Abstract
Green areas, in particular lawns, play important roles in cities. Unfortunately, they are often arranged in sites contaminated with heavy metals. This study analyzed soils and lawn swards in three districts of the city of Wrocław. Three different categories of lawns were examined: residential areas, street lawns and parks. Particular focus was placed on soil contamination with the metals Zn, Cu, Cd and Pb and their accumulation in the aboveground biomass of the perennial ryegrass Lolium perenne, the main grass species, and in the biomass of other components of the lawn sward. The research revealed local occurrence of elevated metal concentrations in soils, although in most of the studied sites, they did not exceed the safe values set byPolish law. The accumulation of metals in the aboveground parts of ryegrass and other plants forming the lawn sward depended primarily on the type of metal. The study confirmed the high phytoavailability of cadmium and zinc and the very low phytoavailability of lead. Perennial ryegrass accumulated considerably lower amounts of lead and copper compared with other components of the lawn sward, which indicates the potential suitability of this species for the phytostabilization and sustainable development of areas contaminated with these two metals.
Keywords:
soil; sustainable city; green area; lawn; sward; grass; phytoavailability; phytostabilization; copper; lead 1. Introduction
In the 20th century, the share of people living in cities increased significantly. Almost 70% of the world’s population is expected to live in cities by 2050 [1,2,3]. In addition to basic economic and cultural functions, cities must also provide residents with opportunities for rest and recreation. Green areas are therefore very important in cities because, on the one hand, they allow residents to relax in nature-based conditions and, at the same time, they also provide important ecosystem services [4,5,6,7] that contribute to their sustainable development.
Lawns are important elements of green areas in cities. They are arranged within larger green spaces, such as parks, but can also function as separate forms of landscaping along streets and within housing estates [3,4]. The selection of grass species for arranging the lawns is determined mainly by esthetic considerations, but an additional factor that definitely should be taken into account is the resistance of plants to various stresses, in particular to soil contamination. In cities, soils contaminated with potentially toxic metals originating from various sources are very common [8,9,10,11]. In such sites, plants with high tolerance should be used to ensure that they will grow well and that they will not show symptoms of stress. An additional important factor that should be considered when choosing plant species for greening contaminated sites in urban areas is their suitability for the phytostabilization of contaminants. Potentially toxic elements should not enter the biogeochemical cycle. On the contrary, they should be immobilized in the soil. Therefore, when arranging lawns in polluted urban areas, the selection of plant species and varieties should be based on the physiological group of excluders so that the concentrations of pollutants in the aboveground biomass of plants are as low as possible [12,13,14].
One of the grass species commonly used for greening barren spaces is the perennial ryegrass Lolium perenne, L., which is usually sown as the main ingredient in various mixtures. Many scientists and practitioners consider this species a great candidate for the phytoremediation of contaminated sites because of its tolerance for some metals, hardiness under unfavorable conditions, and rapid establishment that allows for the development of quick ground cover once sown [15,16,17,18,19]. It has a very wide ecological spectrum and can grow in different habitats and climatic conditions [16,17,18,19,20,21]. However, it should be added that perennial ryegrass has high requirements regarding the supply of nutrients in the soil and regular fertilization [20,21,22]. Some authors have proven that L. perenne has the ability to greatly accumulate certain metals [23,24,25], which would be unfavorable for the phytostabilization of contaminated soils. However, most papers emphasize that grasses, including L. perenne, usually accumulate metals in their roots and translocate them very poorly to the aboveground parts [26,27,28,29,30].
Due to the fact that the literature provides inconsistent information on the uptake of potentially toxic metals by grasses, in particular by the perennial ryegrass, grown in contaminated soils, this research was planned to contribute to the knowledge regarding this issue. The aim of the study was to determine the accumulation of selected potentially toxic metals in the aboveground parts of plants forming the lawn sward in variously polluted soils within the city, with particular emphasis on the main component of vegetation, i.e., the ryegrass L. perenne. The research included lawns that served various functions in the urban space and that were located in different parts of the city. Special attention was paid to the comparison of the ability to accumulate various metals by L. perenne and other sward components. The research hypothesis assumed that this species has a lower ability to bioaccumulate metals than other sward components, which should be reflected by the calculated values of the bioaccumulation factor BAF [29,30].
