Abstract
The growth of forest trees in the relatively young soils of Europe has historically been limited by nitrogen (N). The high anthropogenic N deposition and intense forest management during the last century, however, have caused forest trees in large areas to show signs of being limited by phosphorus (P) or base cations. This indicates that the current situation is not sustainable. The net addition of N to forests here stems from deposition and N fixation, both processes that add N to the topsoil. Phosphorus and cations are released by weathering in the mineral soil. Do European trees have what it takes to efficiently take up P and cation nutrients when they become scarce? Important factors influencing their capacity to take up P and cations are their roots’ distribution and function, mycorrhizal distribution and type, and the response of their root and mycorrhizal growth and function to N depositions and nutrient deficiencies. The literature shows that the ability to be limited by something other than nitrogen will be hardest for shallow-rooted conifer trees, followed by ectomycorrhizal deciduous trees; arbuscular mycorrhizal trees will handle the shift best. This knowledge should be incorporated into forest growth models to promote sustainable management decisions.
1. Background
Forest management is required in most countries to be sustainable, meaning that the next generation of trees should have the same resource availability and, thereby, production as the current forest. Forest sustainability is affected by management as well as abiotic and biotic factors. One factor important for maintaining the growth and health of the forest is a balanced nutrient supply for the trees. Nutrients are added to the available pool, via mainly deposition, decomposition and weathering, and removed via leaching, harvesting and erosion. The input and output of nutrients must be in balance for the forest’s growth to be sustainable [1]. There are indications that this is not the case in most areas in Europe [2]. European forests grow on a range of soils, from deep sediment soils to shallow poor soils. At higher altitudes, and from central Germany further north, the last Ice Age ended less than 10,000 years ago [3]. This reset the soil-forming process, creating relatively young soils [4]. In pre-industrial times, most soils in Europe were N-limited [5]. In particular, the soils that had been reset during the last Ice Age had not yet reached their terminal P-limited stage [6]. In recent decades, however, the nutrient status of these trees has changed. This indicates that other nutrients are starting to limit forest growth. High N concentrations in foliage are more frequent at sites where the critical load of N is exceeded [7,8,9,10,11]. The N:P ratios of tree foliage in Europe increased by 3 to 8% between 1990 and 2000, as the P content in the foliage decreased more than the N content did [12]. This trend has then continued, as the fraction of stands with a P limitation increased from 25% in 1995 to 33% in 2017 [11]. The N:P ratios suggest that more stands of Fagus sylvatica, Quercus petraea and Quercus robur are P-limited than conifer stands (Picea abies and Pinus sylvestris) [12]. The fewer N-limited conifer stands can partly be explained by their more northern distribution (where nitrogen deposition is lower). Binkley and Högberg [5] stated that most Swedish forests are still strongly N-limited; an exception is more southern areas, which show signs of P limitation [5,13,14,15]. The frequent occurrence of P limitation in central European forests has been confirmed by growth responses in several fertilization experiments [12] and by examining changes in foliage nutrient concentrations after fertilization [16]. The shift to P limitation reflects a higher demand for P than the supply. This interpretation is supported by the negative trend in foliar P concentrations being the strongest in stands with an already low P status [12,17,18]. Moreover, the N:K ratio has increased in Quercus petraea and Picea abies [12], as well as in Fagus sylvatica, in Switzerland [18], indicating that nutrients other than N and P can limit growth. Many studies have reported K limitations [19], but in Europe these are mainly on calcareous bedrock and on peatlands [20,21]. In Fagus sylvatica, the Ca and Mg concentrations of the foliage have also decreased, and about 1/3 of the stands had foliage concentrations indicating a Ca and/or Mg deficiency [17].
The high input of N relative to P globally has shifted the N:P ratio in air, water and organisms [22]; despite decreasing N deposition in Europe, the N:P ratio is not responding because large N pools have been built up [23]. The nutrient status of trees reflects the amounts of nutrients available in the soil. Sites in which the critical N load is exceeded have more NO3− in their mineral soil compared to sites with less N deposition [7,8], pointing to high N availability. In Europe, the foliar P concentration of Fagus sylvatica is negatively correlated to the N:P ratio in the soil and N deposition [17,18]. A negative correlation with N deposition was non-existent in Fagus sylvatica in Switzerland in the mid-1980s, when P concentrations were still above the lower thresholds for normal nutrition [24], but the correlation was strong in 2015 [18]. Even foliar N was negatively correlated with N deposition in 2015, probably because the lower P concentration drags the N concentration down (as there is a limit to how wide the N:P ratio can be) [18]. Moreover, in Germany—the area with the highest N deposition in Europe—the base saturation has been found to be lower in plots where the critical N load is exceeded [7,25]. This is in line with the fact that Mg deficiency is more common at sites where the critical load for N in the soil solution is exceeded [8,18]. In southern Sweden, base saturation has decreased at least since 1949 [26,27,28,29], but the decrease has now stabilized and the most acidified soils have somewhat recovered, although most soils there still have a low base saturation [30,31]. In Central Europe, the decrease in base saturation during the second half of the 20th century can be linked to the leaching of base cations caused by acid deposition, which peaked in the 1970s [32]. Since the 1970s, acid deposition has decreased by 70–90% [33]. This is evident in the monitoring sites of the International Co-operative Program on Assessment and Monitoring of Air Pollution Effects on Forests (ICP-forests), where base saturation has typically declined by more than 20% from 1995 to 2012. Conversely, values have increased in those soils that had a base saturation below 20% in 1995 [30,34]. Until 2020, the base cation/Al3+ ratio was still decreasing in Switzerland [25]. The decrease in base cations is also an effect of the increased production in forests during this time due to N deposition, increased temperatures and more efficient management techniques [35,36,37,38]. Harvesting, especially the harvesting of slash and even stumps for biofuel, removes large amounts of nutrients from the system instead of returning them to the soil via decomposition [39,40,41]. Removing nutrients faster than they weather, without fertilizing, is not sustainable.
Thus, nitrogen deposition and acid deposition, in combination with an increased growth rate and nutrient removal via harvest, have shifted the limitation from clearly N-limited to often P- or base cation-limited. The lower availability of base cations and P in the soil, combined with an increase in their demand, will force trees to change their nutrient uptake strategy. They now need to focus on P or base cations instead of N.
