1. Introduction
Copper plays a crucial role in paving the way toward a sustainable future, particularly in the realm of low-carbon technologies and renewable energy [
1,
2]. The production of energy from renewable sources heavily relies on copper, with offshore wind farms necessitating five times more copper than coal-fired power plants. Copper is utilized in the production of cables for wind turbines, facilitating the integration and transmission of power, including deep sea cables. The exploration and operation of large-scale copper mines, such as those conducted by «KazMinerals» in Kazakhstan, prove to be relevant in this context.
The supply of copper is pivotal in achieving a sustainable future and bolstering the global shift to a green economy [
3,
4,
5]. Projections indicate an impending shortage in the copper market as production from existing mines declines, while demand increases from both conventional and emerging sources. Sufficient copper supplies are necessary to foster a sustainable future, serving purposes such as improving living standards in developing countries through energy and transportation infrastructure, supporting the adoption of renewable energy production, and facilitating the utilization of low-emission transportation technologies, including electric vehicles.
Copper producers, like «Kazminerals», play a decisive role in driving industrial growth, bolstering export profits, GDP, and employment opportunities through projects like Bozshakol and Aktogay. Currently, metallurgical projects are contributing to sustainable development by reducing the impact of production activities on the environment and fostering environmentally conscious attitudes among their employees, contractors, and suppliers. These copper industries must adhere to environmental protection policies, including responsible practices in emissions and waste reduction, as well as efficient utilization of energy and water resources [
6].
The principles of sustainable development are upheld by deposits like Aktogay, which exhibit a low overburden coefficient, meaning that a limited amount of waste rock is produced per ton of extracted ore, thereby enhancing efficiency [
7].
The extraction method is a contemporary technique utilized for the extraction, concentration, and separation of various metals. This method has extensive industrial applications worldwide for extracting metals such as nickel [
8], cobalt [
9], copper [
10], uranium [
11], rhenium [
12,
13], indium [
14], and rare earth elements [
15] from complex solutions. During the extraction process, the formation of intermediate phases, known as interphase suspensions, occurs. These suspensions accumulate either at the interface between the aqueous and organic phases or within the organic phase, resulting from the mixing of liquids with limited mutual solubility. Consequently, droplets of a denser liquid become dispersed within a lighter one [
16]. In most cases, interphase formations manifest as stable gelatinous suspensions containing an extractant and an aqueous solution [
17].
The factors influencing the formation of interphase suspensions, as revealed by scientific studies [
8], include the type of ore, composition of the initial solution, choice of extractant and diluent, equipment design, and the type of phase mixing. In a related scientific work [
11], the author identifies several causes for the formation of interphase suspensions, such as the presence of iron and titanium in the solution, silicon and humic substances, dust, sludge, hydrolysis of certain compounds during the extraction process, and the aging of solutions. It is crucial and urgent to conduct research and explore novel technological solutions for diminishing the formation of a third phase, commonly referred to as crud, during copper extraction operations. Typically, the constituents of crud primarily consist of silicic acid, present as colloidal particles that are not easily separated even through meticulous filtration. Additionally, solid compounds including ore material, metal oxides, and hydroxides [
18,
19,
20,
21,
22,
23], along with multiply charged ions (Zr, Sc, Fe, Al, rare earth elements, etc.), found in the original aqueous solutions, form sparsely soluble complexes with specific extractants and their hydrolysis products. Moreover, degradation products of the extractants and diluents accumulate in the organic phase during operation.
The mentioned works [
18,
19] discovered that interphase formations are released during the extraction of uranium, specifically during the washing operation of the extract. These formations consist of silica (quartz) and inorganic sulfates. The presence of suspended particles during the extraction operation is attributed to solid ore particles present in sulfuric acid solutions resulting from the leaching of raw materials. These particles are further abraded into smaller particles when the solutions are pumped with pumps. Additionally, interphase formations are formed from ammonia solutions of ammonium sulfate at low pH values. This leads to the formation of a yellow precipitate known as ammonium diuranate (NH
4)
2U
2O
7, which becomes entrapped within the extractant.
Interphase suspensions in technological schemes involving uranium, copper, nickel, and cobalt often contain humic acids and lignin [
19]. For instance, in factories where extraction is employed to extract uranium, using phosphoric acid as an extractant leads to the formation of abundant wax-like precipitates. According to the aforementioned author [
20], the formation of these precipitates is associated with the content of humic acid in the composition of phosphoric acid.
In [
21], the interaction in the D2EHPA–In
2(SO
4)
3–Fe
2(SO
4)
3–Ti
2(SO
4)
3–ZnSO
4–H
2O system was studied, showing that the basis of cruds is the salt InR3, which results from the secondary interaction of indium ions in solution with compounds formed in the extract. In [
22], the formation of interphase suspensions containing osmium during the extraction of rhenium from acid wash solutions in copper production was examined. Secondary interaction of salts with compounds formed in the extract also occurs when the extractant contains iron and titanium [
23,
24,
25,
26,
27]. This interaction greatly contributes to crud formation in the raffinate when in contact with the original indium-containing solution.