2. Materials and Methods
2.1. Study Sites
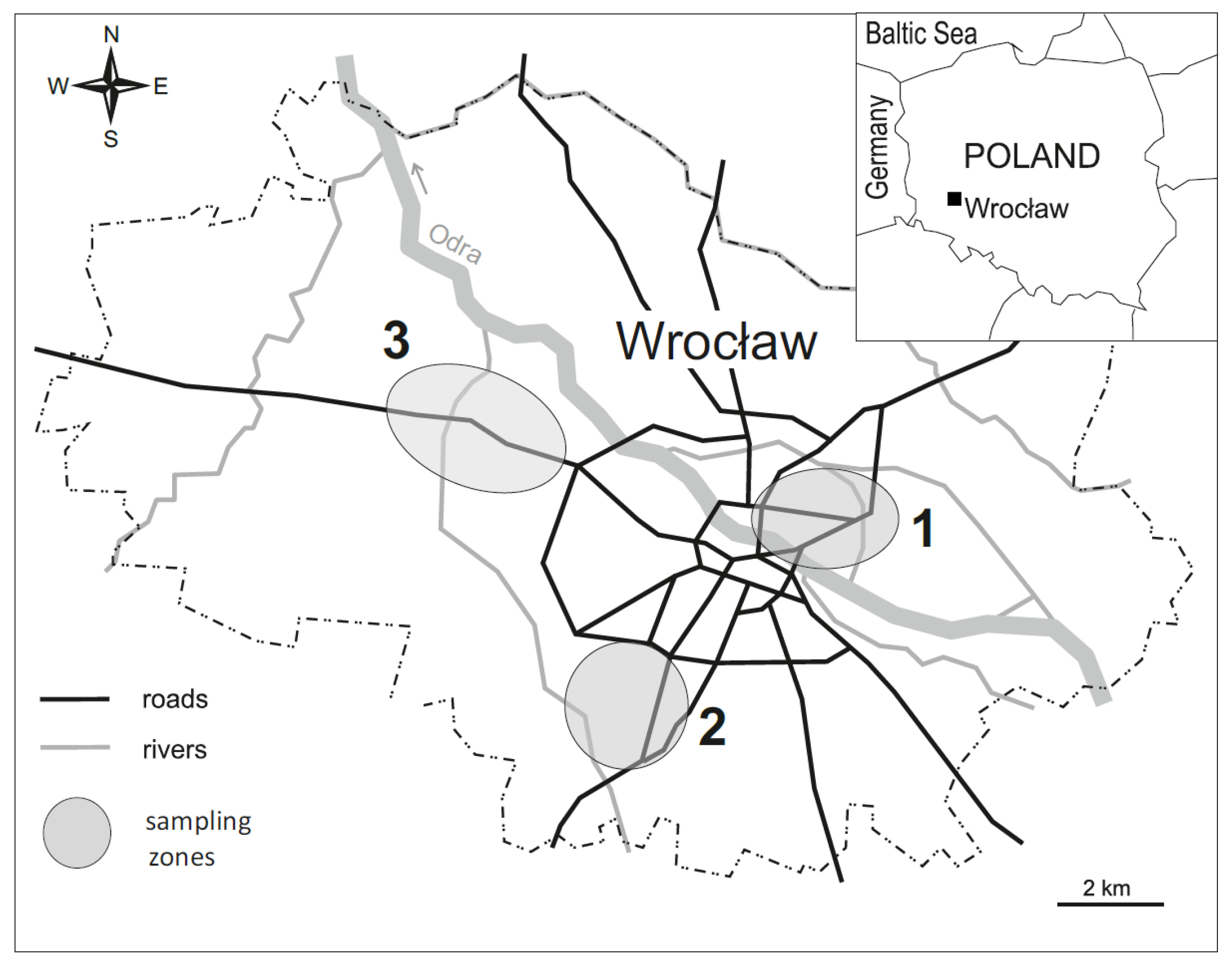
The study was carried out in Wrocław, the third largest city in Poland and the capital of Lower Silesia (Figure 1), which has over 600,000 inhabitants. Taking into account the results of previous studies [31,32,33], research sites were chosen in three parts of the city: eastern (1), southern (2) and western (3), as shown in Figure 1.
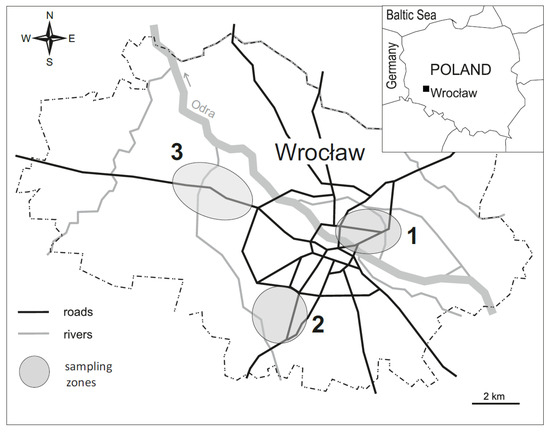
Figure 1.