Do the dominant tree species in Europe have the ability to efficiently take up enough of these new limiting nutrients? Where are the nutrients, what strategies do the trees need to take them up and what is the trees’ response to different nutrient limitations and their direct response to high N depositions? These key questions must be answered to better model the nutrient budgets and growth of European forests and to develop management methods that are sustainable from a nutrient perspective. In this paper I gone through the literature trying to find answers.
2. The Spatial Distribution of Available Nutrients
Nutrient uptake is a combination of available nutrients, the presence of roots and mycorrhiza and their ability to take up specific nutrients. Trees have most of their roots in the upper layer of the soil, and root density decreases with increasing root depth [42,43,44]. The reason why most roots are in the top layers is because most nutrients are mobilized by the decomposition of organic matter added to the topsoil [45]. Importantly, trees grow not solely by mineralizing the organic material they themselves produce. In the long term, nutrient limitation is determined by the net input to the tree’s nutrient cycling [46]. The net input of N comes from deposition and fixation, which mainly occur in the top layer. The weathering of N-rich minerals and N fixation through symbiosis can also contribute to a net N addition in deeper soil layers, although these processes are slow in European forests [47]. In contrast, the net input of P and base cations stem mainly from weathering, which is most prevalent in the top layer [48]. Nonetheless, the amount of easily weatherable material increases with soil depth, explaining why the realized weathering increases down to about a 50 cm soil depth [49,50]. In even deeper soil layers, a low weathering capacity prevents an increase in weathering (pers comm. Cecilia Axelsson). However, even if the weathering rate in deep soils is slow, large amounts of available or potentially available nutrients (e.g., CaPO4) can be present in deep soil layers due to long-term accumulation and low uptake [43]. When N limitation changes to a base cation or P limitation, then the main net input of the limiting resource shifts from the topsoil to deeper layers. This makes it even more important for trees to have efficient nutrient uptake in deep soil layers.
3. Root and Mycorrhiza’s Distribution and Growth
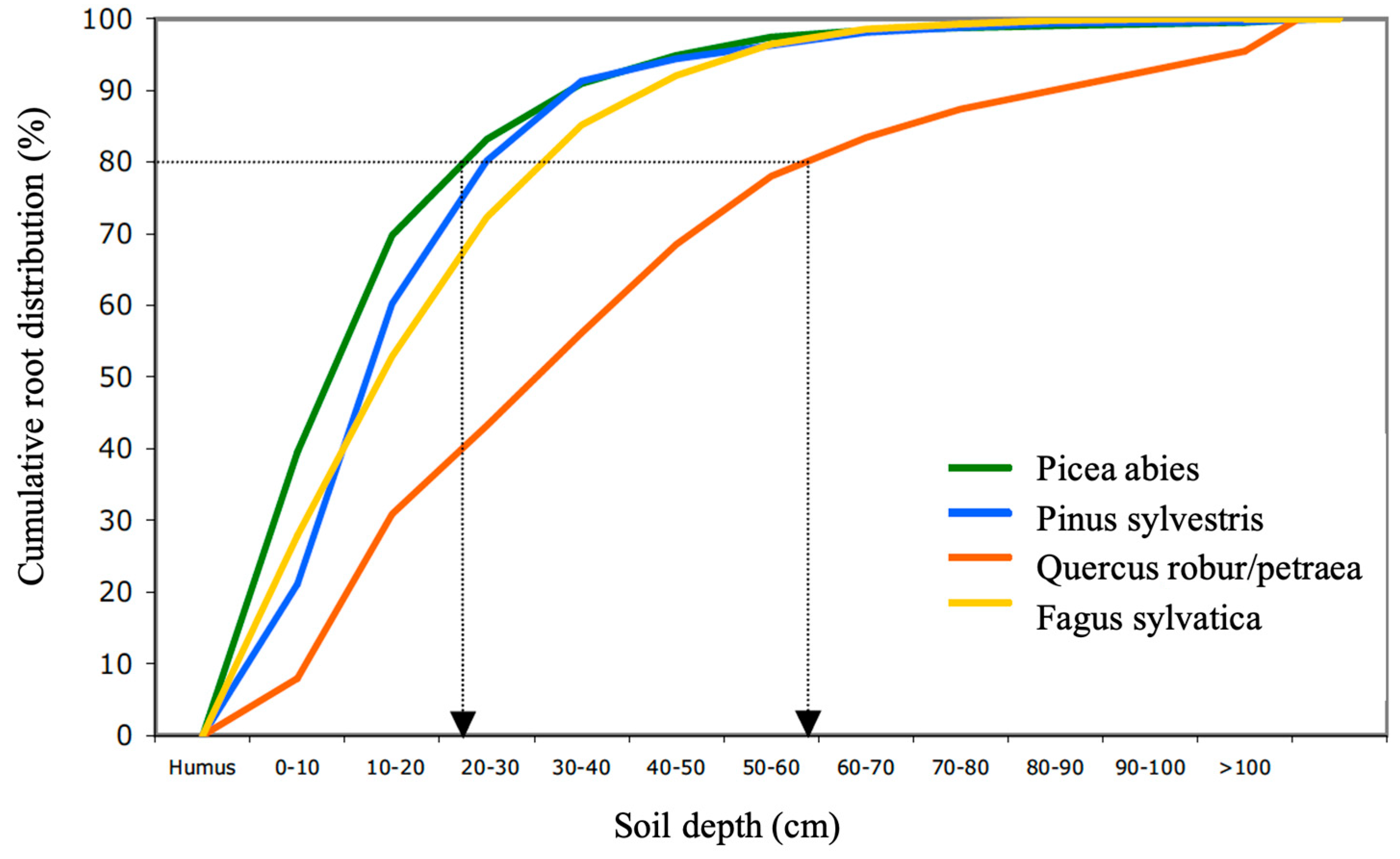
Different tree species have different root architectures and different amounts of mycorrhiza mycelia. This makes the soil volume from which they can take up nutrients (their uptake zone) different [42,43,44,51,52,53]. Picea abies has the shallowest root system of the dominant tree species in Europe. On average, 80% of its fine root biomass is located in the humus layer and the top 25 cm of the mineral soil. Quercus robur/petraea, in turn, which exhibits deep root systems, has 80% of its roots in the top 60 cm of mineral soil. Fagus sylvatica and Pinus sylvestris take a middle position, with 80% of their roots at a 25–35 cm depth (Figure 1) [51]. The distribution of ectomycorrhiza (EM) follows the root distribution in most stands [52,54,55]. The same seems to be true for arbuscular mycorrhiza (AM) [56,57]. Picea abies, Pinus sylvestris and Fagus sylvatica together cover 59% of the forests in Europe [58]. Accordingly, approximately 80% of the fine roots and mycorrhiza in most forests are located in the top 30 cm of soil. Their rooting depth may, however, vary depending on the soil type, moisture, nutrient availability, pH, stoniness and other factors [42,44]. For example, the maximum rooting depth of Picea abies may vary between 150 cm and 25 cm, depending on site conditions [59].
