Chemical and Physical Characterization of Three Oxidic Lithological Materials for Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxidic Lithological Material
2.2. Chemical Analysis
3. Results and Discussion
3.1. Characterization of Raw Materials
3.2. Radioactivity Analysis
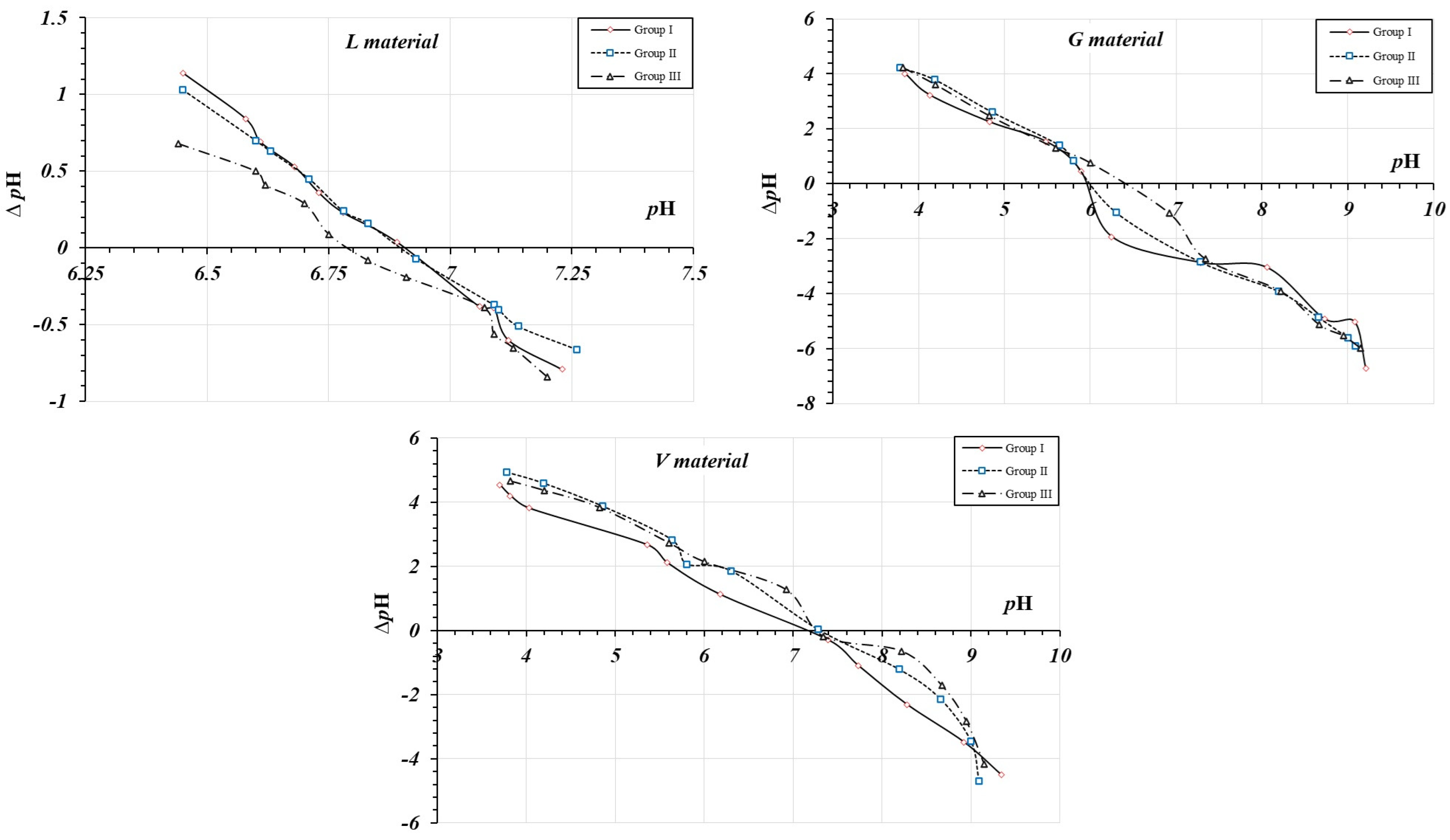
3.3. Point of Zero Charge—PCZ
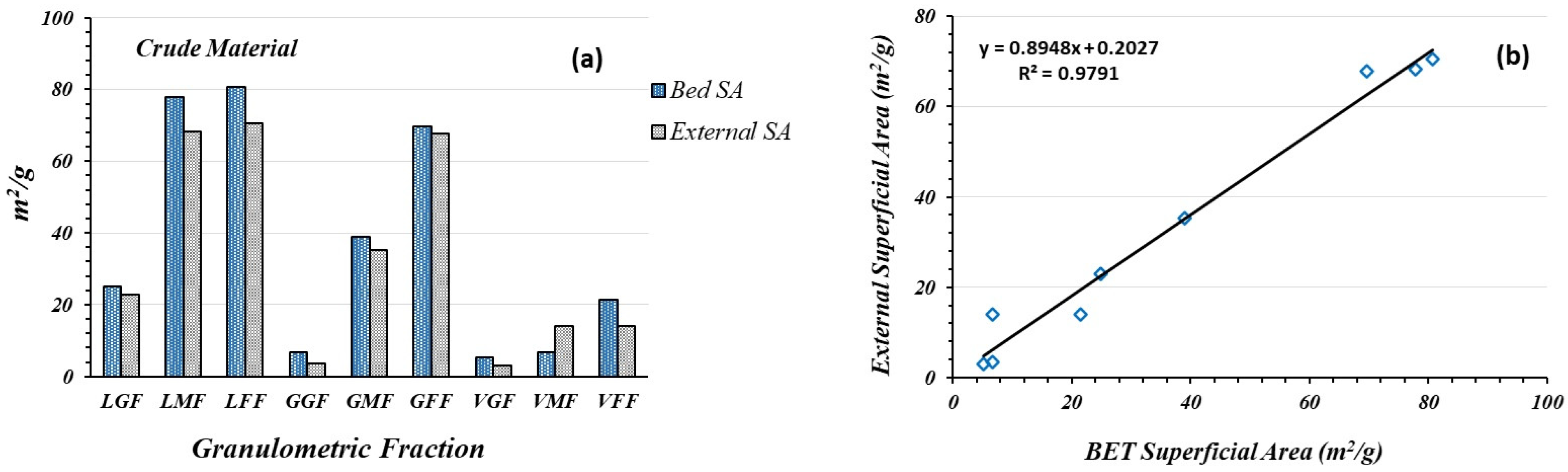
3.4. Specific Surface of Raw Granulometric Fractions
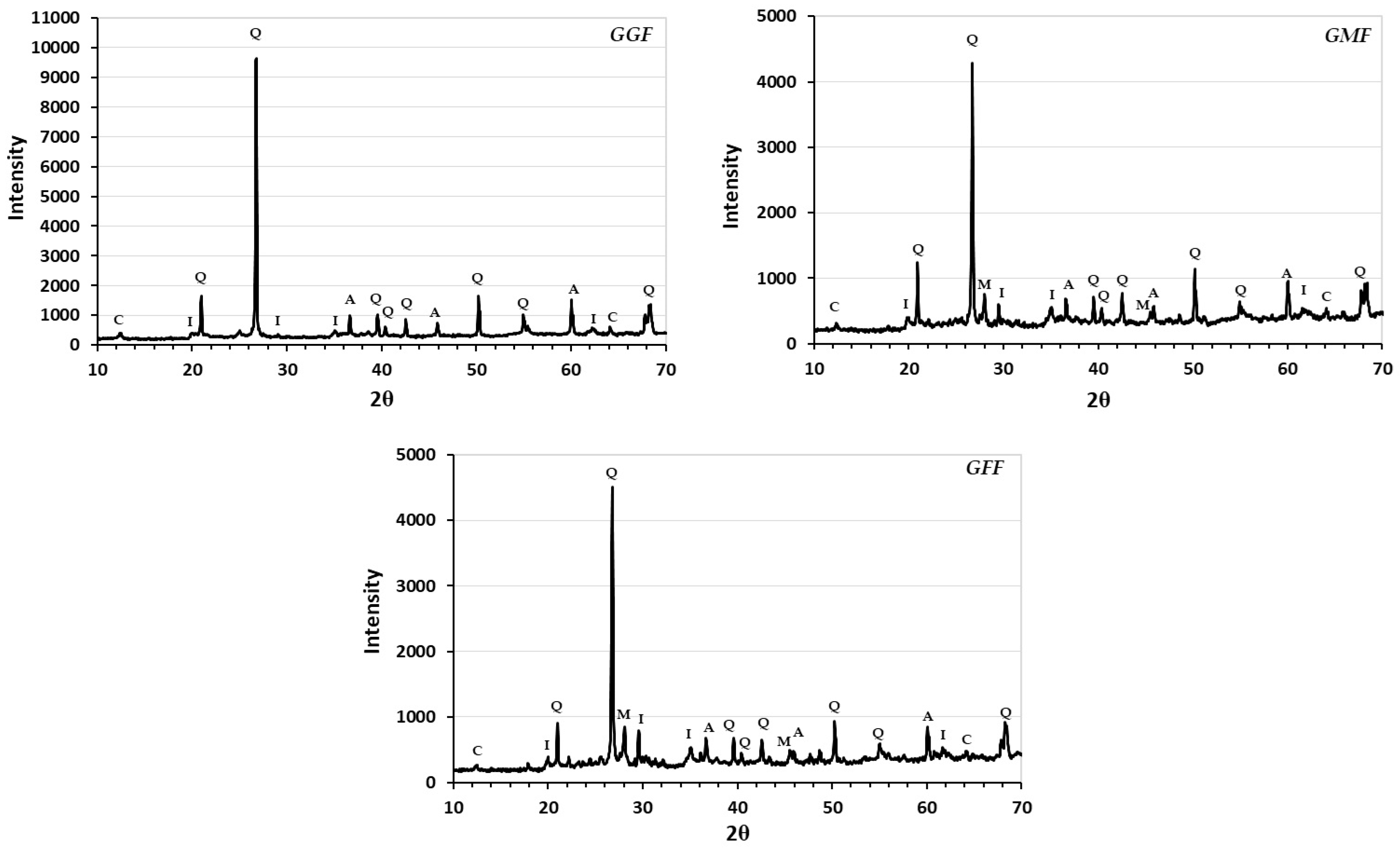
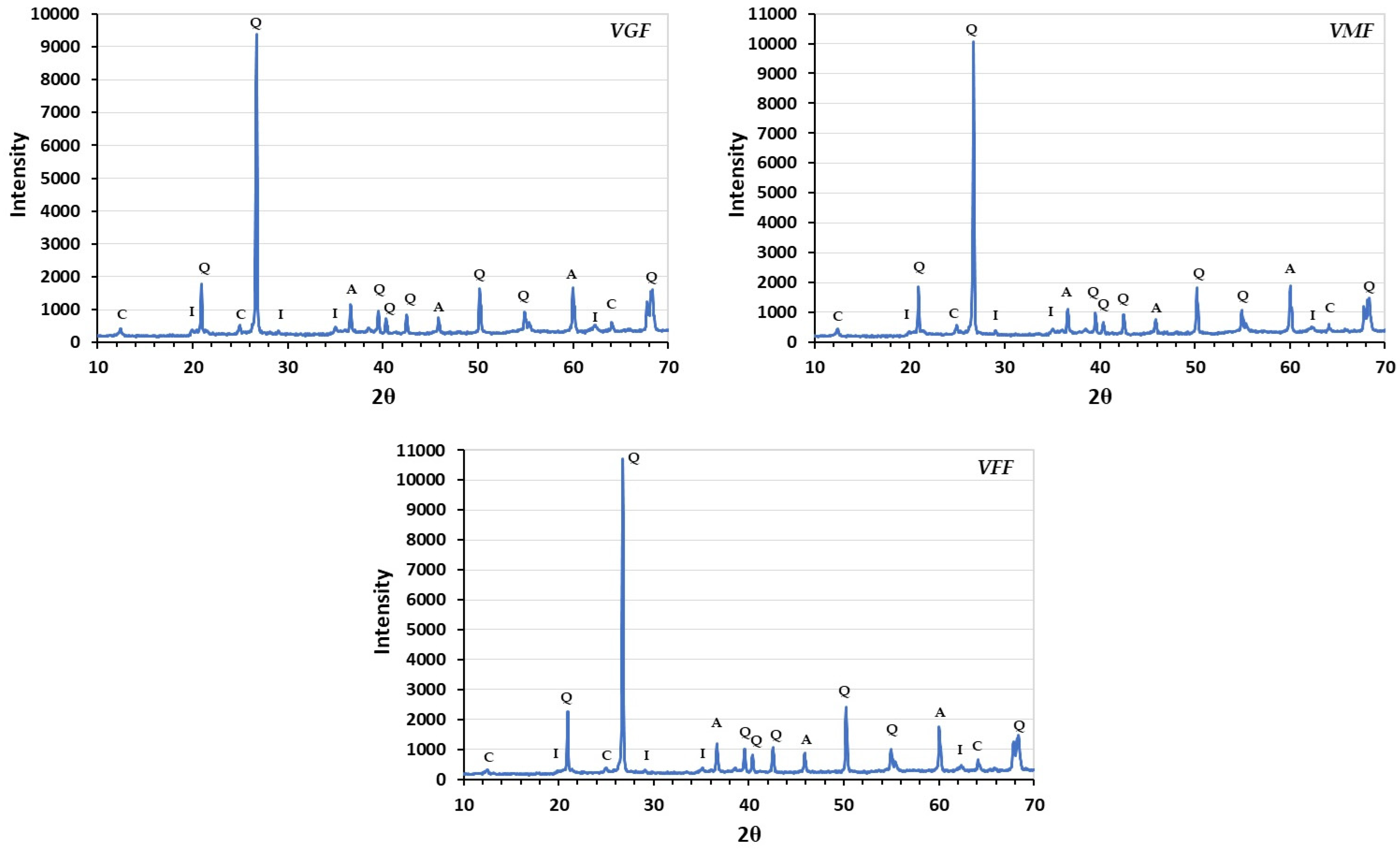