Location of the study areas.
The research covered lawns performing various functions, having different histories and subjected to various types of human pressure. The following sampling sites were represented: treeless glades located in the inner parts of the old parks (P), lawns within housing estates in residential areas (R) and roadside lawns adjacent to the streets (S). Although most industrial plants that operated in Wrocław in the 20th century were located in the western districts of the city, differentially contaminated soils were also spread out in other parts of the city. In each of the areas (1, 2, 3), four replicate sites representative for each type of lawn (P, R, S) were designated. Those replicates were randomly placed on the lawns within areas 1, 2 and 3. In total, the research covered 36 sampling sites. In the third 10-day block of May, a visual assessment of the lawn sward was performed, and soil samples and samples of the aboveground parts of the lawn sward were collected.
2.2. Soil and Plant Sampling and Preparation
Soil samples were collected from the 0–15 cm deep layer after removing the turf. Ten subsamples were taken with a core sampler from an area of ca. 2 m2, which made up an average sample of about 3 kg. The soil samples were transported to the laboratory, air-dried and homogenized prior to analysis. The aboveground part of the plants comprising the lawn sward was taken from the same area by cutting at a height of 1 cm above the ground surface. The material was carefully divided into individual species, with particular emphasis on the perennial ryegrass Lolium perenne. The plant material was washed with tap water and distilled water prior to subsequent drying. The share of individual species in the sward biomass was determined using the botanical-weight method after drying the samples at 50 °C for 48 h.
2.3. Soil Analysis
Basic soil properties were determined according to the standard methods described by Tan 2005 [34]. The soil texture (grain size distribution) was determined by a combined sieve and hydrometer method, with special focus on the soil fractions responsible for cation sorption capacity, i.e., fine soil FS (<0.02 mm) and the clay fraction (<0.002 mm). The content of organic carbon (OC) was measured by a dry combustion method (Vario MacroCube, Elementar, Stockport, UK), and soil pH was determined potentiometrically in the suspension in 1 M KCl, (1:2.5, m:v). Total concentrations of the metals Zn, Cu, Cd and Pb in the soils were measured by ICP-AES (iCAP 7400, Thermo Fisher Scientific, Waltham, MA, USA) after microwave digestion of the samples with aqua regia (concentrated HCl + HNO3 in a 3:1 ratio) [35]. Validation of the analytical processes included the use of blanks, blind duplicates and commercial reference materials certified for aqua-regia-extracted elements (CNS 392 and CRM 027, supplied by Sigma-Aldrich, Saint Louis, MO, USA) as well as internal reference materials. The recovery of all four metals in the reference materials was within acceptable limits, i.e., in the range of 96–102% of certified values.
2.4. Plant Analysis
The concentrations of heavy metals (Zn, Cu, Cd and Pb) in the plant material were determined separately for the isolated perennial ryegrass L. perenne and for the mixture of all other sward components (Mix). The dried plant material was cut and ground and subjected to microwave digestion (CEM-MARS Xpres, Matthews, NC, USA) with concentrated HNO3, which was preceded by oxidation with 30% perhydrol [28]. The concentrations of elements in the analytes were determined using ICP-AES, as in the case for the soil digests. To validate the analytical method, two plant-certified plant reference materials were used: BCR-414 and DC-73349. Similarly to the results of the soil analyses, metal recovery from the plant reference materials was acceptable, falling within 96–104% of the certified values.
2.5. Statistics
Statistical analysis involved the comparison of soil and plant properties between various kinds of usage and different locations in the city. Special attention was given to metal concentrations in the soils and the collected plant material. Similarly, the concentrations of metals in the isolated shoots of ryegrass were compared with those in the mixture of other sward components. The significance of the differences between the means, set at p < 0.05, was tested by one-way ANOVA, followed by Tukey’s test. Additionally, principal component analysis (PCA) was performed in order to examine the multivariate relationships between the parameters that affected the concentration of the metals in the plant material. All the statistical calculations were performed using Excel 2003 (Microsoft, Redmond, WA USA) and Statistica 13.0 (TIBCO, Santa Clara, CA, USA) software, and the results have been presented in the form of graphs.
3. Results and Discussion
The soils under study had a relatively light texture, and their content of clay fraction did not exceed 8%, with a mean value as low as 3% (Table 1). The mean content of the silt and sand fractions in the soils was 23 and 73%, respectively. The vast majority of soils belonged to the textural groups of sands and loamy sands, while some were classified as sandy loams. The content of organic carbon OC was in the range of 16–54 g/kg, and the pH of the vast majority of the samples was neutral, with a median pH value of 6.8. Only in one single sample taken from the housing estate area and in three samples collected from parks was the pH value below 6.0. Fortunately, none of those samples were significantly contaminated with heavy metals. The concentrations of heavy metals in the soils varied greatly, and in most of the samples, they remained below the permissible values (PVs) set by Polish law for residential and recreational areas (Table 1); however, for some objects examined in their individual sites, the metal concentrations in the soils exceeded those values. The most heavily polluted soils occurred in two single sites in the park (P) in the western part of the city (3), where we found co-occurring high concentrations of all four metals.

