Abstract
One of the challenges of our time is replacing the existing fossil fuel-based economy with a green one in the framework of developing a sustainable society. The biological synthesis of nanoparticles from plant extracts is currently under study for developing environmentally compatible nanoparticle synthesis and avoiding adverse effects. The environmental impacts of emissions, energy requirements, and energy losses are calculated to comparatively evaluate the effects of the traditional process, in addition to any new one, in obtaining TiO2 nanoparticles from the life cycle point of view. The two methods are syntheses via green chemistry (using an aqueous extract of Cymbopogon citratus) and via the chloride route, which are some of the most used methods for the synthesis of TiO2 nanoparticles owing to them having the most advanced production processes. The life cycle analysis was carried out using OpenLCA software, which showed that the production of chemically synthesized TiO2 contributes to greenhouse gas emissions and respiratory effects caused by inorganic substances. On the other hand, green synthesis contributes to reductions in toxicity and greenhouse gas emissions.
1. Introduction
Environmental sustainability has been defined as one of the Millennium Development Goals of the United Nations. From an environmental point of view, anthropogenic causes, such as the combustion of fuels derived from fossil resources, started modifying the carbon cycle once we rapidly emitted CO2 into the atmosphere, using a source of carbon sequestered away hundreds of millions of years ago; some unexpected consequences on a planetary scale may result. This is called anthropogenic climate change. One of the most promising approaches to achieving environmental sustainability is the concept of a “Green Economy”, which “improves human well-being and social equity, while significantly reducing environmental risks and ecological scarcities” [1].
Currently, the search for new materials that circumvent the current problems of environmental deterioration must be based on sustainable development, with strategies focused on the circular economy. The aim is to generate fewer toxic products, reduce waste to a minimum, use renewable resources, and reduce energy consumption. This is part of the 12 principles of green chemistry, which include the use of “green” solvents, ecological catalysis, selective transformations, soft synthesis, and green synthesis (also called biosynthesis) [2]. The last concept includes the use of bacteria, yeasts, fungi [3,4,5], algae [6], actinomycetes, natural organic substances [7], and plant extracts obtained from any part of a plant, from roots to fruits, skin, or seeds [8,9,10]. For the synthesis of titanium dioxide (TiO2) nanoparticles, a semiconductor with multiple applications, including photocatalysis, as well as many plant extracts have been used. Bahri et al. [8] have presented an exhaustive review of the biosynthesis of TiO2, using plant extracts obtained with solvents of different polarities and under different synthesis conditions (pH, temperature, stirring speed, and reaction time, among others) that influence the morphology and structural characteristics of the particles obtained and, consequently, the possible applications of the synthesized material.
In the last decade, green technologies have gained great relevance due to the rapid depletion of natural resources. These technologies offer sustainable solutions for manufacturing nanomaterials, with applications in sectors such as medicine, energy, sensors, and the food industry. Although numerous chemical and physical methods exist to produce nanoparticles and metal oxides, many of them involve high costs and the use of toxic chemicals that pose health and environmental risks. In this context, green synthesis stands out by using natural agents, such as extracts from plants, fruits, flowers, algae, and microorganisms, which eliminates the need for high-pressure reactors and high temperatures. A crucial point of this approach is the reduction of polluting residues and the generation of biodegradable products. Several studies have shown that plant extracts are particularly suitable for producing nanoparticles, even at a pilot scale, facilitating the transition to more sustainable production [11,12,13,14,15]. Among the various metal oxides, the following are the most common.
Among the various metal oxides, titanium dioxide (TiO2) has captured the interest of the scientific community due to its electronic, chemical, and physical properties. There are two main methods for its production: the chloride process and the sulfate process. While the former is used for the rutile phase of TiO2 and is considered more energy-efficient and environmentally friendly, the sulfate process is used for minerals such as ilmenite. Although 60% of the world’s TiO2 production uses the chloride route, its environmental impacts and long-term benefits have not yet been fully evaluated. Therefore, it is crucial to perform a comparative analysis of both methods, from extraction to production and disposal, to understand their true environmental impacts [16,17,18,19,20].
To highlight the benefits of green synthesis in nanoparticle production, several relevant studies will be presented. These examples highlight how the use of plant extracts as reducing and stabilizing agents not only minimizes the use of toxic chemicals but also offers sustainable and efficient methods for the fabrication of nanomaterials. Saad et al. [21] demonstrated the importance of green synthesis, a methodology that avoids the use of toxic chemicals, by employing plant extracts to produce nanoparticles. In this case, titanium dioxide nanoparticles (TiO2 NPs) were synthesized using an extract of green tea leaves, demonstrating a greener and more sustainable approach. Suja Joseph and co-workers [22] highlight in their study the importance of green synthesis by using green tea extract to produce ZnO nanoparticles, TiO2, and a TiO2-ZnO nanocomposite. This sustainable approach minimizes the use of toxic chemicals, offering an environmentally friendly and efficient alternative. The research demonstrates that the synthesized materials possess diverse useful morphologies, with outstanding applications in coatings for dental implants, highlighting the potential of green synthesis for innovations in nanotechnology and biomedicine. Nabi and co-workers [23] developed a green method to synthesize titanium dioxide (TiO2) nanoparticles (NPs) using lemon peel extract. The flavanol hesperidin in the extract acts as a reducing and capping agent. In photocatalytic tests, TiO2 NPs demonstrated more than 70% efficiency in the degradation of rhodamine B (RhB), which is superior to commercial particles. This green method is not only environmentally friendly but also produces particles with excellent properties for environmental applications. Vijayakuma and co-workers [24], employed Ocimum americanum L. leaf extract to synthesize TiO2 in order to investigate its antimicrobial, antiproliferative, and photocatalytic properties, which could offer new perspectives in environmental and therapeutic applications. Goutam and co-workers [25], synthesized titanium dioxide (TiO2) nanoparticles using Ocimum americanum L. leaf extract, employing an eco-friendly method. The nanoparticles with an average size of 25 nm showed excellent antimicrobial properties against various pathogens and high antiproliferative activity on skin cancer cells. In addition, they demonstrated a photocatalytic efficiency of 91.1% in the degradation of methylene blue dye. This green approach is not only sustainable and cost-effective but also provides materials with broad environmental and therapeutic applications.