3.5. Mineralogy, XRD Analysis
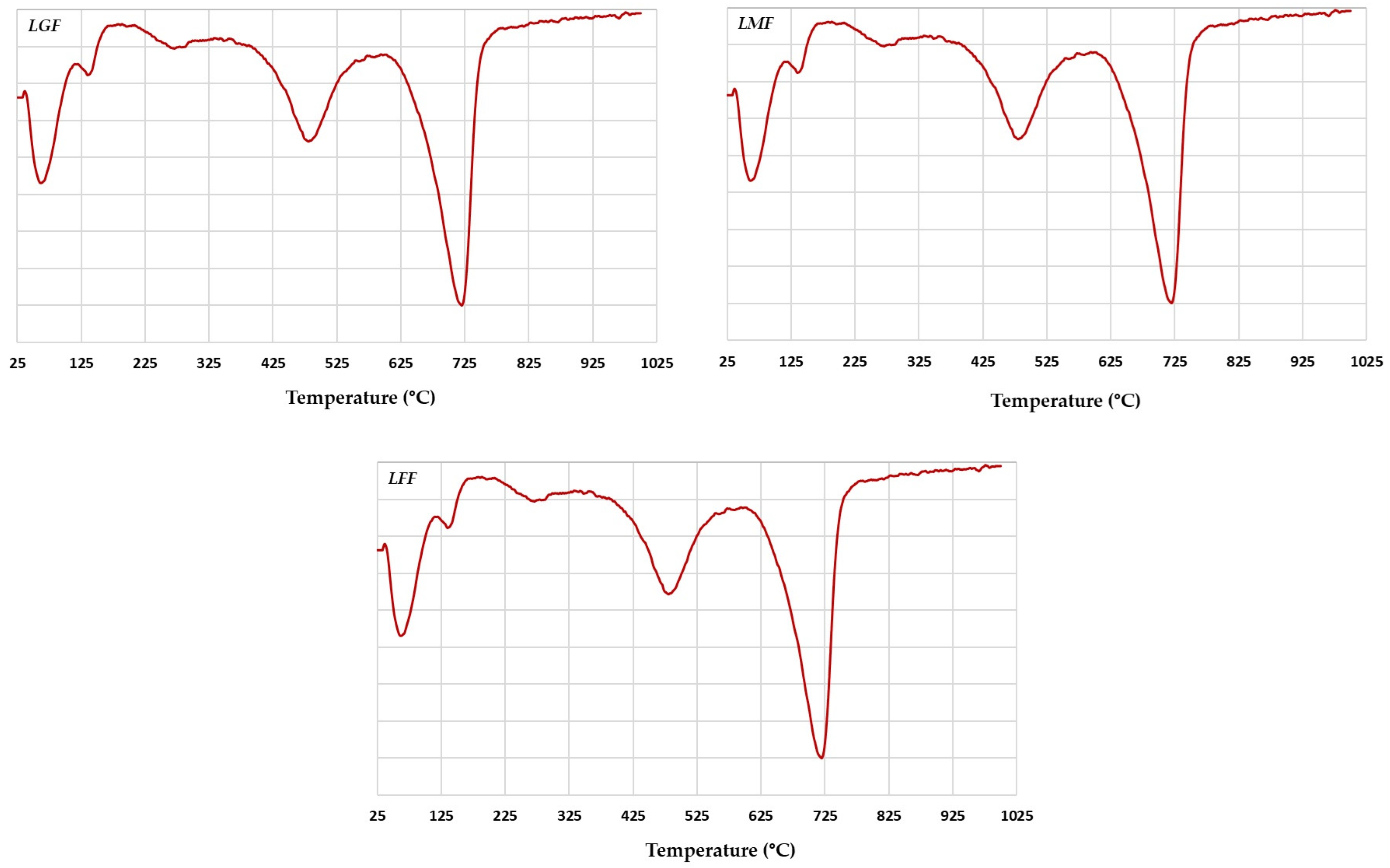
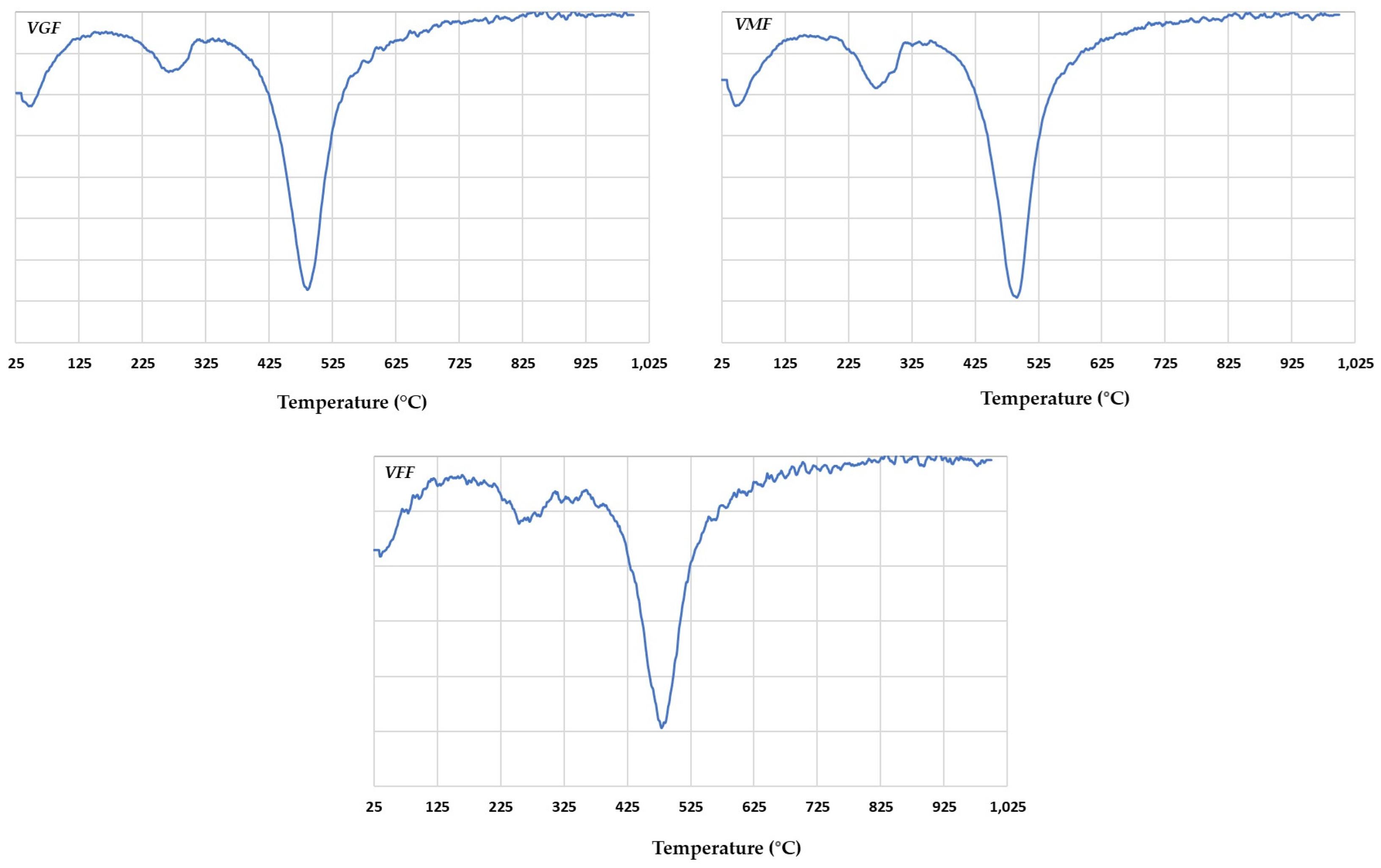
3.6. TDA Analysis
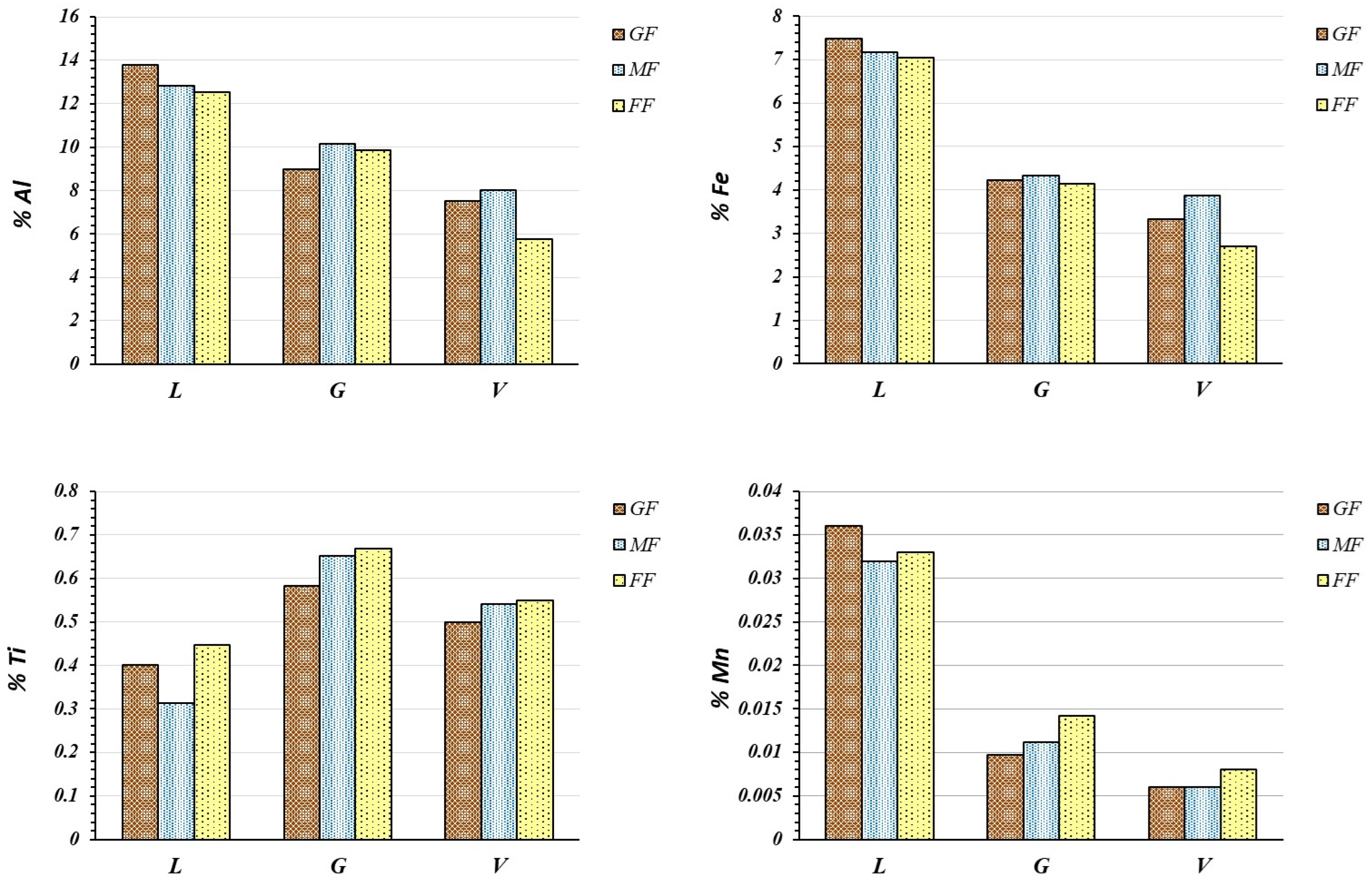
3.7. Metals Forming Amphoteric Oxides
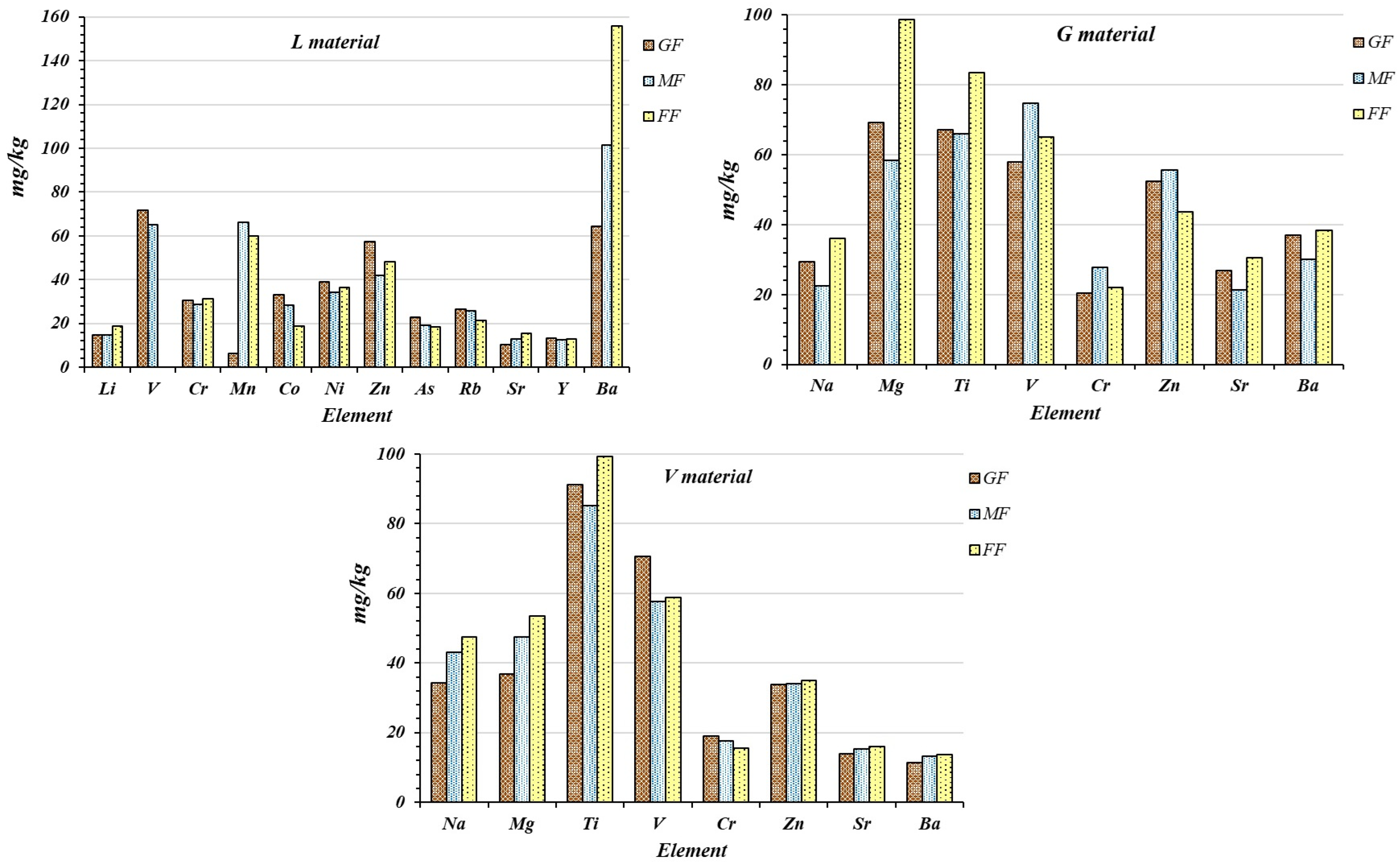
3.8. Other Metallic Species
3.9. IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, H.N. Adsorption Technology for Water and Wastewater Treatments. Water 2023, 15, 2857. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, G.; Zhang, Q.; Zhao, W. Synergistic