]
- Barrero, J.M.; Bloom, N.; Davis, S.J. COVID-19 Is Also a Reallocation Shock; NBER: Cambridge, MA, USA, 2020. [Google Scholar]
- Kozlowski, J.; Veldkamp, L.; Venkateswaran, V. Scarring Body and Mind: The Long-Term Belief-Scarring Effects of Covid-19; NBER: Cambridge, MA, USA, 2020. [Google Scholar]
- Alvarez, F.; Argente, D.; Lippi, F. A simple planning problem for COVID-19 lock-down, testing, and tracing. Am. Econ. Rev. Insights 2021, 3, 367–382. [Google Scholar] [CrossRef]
- Berger, D.W.; Herkenhoff, K.F.; Mongey, S. An SEIR Infectious Disease Model with Testing and Conditional Quarantine; NBER: Cambridge, MA, USA, 2020. [Google Scholar]
- Chari, V.V.; Kirpalani, R.; Phelan, C. The hammer and the scalpel: On the economics of indiscriminate versus targeted isolation policies during pandemics. Rev. Econ. Dyn. 2021, 42, 1–14. [Google Scholar] [CrossRef]
- Ren, X. Pandemic and lockdown: A territorial approach to COVID-19 in China, Italy and the United States. Eur. Geo. Econ. 2020, 61, 423–434. [Google Scholar] [CrossRef]
- La Torre, D.; Liuzzi, D.; Marsiglio, S. Epidemic outbreaks and the optimal lockdown area: A spatial normative approach. Econ. Theory 2024, 77, 349–411. [Google Scholar] [CrossRef]
- Conant, G.C.; Wolfe, K.H. Self-tests for COVID-19: What is the evidence? A living systematic review and meta-analysis (2020–2023). PLoS Glob. Public Health 2024, 4, e0002336. [Google Scholar]
- Gail, H.R.; Hantler, S.L.; Taylor, B.A. Spectral analysis of M/G/1 and G/M/1 type markov chains. Adv. Appl. Probab. 1996, 28, 114–165. [Google Scholar] [CrossRef]
- Ross, S.M. Introduction to Probability Models, 11th ed.; Academic Press: San Diego, CA, USA, 2014; pp. 1–784. [Google Scholar]
- Ellison, G. Implications of heterogeneous SIR models for analyses of COVID-19. Rev. Econ. Des. 2024, 1–37. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics IV: Analysis of experimental epidemics of the virus disease mouse ectromelia. Epidemiol. Infect. 1937, 37, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Piunovskiy, A.B.; Clancy, D. An explicit optimal intervention policy for a deterministic epidemic model. Optim. Contr. Appl. Met. 2008, 29, 413–428. [Google Scholar] [CrossRef]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklöv, J. The effective reproduction number for the omicron SARS-CoV-2 variant of concern is several times higher than Delta. J. Travel Med. 2022, 29, taac037. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).