On the other hand, Cymbopogon citratus, known as lemongrass, is a grass that does not require much care in its cultivation; its phytochemical composition includes tannins, saponins, phenols, anthraquinones, flavonoids, and alkaloids: components to which analgesic, anti-inflammatory, antipyretic and antioxidant activities, among others, are attributed and which, during green synthesis, function as reducing and/or stabilizing agents [25,26,27,28]. Lemongrass leaf extract has been used to synthesize silver nanoparticles [29], iron oxides [30], copper oxides [31], and zinc oxides [32]. Saavedra et al. [33] synthesized TiO2 nanoparticles with crystal sizes of 20 and 18.7 nm using green chemistry methods, including those that are ultrasound-assisted, using an alcoholic extract of Cymbopogon citratus.
The objective of this study was to perform a life cycle analysis (LCA) that would comparatively evaluate the environmental impact of TiO2 nanoparticles synthesized using green chemistry (using the aqueous extract of Cymbopogon citratus) as a third route. This green chemistry process was compared to the traditional routes of TiO2 production, specifically the chloride and sulfate routes. To that end, a detailed life cycle analysis was carried out, quantifying the energy consumed and emissions generated during the TiO2 synthesis process. This study focused on the collection and analysis of life cycle inventory data to accurately compare the environmental impacts of the different production routes and evaluate the feasibility of the green chemistry-based method.
2. Methodology
To compare the environmental impact of the synthesis of titanium dioxide (TiO2) nanoparticles using the chloride route and green chemical synthesis, a life cycle analysis (LCA) was performed that evaluated the raw materials, synthesis processes, and forms of crystallization of TiO2. The chloride route, widely used in industry for its lower energy consumption and emissions, was compared to the proposed green synthesis, which employs fewer toxic reagents and more environmentally benign conditions. Although the chloride route accounts for more than 60% of global TiO2 production, green synthesis presents itself as a promising alternative in terms of reduced environmental impact by minimizing the use of hazardous chemicals. The sulfate route was not considered in this analysis because its industrial use for TiO2 production has not yet reached a significant scale, given the complexity and high cost of its process [32].
To perform the life cycle analysis (LCA) and compare the environmental impact of the synthesis of titanium dioxide nanoparticles (TiO2), using the chloride route and green chemical synthesis, the following structured methodology was followed that allows evaluation of each stage of the production process. The main objective of this LCA is to quantify and compare the environmental impacts associated with both routes, considering their raw materials, synthesis processes, and operating conditions. The methodology is developed in four key steps: first, the scope and boundaries of the analysis are defined, establishing the processes and resources to be evaluated; second, a life cycle inventory is conducted, which consists of collecting data on inputs, energy, emissions, and waste from each route; third, a life cycle impact assessment is carried out, where environmental burdens are quantified in categories such as climate change, energy consumption and toxicity; and finally, the results are interpreted, providing a clear view of the environmental benefits and drawbacks of each process. These steps are described in detail below.
2.1. LCA Methodology
ISO Standard number 14040 stresses that life cycle analysis is a systematic set of procedures for collecting and examining material and energy inputs and outputs as well as environmental impact. It often consists of four steps: definition of objectives and scope, life cycle inventory analysis, impact assessment, and interpretation of results [34].
This assesses inputs and outputs of materials and energy and the associated environmental impacts directly attributable to the operation of a product throughout its life cycle. LCA has been used to assess the environmental impacts of products and services, accounting for emissions and the resources used during production, distribution, use and disposal. The importance of life cycle analysis lies in obtaining a holistic perspective of the environmental aspects associated with a service or product derived from a system. Understanding the interrelationships that exist throughout the entire life cycle makes this analysis a solid and relevant tool that allows us to identify the consequences more clearly [33,35].
2.1.1. Objectives and Parameters of This Study
The purpose of this study was to determine the environmental effect of obtaining TiO2 nanoparticles using green chemistry synthesis. In addition, we sought to provide information on this new synthesis route and compare it to the existing information on the chloride and sulfate routes. Figure 1 shows the simplified flow diagram for the new process. Table 1 compares the main characteristics of the processes of obtaining TiO2 to provide an understanding of them, their impacts, and possible alternatives to reduce the environmental impact.