Methods for selecting extractants of the ACORGA class are discussed in works [
22,
23,
24,
25,
26], taking into consideration the suppression of iron and silicon impurities [
27,
28]. These works propose mechanisms for silicon polymerization, albeit with different initial solution compositions and preparation histories than those considered in the present study.
In [
29], the authors developed a technology to reduce sulfuric acid consumption, increase copper extraction, decrease copper losses from waste solutions, and improve the quality of cathode copper by utilizing an extractant of the LIX 984N type. Comparative data on extractants of the LIX and ACORGA types, as presented in [
30,
31,
32,
33,
34], are of interest.
To minimize and prevent crud formation, researchers have employed various methods, including the selection of organic phases with the addition of different modifiers [
35,
36,
37], selective precipitation, removal of harmful metal impurities [
38,
39,
40,
41,
42], addition of special surfactants, inclusion of flocculants, air blowing to prevent the formation of dense suspension, and flow rate adjustments of organic and aqueous phases, among others [
43,
44]. Equipment reconstruction [
45] and mechanical removal of crud [
40,
41,
42,
43,
44] have also been explored. It has been found in [
46,
47,
48] that an increase in the aromaticity of the diluent and the addition of an aqueous phase can reduce the adsorption of the extractant on the main component of crud-silicon dioxide potentially resulting in decreased crud formation and extractant loss. However, an increase in the composition of the organic phase with aromatic hydrocarbons is undesired due to their propensity for substitution reactions and high polarity, as they can lead to extractant degradation.
To reduce extractant costs resulting from crud formation, periodic processing of the interfacial suspension is recommended [
49,
50,
51], followed by treatment with an acidic solution to extract the extractant and return it to the extraction process. The use of polysaccharides [
52,
53], such as guar gum, xanthan gum, gum arabic, and starch, has also been investigated. The results indicate that guar gum is an effective additive enabling the extraction of the organic phase from gum with a yield of up to 95%.
In summary, an analysis of publications reveals that the formation and composition of crud are influenced by the composition of the solutions from which the target metal is extracted, as well as the nature and purity of the extractant components [
54]. Therefore, there is a need to explore new solutions that can effectively reduce crud formation during copper extraction using various classes of extractants and additives, especially considering the progressive decrease in copper concentration in ores, such as those found in the Aktogay deposit.
The objective of this study was to investigate the process of reducing crud formation during the liquid extraction of copper from productive copper-containing sulfate solutions obtained during the processing of ore from the Aktogay deposit. Various classes of extractants and additives were utilized, specifically in the form of a crud suppressor.
2. Materials and Methods
Materials. The research focused on the investigation of copper extraction processes employing different extractants. The main object of study was a productive copper-containing sulfate solution, commonly referred to as PLS (Pregnant Leach Solution). This solution was obtained through the processing of ore derived from the Aktogay deposit, with a solution pH of approximately 1.7.
The results of chemical analysis of a sample of the productive solution are presented in
Table 1.
As shown in
Table 1, the concentration of copper in the solution is 1.25 g/dm
3, while sulfuric acid has a concentration of 2.22 g/dm
3. The concentrations of aluminum, magnesium, total iron, calcium, and phosphorus are 29.1, 12.98, 4.23, 0.69, and 0.55 g/dm
3, respectively. Silicon is also present in the solution with a concentration of 0.51 g/dm
3. Other elements in the solution have relatively low levels.
To determine the composition of the solid residue obtained after evaporating the sulfuric acid solution to wet salts, X-ray fluorescence analysis (XRF) was performed.
Table 2 presents the results of the XRF analysis of the solid residue.