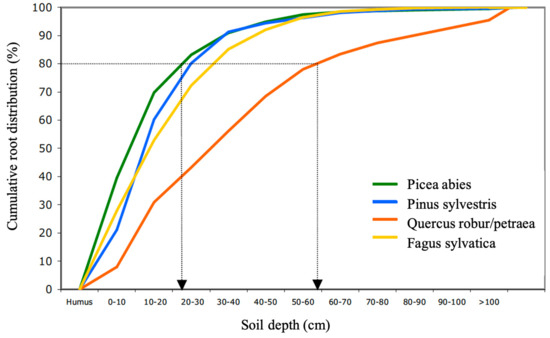
Figure 1.
Mean cumulative root distribution over soil depth for Picea abies, Pinus sylvestris, Fagus sylvatica and Quercus robur/Quercus petraea (merged).
The distribution of roots and mycorrhiza in the soil is also affected by nitrogen deposition and shifts in limiting nutrients. A shortage of N, P and S stimulates root growth and thereby increases the uptake zone [60,61]. In contrast, a deficiency of K, Mg or Mn slows root growth [60,61,62]. Mycorrhiza has been found to respond similarly by increasing the growth of mycelia under N and P deficiencies, but not under K, Ca or Mg deficiencies [63]. Accordingly, a tree limited by base cations does not necessarily respond by producing more roots and mycorrhiza to overcome the limitation. More likely, the opposite is true: the nutrient balance will not be restored because the resource allocation in the tree will increase the uptake of the limiting nutrient. The multiple limitation hypothesis suggests that resources should be allocated to mitigate nutrient limitation [64]. This might be valid for N and P limitations, but not for K, Ca and Mg limitations.
A high N availability would lead to lower allocation to the roots; at the same time, an increasing P limitation would lead to a higher allocation. This would represent two contradicting responses. However, a meta-analysis revealed that increasing the amount and duration of N deposition increases the total root biomass but decreases fine root biomass and increases fine root turnover [65,66,67]. Accordingly, this response is less a question of allocation than of a change in morphology. Fine root biomass decreases with the duration of N deposition, and the decrease is faster at higher N loads [66]. Thus, even at very high N loads that would lead to a P limitation, there is no increase in fine root length—the common response to low P availability [68]. The high root turnover during high N availability could, however, increase the P uptake because new root production enables new soil volumes to be harvested for P [69]. Nonetheless, in general, high N deposition does not induce more fine roots, as expected with a P limitation.
Importantly, the root response to N deposition differs between trees associated with AM and EM [67]. The general trend (decreased fine roots) was valid for EM trees but not for AM trees, which rather tended to increase their amount of fine roots with high N deposition. In response to N-deposition acid phosphatase exudation also increased seven times more in AM than EM trees [67].
In both AM and EM trees, external mycelia and colonization decrease with increasing N deposition. The community composition also changes [67,70,71,72,73]. This might reflect less carbon allocation to the mycorrhiza, but also a shift in the ectomycorrhizal community to shorter exploration types [70,73]. Moreover, EM does not increase its P mobilization when subject to high N deposition in conifer forests. The critical N load to affect the mycorrhiza differs between studies, but conifer stands (EM) generally have a lower critical load than deciduous trees associated with EM [70]. The available literature is insufficient to determine the critical loads for AM [73], but Ma et al. (2021) [67] reported an even larger decrease in AM than EM abundance with high N depositions. Even so, in North America, an increasing N deposition was associated with a higher relative abundance of AM trees, higher growth rates and higher recruitment [74]. Furthermore, the basal area, growth and recruitment curves of EM and AM trees cross. The combination of crossing temporal demographic responses and N deposition means that lengthier periods with high N deposition rates will transform EM forests to AM forests [74]. A better root growth response and P mobilization response to N deposition (rather than a more N deposition-resistant AM) could partly explain the success of AM versus EM trees under high N depositions.
4. Root and Mycorrhiza Uptake Efficiency
The presence of root or mycorrhiza hyphae at a certain point in the soil does not necessarily mean that they can take up the available nutrients there. In tropical areas, the correlation between root depth and nutrient uptake is good [75,76]. In Europe, the uptake from deep soil layers has, however, been greater than expected based on the trees’ root distribution [77,78]. This relatively higher uptake is due to the overlapping of the uptake cylinders around the roots and mycorrhiza, from which nutrients can be taken up [79]. Based on root distribution and diffusion coefficients, the uptake of K from a 50 cm soil depth was 78% of that at 5 cm, even though the root length at 50 cm was only 23% of that at 5 cm [78]. For phosphorus, which is less mobile, the overlap of the uptake cylinders around the roots and mycorrhiza is smaller [79]. Its corresponding uptake from a 50 cm soil depth (versus 5 cm) was estimated to be only 25% [78]. In a field study, Göransson [54] used analogs and isotopes to determine that the contribution to the total uptake from a 50 cm versus 15 cm soil depth in oak was 50% for Cs (as an analog for K) and 25% for 15N, but only 4% for radioactive P (not compensating for dilution and discrimination). Brandtberg, Bengtsson and Lundkvist [77] injected radioactive Ca and P under Norway spruces at 2 and 35 cm depths, and found the relative contribution from the 35 cm soil depth for Ca was 14%, while it was only 7% for P (not compensating for dilution). As the distribution of the external mycorrhizal mycelia correlates with root density [54,80], the overlap should be even higher. This is because, based on length, the mycorrhiza mycelia in the soil by far exceed the root length [52,81].