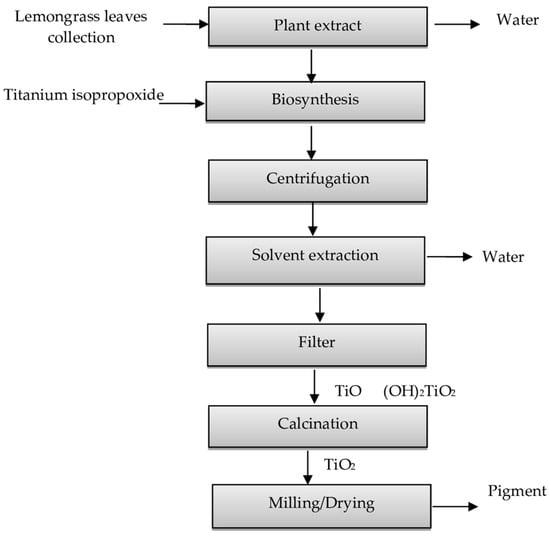
Figure 1.
Simplified diagram of the titanium oxide nanoparticle process using green synthesis.

Table 1.
Comparison of the main characteristics of the processes of obtaining TiO2 [11].
For the life cycle analysis (LCA), Eco-indicator 99, which allows emissions to be quantified and categorized into various impact categories, was chosen as the impact assessment method, with ten relevant categories selected to show the functional unit. The software used to carry out the environmental impact assessment was OpenLCA, a free, open-source software used for LCA and sustainability assessment. Eco-invent is a European database that is available in OpenLCA and free of charge for the classified countries of the ONU [36,37,38]. All the information obtained was processed and included. The results from the database and bibliographic sources were compared.
Limits in the System
No final scenario in which synthesis by-products would be landfilled, treated, incinerated, or recycled was included due to the wide range of possibilities and number of assumptions involved. However, the climate change perspective is included here so that we can measure emissions and impacts throughout the life cycle.
2.1.2. Life Cycle Inventory
The objective of this analysis was to calculate the cumulative demand for CO2 emissions from the biosynthesis process, using 1 kg of TiO2 as a functional unit. The process flow diagram was based on the experimental parameters used in the research developed at the Polytechnic University of the State of Morelos (UPEMOR). The electrical energy data were determined based on the energy required by each piece of equipment in the process.
To analyze and compare the impacts of the two scenarios, the processes corresponding to each input were selected from the available life cycle inventory database, as shown in Table 2. The inputs considered were hydrochloric acid (HCl), on-site steam, and electricity. Except for electricity, all data were obtained from laboratory investigations.

Table 2.
Data collection of green synthesis processes and the chloride route.
In this study, only two routes were compared; as the chloride route has a greater impact on titanium dioxide production than the sulfate one, the chloride route and the green synthesis will be compared. To evaluate the impact, the Eco-indicator 99 methodology was used. The method of assessing resource depletion was based on the additional energy that would have been required in the future to extract the resource due to its present depletion.
Various bibliographic sources were consulted to determine the total damage in each category, complementing this information. These data allow a direct comparison of the inputs within each impact category. In order to compare the impacts, the values were normalized for each category. In the life cycle analyses, the phases of packaging, transport, application, and final disposal after useful life were not included.
To determine CO2 emissions, the biosynthesis process was modeled using the OpenLCA 2.0 software.
Table 3 shows the process inputs and their values, which were used to perform the life cycle analysis in each of the scenarios proposed in this work. The results of the analysis presented here represent a realistic scenario; due to the lack of complete data, the search for information from other sources, in the best-case scenario, will reduce uncertainty in the analysis and its interpretation [39].

Table 3.
Inputs and outputs evaluated in green synthesis.
2.1.3. Life Cycle Impact Assessment (LCIA)
This section focuses on each method of analysis separately. The analysis of the existing interrelationships in the processes revealed that these impacts are linked to assumed production volume. Forestry operations and transportation showed a moderate contribution to most impacts, except for the indicators of agricultural land occupation and eutrophication, while the life cycle assessment emphasized the environmental impact of emissions. The results of these methods need not coincide. We compared the results to see what conclusions could be drawn. The analysis focused on titanium isopropoxide extracted from biosynthesis using Cymbopogon citratus extract as a precursor to obtain TiO2.
The inventory data were obtained from research developed at the Polytechnic University of the State of Morelos (UPEMOR); these are available upon request [40] and from other articles [41,42,43]. They were subsequently analyzed to quantify environmental effects. For the life cycle analysis, they were modeled in the OpenLCA program, version 2.0. Once the values of the indicators were obtained, each impact was evaluated.
Impacts can have various implications regarding titanium oxide production through biosynthesis, although they can be direct and indirect and depend on several specific factors.
The production of Cymbopogon citratus, a plant that includes several species known as lemongrass, can have both positive and negative environmental impacts depending on how the plant is cultivated or managed. A sustainable harvest of Cymbopogon citratus, in which the leaves or stems are harvested in a controlled manner and recovery periods are allowed for the plant, can positively impact local biodiversity and the conservation of the species. On the other hand, the cultivation of these plants can contribute to the biodiversity of beneficial insects, such as bees and ladybugs, which help in pollination and pest control.
Figure 2 shows the relative results of the biosynthesis life cycle analysis impact assessment indicators using the Recipe Midpoint 2014 indicator, in which the calcination, drying, and filtration stages show consistently high impacts on multiple indicators, including climate change, fossil fuel depletion, and human toxicity.
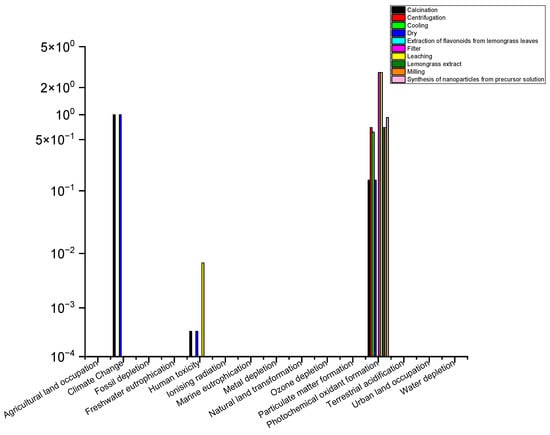
Figure 2.