According to
Table 2, the residue contains significant amounts of aluminum (4.9%), magnesium (3.8%), and iron (5.1%). The sulfur content is approximately 15%, which can be attributed to the presence of metal sulfates in the solution. The presence of the SO
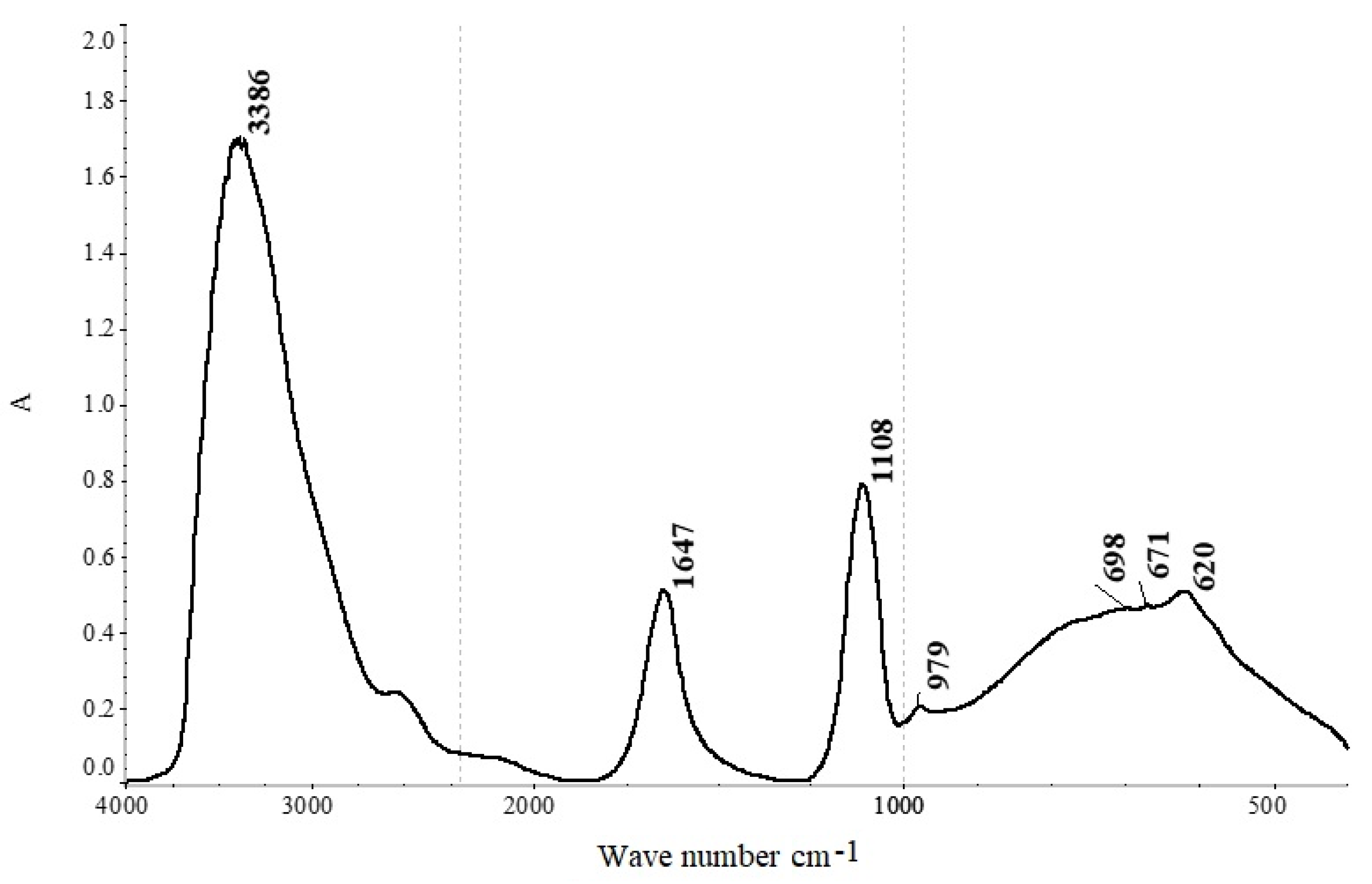
42- group is confirmed by infrared (IR) analysis of a sample of the productive solution (
Figure 1).
In the spectrum (
Figure 1), absorption bands corresponding to water are observed at wave numbers of ν(OH) 3368 cm
−1 and δ(HOH) 1647 cm
−1 [
48]. The presence of the SO
42− group is evidenced by absorption bands at wave numbers 1108, 979, 671, and 620 cm
−1 [
30]. The band with a peak at a wave number of 698 cm
−1 corresponds to the manifestation of deformation vibrations of Me-O-H [
55].
The presence of deformation vibrations of Me-O-H suggests that aluminum and iron salts undergo hydrolysis in aqueous solutions. In an aqueous solution, the aluminum ion is hydrated, meaning it attracts the negatively charged ends of water molecules and forms strong bonds with several water molecules. This results in the formation of a hexahydrate with the composition Al(OH
2)
63+, where six water molecules are arranged octahedrally around the aluminum ion [
55].
Based on the results obtained from chemical, infrared spectroscopic, and X-ray fluorescence (XRF) analyses, it can be concluded that the productive solution contains silicates of aluminum, magnesium, iron, and calcium, in addition to sulfates. These silicates are the primary components responsible for the formation of impurities. However, the solid residue has a relatively low silicon content of approximately 0.3%. This suggests that insoluble hydroxides, such as Al(OH)3 and Fe(OH)3, which are generated through the hydrolysis of metal salts in the original sulfuric acid solution, may also contribute to the presence of impurities.
Methods of analysis. Analytical methods were employed to analyze the samples in this study. The quantitative determination of major elements in solution was conducted using an Optima 8300DV inductively coupled plasma atomic emission spectrometer and a PFP 7 flame photometer (Jenway, London, UK).
Infrared absorption spectra were recorded using an Avatar 370 IR-Fourier spectrometer across the range of 400–4000 cm−1 from capillary layers in KRS-5 windows. The experimental setup included the «Transmission E.S.P.» attachment. Processing of the collected data was performed utilizing OMNIC 6 software, along with electronic libraries including HR Aldrich FT-IR Collection Edition II (containing 18,454 spectra) and Aldrich Organometallic, Inorganic, Boron, Deuterium Compounds (consisting of 632 spectra) published by Nicolet Instrument Corp. (Madison, WI, USA) in 1995. In cases where information was not available in the electronic libraries, literary sources were consulted.