The overlap of the uptake cylinders around the roots and hypha enables mobile nutrients such as K and NO3− to be taken up even at low root densities. Therefore, the uptake zone for mobile ions may perhaps be set down to the soil depth that encompasses 95% of the roots. For P, which is very immobile, the relative fine root biomass at different soil depths may be a better estimate.
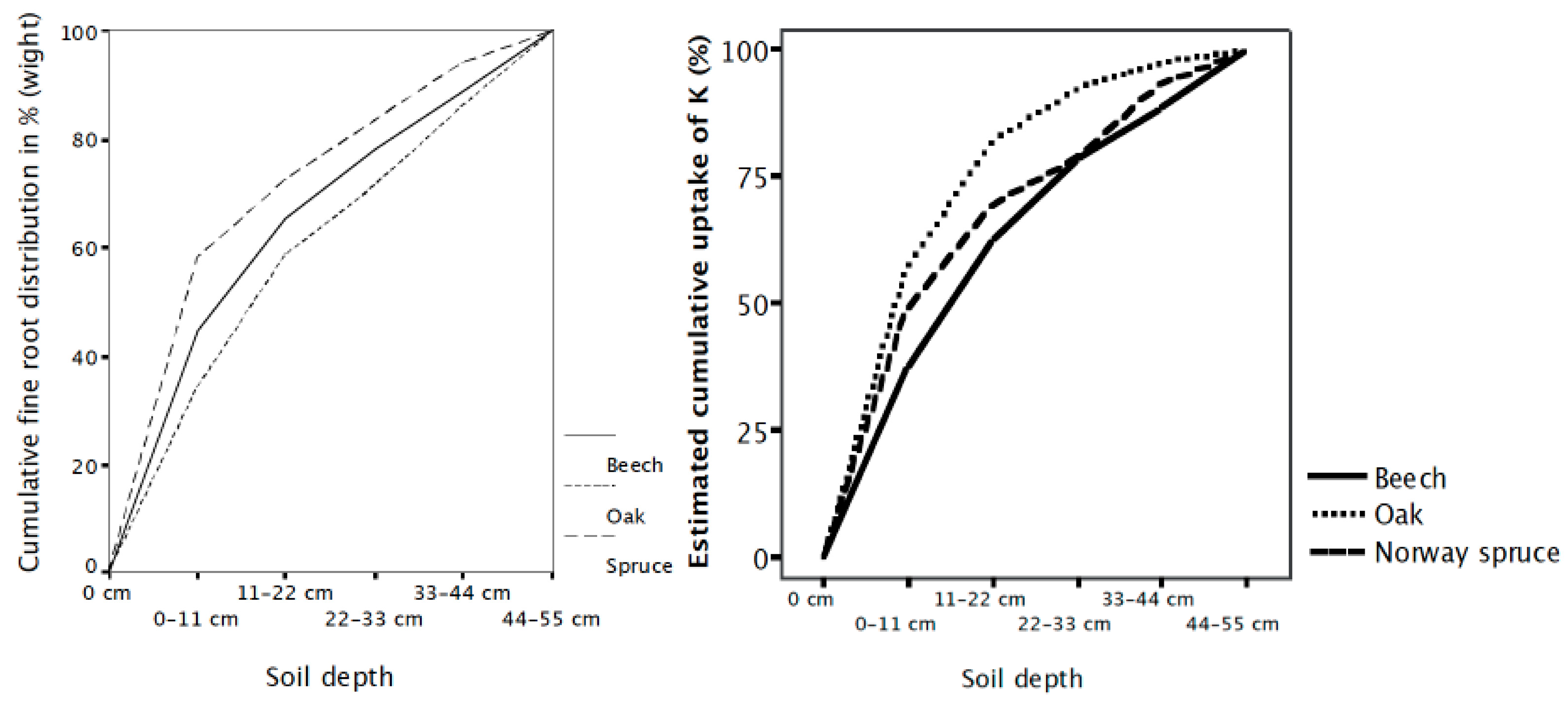
Roots at different depths may also differ in their capacity to take up nutrients [55,82,83]. This capacity can especially influence the uptake of mobile nutrients transported to the roots by mass flow. The uptake capacity of Quercus robus roots decreased with increasing soil depth for K (measured as 86Rb) and tended to do the same for NH4 but not for H2PO4− [83]. Göransson [54] estimated the cumulative uptake capacity of K with increasing soil depth for Quercus robur, Fagus sylvatica and Picea abies, taking both root distribution and root nutrient uptake capacity into account. The results showed that Quercus robur, which has the deepest root system, had the shallowest uptake of K due to the low uptake capacity of K by the oak roots in deep soil layers (Figure 2) [54]. This low capacity of Q. robur was confirmed by injecting Cs, an analog to K, at different depths in the same stand [78]. A possible explanation is that physiological differences in roots at different soil depths have developed during evolution. Quercus robur is one of the most deep-rooted species, explaining why it is more likely to have evolved differences in its uptake with soil depth than Fagus sylvatica or Picea abies. Different uptake capacities with soil depth have also been reported in the deep-rooted species sugar maple and eucalyptus [82,84]. Moreover, K circulates rapidly due to leaching from the leaves [85]. This explains why K uptake mainly takes place in the topsoil, whereas P is more strongly retained by the trees and therefore has a relatively greater uptake from deep soil layers than K. From an evolutionary perspective, K has seldom limited growth. Thus, as an uptake at depth has never been needed for K, it would be carbon efficient for Quercus robur to down-regulate its uptake capacity of K from deep soil layers [83].
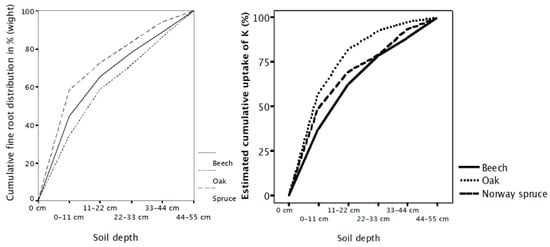
Figure 2.