Relative results of the indicators in each of the stages of the TiO2 green synthesis manufacturing process.
The electricity required is a major contributor to various types of impacts, especially considering heat needs. The effects of transforming energy into electricity are significant in terms of fossil resource depletion and ozone depletion. The impact that is generated by electricity is attributed to it when it is obtained, not when it is used in the synthesis, as very little is used in each of the reactions, but it is important to analyze the impacts that are generated at this point because they also have significant effects on the depletion of fossil resources: problems that have arisen or been given greater importance in recent days. Figure 3 shows the environmental impacts related to climate change, expressed in terms of CO2 equivalent (kg CO2 eq), associated with the different stages of the green synthesis process for obtaining titanium dioxide (TiO2) using green tea leaves as precursor. The data show that the calcination and drying stages are the most relevant in terms of CO2 emissions, while other stages of the process have minor but significant impacts. The impacts of each stage, according to the results of the life cycle analysis (LCA), are detailed below.
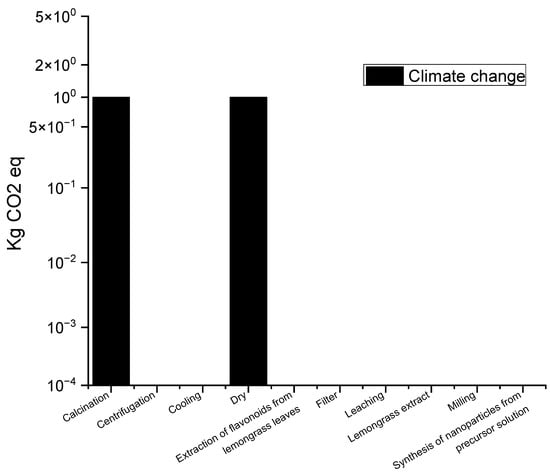
Figure 3.
Contribution of CO2 emissions to the TiO2 manufacturing process that uses green synthesis.
The calcination and centrifugation processes are the main stages responsible for the increase in CO2 emissions. Calcination, a process that involves heating at high temperatures to crystallize TiO2 nanoparticles, generates large amounts of emissions due to high energy consumption, which explains the high value in kg CO2 eq. Centrifugation, for its part, is also an energy-intensive process, particularly if high-power equipment is used for phase separation, which contributes to the increase in emissions.
In the drying stage, which refers to the removal of water or solvents from the processed material, the use of ovens or other heating equipment involves considerable energy consumption. This process, together with calcination, is one of the main sources of CO2 emissions due to the energy required to evaporate the liquids.
The extraction of bioactive compounds such as polyphenols, which act as reducing agents in the synthesis of nanoparticles, has a relatively minor environmental impact compared to the previous stages. This process does not require a significant amount of energy, which is reflected in the low CO2 emissions.
In addition to these four key stages, other process steps, such as filtration, synthesis of nanoparticles from the precursor solution, washing, and milling, have almost negligible emission values. This indicates that these activities are not energy intensive and, therefore, their impact on greenhouse gas emissions is minimal [16,44,45,46,47,48].
Figure 4 shows the eco-indicator results of toxicity at various stages of the titanium dioxide (TiO2) production process using lemon tea leaves as precursor, expressed in terms of 1,4-dichlorobenzene equivalents (1,4-DB eq). The results reveal that the leaching step has the highest impact in terms of ecotoxicity compared to other process steps. This analysis is based on the evaluation of the potential effects of each stage on the environment and human health.
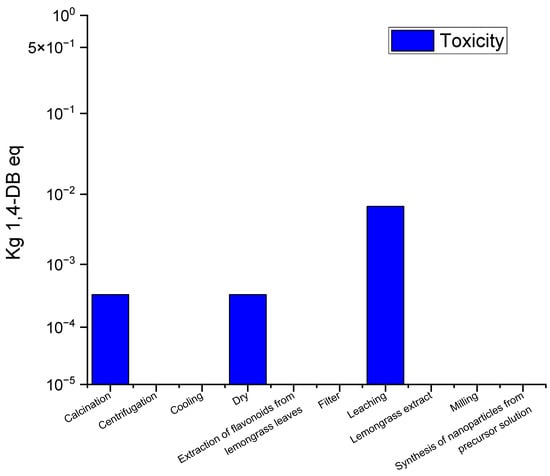
Figure 4.
Ecotoxicity profile for the TiO2 manufacturing process using green synthesis.
Leaching is the main source of ecotoxicity in the process. Leaching involves the extraction of bioactive compounds, such as flavonoids and antioxidants, from lemon tea leaves. Although these leaves are natural, the process can generate liquid residues containing organic compounds that, if not handled properly, can be released into the environment and affect water and soil quality. These residues can cause acidification and eutrophication, negatively impacting both aquatic and terrestrial ecosystems. In addition, the chemicals released can have adverse effects on human health.
The filtration process, which separates solids from the liquid after extraction, also contributes to ecotoxicity, although to a lesser extent than leaching. During this phase, the remains of the lemon tea leaves and the solvents used may contain traces of TiO2 nanoparticles and organic compounds. If these residues are not properly managed, they could generate ecological impacts, mainly affecting water and soil.