X-ray fluorescence analysis was carried out using an Axios PANalytical wavelength dispersive spectrometer (fluorescent x-ray spectrometer with wave dispersion (WDXRF) Axios MAX, PANalytical, Lelyweg 1, 7602 EA Almelo, The Netherlands, 2011.
X-ray phase analysis was performed using a D8 ADVANCE diffractometer “BRUKER AXS GmbH” (Karlsruhe, Germany) equipped with α-C radiation, a tube voltage of 40 kV, and a current of 40 mA. Data processing of the obtained diffraction patterns and calculation of interplanar distances were accomplished using EVA software. Sample interpretation and phase identification were accomplished using the search/match program utilizing the PDF-2 database from the International Center for Diffraction Data ICDD (USA).
Extraction experiment procedure. Extraction experiments were conducted under controlled room temperature conditions of 20 ± 5 °C. Temperature stability was ensured using a LOIP-105A thermostat. The investigation focused on studying the volume ratio of the organic and aqueous phases, ranging from 1:2 to 1:10 with a step size of 2. The contact time between phases was determined based on the time-dependent behavior of the copper distribution coefficient (DCu), set at 5 min. The extraction process was carried out in conventional separatory funnels, followed by phase separation through settling. Aqueous solutions were filtered through a designated paper filter labeled as “red ribbon” before sampling for analysis. Mechanical mixing was employed to achieve homogenization of the phases. Depending on the experimental objectives, the resulting solutions were analyzed for their copper content.
To further investigate the process and optimize the extraction of copper, different classes of extractants were tested, including ketoximes (derivatives of ketones) and aldoximes (derivatives of aldehydes). The goal was to identify the most effective extractant for suppressing the formation of copper during the extraction process.
3. Results
Selecting an extractant to reduce crud formation during the extraction of copper from sulfuric acid solutions. The selection of an extractant during the extraction process is influenced by several factors, including its extraction capacity and the initial concentration of the metal in the process solutions. Other considerations include the cost and availability of the extractants, their class and application, extraction and re-extraction kinetics, selectivity, net copper transfer, ease of operation, composition purity, low viscosity, and chemical stability. The choice of extractants for effective extraction technology is discussed in [
56], which highlights the importance of these parameters.
Two classes of extractants that have gained commercial recognition for the extraction of copper from acidic leach solutions are ketoximes and aldoximes [
57,
58,
59]. In this study, chemically pure LIX 984N, ACORGA M5774, and ACORGA M5640 were utilized to identify the optimal extractant. Typically, the extractant-to-diluent volume ratio ranges from 10–20% to 80–90% [
60]. Aliphatic kerosene was employed as the diluent, and the organic phase consisted of a 10 vol. % solution of the investigated extractants in purified kerosene.
LIX 984N, a ketoxime derived from a ketone, was one of the extractants studied. In the IR spectrum of LIX 984N, stretching vibrations of C-H in aliphatic hydrocarbons were observed at 2957, 2926, 2871, and 2856 cm
−1, while bending vibrations of aliphatic hydrocarbons (δ(CH
3), δ(CH
2)) were observed at 1464 and 1378 cm
−1 [
57]. Stretching vibration of ν(OH) appeared at 3374 cm
−1. Stretching vibrations of C-H in aromatic compounds occurred at 3058 and 3034 cm
−1 [
48]. The bands at wave numbers 1623, 1585, and 1496 cm
−1 correspond to stretching vibrations of C=C aromatic rings and stretching vibrations of the C=N bond of the azomethine group in oximes [
48]. Vibrations of the C–O–H group were observed in the range of 1400–1000 cm
−1. A band at a wavenumber of 1268 cm
−1 corresponds to –C–N– vibrations in compounds of the type: ArNHR [
57].
ACORGA M5774 and ACORGA M5640 (manufactured by Syensqo, Belgium/USA) are modified aldoxime extractants that have widespread use in the copper industry, specifically in the largest copper mines around the world.
The IR spectrum of ACORGA M5774, a representative of modified aldoximes (specifically 5-nonyl salicylaldoxime), was examined. Similar bands, characteristic of LIX 984N, were observed in the IR spectrum of ACORGA M5774, albeit slightly shifted to higher frequencies. For instance, the C-H stretching vibrations in aliphatic hydrocarbons appeared at 2967, 2933, and 2875 cm
−1, while the bending vibrations (δ(CH3), δ(CH2)) were observed at 1469 and 1388 cm
−1. The stretching vibration of ν(OH) occurred at 3398 cm
−1 [
55]. C-H stretching vibrations in aromatic compounds were observed at 3082, 3064, and 3033 cm
−1. The bands at wave numbers 1624, 1584, and 1497 cm
−1 corresponded to the stretching vibrations of C=C aromatic rings and the C=N bond in the azomethine group of oximes [
55].
