Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China
Abstract
1. Introduction
2. Materials and Methods
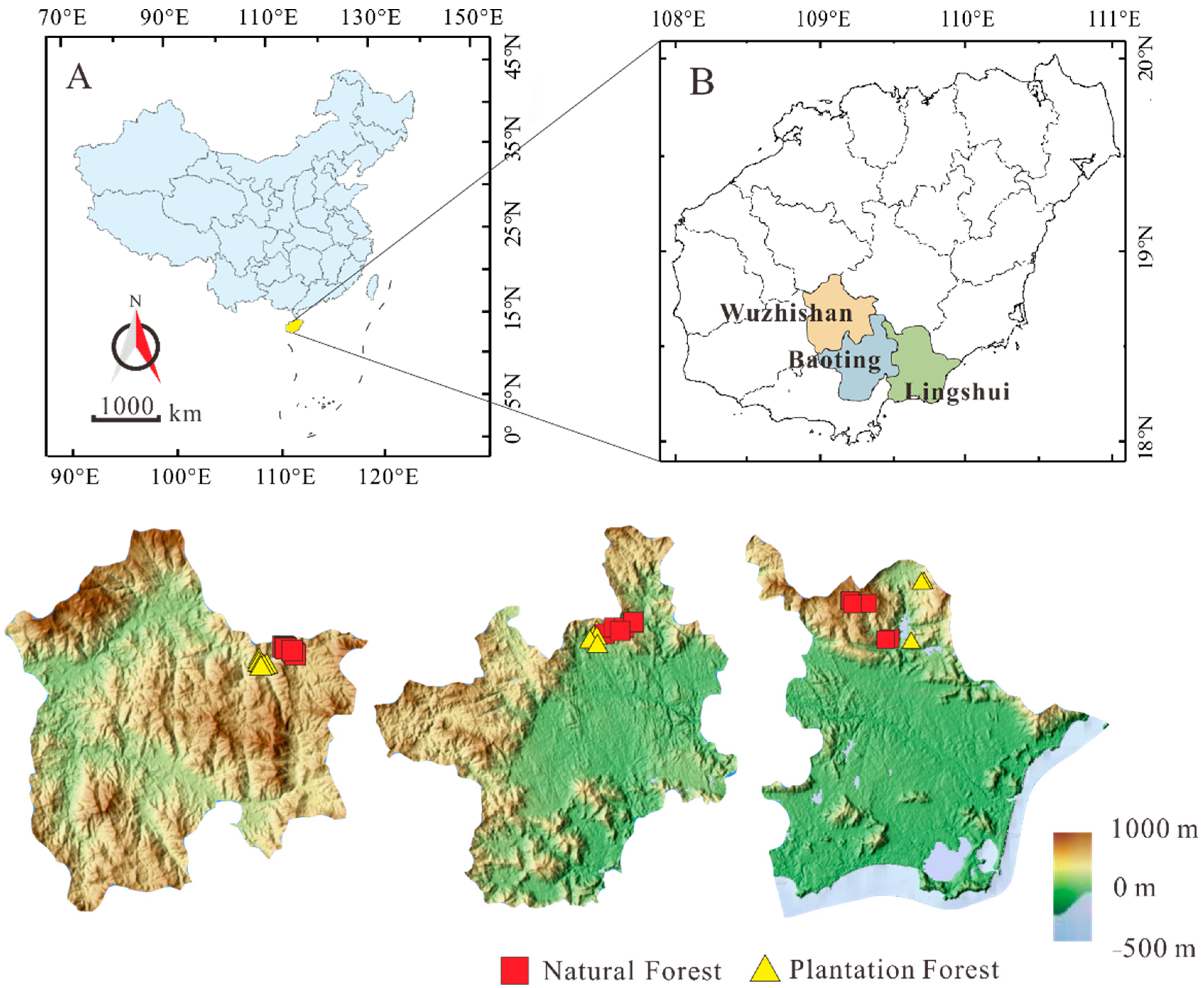
2.1. Experimental Site
2.2. Sample Sources and Pre-Treatment
2.3. Analytical Methods
3. Results and Discussion
3.1. Spatial Trend of LOC in Two Different Forest Types
3.2. Vertical Distribution Trend of LOC
3.3. Comparison of LOC/TOC Ratio
3.4. Correlation between LOC Concentration and Selected Soil Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prietzel, J.; Christophel, D. Organic carbon stocks in forest soils of the German Alps. Geoderma 2014, 221–222, 28–39. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, P.; Fu, B.; Wu, D.; Zhao, Z. Soil organic carbon distribution and factors affecting carbon accumulation in natural and plantation forests in tropical China. Ecol. Indic. 2023, 148, 110127. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Chang. 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Meinshausen, M.; Arora, V.K.; Jones, C.D.; Anav, A.; Liddicoat, S.K.; Knutti, R. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 2014, 27, 511–526. [Google Scholar] [CrossRef]
- Pacala, S.; Socolow, R. Stabilization Wedges: Solving the Climate Problem for the Next 50 Years with Current Technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Smith, P. Soils and climate change. Curr. Opin. Environ. Sustain. 2012, 4, 539–544. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.d.R.d.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Chadwick, D.; Jones, D.L.; Evans, C.; Jones, M.B.; Rees, R.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef]
- Esmaeilzadeh, J.; Ahangar, A.G. Influence of soil organic matter content on soil physical, chemical and biological properties. Int. J. Plant. Sci. 2014, 4, 244–252. [Google Scholar]
- Khatoon, H.; Solanki, P.; Narayan, M.; Tewari, L.; Rai, J.; Hina Khatoon, C. Role of microbes in organic carbon decomposition and maintenance of soil ecosystem. Int. J. Chem. Eng. 2017, 5, 1648–1656. [Google Scholar]
- Cai, Y.; Chang, S.X. Disturbance effects on soil carbon and greenhouse gas emissions in forest ecosystems. Forests 2020, 11, 297. [Google Scholar] [CrossRef]
- Grasset, C.; Rodriguez, C.; Delolme, C.; Marmonier, P.; Bornette, G. Can soil organic carbon fractions be used as functional indicators of wetlands? Wetlands 2017, 37, 1195–1205. [Google Scholar] [CrossRef]