Activation of Sulfidized Hemimorphite with Copper-lead Species for Improving Surface Hydrophobicity and Floatability. Sep. Purif. Technol. 2024, 332, 125854. [Google Scholar] [CrossRef]
- Melliti, E.; Van der Bruggen, B.; Elfil, H. Chemical Inhibition of Combined Gypsum and Iron Oxides Membrane Fouling during Reverse Osmosis Desalination Process: Prevention and Regeneration of Membranes. Desalination 2023, 551, 116414. [Google Scholar] [CrossRef]
- Tan, X.; Saha, P.; Klausner, J.; Abbasi, B.; Benard, A. Modeling and Experimental Validation of Direct Contact Crossflow Packed Beds Condenser used in HDH Desalination Systems. Desalination 2023, 551, 116414. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in Depressants for Flotation Separation of Cu-Fe Sulfide Minerals at Low Alkalinity: A critical Review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Nazari, M.T.; Schnorr, C.; Rigueto, C.V.T.; Alessandretti, A.; Melara, E.; da Silva, N.F.; Crestani, L.; Ferrari, V.; Vieillard, J.; Dotto, G.L.; et al. A Review of the Main Methods for Composite Adsorbents Characterization. Environ. Sci. Pollut. Res. 2022, 29, 88488–88506. [Google Scholar] [CrossRef]
- Prato, J.G.; Millán, F.; Rangel, M.; Márquez, A.; González, L.C.; Ríos, I.; García, C.; Rondón, C.; Wang, E. Adsorption of Pb (II) Ions on Variable Charge Oxidic Calcined Substrates with Chemically Modified Surface. F1000Research 2024, 12, 747. [Google Scholar] [CrossRef]
- Owens, P.R.; Libohova, Z. Soil Morphology. In Encyclopedia of Soils in the Environment, 2nd ed.; Goss, M.J., Oliver, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 149–170. [Google Scholar] [CrossRef]
- Foth, H.D. Fundamentals of Soil Science, 8th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Fassbender, H.W.; Bornemisza, E. Química de Suelos con Énfasis en Suelos de América Latina, 2nd ed.; Instituto Interamericano de Ciencias Agrícolas—IICA: San José, Costa Rica, 1994; pp. 7–156. Available online: https://repositorio.iica.int/handle/11324/6801 (accessed on 7 July 2024).