One notable feature in the IR spectrum of ACORGA M5774 is the presence of a stretching vibration of the carbonyl group ν(C=O) at 1736 and 1712 cm
−1 [
30]. Vibrations of the C-O-H group appeared in the range of 1400–1000 cm
−1 [
30]. The band at a wavenumber of 1271 cm
−1 fell within the range of –C–N– vibrations in compounds of the ArNHR type. The bands observed at 828, 795, 743, 712, and 663 cm
−1 corresponded to the out-of-plane deformation vibrations of CH in aromatic compounds. Furthermore, bands in the range from 650 to 250 cm
−1 indicated the presence of substituent groups in benzene derivatives [
55].
The selection of the optimal extractant was studied using a productive sulfuric acid solution with concentrations of 1.25 g/dm
3 Cu and 2.22 g/dm
3 H
2SO
4.
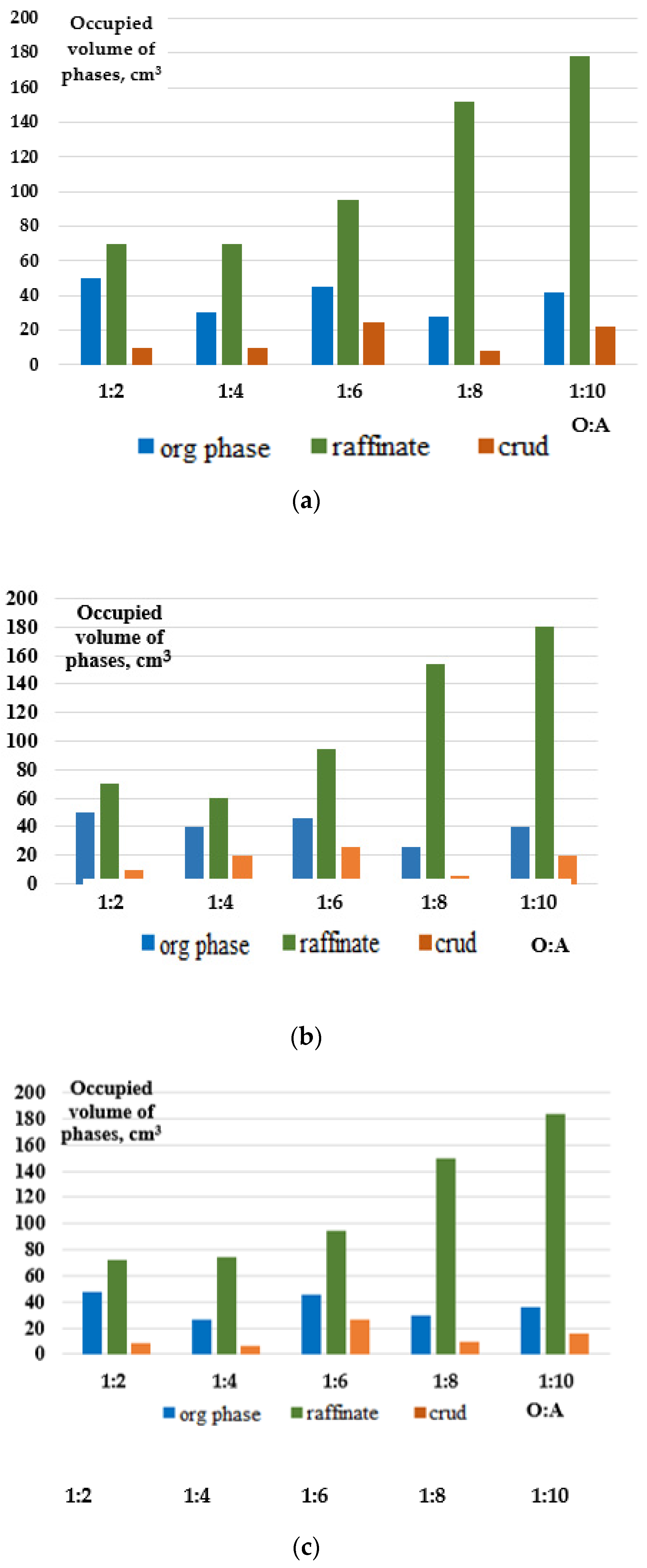
Table 3 presents the copper extraction figures for different classes of extractants and varying O:A ratios from an industrial solution. The numerical variations in the values are attributed to determination method error and the human factor.
It was found that during the extraction of copper from the productive solution using LIX 984N (a ketoxime) and ACORGA M5774 and M5640 (aldoximes), at a phase ratio of O:A = 1:2, the copper extraction rates into the extract were approximately 48.4%, ~67%, and ~55%, respectively. However, increasing the O:A ratio toward the aqueous phase during extraction significantly decreased the degree of copper extraction. For instance, the use of LIX 984N resulted in a copper extraction as low as 13.36% at certain O:A ratios. By comparison, ACORGA M5774’s extraction efficiency decreased to 17.44% at an O:A ratio of 1:4.
Visual analysis of the copper extraction process using LIX 984N, ACORGA M5774, and ACORGA M5640 extracts revealed important observations. It should be noted that the extraction process involves the formation of three phases: raffinate, extract, and crud, with the crud concentrated as a separate phase within the extract.