- Jinbo, Z.; Changchun, S.; Wenyan, Y. Land use effects on the distribution of labile organic carbon fractions through soil profiles. Soil. Sci. Soc. Am. J. 2006, 70, 660–667. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Han, X.; Zou, W.; Chen, X.; Lu, X.; Feng, Y. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Z.; Lu, X. Changes of soil labile organic carbon fractions and their relation to soil microbial characteristics in four typical wetlands of Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 82, 381–389. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Wang, L.; Guo, J.; Feng, J.; Wu, H.; Lin, G. Distribution patterns and controlling factors for the soil organic carbon in four mangrove forests of China. Glob. Ecol. Conserv. 2019, 17, e00575. [Google Scholar] [CrossRef]
- Tipping, E.; Woof, C. Humic substances in acid organic soils: Modelling their release to the soil solution in terms of humic charge. J. Soil Sci. 1990, 41, 573–586. [Google Scholar] [CrossRef]
- Akpa, S.I.C.; Odeh, I.O.A.; Bishop, T.F.A.; Hartemink, A.E.; Amapu, I.Y. Total soil organic carbon and carbon sequestration potential in Nigeria. Geoderma 2016, 271, 202–215. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Hobley, E.; Wilson, B.; Wilkie, A.; Gray, J.; Koen, T. Drivers of soil organic carbon storage and vertical distribution in Eastern Australia. Plant Soil 2015, 390, 111–127. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Yang, S.; Jansen, B.; Kalbitz, K.; Chunga Castro, F.O.; van Hall, R.L.; Cammeraat, E.L.H. Lithology controlled soil organic carbon stabilization in an alpine grassland of the Peruvian Andes. Environ. Earth Sci. 2020, 79, 66. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Z.; Fu, B.; Wang, J.; Tang, W. Characteristics of soil organic carbon fractions under different land use patterns in a tropical area. J. Soils Sediments 2021, 21, 689–697. [Google Scholar] [CrossRef]
- Carter, M.; Angers, D.; Gregorich, E.; Bolinder, M. Characterizing organic matter retention for surface soils in eastern Canada using density and particle size fractions. Can. J. Soil Sci. 2003, 83, 11–23. [Google Scholar] [CrossRef]
- Mitchard, E.T. The tropical forest carbon cycle and climate change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Gloor, E.; Bååth, E.; Meir, P. Soil carbon and microbes in the warming tropics. Funct. Ecol. 2022, 36, 1338–1354. [Google Scholar] [CrossRef]