Cumulative root distribution and cumulative uptake of K with increasing soil depth based on their root distribution and the nutrient uptake capacity of their roots at different soil depths by Fagus sylvatica, Quercus robur and Picia abies. Palsgard, Denmark.
Finally, different species of mycorrhiza have different abilities to mobilize nutrients from different inorganic and organic compounds, which is why high mycorrhizal diversity could increase nutrient uptake [86,87]. The mycorrhiza species differ at different soil depths [88,89]. Their different uptake capacities of various nutrients [90,91,92,93] may also influence the relative uptake at different depths. How the uptake capacity of roots and mycorrhiza differs with root depth is still mostly unknown. More research is needed to incorporate this aspect into estimating the relative uptake of different nutrients from different soil depths.
5. Implications for Modeling Forest Nutrition
In ecosystem models, the uptake from different soil depths is usually a function of soil nutrient concentrations and relative root distribution; alternatively, a root depth is defined and this is considered to represent the uptake zone [94,95,96]. Nonetheless, the fact that these overlapping effects differ between nutrients, as well the distributions of mycorrhiza or the different uptake efficiencies of roots and mycorrhiza at different depths, are usually not included in models of the nutrient budgets of forest soils [44,94,95]. This needs to be rectified because, as shown above, these factors can substantially affect the estimations of nutrient budgets in the soil [94,96]. Changing the depth of the uptake zone for a modeled tree species from 30 to 40 cm can make the difference between whether there are enough base cations or P to sustain growth or not [37,97] and, thus, whether the current management method is sustainable or not. For very immobile nutrients, a more linear correlation to the root distribution over soil depth may be appropriate (as mycorrhiza distribution follows the root distribution). For very mobile nutrients, uptake begins to decline only when the overlap starts to decline. The amount and type of mycorrhiza must be considered because tree species with abundant mycorrhiza will exhibit a greater overlapping of their uptake cylinders than species with less mycorrhiza. Finally, different uptake capacities with soil depth need to be considered. Good measurements of the distribution of the mycorrhiza mycelia in the soil, as well as good data on their nutrient uptake capacities, are still lacking. A better use of the vertical distribution of roots and mycorrhiza, as well as a distinction between different types of fine roots (transporting and absorbing) and mycorrhiza types, can improve this modeling [96].
6. Conclusions
European forests are not sustainable from a nutrient perspective, as we are seeing a shift in nutrient limitation from nitrogen to P and base cations. How well the trees will handle being limited by P and cations depends, to a large degree, on their capacity to take them up from deep soil layers and the response in their root and mycorrhizal growth. Oak can probably better utilize the P in deep soil layers than beech and especially spruce due to its deeper root system and associated mycorrhiza. Trees are, in general, poorly prepared to be limited by cations because there is no positive root or mycorrhizal growth response to this limitation. Even if base cations are rather mobile, the uptake capacity of deeper roots seems to be poor, at least for K. Further, a high diversity of mycorrhiza increases the trees’ chance to mobilize nutrients from different organic and inorganic compounds. Their response to the high availability of N will also play a role. The decrease in root growth and mycorrhiza at high N availabilities in EM trees will decrease their capacity to take up other nutrients; this will become limiting for growth, especially regarding P. This effect will be strongest in conifers, whose mycorrhiza seem to be more sensitive to N than the EM of deciduous trees. The increase in root growth and strong increase in phosphatase exudation in AM trees at high N availabilities will make them more competitive. Their increase will be at the expense of EM trees, as already shown in the US. Adapting to being limited by something other than nitrogen will be hardest for shallow-rooted conifer trees, followed by EM deciduous trees; AM trees will handle the shift best. More research is needed to improve our estimates of trees’ nutrient uptake at different soil depths and how this is affected by a potential change in nutrient limitation. This is a crucial step in more reliably predicting soil nutrient budgets. Without reliable models on how tree species affect soil nutrient budgets, we cannot develop more sustainable management regimes.
Funding
University of Natural Resources and Life Sciences Vienna.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sverdrup, H. Nutrient sustainability for Swedish forests. In Developing Principles and Models for Sustainable Forestry in Sweden; Sverdrup, H., Stjernquist, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 5. [Google Scholar]
- Krüger, I.S.T.; Potočić, N.; Ukonmaanaho, L.P.R. Increased Evidence of Nutrient Imbalances in Forest Trees across Europe, ICP Forests Brief 4; Thünen Institute of Forest Ecosystems: Braunschweig, Germany, 2020. [Google Scholar]
- Svenning, J.C.; Skov, F. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecol. Biogeogr. 2007, 16, 234–245. [Google Scholar] [CrossRef]