In the case of the LIX 984N extractant, phase separation occurs rapidly at O:A ratios of 1:2 and 1:4. However, further separation becomes more challenging and requires a longer settling time, up to 1 day. As the O:A ratio increases from 1:2 to 1:10, there is a gradual increase in the formation of crud, which is initially smeared along the walls of the separating funnel and then settles more substantially at the bottom.
For the ACORGA M5774 extractant, irrespective of the O:A ratio, the phases separate quickly, and the process of crud formation is similar to that observed with LIX 984N. However, at higher O:A ratios, the amount of waste in the organic phase decreases, resulting in very little sediment formation at an O:A ratio of 1:10.
The behavior of the ACORGA M5640 extractant is different. Phase separation becomes difficult even at an O:A ratio of 1:2, and the process of crud formation is similar to that observed with LIX 984N. However, it should be noted that at an O:A ratio of 1:8, the behavior of ACORGA M5640 changes. In this case, a small amount of finely dispersed sediment forms, which passes through the filter when filtering the organic phase and enters the raffinate.
Based on visual observations, it was also noted that as the O:A ratio increases from 1:1 to 1:10, the color of the raffinate takes on a brown or dark brown tint. According to the literature references [
26,
27,
28], at higher pH values, the solution turns yellow due to hydrolysis. If the pH exceeds 2–3, further condensation occurs, leading to the formation of colloidal gels, ultimately resulting in the precipitation of reddish-brown hydrated iron oxide (III).
According to the results obtained, the extractants can be ranked in the following order based on their effectiveness in reducing crud formation:
The extractant ACORGA M5774 demonstrated the most favorable results, followed by LIX 984N and then ACORGA M5640, in terms of their ability to reduce crud formation. Further research is focused on investigating the material composition of the cruds formed within the volume of the organic phase during the extraction process using the aforementioned extractants.
Characterization of the material composition of crud. Following the extraction process, the cruds were collected in a distinct vessel, filtered, and subsequently dried at a temperature of 105 °C. The collected crud sample was then subjected to chemical analysis, X-ray diffraction (XRD), and infrared (IR) spectroscopic analysis. The phase distribution of the crud sample is depicted in
Figure 2.
Chemical analysis of the crud sample is presented in
Table 4.
According to the data presented in
Table 4, the primary constituents found in the crud are as follows (percentage by weight): silicon (17.58%), nickel (3.13%), total iron (2.31%), aluminum (2.14%), magnesium (2.023%), and others. The remaining elements have relatively low levels of concentration.
Table 5 presents the results of the X-ray phase analysis method.
Based on the data presented in
Table 4, the crud sample primarily consists of two compounds: alunogen [Al(H
2O)
6]
2(SO
4)
3(H
2O)
5 at 28.3% and quartz SiO
2 at 28.1%. It is possible that quartz enters the crud sample through a solution produced by leaching copper ore. Previous research [
18] has shown that silica (quartz) and inorganic sulfates are released during the extraction of uranium, which involves washing the extract. Solid particles of ore are present in sulfuric acid solutions used for leaching, and when the solutions are pumped, these particles are further worn down into smaller particles, ultimately resulting in the presence of suspension in the extraction process.
Additionally, the crud sample contains a small amount of nuyakasite (7.6%), a complex silicate of iron and aluminum with variable composition of sodium and silicon oxide (3.9%).
IR spectroscopic analysis of the crud sample reveals the presence of intense bands associated with the methylene (CH
2) and methyl (CH
3) groups of aliphatic hydrocarbons. These bands correspond to stretching vibrations ν(CH
2, CH
3) at 2955, 2924, 2870, and 2855 cm
−1, deformation vibrations δ(CH
2, CH
3) at 1459 and 1377 cm
−1, and pendulum vibrations ρ(CH
2) at 722 cm
−1 [
55].
Furthermore, bands observed at wave numbers 1647, 1611, and 1542 cm
−1 align with the expected range for stretching vibrations of C=C aromatic rings and stretching vibrations of the C=N bond of the azomethine group of oximes [
55].
The IR spectroscopic analysis data provides evidence supporting the occurrence of extractant loss during the copper extraction operation, leading to the formation of crud. Based on this information, it can be concluded that the primary components of the crud in most cases are silicic acid, present in the form of colloidal particles that cannot be effectively removed even through careful filtration, as well as inorganic sulfates. The aqueous solutions also contain solid compounds such as ore material, metal oxides, and hydroxides, among others.
The subsequent phase of the research entails investigating alternative methods and conditions that can help inhibit the formation of crud during the copper extraction process. In this regard, the use of the CR 60 additive will be examined as a potential approach.
Study of the physical and chemical composition of the CR 60-mitigation reagent. The Solvay reagent, specifically from the ACORGA® CR60 series, is widely employed on an industrial scale in numerous factories across North and Latin America, as well as in production facilities including the Democratic Republic of Congo. The primary advantage of utilizing ACORGA CR60 (hereafter referred to as the additive) is its efficacy in minimizing the formation of crud within settling tanks. This reduction results in increased capacity for productive sulfuric acid solutions (PLS) and organics, directly translating to enhanced plant productivity. Notably, the presence of silicon dioxide in PLS solutions can complicate the liquid extraction process. However, the introduction of the ACORGA CR60 reagent has been observed to enable a stable operational process.