- Cox, P.M.; Pearson, D.; Booth, B.B.; Friedlingstein, P.; Huntingford, C.; Jones, C.D.; Luke, C.M. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 2013, 494, 341–344. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Chen, G.; Yin, Y.; Gao, R.; Lin, C. Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 2009, 323, 153–162. [Google Scholar] [CrossRef]
- Wang, Q.-K.; Wang, S.-L.; Zhong, M.-C. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Franklin, J.; Arroyo-Rodríguez, V.; Field, R.; Aguilar, S.; Aguirre, N.; Ahumada, J.; Aiba, S.-I.; Alves, L.F.; Anitha, K.; et al. Phylogenetic classification of the world’s tropical forests. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 115, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fellowes, J.R.; Jiang, X.; Wang, W.; Chan, B.P.L.; Ren, G.; Zhu, J. Degradation of tropical forest in Hainan, China, 1991–2008: Conservation implications for Hainan Gibbon (Nomascus hainanus). Biol. Conserv. 2010, 143, 1397–1404. [Google Scholar] [CrossRef]
- Fu, B.; Liu, Y.; Zhao, Z.; Zhang, L.; Wu, D.; Liu, H. Dissolved palladium in heavy traffic location runoff: Dynamic variation and influencing factors. Urban Water J. 2018, 15, 880–887. [Google Scholar] [CrossRef]
- Tang, L.; Mao, L.; Shu, J.; Li, C.; Shen, C. Overview of Modern and Quaternary Vegetation in China. In Atlas of Quaternary Pollen and Spores in China; Science Press: Beijing, China, 2020; pp. 1–87. [Google Scholar]
- Shi-Yong, D.; Zhen-Chuan, C.; Xian-Chun, Z. Biodiversity and conservation of pteridophytes from Diaoluo Mountain, Hainan Island. Biodivers. Sci. 2003, 11, 422. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Ladha, J. Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil. Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar]
- Rossi, J.; Govaerts, A.; De Vos, B.; Verbist, B.; Vervoort, A.; Poesen, J.; Muys, B.; Deckers, J. Spatial structures of soil organic carbon in tropical forests—A case study of Southeastern Tanzania. Catena 2009, 77, 19–27. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, X.; Xiao, W.; Wang, L.; Sun, Y.; Guan, Q.; Meshack, A.O. Comparing soil organic carbon stock and fractions under natural secondary forest and Pinus massoniana plantation in subtropical China. Catena 2022, 212, 106092. [Google Scholar] [CrossRef]