- Bernasconi, S.M.; Bauder, A.; Bourdon, B.; Brunner, I.; Bünemann, E.; Chirs, I.; Derungs, N.; Edwards, P.; Farinotti, D.; Frey, B.; et al. Chemical and biological gradients along the Damma Glacier soil chronosequence (Switzerland). Vadose Zone J. 2011, 10, 867–883. [Google Scholar] [CrossRef]
- Binkley, D.; Högberg, P. Tamm Review: Revisiting the influence of nitrogen deposition on Swedish forests. For. Ecol. Manage. 2016, 368, 222–239. [Google Scholar] [CrossRef]
- De Vries, W.; Posch, M. Modelling the impact of nitrogen deposition, climate change and nutrient limitations on tree carbon sequestration in Europe for the period 1900–2050. Environ. Pollut. 2011, 159, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Bolte, A.; Holzhausen, M.; Wolff, B. Exceedance of critical loads of nitrogen and sulphur and its relation to forest conditions. Eur. J. For. Res. 2005, 124, 289–300. [Google Scholar] [CrossRef]
- Waldner, P.; Thimonier, A.; Pannatier, E.G.; Etzold, S.; Schmitt, M.; Marchetto, A.; Rautio, P.; Derome, K.; Nieminen, T.M.; Nevalainen, S. Exceedance of critical loads and of critical limits impacts tree nutrition across Europe. Ann. For. Sci. 2015, 72, 929–939. [Google Scholar] [CrossRef]
- De Vries, W.; Vel, E.; Reinds, G.; Deelstra, H.; Klap, J.; Leeters, E.; Hendriks, C.; Kerkvoorden, M.; Landmann, G.; Herkendell, J. Intensive monitoring of forest ecosystems in Europe: 1. Objectives, set-up and evaluation strategy. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Sardans, J.; Alonso, R.; Janssens, I.A.; Carnicer, J.; Vereseglou, S.; Rillig, M.C.; Fernández-Martínez, M.; Sanders, T.G.; Peñuelas, J. Foliar and soil concentrations and stoichiometry of nitrogen and phosphorous across European Pinus sylvestris forests: Relationships with climate, N deposition and tree growth. Funct. Ecol. 2015, 30, 676–689. [Google Scholar] [CrossRef]
- Du, E.; van Doorn, M.; de Vries, W. Spatially divergent trends of nitrogen versus phosphorus limitation across European forests. Sci. Total Environ. 2021, 771, 145391. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P. Tree mineral nutrition is deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Thelin, G. Ash Recycling to Spruce and Beech Stands Effects on Nutrients, Growth, Nitrogen Dynamics and Carbon Balance; Report 965; Värmeforsk Service AB: Stockholm, Sweden, 2006. (In Swedish) [Google Scholar]
- Akselsson, C.; Westling, O.; Alveteg, M.; Thelin, G.; Fransson, A.-M.; Hellsten, S. The influence of N load and harvest intensity on the risk of P limitation in Swedish forest soils. Sci. Total Environ. 2008, 404, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Ostertag, R.; DiManno, N.M. Detecting terrestrial nutrient limitation: A global meta-analysis of foliar nutrient concentrations after fertilization. Front. Earth Sci. 2016, 4, 23. [Google Scholar] [CrossRef]
- Talkner, U.; Meiwes, K.J.; Potočić, N.; Seletković, I.; Cools, N.; De Vos, B.; Rautio, P. Phosphorus nutrition of beech (Fagus sylvatica L.) is decreasing in Europe. Ann. For. Sci. 2015, 72, 919–928. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Foliar nutrient concentrations of European beech in Switzerland: Relations with nitrogen deposition, ozone, climate and soil chemistry. Front. For. Glob. Chang. 2020, 3, 33. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Wang, L.; Katzensteiner, K.; Schume, H.; Van Loo, M.; Godbold, D.L. Potassium fertilization affects the distribution of fine roots but does not change ectomycorrhizal community structure. Ann. For. Sci. 2016, 73, 691–702. [Google Scholar] [CrossRef]
- Sarkkola, S.; Ukonmaanaho, L.; Nieminen, T.M.; Laiho, R.; Laurén, A.; Finér, L.; Nieminen, M. Should harvest residues be left on site in peatland forests to decrease the risk of potassium depletion? For. Ecol. Manag. 2016, 374, 136–145. [Google Scholar] [CrossRef]
- Penuelas, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Chang. Biol. 2020, 26, 1962–1985. [Google Scholar] [CrossRef]
- Schmitz, A.; Sanders, T.G.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 2019, 244, 980–994. [Google Scholar] [CrossRef]
- Göttlein, A. Grenzwertbereiche für die ernährungsdiagnostische Einwertung der Hauptbaumarten Fichte, Kiefer, Eiche, Buche. Allg. Forst. Jagdztg. 2015, 186, 110–116. [Google Scholar]
- Braun, S.; Tresch, S.; Augustin, S. Soil solution in Swiss forest stands: A 20 year’s time series. PLoS ONE 2020, 15, e0227530. [Google Scholar] [CrossRef] [PubMed]
- Falkengren-Grerup, U.; Linnermark, N.; Tyler, G. Changes in acidity and cation pools of southern Sweden soils between 1949 and 1985. Chemosphere 1987, 16, 2239–2248. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Brunet, J.; Diekmann, M. Nitrogen mineralisation in deciduous forest soils in south Sweden in gradients of soil acidity and deposition. Environ. Pollut. 1998, 102, 415–420. [Google Scholar] [CrossRef]
- Jönsson, U.; Rosengren, U.; Thelin, G.; Nihlgård, B. Acidification-induced chemical changes in coniferous forest soils in southern Sweden. Environ. Pollut. 2003, 123, 75–83. [Google Scholar] [CrossRef]
- Göransson, H.; Rosengren, U.; Thelin, G.; Nihlgård, B. Effects on soil chemistry and nutrient uptake by admixture of birch in Norway spruce stands. In Sustainable Forestry in Temperate Regions; Lund University: Lund, Sweden, 2002. [Google Scholar]
- Johnson, J.; Graf Pannatier, E.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Naturvårdsverket. Bara Naturlig Föryngring; Naturvårdsverket: Stockholm, Sweden, 2022; ISBN 978-91-620-7069-4.