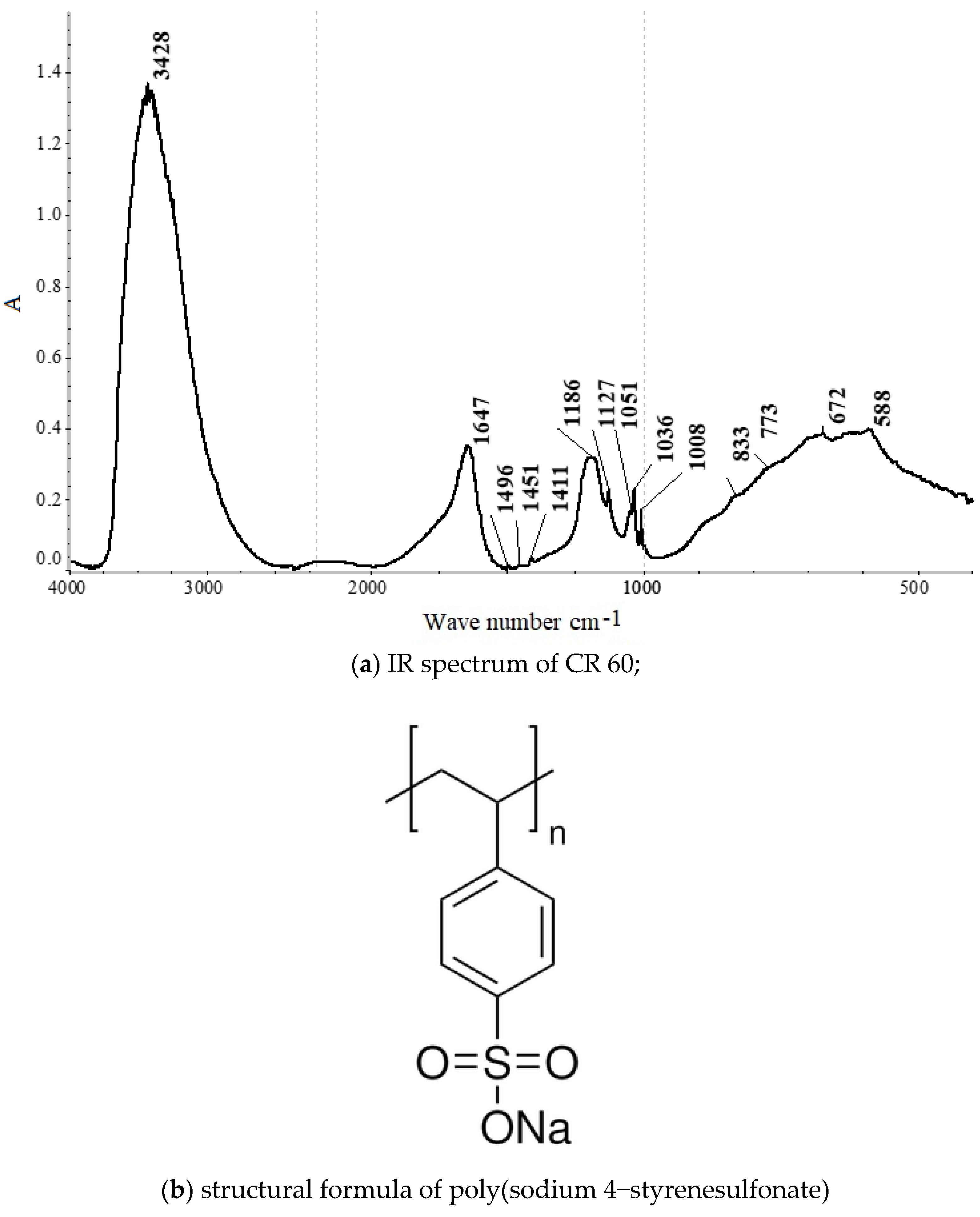
IR spectrum of CR 60 is depicted in
Figure 3a. IR spectrum exhibits characteristic water absorption bands at ν(OH)—3428 cm
−1 and δ(HOH)—1647 cm
−1 [
48]. Additionally, sulfonate ion vibrations are detected at RSO
2O—1186, 1127, 1051, 1036, 1008, 672, and 588 cm
−1 [
48]. The presence of poly(sodium 4-styrenesulfonate) is evident in the spectrum, as indicated by peaks at—1496, 1451, 1411, 1186, 1127, 1036, 1008, 833, 773, 672, and 588 cm
−1 [
48].
The structural formula of poly(sodium 4-styrenesulfonate) is depicted in
Figure 3b, with its chemical formula defined as (H
2C=CHC
6H
4SO
3Na)n. This compound is a monomeric polymer, characterized by the sulfonate ion functional group S(=O)
2-O−. The general formula RSO
2O− denotes the radical R. Surfactants such as alkali metal sulfonates (primarily sodium alkylsulfonates) are widely utilized, and poly(sodium 4-styrenesulfonate) falls into this category.