- Sheng, H.; Zhou, P.; Zhang, Y.; Kuzyakov, Y.; Zhou, Q.; Ge, T.; Wang, C. Loss of labile organic carbon from subsoil due to land-use changes in subtropical China. Soil. Biol. Biochem. 2015, 88, 148–157. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma 2002, 107, 109–141. [Google Scholar] [CrossRef]
- Lyu, M.; Noormets, A.; Ukonmaanaho, L.; Li, Y.; Yang, Y.; Xie, J. Stability of soil organic carbon during forest conversion is more sensitive in deep soil than in topsoil in subtropical forests. Pedobiologia 2021, 84, 150706. [Google Scholar] [CrossRef]
- Yang, J.; Li, A.; Yang, Y.; Li, G.; Zhang, F. Soil organic carbon stability under natural and anthropogenic-induced perturbations. Earth Sci. Rev. 2020, 205, 103199. [Google Scholar] [CrossRef]
- Diao, J.; Liu, J.; Zhu, Z.; Wei, X.; Li, M. Active forest management accelerates carbon storage in plantation forests in Lishui, southern China. For. Ecosyst. 2022, 9, 100004. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, X.; Wei, X.; Shao, M.; Li, T.; Yu, Q. Total soil organic carbon increases but becomes more labile after afforestation in China’s Loess Plateau. For. Ecol. Manag. 2020, 461, 117911. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Geraei, D.S.; Hojati, S.; Landi, A.; Cano, A.F. Total and labile forms of soil organic carbon as affected by land use change in southwestern Iran. Geoderma Reg. 2016, 7, 29–37. [Google Scholar] [CrossRef]
- Yu, P.; Han, K.; Li, Q.; Zhou, D. Soil organic carbon fractions are affected by different land uses in an agro-pastoral transitional zone in Northeastern China. Ecol. Indic. 2017, 73, 331–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Xiao, M.; Feng, Z.; Yu, Y.; Yao, H. Effects of microplastics on soil carbon dioxide emissions and the microbial functional genes involved in organic carbon decomposition in agricultural soil. Sci. Total Environ. 2022, 806, 150714. [Google Scholar] [CrossRef] [PubMed]
- Bashir, O.; Ali, T.; Baba, Z.A.; Rather, G.; Bangroo, S.; Mukhtar, S.D.; Naik, N.; Mohiuddin, R.; Bharati, V.; Bhat, R.A. Soil organic matter and its impact on soil properties and nutrient status. In Microbiota and Biofertilizers; Volume 2—Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Cham, Switzerland, 2021; pp. 129–159. [Google Scholar]
- Li, Q.; Wang, L.; Fu, Y.; Lin, D.; Hou, M.; Li, X.; Hu, D.; Wang, Z. Transformation of soil organic matter subjected to environmental disturbance and preservation of organic matter bound to soil minerals: A review. J. Soils Sediments 2023, 23, 1485–1500. [Google Scholar] [CrossRef]