- Mylona, S. Sulphur dioxide emissions in Europe 1880–1991 and their effect on sulphur concentrations and depositions. Tellus B 1996, 48, 662–689. [Google Scholar] [CrossRef]
- Fowler, D.; Smith, R.; Muller, J.; Cape, J.N.; Sutton, M.; Erisman, J.W.; Fagerli, H. Long term trends in sulphur and nitrogen deposition in Europe and the cause of non-linearities. Water Air Soil Pollut. Focus 2007, 7, 41–47. [Google Scholar] [CrossRef]
- Cools, N.; De Vos, B. Availability and evaluation of European forest soil monitoring data in the study on the effects of air pollution on forests. Iforest-Biogeosci. For. 2011, 4, 205. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef]
- Solberg, S.; Dobbertin, M.; Reinds, G.J.; Lange, H.; Andreassen, K.; Fernandez, P.G.; Hildingsson, A.; de Vries, W. Analyses of the impact of changes in atmospheric deposition and climate on forest growth in European monitoring plots: A stand growth approach. For. Ecol. Manag. 2009, 258, 1735–1750. [Google Scholar] [CrossRef]
- Sverdrup, H.; Stjernquist, I. Developing Principles and Models for Sustainable Forestry in Sweden; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Kint, V.; Aertsen, W.; Campioli, M.; Vansteenkiste, D.; Delcloo, A.; Muys, B. Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Chang. 2012, 115, 343–363. [Google Scholar] [CrossRef]
- Wall, A. Risk analysis of effects of whole-tree harvesting on site productivity. For. Ecol. Manag. 2012, 282, 175–184. [Google Scholar] [CrossRef]
- Clarke, N.; Kiær, L.P.; Kjønaas, O.J.; Bárcena, T.G.; Vesterdal, L.; Stupak, I.; Finér, L.; Jacobson, S.; Armolaitis, K.; Lazdina, D. Effects of intensive biomass harvesting on forest soils in the Nordic countries and the UK: A meta-analysis. For. Ecol. Manag. 2021, 482, 118877. [Google Scholar] [CrossRef]
- Roy, S.; Leban, J.-M.; Zeller, B.; van der Heijden, G.; Reichard, A.; Gehin, M.-C.; Santenoise, P.; Saint-Andre, L. Removing harvest residues from hardwood stands affects tree growth, wood density and stem wood nutrient concentration in European beech (Fagus sylvatica) and oak (Quercus spp.). For. Ecosyst. 2022, 9, 100014. [Google Scholar] [CrossRef]
- Rosengren, U.; Göransson, H.; Jönsson, U.; Stjernquist, I.; Thelin, G.; Wallander, H. Functional Biodiversity Aspects on the Nutrient Sustainability in Forests—Importance of Root Distribution. J. Sustain. For. 2005, 21, 75–98. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.-D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Germon, A.; Laclau, J.-P.; Robin, A.; Jourdan, C. Tamm Review: Deep fine roots in forest ecosystems: Why dig deeper? For. Ecol. Manag. 2020, 466, 118135. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Uhlig, D.; Amelung, W.; Von Blanckenburg, F. Mineral nutrients sourced in deep regolith sustain long-term nutrition of mountainous temperate forest ecosystems. Glob. Biogeochem. Cycles 2020, 34, e2019GB006513. [Google Scholar] [CrossRef]
- Holloway, J.M.; Dahlgren, R.A. Nitrogen in rock: Occurrences and biogeochemical implications. Glob. Biogeochem. Cycles 2002, 16, 65-1–65-17. [Google Scholar] [CrossRef]
- Augusto, L.; Turpault, M.-P.; Ranger, J. Impact of forest tree species on feldspar weathering rates. Geoderma 2000, 96, 215–237. [Google Scholar] [CrossRef]
- Stendahl, J.; Akselsson, C.; Melkerud, P.-A.; Belyazid, S. Pedon-scale silicate weathering: Comparison of the PROFILE model and the depletion method at 16 forest sites in Sweden. Geoderma 2013, 211, 65–74. [Google Scholar] [CrossRef]
- Sverdrup, H.; Warfvinge, P. Calculating field weathering rates using a mechanistic geochemical model PROFILE. Appl. Geochem. 1993, 8, 273–283. [Google Scholar] [CrossRef]
- Rosengren, U.; Stjernquist, I. Gå på Djupet: Om Rotdjup och Rotproduktion i Olika Skogstyper; SUFOR Report; Plant Ecology and Systematics: Lund, Sweden, 2004. [Google Scholar]
- Wallander, H.; Göransson, H.; Rosengren, U. Production, standing biomass and ∂15N/∂13C abundance of ectomycorrhizal mycelia at different soil depths in spruce forests and mixed (spruce-oak) forests in southern Sweden. Oecologia 2004, 139, 89–97. [Google Scholar] [CrossRef]
- Leake, J.R. Is diversity of ectomycorrhizal fungi important for ecosystem function? New Phytol. 2001, 152, 1–3. [Google Scholar] [CrossRef]
- Göransson, H. The Vertical Distribution of Roots, Mycorrhizal Mycelia and Nutrient Acquisition in Mature Forest Trees; Lund University: Lund, Sweden, 2006. [Google Scholar]
- Göransson, H.; Wallander, H.; Ingerslev, M.; Rosengren, U. Estimating the relative nutrient uptake from different soil depth of Quercus robur, Fagus sylvatica and Picea abies (L.) Karst. Plant Soil 2006, 286, 87–97. [Google Scholar] [CrossRef]
- Becerra, A.; Bartoloni, N.; Cofré, N.; Soteras, F.; Cabello, M. Arbuscular mycorrhizal fungi in saline soils: Vertical distribution at different soil depth. Braz. J. Microbiol. 2014, 45, 585–594. [Google Scholar] [CrossRef]
- de Araujo Pereira, A.P.; Santana, M.C.; Bonfim, J.A.; de Lourdes Mescolotti, D.; Cardoso, E.J.B.N. Digging deeper to study the distribution of mycorrhizal arbuscular fungi along the soil profile in pure and mixed Eucalyptus grandis and Acacia mangium plantations. Appl. Soil Ecol. 2018, 128, 1–11. [Google Scholar] [CrossRef]
- Köble, R.; Seufert, G. Novel maps for forest tree species in Europe. In Proceedings of the 8th European Symposium on the Physico-Chemical Behaviour of Air Pollutants: “A Changing Atmosphere”, Torino, Italy, 17–20 September 2001. [Google Scholar]
- von Zoth, R.; Block, J. Untersuchungen anWurzelballen sturmgeworfener Bäume in Rheinland-Pfalz. Forst Holz 1992, 47, 566–571. [Google Scholar]
- Ericsson, T. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 168–169, 205–214. [Google Scholar] [CrossRef]
- Ericsson, T.; Rytter, L.; Vapaavuori, E. Physiology of carbon allocation in trees. Biomass Bioenergy 1996, 11, 115–127. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, A.; Wallander, H.; Carlsson, M.L.; Huss-Danell, K. Fungal Biomass in Roots and Extramatrical Mycelium in Relation to Macronutrients and Plant Biomass of Ectomycorrhizal Pinus sylvestris and Alnus incana. New Phytol. 1995, 131, 443–451. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants--an economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, Q.; Huang, L.; Lin, Q.; Wu, J.; Liu, F. Fine-root functional trait response to nitrogen deposition across forest ecosystems: A meta-analysis. Sci. Total Environ. 2022, 844, 157111. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, B.; Nie, Y.; Liu, Y.; Kuzyakov, Y. Root and mycorrhizal strategies for nutrient acquisition in forests under nitrogen deposition: A meta-analysis. Soil Biol. Biochem. 2021, 163, 108418. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Steingrobe, B. A sensitivity analysis for assessing the relevance of fine-root turnover for P and K uptake. J. Plant Nutr. Soil Sci. 2005, 168, 496–502. [Google Scholar] [CrossRef]