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil Constraints in an Arid Environment—Challenges, Prospects, and Implications. Agronomy 2023, 13, 220. [Google Scholar] [CrossRef]
- Prato, J.G.; Millán, F.; Ríos, A.; González-Ramírez, L.C. Uso de Materiales Litológicos Oxídicos para la Reducción de la Dureza en Aguas Naturales. Inf. Tecnol. 2022, 33, 145–156. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Tiwari, D.; Wang, H. Effect of Ionic Strength on Adsorption of As(III) and As(V) on variable charge soils. J. Environ. Sci. 2009, 21, 927–932. [Google Scholar] [CrossRef]
- Millán, F.; Prato, J.G.; González, L.C.; Márquez, A.; Djabayan, P. Cu (II) Chemisorption on Calcined Substrates made with an Oxidic Refractory Variable Charges Lithological Material. Rev. Tec. Ing. Univ. Zulia 2019, 42, 10–18. [Google Scholar] [CrossRef]
- Sadeghalvad, B.; Khorshidi, N.; Azadmehr, A.; Sillanpää, M. Sorption, Mechanism, and Behavior of Sulfate on Various Adsorbents: A Critical Review. Chemosphere 2021, 263, 12806. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.K.; Qafoku, N.P.; Van Ranst, E.; Li, J.Y.; Jiang, J. Adsorption Properties of Subtropical and Tropical Variable Soils: Implication from Climate Change and Biochard Amendment. Adv. Agron. 2016, 135, 1–58. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Xu, R.K.; Tiwari, D. Phosphate Adsorption at Variable Charge Soils/water Interfaces as Influenced by Ionic Strength. Aust. J. Soil Res. 2009, 47, 529–536. [Google Scholar] [CrossRef]
- Prato, J.G.; Millán, F.C.; González, L.C.; Ríos, A.C.; López, E.; Ríos, I.; Navas, S.; Márquez, A.; Carrero, J.C.; Díaz, J.I. Adsorption of Phosphate and Nitrate Ions on Oxidic Substrates Prepared with a Variable-Charge Lithological Material. Water 2022, 14, 2454. [Google Scholar] [CrossRef]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; pp. 406–407. [Google Scholar]
- Sposito, G. The Operational Definition of the Zero Point of Charge in soils. Soil Sci. Soc. Am. J. 1981, 45, 292–297. [Google Scholar] [CrossRef]
- Marcano-Martínez, E.; McBride, M.B. Comparison of the Titration and Ion Adsorption Methods for Surface Charge Measurement in Oxisols. Soil Sci. Soc. Am. J. 1989, 53, 1040–1045. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 551–656. [Google Scholar] [CrossRef]
- McKean, S.J. Manual de Análisis de Suelo y Tejido Vegetal; Centro Internacional de Agricultura Tropical: Bogotá, Colombia, 1993; pp. 95–99. [Google Scholar]
- Adams, M. Fundamentos de Química de Suelos; Consejo de Desarrollo Científico y Humanístico—Universidad Central de Venezuela: Caracas, Venezuela, 1995; pp. 230–257. [Google Scholar]
- Tan, K.H. Principles of Soil Chemistry, 4th ed.; CRC Press: New York, NY, USA, 2011; pp. 133–241. [Google Scholar]
- Freitas, J.M.; Netto, A.M.; Correa, M.M.; Xavier, B.T.; Assis, F.X. Potassium Adsorption in Soil Cultivated with Sugarcane. An. Acad. Bras. Cienc. 2018, 90, 541–555. [Google Scholar] [CrossRef]
- Renne, P.R.; Mundil, R.; Balco, G.; Min, K.; Ludwig, K.R. Joint Determination of 40K Decay Constants and 40Ar∗/40K for the Fish Canyon Sanidine Standard, and Improved Accuracy for 40Ar/39Ar Geochronology. Geochim. Cosmochim. Acta 2010, 74, 5349–5367. [Google Scholar] [CrossRef]
- Cinelli, G.; De Cort, M.; Tollefsen, T. (Eds.) European Atlas of Natural Radiation; Publication Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Macht, F.; Eusterhues, K.; Pronk, G.J.; Totsche, K.U. Specific Surface Area of Clay Minerals: Comparison between Atomic Force Microscopy Measurements and Bulk-gas (N2) and -Liquid (EGME) Adsorption Methods. Appl. Clay Sci. 2011, 53, 20–26. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, W. Rare Earth Elements (REEs) Recovery and Porous Silica Preparation from Kaolinite. Powder Technol. 2021, 391, 522–531. [Google Scholar] [CrossRef]
- Conconi, M.S.; Guana, M.R.; Serra, M.F.; Suarez, G.; Aglietti, E.F.; Rendtorff, N.M. Quantitative Firing Transformations of a Triaxial Ceramic by X-ray Diffraction Methods. Cerâmica 2014, 60, 524–531. [Google Scholar] [CrossRef]
- Dah-Traoré, Y.; Zerbo, L.; Seynou, M.; Ouedraogo, R. Mechanical, Microstructural and Mineralogical Analyses of Porous Clay Pots Elaborated with Rice Husks. JMMCE 2018, 6, 257–270. [Google Scholar] [CrossRef][Green Version]
- Allali, A.; Monsif, M.; Idrissi Kandri, N.; Zerouale, A. Caractérisation Physico-Chimique des Enduits Anciens et de Restauration de la Muraille (Al Moahades) Bab Chaâfa de la Médina de Salé: Etude Comparative. J. Mater. Environ. Sci. 2014, 5, 2205–2211. [Google Scholar]
- Sedmale, G.; Randers, M.; Grase, L.; Kostjukovs, J. Use of Differential Treatment of Illite to Modify Their Structure and Properties. Mater. Sci. Appl. Chem. 2015, 32, 19–22. [Google Scholar] [CrossRef][Green Version]
- Burduhos Nergis, D.D.; Abdullah, M.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef]
- Manoharan, C.; Sutharsan, P.; Dhanapandian, S.; Venkatachalapathy, R. Characteristics of Some Clay Materials from Tamilnadu, India, and their Possible Ceramic Uses. Cerâmica 2012, 58, 412–418. [Google Scholar] [CrossRef]