In the extraction of flavonoids from lemon tea leaves, the focus is on the extraction of antioxidants and other compounds that act as reducing agents in the formation of TiO2 nanoparticles. Although this process is relatively natural, the generation of organic residues, although minimal, could have a moderate impact on ecotoxicity, depending on the handling of the solvents and by-products.
The calcination stage is a process that demands considerable energy use; calcination has a relatively low impact on ecotoxicity. This is because burning the organic materials present in the lemon tea leaves at high temperatures does not generate significant toxic products that can be released into the environment.
The cooling, milling, and nanoparticle synthesis steps have minimal or negligible impact on ecotoxicity, indicating that they do not generate large amounts of toxic residues or contribute significantly to water or soil contamination.
Figure 5 illustrates the formation of photochemical oxides in the titanium dioxide (TiO2) green synthesis process, measured in terms of kilograms of non-methane volatile organic compounds (NMVOCs). The filtration and leaching stages present the highest emissions of photochemical oxidants, reaching approximately 2.5 kg of NMVOCs. In contrast, other stages of the process, such as flavonoid extraction, grinding, centrifugation, cooling, and preparation of the precursor solution, show moderate emissions, ranging between 0.4 and 0.8 kg of NMVOCs. The calcination and drying stages, on the other hand, show minimal emissions, almost negligible, compared to the other stages of the process.
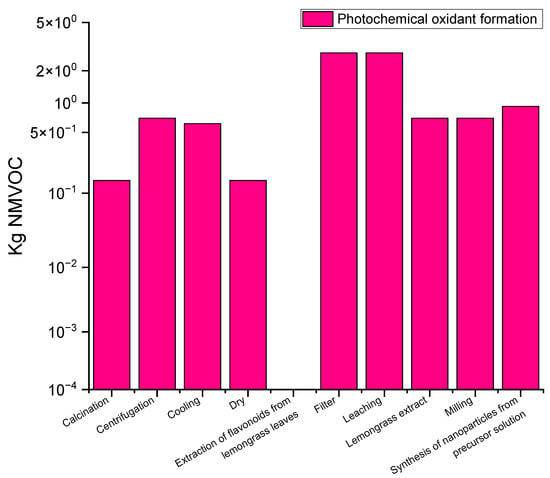
Figure 5.
Photochemical oxidant formation profile for the TiO2 fabrication process using green synthesis.
The process of TiO2 synthesis from lemongrass leaves is detailed below, describing the NMVOC emissions at each stage: filtration and leaching stages generate the highest NMVOC emissions. During filtration, lemongrass leaves are processed to extract soluble compounds, followed by leaching, where flavonoids and other bioactive compounds are obtained. The decomposition of organic compounds in these stages results in a high production of photochemical oxidants.
In the flavonoid extraction stage, flavonoids and other compounds of interest are extracted using solvents. The moderate emissions of NMVOCs at this stage (0.4 to 0.8 kg) are due to the volatilization of the organic compounds present in the lemongrass leaves.
The stages of the titanium dioxide (TiO2) synthesis process from lemongrass leaves, including grinding, centrifugation, cooling, preparation of the precursor solution, calcination, and drying, present a moderate environmental impact in terms of emissions of non-methane volatile organic compounds (NMVOCs). During grinding, the leaves are crushed, releasing volatile compounds that contribute to moderate emissions. In centrifugation, solids and liquids are separated, generating emissions due to the extracted compounds. Subsequent cooling, although it stabilizes the solution, has a minor impact on the formation of photochemical oxidants. The preparation of the precursor solution also moderately emits NMVOCs due to the volatilization of organic compounds. The calcination and drying stages, being mainly inorganic and thermal processes, have minimal emissions, suggesting that these final stages have a negligible environmental impact.
Table 4 presents a comparative analysis of the ecological profile in the production of titanium dioxide (TiO2) by two methods: green synthesis and conventional synthesis. In general, green synthesis shows significant environmental advantages, especially in the reduction of greenhouse gas emissions, which contributes to mitigating climate change. This method minimizes the use of toxic substances, reduces energy consumption, and optimizes the use of raw materials, which improves yields and reduces waste generation. In the acidification and eutrophication category, green synthesis has a lower impact, with a value of 0.00644, while conventional synthesis reaches 0.0266. However, the levels of eutrophication associated with green synthesis are linked to the inadequate disposal of the waste generated, an aspect that still requires control. In the case of conventional synthesis, the impact in this category comes mainly from the production of hydrochloric acid and the disposal of the waste generated in its manufacture. In terms of ecotoxicity, green synthesis has a value of 9.34 × 10−6, higher than that of conventional synthesis, which is 4.78 × 10−8. This indicates that, despite the benefits in other areas, green synthesis has a greater impact on environmental toxicity, which refers to the use of pesticides in plant production. In terms of carcinogenicity, green synthesis generates no impact (0), while conventional synthesis shows a minimal impact of 3.92 × 10−10. This highlights an important benefit of green synthesis, which avoids the generation of carcinogenic substances. Regarding climate change, green synthesis has a lower impact with a value of 5.27 × 10−8, compared to 7.69 × 10−7 for conventional synthesis, which reinforces the idea that the green method is more sustainable in terms of greenhouse gas reduction. In the ozone depletion category, the green synthesis has no impact (0), while the conventional synthesis presents a very low value of 1.50 × 10−11. For respiratory effects caused by inorganic substances, green synthesis has a lower impact (1.37 × 10−7) compared to conventional synthesis (1.20 × 10−6). Similarly, respiratory effects caused by organic substances are lower in green synthesis (1.65 × 10−7) compared to conventional synthesis (8.26 × 10−7). Finally, in the mineral category, consumption is slightly higher in green synthesis (0.00166) compared to conventional synthesis (0.00112). This is due to the use of mineral resources in the green synthesis process, reflecting a higher consumption of raw materials

Table 4.