Consequently, the ACORGA CR60 additive, being a surfactant, presents a noteworthy prospect for future investigations aimed at studying the reduction of crud formation using the CR60 reagent.
Investigation into the effect of CR 60 additive on reducing the formation of crud during copper extraction using various extractants. Experimental investigation of copper extraction using LIX 984 N, ACORGA M5774, and ACORGA M5640 extractants at a concentration of 10 vol. %, supplemented with 5 cm3 of CR60 additive.
The research findings are outlined in
Table 6.
Table 5 presents the results of the copper extraction study, indicating that ACORGA M5640 exhibited the highest copper extraction rate at 24%, followed by ACORGA M5774 at 15%.
Comparing the data with and without the CR60 additive, a disparity in copper extraction into the extract is evident. Without the additive, the copper extraction rates were higher compared to those with the addition of CR60, as shown in
Table 6. It is hypothesized that the CR60 additive may influence the copper recovery rate, while concurrently decreasing the formation of cruds by a factor of three.
Visual examination of the crud formation process during copper extraction from the productive solution, regardless of the extractant used, revealed that in the presence of the CR60 additive, the phases separated rapidly, but the raffinates remained turbid. Consequently, the raffinates were allowed to settle for one day and were subsequently filtered using a red ribbon filter. The resulting raffinates displayed pure, transparent solutions without sediment, exhibiting a light brown color with a yellowish tint. Adding a 5 cm3 quantity of ACORGA CR60 reagent into the productive sulfuric acid solution prior to the liquid extraction operation significantly reduced the formation of interfacial cruds.
The research findings suggest that the application of surfactants for crud processing is a promising avenue. This approach offers several advantages, including the opportunity to return the extractant and copper-containing solution to circulation, as well as reducing the quantity of cruds sent for disposal. However, it is imperative to consider the influence of surfactants on the level of copper extraction and subsequently optimize the conditions for the liquid extraction process.
Optimization of technological extraction parameters to minimize interphase waste (crud) formation.
Optimization of Technological Parameters to Minimize Interphase Waste (Crud) Formation in Copper Extraction
Based on the findings of this study, the following conditions have been identified as the optimal technological parameters for minimizing the formation of interphase waste (crud) during copper extraction:
These optimized parameters have been determined based on their efficacy in reducing the formation of interphase waste during the copper extraction process.
Based on the results obtained in this study, the identified optimal technological parameters for copper extraction can effectively reduce the formation of interphase waste (crud) by 2–3 times. By implementing these conditions, it is possible to mitigate the development of a viscous, polymerized structure of the silicon component in the interphase waste (crud).
Furthermore, maintaining control over the solution pH within the range of 1.7 to 2.8 enables unimpeded phase separation and reduces any adverse impact on extraction efficiency.
The proposed method and extraction conditions, alongside the recommended practices for minimizing interphase waste formation, can serve as a universal approach for similar solutions found in heap-leaching processes at the Aktogay deposit.
A research study was conducted to enhance the energy efficiency of copper production activities at «Kazminerals» Aktogay through the reduction of crud. The research findings demonstrate that by reducing waste mass and minimizing impurities, a sustainable long-term increase in energy efficiency can be achieved. Furthermore, the transition from underground mining to open-pit mining at the Aktogay field has directly contributed to improving energy efficiency and promoting sustainable development.
The evaluation of energy efficiency improvement in the production activity of Aktogay involves considering energy usage per ton of processed sulfide ore. By incorporating the utilization of suppressive reagents, such as cradles, the amount of waste generated is reduced, resulting in decreased electricity consumption, diesel fuel consumption, gasoline usage, and overall heat consumption. This reduction in energy consumption leads to a more favorable energy efficiency. To present a comprehensive overview of the energy efficiency indicators, we have included summary data from
Table 7 and
Table 8, which highlight the improvements achieved by employing mitigation reagent CR 60, an effective flotation method, and the proposed extraction method.
The large-scale production at Aktogay has led to significant resource savings through the implementation of modern crushing and flotation technologies, as well as the usage of crud mitigation reagents, which further enhance efficiency by reducing energy consumption per ton of processed ore. This development proposal also aligns with the concept of climate improvement.
The proposed extraction technique, coupled with crud formation reduction, is comparable to greenhouse gas (GHG) emissions on a production scale. The calculations of CO2 emissions per thousand tons, distributed according to production types, indicate that reducing waste mass also leads to a decrease in specific carbon dioxide (CO2) emissions. Additionally, it was observed that a 25% reduction in waste mass corresponded to a 13% decrease in CO2 emissions, which corresponded to 732 tons in total in KAZ Minerals enterprises. Simultaneously, the implementation of mitigation reagents resulted in an 18% increase in revenues.
The overall objective of developing an extraction method using reagents that inhibit the formation of cruds is to minimize energy consumption at production sites, further promoting energy efficiency and sustainability. The overall objective of developing an extraction method using.