- Bahr, A.; Ellström, M.; Akselsson, C.; Ekblad, A.; Mikusinska, A.; Wallander, H. Growth of ectomycorrhizal fungal mycelium along a Norway spruce forest nitrogen deposition gradient and its effect on nitrogen leakage. Soil Biol. Biochem. 2013, 59, 38–48. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Wallander, H. Production of external mycelium by ectomycorrhizal fungi in Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol. 2003, 158, 409–416. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Bååth, E.; Falkengren-Grerup, U.; Wallander, H. Growth of ectomycorrhizal mycelia and composition of soil microbial communities in oak forest soils along a nitrogen deposition gradient. Oecologia 2007, 153, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lilleskov, E.A.; Kuyper, T.W.; Bidartondo, M.I.; Hobbie, E.A. Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review. Environ. Pollut. 2019, 246, 148–162. [Google Scholar] [CrossRef]
- Averill, C.; Dietze, M.C.; Bhatnagar, J.M. Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Glob. Chang. Biol. 2018, 24, 4544–4553. [Google Scholar] [CrossRef]
- Lehmann, J. Subsoil root activity in tree-based cropping systems. Plant Soil 2003, 255, 319–331. [Google Scholar] [CrossRef]
- Soethe, N.; Lehmann, J.; Engels, C. The vertical pattern of rooting and nutrient uptake at different altitudes of a south Ecuadorian montane forest. Plant Soil 2006, 286, 287–299. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Bengtsson, J.; Lundkvist, H. Distribution of the capacity to take up nutrients by Betula spp. and Picea abies in mixed stands. For. Ecol. Manag. 2004, 198, 193–208. [Google Scholar] [CrossRef]
- Göransson, H.; Ingerslev, M.; Wallander, H. The vertical distribution of N and K uptake in relation to root distribution and root uptake capacity in mature Quercus robur, Fagus sylvatica and Picea abies stands. Plant Soil 2008, 306, 129–137. [Google Scholar] [CrossRef]
- Newman, E.I.; Andrews, R.E. Uptake of phosphorus and potassium in relation to root growth and root density. Plant Soil 1973, 38, 49–69. [Google Scholar] [CrossRef]
- Göransson, H.; Rosengren, U.; Wallander, H.; Fransson, A.-M.; Thelin, G. Nutrient acquisition from different soil depths by Pedunculate oak. Trees 2005, 20, 292–298. [Google Scholar] [CrossRef]
- Thelin, G.; Sverdrup, H.; Holmqvist, J.; Rosengren, U.; Linden, M. Sustainability in spruce and mixed-species stands. In Developing Principles and Models for Sustainable Forestry in Sweden; Sverdrup, H., Stjernquist, I., Eds.; Managing forest ecosystems; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 5, pp. 337–354. [Google Scholar]
- da Silva, E.V.; Bouillet, J.P.; de Moraes Gonçalves, J.L.; Junior, C.H.A.; Trivelin, P.C.O.; Hinsinger, P.; Jourdan, C.; Nouvellon, Y.; Stape, J.L.; Laclau, J.P. Functional specialization of Eucalyptus fine roots: Contrasting potential uptake rates for nitrogen, potassium and calcium tracers at varying soil depths. Funct. Ecol. 2011, 25, 996–1006. [Google Scholar] [CrossRef]
- Göransson, H.; Fransson, A.-M.; Jönsson-Belyazid, U. Do oaks have different strategies for uptake of N, K and P depending on soil depth? Plant Soil 2007, 297, 119–125. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Laskowski, M.J.; Burton, A.J.; Lessard, V.C.; Zak, D.R. Variation in Sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Bergkvist, B.; Folkeson, L. The influence of tree species on acid deposition, proton budgets and element fluxes in south Swedish forest ecosystems. Ecol. Bull. 1995, 44, 90–99. [Google Scholar]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef]
- Rosling, A.; Landeweert, R.; Lindahl, B.D.; Larsson, K.-H.; Kuyper, T.W.; Taylor, A.F.S.; Finlay, R.D. Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytol. 2003, 159, 775–783. [Google Scholar] [CrossRef]
- Dickie, I.A.; Xu, B.; Koide, R.T. Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T-RFLP analysis. New Phytol. 2002, 156, 527–535. [Google Scholar] [CrossRef]
- Dighton, J.; Mason, P.P.; Poskitt, J.M. Field use of 32P to measure phosphate uptake by birch mycorrhizas. New Phytol. 1990, 116, 655–661. [Google Scholar] [CrossRef]
- Dighton, J.; Poskitt, J.M.; Brown, T.K. Phosphate influx into ectomycorrhizal and saprophytic fungal hyphae in relations to phosphate supply: A potential method for selection of efficient mycorrhizal species. Mycol. Res. 1993, 97, 355–358. [Google Scholar] [CrossRef]
- Wallander, H.; Wickman, T.; Jacks, G. Apatite as a P source in mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Plant Soil 1997, 196, 123–131. [Google Scholar] [CrossRef]
- Mensah, J.A.; Koch, A.M.; Antunes, P.M.; Kiers, E.T.; Hart, M.; Bücking, H. High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 2015, 25, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Hanson, P.J.; Iversen, C.M.; Kumar, J.; Walker, A.P.; Wullschleger, S.D. Root structural and functional dynamics in terrestrial biosphere models–evaluation and recommendations. New Phytol. 2015, 205, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Sverdrup, H.; Thelin, G.; Robles, M.; Stjernquist, I.; Sörensen, J. Assesing nutrient sustainability of forest production for different tree species considering Ca, Mg, K, N and P at Björnstorp Estate, Sweden. Biogeochemistry 2006, 81, 219–238. [Google Scholar] [CrossRef]
- Wang, B.; McCormack, M.L.; Ricciuto, D.M.; Yang, X.; Iversen, C.M. Embracing fine-root system complexity in terrestrial ecosystem modeling. Glob. Chang. Biol. 2023, 29, 2871–2885. [Google Scholar] [CrossRef]
- Wallman, P.; Svensson, M.G.E.; Sverdrup, H. ForSAFE-an integrated process-oriented forest model for long-term sustainability assessments. For. Ecol. Manag. 2005, 207, 19–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).