Significant environmental effects to produce TiO2 nanoparticles.
When assessing environmental impact from a life cycle perspective (LCA), it is important to consider not only operational parameters but also raw material extraction, energy use, by-products, and end-of-life of the product. Table 5 shows the yield, purity, and cost data in the green synthesis versus the chloride route for TiO2 production. The green synthesis of TiO2 nanoparticles, using lemongrass as a precursor, shows clear advantages over the traditional chloride route method both in terms of sustainability and economic costs.

Table 5.
Evaluation of yield, purity, and costs in green synthesis versus chloride route for TiO2 production.
From the comparative table, it is observed that the green synthesis offers a higher yield (92% vs. 74%), which implies a more efficient use of materials, generating fewer losses during the process. This also translates into a lower production cost, with the green route being more economical (83.6 units) compared to the chloride route (88.88 units). Both techniques achieve 100% purity in the final product, which guarantees the quality of the TiO2 obtained, but the green synthesis features a shorter reaction time (3 h versus 3.25 h), which reduces energy consumption and, therefore, greenhouse gas (GHG) emissions associated with the process. In addition, neither process generates direct solid waste, but green synthesis has the advantage of not producing toxic by-products, such as hydrochloric acid (HCl), which is generated in the chloride route.
From a life cycle perspective (LCA), green synthesis not only uses renewable feedstocks, such as lemongrass, which reduces dependence on non-renewable resources, but also reduces the potential toxicity and environmental risks associated with hazardous waste management. In comparison, the chloride route requires additional treatments to mitigate the effects of hazardous by-products such as TiCl4, which increases its environmental footprint and waste management costs. On the cost side, green synthesis is more economically competitive, making it an attractive option for industry. As demand for sustainable technologies continues to grow and environmental regulations become more stringent, this alternative could gain market acceptance, especially in sectors where sustainability is a priority. In addition, the use of natural raw materials could help foster local economies and support sustainable agriculture, generating additional socioeconomic benefits. Political support and environmental regulations play a key role in the adoption of technologies such as green synthesis. As governments implement policies that favor sustainability and carbon footprint reduction, clean technologies such as this can receive incentives and funding, facilitating their large-scale implementation. Furthermore, in socioeconomic terms, green synthesis offers an opportunity to develop cleaner value chains, minimizing the impact on human health and improving working conditions since the hazards associated with toxic chemicals are eliminated [16].
3. Conclusions
The green synthesis of titanium dioxide (TiO2), using natural precursors, such as lemon tea leaves, represents a significant advance in the transition to more sustainable industrial processes. Throughout this research, it has been demonstrated that this approach significantly reduces the use of aggressive chemical reagents, such as hydrochloric acid, and decreases the generation of toxic by-products, thus mitigating the environmental impact of TiO2 production. In a global context where the reduction of greenhouse gases is crucial to curb climate change, green synthesis is positioned as an environmentally friendly and viable alternative. Despite the environmental benefits, one of the most critical challenges is energy consumption in stages such as calcination, centrifugation, and drying, which continue to contribute to CO2 emissions. While this is an aspect that cannot be ignored, it is important to note that the reduction in the use of toxic chemicals and the incorporation of natural compounds, such as green tea antioxidants, contribute significantly to reducing the overall environmental footprint of the process. This marks a fundamental difference from traditional methods of TiO2 synthesis, where there is a heavy reliance on harsh chemicals with a considerably higher environmental impact. Another crucial aspect identified in this research is the importance of proper management of organic wastes derived from lemon tea leaves. Although these steps may generate some ecotoxicity, the use of a natural precursor minimizes this effect when compared to conventional processes, in which the waste generated is more difficult to handle and possesses greater hazardousness. This finding reinforces the premise that green synthesis, despite its challenges, offers lower environmental risk and is, therefore, a preferred option for the industrial production of TiO2. The sustainability of the process depends not only on the synthesis technique but also on the agricultural practices used to obtain the natural precursors. Proper management of pesticides and fertilizers, as well as the conservation of native vegetation around lemon tea plantations, are key factors in maximizing the environmental benefits of this methodology. A comprehensive approach that incorporates sustainable agricultural practices ensures that the benefits of green synthesis are not compromised by indirect impacts associated with the production of the natural precursors. Finally, while the energy cost of green synthesis remains a constraint, the results obtained in this research suggest that the combination of natural methods and advanced technological processes has the potential to reduce the overall environmental impact of the production of TiO2. Not only is this process capable of minimizing greenhouse gas emissions and the use of hazardous reagents, it also offers a pathway to more environmentally friendly industrial production. With improvements in energy efficiency and process optimization, green synthesis can become a key solution in the transition to a circular, low-carbon economy. In conclusion, green TiO2 synthesis, despite the challenges associated with its implementation, presents a sustainable framework for the production of advanced materials. This approach contributes directly to the reduction of environmental impact and, if complemented by responsible agricultural practices and energy-efficient technologies, can play an essential role in the fight against climate change and in innovation within the materials industry.