- Damian, G.; Damian, F.; Szakács, Z.; Iepure, G.; Aştefanei, D. Mineralogical and Physico-Chemical Characterization of the Oraşu-Nou (Romania) Bentonite Resources. Minerals 2021, 11, 938. [Google Scholar] [CrossRef]
- Kirsten, M.; Mikutta, R.; Vogel, C.; Thompson, A.; Mueller, C.W.; Kimaro, D.N.; Bergsma, H.L.T.; Feger, K.H.; Kalbitz, K. Iron Oxides and Aluminous Clays Selectively Control Soil Carbon Storage and Stability in the Humid Tropics. Sci. Rep. 2021, 11, 5076. [Google Scholar] [CrossRef]
- Kgabi, D.P.; Ambushe, A.A. Characterization of South African Bentonite and Kaolin Clays. Sustainability 2023, 15, 12679. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation and Analysis, 2nd ed.; CRC Press: New York, NY, USA, 2005; pp. 198–470. [Google Scholar] [CrossRef]
- Húlan, T.; Trník, A.; Medved, I. Kinetics of Thermal Expansion of Illite-based Ceramics in the Dehydroxylation Region during Heating. J. Therm. Anal. Calorim. 2017, 127, 291–298. [Google Scholar] [CrossRef]
- Qafoku, N.P.; Van Ranst, E.; Noble, A.; Baert, G. Variable Charge Coils: Their Mineralogy, Chemistry and Management. Adv. Agron. 2004, 84, 159–214. [Google Scholar] [CrossRef]
- Schindler, P.W.; Stumm, W. The Surface Chemistry of Oxides, Hydroxides and Oxide Minerals. In Environmental Science and Technology. Aquatic Surface Chemistry. Chemical Processes at the Particle-Water Interface, 1st ed.; Stumm, W., Ed.; Wiley: New York, NY, USA, 1987; pp. 83–110. [Google Scholar]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, O.; Hu, Y.; He, Z. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Macarovici, G.; Macarovici, D. Chimia Oxizilor Dubli si Utilizarea, 1st ed.; Editura Academiei Socialiste Romania: Bucuresti, Romania, 1975; pp. 100–194. [Google Scholar]
- Millán, F.; Prato, J.G.; Montilla, T.; Tănăselia, C. Using of Variable Charge Adsorption Beds for Filtration of Residual Waters. Rev. Tec. Ing. Univ. Zulia 2018, 41, 98–110. [Google Scholar]
- González Martínez, S.P. Caracterización de la Fracción Arcilla en algunos Suelos Derivados de Cenizas Volcánicas de Costa Rica. Tesis de Grado de Magister Scientiae, Instituto Interamericano de Ciencias Agrícolas de la OEA, San José, Costa Rica, 1972. [Google Scholar]
- Fagundo, J.R.; Valdés, J.J.; Pajón, J.M. Determinación del Contenido de los Minerales Cuarzo y Caolinita en Sedimentos Mediante Espectroscopia Infrarroja. Rev. Cienc. Químicas 1984, 15, 327–333. [Google Scholar]








| Material | Color * | Texture (%) | dap (g/mL) | Organic Matter (%) | pH | C.E.C cmol (+)/kg | EC (dS/m) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||
| L | 10R 3/6 | 54 | 44 | 2 | 1.18 | 0.40 | 7.66 | 13.40 | 0.18 |
| G | 10YR 8/1 | 74 | 24 | 2 | 1.28 | 0.35 | 6.57 | 6.76 | 0.23 |
| V | 10YR 8/2 | 45 | 33 | 22 | 1.29 | 0.25 | 4.63 | 13.77 | 0.07 |
| Sample | Mass (g) | U | Th | Ra | K | I(r) |
|---|---|---|---|---|---|---|
| L | 11.33 | 73.1 | 47.2 | 71.5 | 878 | 0.79 |
| G | 12.00 | 45.7 | 55.8 | 63.4 | 308 | 0.59 |
| V | 12.53 | 87.1 | 56.2 | 83.3 | 253 | 0.63 |
| Quartz | (1100–800) cm−1 Si–O valence vibration G: 1101.87, 1033.42, 1008.12, and 913 cm−1 V: 1007.43, 1033.42, and 1104 cm−1 (800–600) cm−1 Si–Si valence vibration G: 798.01, 779.08, and 694.70 cm−1 V: 798.41, 779.44, and 694.81 cm−1 (460–430) cm−1 Si–O–Si distortion G: 431.00 and 470.12 cm−1 V 430.68 and 469.75 cm−1 |
| Kaolinite | (3600–3700) cm−1 O–Al–OH and O–Si–OH stretching G: 3697.78, 3625.06, and 3622.35 cm−1 V: 3698.02, 3653.25, and 3622.83 cm−1 (1120–1003) cm−1 Si–O valence vibration G: 1008.12, 1033.42, and 1101.87 cm−1 V: 1007.43, 1033.42, and 1104.47 cm−1 (936–818) cm−1 M–O–H distortion G: 913 cm−1 V: 913.21 cm−1 (587–430) Si–O–M and M–O–H vibration G: 431, 470.12, and 536.68 cm−1 V: 430.68, 469.75, and 536.70 cm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prato, J.G.; Millán, F.; Senila, M.; Levei, E.A.; Tănăselia, C.; González, L.C.; Ríos, A.C.; Sagñay Yasaca, L.; Dávalos, G.E. Chemical and Physical Characterization of Three Oxidic Lithological Materials for Water Treatment. Sustainability 2024, 16, 7902. https://doi.org/10.3390/su16187902
Prato JG, Millán F, Senila M, Levei EA, Tănăselia C, González LC, Ríos AC, Sagñay Yasaca L, Dávalos GE. Chemical and Physical Characterization of Three Oxidic Lithological Materials for Water Treatment. Sustainability. 2024; 16(18):7902. https://doi.org/10.3390/su16187902
Chicago/Turabian StylePrato, José G., Fernando Millán, Marin Senila, Erika Andrea Levei, Claudiu Tănăselia, Luisa Carolina González, Anita Cecilia Ríos, Luis Sagñay Yasaca, and Guillermo Eduardo Dávalos. 2024. "Chemical and Physical Characterization of Three Oxidic Lithological Materials for Water Treatment" Sustainability 16, no. 18: 7902. https://doi.org/10.3390/su16187902
APA StylePrato, J. G., Millán, F., Senila, M., Levei, E. A., Tănăselia, C., González, L. C., Ríos, A. C., Sagñay Yasaca, L., & Dávalos, G. E. (2024). Chemical and Physical Characterization of Three Oxidic Lithological Materials for Water Treatment. Sustainability, 16(18), 7902. https://doi.org/10.3390/su16187902