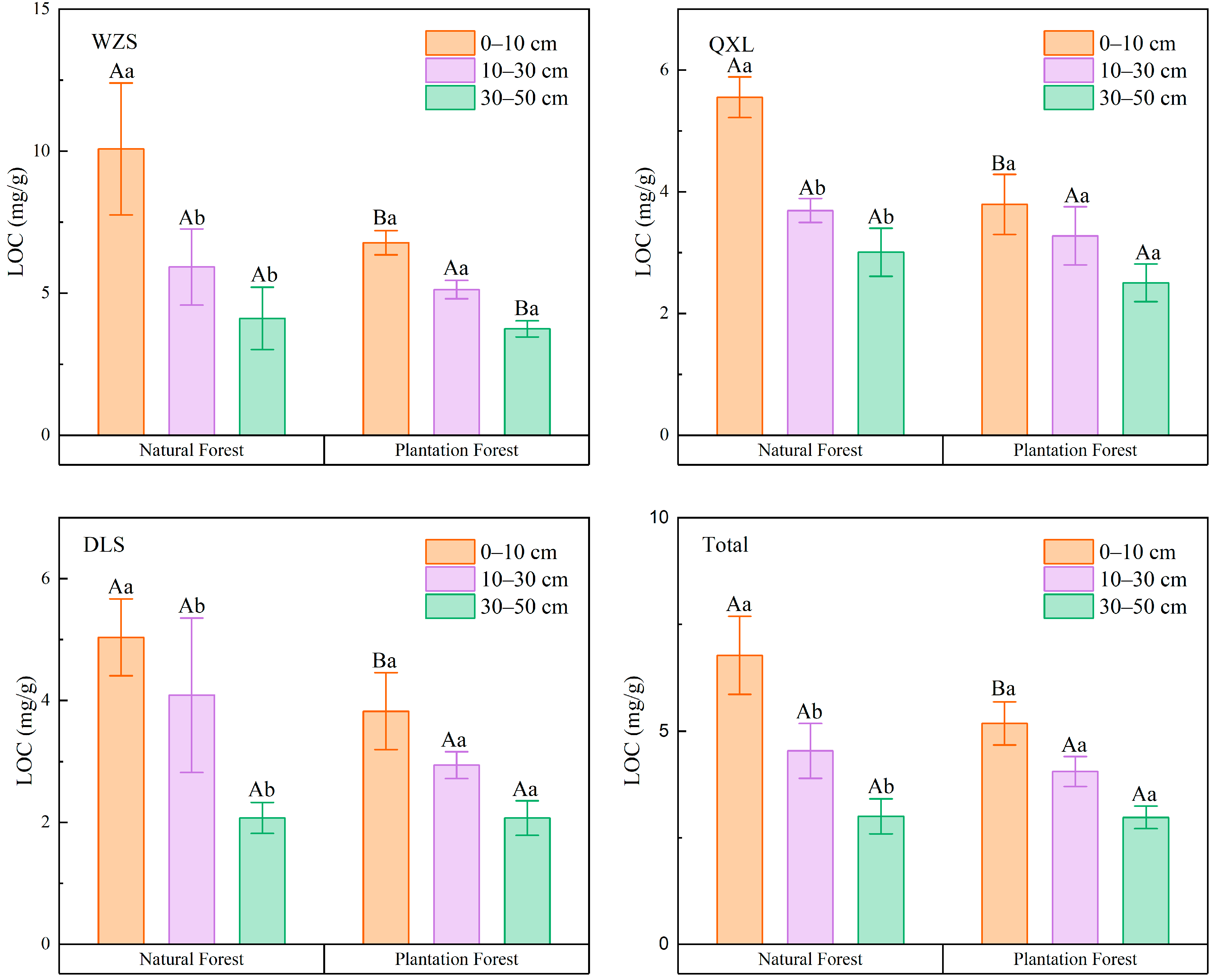


| Sampling Site | Forest Type | Mean ± SE(m) 1 (mg/g) | Minimum (mg/g) | Maximum (mg/g) | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| WZS | Plantation forest | 5.22 ± 0.36 Ab 2,3 | 2.76 | 8.14 | 28.93 |
| Natural forest | 7.64 ± 1.40 Aa | 2.25 | 21.67 | 73.30 | |
| QXL | Plantation forest | 3.19 ± 0.28 Bb | 1.79 | 5.27 | 30.09 |
| Natural forest | 4.08 ± 0.33 Ba | 1.93 | 6.70 | 31.86 | |
| DLS | Plantation forest | 2.95 ± 0.33 Bb | 1.78 | 4.83 | 33.22 |
| Natural forest | 3.73 ± 0.54 Ba | 1.38 | 10.26 | 61.39 | |
| Total | Plantation forest | 4.07 ± 0.26 b | 1.78 | 8.14 | 38.21 |
| Natural forest | 5.12 ± 0.99 a | 1.38 | 21.67 | 76.37 |
| Site | Forest Type | pH | Clay (%) | Silt (%) | Sand (%) | TOC (g/kg) |
|---|---|---|---|---|---|---|
| WZS | Plantation forest | 5.07 ± 0.02 Ca 1,2 | 0.94 ± 0.03 | 18.85 ± 0.26 | 80.59 ± 0.32 | 119.13 ± 2.65 Aa |
| Natural forest | 4.53 ± 0.02 Cb | 1.36 ± 0.04 | 21.79 ± 0.37 | 77.28 ± 0.37 | 100.71 ± 1.51 Aa | |
| QXL | Plantation forest | 5.93 ± 0.03 Aa | 0.77 ± 0.06 | 19.09 ± 0.40 | 80.84 ± 0.40 | 62.94 ± 0.77 Ba |
| Natural forest | 5.87 ± 0.04 Aa | 0.79 ± 0.03 | 17.85 ± 0.30 | 81.80 ± 0.32 | 59.33 ± 0.55 Aa | |
| DLS | Plantation forest | 5.60 ± 0.04 Ba | 0.75 ± 0.04 | 17.50 ± 0.58 | 82.08 ± 0.62 | 61.82 ± 1.17 Ba |
| Natural forest | 5.38 ± 0.03 Ba | 0.90 ± 0.03 | 18.13 ± 0.30 | 81.45 ± 0.32 | 48.14 ± 0.54 Ba | |
| Total | Plantation forest | 5.45 ± 0.01 a | 0.85 ± 0.01 | 18.77 ± 0.11 | 80.88 ± 0.12 | 88.61 ± 0.15 a |
| Natural forest | 5.22 ± 0.01 a | 1.00 ± 0.01 | 19.17 ± 0.09 | 80.28 ± 0.10 | 68.73 ± 0.55 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Dong, P.; Fu, B.; Wu, D.; Zhao, Z. Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China. Sustainability 2024, 16, 7836. https://doi.org/10.3390/su16177836
Zhao Z, Dong P, Fu B, Wu D, Zhao Z. Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China. Sustainability. 2024; 16(17):7836. https://doi.org/10.3390/su16177836
Chicago/Turabian StyleZhao, Zeyang, Peng Dong, Bo Fu, Dan Wu, and Zhizhong Zhao. 2024. "Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China" Sustainability 16, no. 17: 7836. https://doi.org/10.3390/su16177836
APA StyleZhao, Z., Dong, P., Fu, B., Wu, D., & Zhao, Z. (2024). Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China. Sustainability, 16(17), 7836. https://doi.org/10.3390/su16177836