Author Contributions
Conceptualization, V.B.-T. and H.M.; methodology, M.d.P.R.-R. and M.Y.D.-C.; software, M.d.P.R.-R.; validation, E.V.-V., H.M. and V.B.-T.; formal analysis, M.d.P.R.-R. and M.Y.D.-C.; investigation, E.V.-V. and H.M.; resources, M.d.P.R.-R., V.B.-T. and H.M.; data curation, M.Y.D.-C. and E.V.-V.; writing—original draft preparation, M.d.P.R.-R., V.B.-T. and E.V.-V.; writing—review and editing, H.M.; visualization, E.V.-V.; supervision, V.B.-T.; project administration, E.V.-V. and H.M.; funding acquisition, M.d.P.R.-R. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by DGAPA-UNAM, Project IN-102222.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the manuscript.
Acknowledgments
María del Pilar Rodriguez Rojas with CVU number 745531 thanks CONAHCyT for a postdoctoral scholarship project number 6578441. H. H. Hinojosa, O. Flores, F. Castillo and J. A. Romero for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Henderson, K.; Loreau, M. A model of Sustainable Development Goals: Challenges and opportunities in promoting human well-being and environmental sustainability. Ecol. Model. 2023, 475, 110164. [Google Scholar] [CrossRef]
- Asiri, A.M. (Ed.) Applications of Nanotechnology for Green Synthesis; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISSN 2523-8035. [Google Scholar]
- Singh, P.; Mijakovic, I. Strong antimicrobial activity of silver nanoparticles obtained by the green synthesis in Viridibacillus sp. extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef] [PubMed]
- Tsekhmistrenko, S.I.; Bityutskyy, V.S.; Tsekhmistrenko, O.S.; Horalskyi, L.P.; Tymoshok, N.O.; Spivak, M.Y. Bacterial synthesis of nanoparticles: A green approach. Biosyst. Divers. 2020, 28, 9–17. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef] [PubMed]
- Thajuddin, N.; Dhanasekaran, D. (Eds.) Algae: Organisms for Imminent Biotechnology; BoD–Books on Demand: Norderstedt, Germany, 2016. [Google Scholar]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-friendly greener synthesis of nanoparticles. Adv. Pharm. Bull. 2020, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.S.; Harun, Z.; Hubadillah, S.K.; Salleh, W.N.W.; Rosman, N.; Kamaruddin, N.H.; Azhar, F.H.; Sazali, N.; Ahmad, R.A.R.; Basri, H. Review on recent advance biosynthesis of TiO2 nanoparticles from plant-mediated materials: Characterization, mechanism and application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1142, 012005. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Niluxsshun MC, D.; Masilamani, K.; Mathiventhan, U. Green synthesis of silver nanoparticles from the extracts of fruit peel of Citrus tangerina, Citrus sinensis, and Citrus limon for antibacterial activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of nanomaterials: Tools and challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of green synthesized metal nanoparticles—A review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Dabirian, E.; Hajipour, A.; Mehrizi, A.A.; Karaman, C.; Karimi, F.; Loke-Show, P.; Karaman, O. Nanoparticles application on fuel production from biological resources: A review. Fuel 2023, 331, 125682. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Ghazvini, M.; Alhuyi Nazari, M.; Ahmadi, M.A.; Pourfayaz, F.; Lorenzini, G.; Ming, T. Renewable energy harvesting with the application of nanotechnology: A review. Int. J. Energy Res. 2019, 43, 1387–1410. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A review of titanium dioxide (TiO2)-based photocatalyst for oilfield-produced water treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Al Malki, J.S.; Hussien, N.A.; Akkad, L.M.; Al Thurmani, S.O.; Al Motiri, A.E. Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation. Sustainability 2023, 15, 11858. [Google Scholar] [CrossRef]
- Dai, Y.; Dong, H.; Sun, L.; Li, J.; Zhang, T.; Geng, Y.; Liu, Z. Life cycle environmental impact assessment of titanium dioxide production in China. Environ. Impact Assess. Rev. 2024, 105, 107412. [Google Scholar] [CrossRef]
- Middlemas, S.; Fang, Z.Z.; Fan, P. Life cycle assessment comparison of emerging and traditional Titanium dioxide manufacturing processes. J. Clean. Prod. 2015, 89, 137–147. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.P.; Saidur, R. A review on the impact of mining and mineral processing industries through life cycle assessment. J. Clean. Prod. 2019, 231, 1200–1217. [Google Scholar] [CrossRef]
- Grubb, G.F.; Bakshi, B.R. Life cycle of titanium dioxide nanoparticle production: Impact of emissions and use of resources. J. Ind. Ecol. 2011, 15, 81–95. [Google Scholar] [CrossRef]
- Saad, A.F.; Selim, Y.A.; Hamada, M.; Elywa, M. Green Synthesis of Titanium Dioxide Nanoparticles Using Ethanolic Extract of Green Tea and Their Antioxidant Activities. J. Appl. Spectrosc. 2023, 90, 1142–1148. [Google Scholar] [CrossRef]
- Joseph, S.; Nallaswamy, D.; Kumar, S.R.; Dathan, P.; Ismail, S.; Rasheed, N.; Munnuswamy, T.; Jose, L. Preparation and Characterization of Titanium Oxide, Zinc Oxide based Nanocomposite Material using Green Tea Extract. Trends Biomater. Artif. Organs 2024, 38, 14–22. [Google Scholar]
- Nabi, G.; Ain, Q.U.; Tahir, M.B.; Nadeem Riaz, K.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2022, 102, 434–442. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vidhya, E.; Anand, G.C.; Nilavukkarasi, M.; Punitha, V.N.; Sakthivel, B. Eco friendly synthesis of TiO2 nanoparticles using aqueous Ocimum americanum L. leaf extracts and their antimicrobial, anti-proliferative and photocatalytic activities. Vegetos 2020, 33, 805–810. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, A. Synthesis and characterization of nanoparticles from neem leaves and banana peels: A green prospect for dye degradation in wastewater. Ecotoxicology 2022, 31, 537–548. [Google Scholar] [CrossRef]
- Haque AN, M.A.; Remadevi, R.; Naebe, M. Lemongrass (Cymbopogon): A review on its structure, properties, applications and recent developments. Cellulose 2018, 25, 5455–5477. [Google Scholar] [CrossRef]
- Nimenibo-Uadia, R.; Nwosu, E.O. Phytochemical, proximate and mineral elements composition of lemongrass (cymbopogon citratus (DC) stapf) grown in ekosodin, Benin city, Nigeria. Niger. J. Pharm. Appl. Sci. Res. 2020, 9, 52–56. [Google Scholar]
- Ajayi, E.; Afolayan, A. Green synthesis, characterization and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus Stapf. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015017. [Google Scholar] [CrossRef]
- Patiño-Ruiz, D.; Sánchez-Botero, L.; Tejeda-Benitez, L.; Hinestroza, J.; Herrera, A. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: Nanotoxicological considerations for potential environmental applications. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100377. [Google Scholar] [CrossRef]
- Cherian, T.; Ali, K.; Saquib, Q.; Faisal, M.; Wahab, R.; Musarrat, J. Cymbopogon citratus functionalized green synthesis of CuO-nanoparticles: Novel prospects as antibacterial and antibiofilm agents. Biomolecules 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Sidik DA, B.; Hairom NH, H.; Ahmad, M.K.; Madon, R.H.; Mohammad, A.W. Performance of membrane photocatalytic reactor incorporated with ZnO-Cymbopogon citratus in treating palm oil mill secondary effluent. Process Saf. Environ. Prot. 2020, 143, 273–284. [Google Scholar] [CrossRef]
- Saavedra, A.; Correa, S.; Nuñez, B.; Patiño, E.; Herrera, A. Improvement of titanium dioxide nanoparticle synthesis with green chemistry methods using lemongrass (Cymbopogon citratus) extract. Mater. Tehnol. 2020, 54, 755–759. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- Fahlman, B.D. What is “materials chemistry”? In Materials Chemistry; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–30. [Google Scholar]
- Nyakudya, P.; Madushele, N.; Madyira, D.M. A Review of Industrial Symbiosis in Influencing Green Manufacturing. In Proceedings of the 2022 IEEE 13th International Conference on Mechanical and Intelligent Manufacturing Technologies (ICMIMT), Cape Town, South Africa, 25–27 May 2022; pp. 80–84. [Google Scholar]
- Pamu, Y.; Alugubelli, S. A comparative study of environmental impacts due to conventional and sustainable concrete. Mater. Today Proc. 2023, 92, 112–120. [Google Scholar] [CrossRef]
- Tragnone, B.M.; Serreli, M.; Arzoumanidis, I.; Pelino, C.A.; Petti, L. Using the product social impact life cycle assessment (PSILCA) database for product comparison: Confetti case study. Int. J. Life Cycle Assess. 2023, 28, 1031–1053. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Miranda-Martínez, E. Degradación Fotocatalítica de Cefalexina, Mediante el uso de Nanopartículas de TiO2 Soportadas en un Biopolímero. Master’s Thesis, Universidad Politécnica del Estado de Morelos, Jiutepec, Mexico, 2023. [Google Scholar]
- Babaizadeh, H.; Hassan, M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater. 2013, 40, 314–321. [Google Scholar] [CrossRef]
- Shah SN, A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorg. Chem. Appl. 2017, 2017, 4101735. [Google Scholar] [CrossRef]
- Di Noi, C.; Ciroth, A.; Srocka, M. openLCA 1.7 Comprehensive User Manual; GreenDeLTa: Berlin, Germany, 2017. [Google Scholar]
- Lokesh, K.; Matharu, A.S.; Kookos, I.K.; Ladakis, D.; Koutinas, A.; Morone, P.; Clark, J. Hybridised sustainability metrics for use in life cycle assessment of bio-based products: Resource efficiency and circularity. Green Chem. 2020, 22, 803–813. [Google Scholar] [CrossRef]
- Osorio-Tejada, J.; Ferlin, F.; Vaccaro, L.; Hessel, V. Life cycle assessment of multistep benzoxazole synthesis: From batch to waste-minimised continuous flow systems. Green Chem. 2022, 24, 325–337. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Z.; Hicks, A.L. Life cycle impact of titanium dioxide nanoparticle synthesis through physical, chemical, and biological routes. Environ. Sci. Technol. 2019, 53, 4078–4087. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Wang, W.; Wang, Y.; Lu, D. Characterization of Waste Biomass Fuel Prepared from Coffee and Tea Production: Its Properties, Combustion, and Emissions. Sustainability 2024, 16, 7246. [Google Scholar] [CrossRef]
- Roy, A.; Bhandari, S.; Sen, T. Room-Temperature Processable TiO2 Solar Paint for Dye-Sensitized Solar Cells. Sustainability 2023, 15, 16610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).