Table 1.
General characteristics of soil properties and metal concentrations in the soils (N = 36).
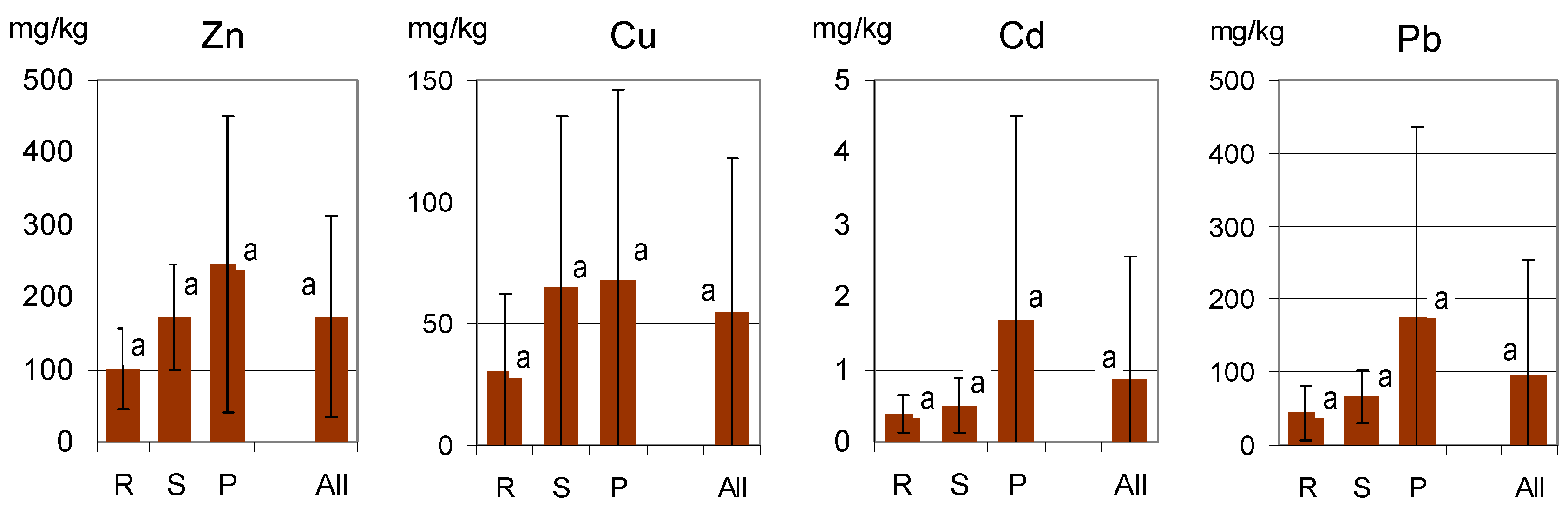
High variations in the metal content of the soils were found in all groups of the study objects, which is illustrated by high values of the standard variation (SD; Figure 2). As the distributions of the values were different from normal (skewed), the data were normalized (logarithmized) prior to statistical analysis. The SD values were calculated for the sets of normalized data. No statistically significant differences were confirmed between the groups of objects R, S and P, and this was true for all the metals examined. The calculated mean concentrations of the metals were lowest in soils of residential lawns (R).
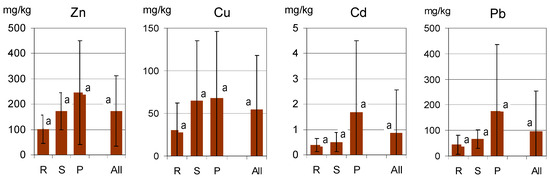
Figure 2.
Metal concentrations in soils of all the study sites (All) and in the groups related to various categories of lawns (R—residential; S—street; P—parks). Columns illustrate the mean values, and error bars represent the standard deviation SD. The same lowercase letters (a) indicate a lack of significant differences (p < 0.05) between the groups of sites.
Such differences in soil contamination are typical for large cities [8,9,10,11,31]. It results from various factors, such as enhanced concentrations of metals in materials used to arrange and fertilize green areas in former post-industrial sites and wastelands. As the city of Wrocław was heavily demolished during World War II, its terrain required leveling. A variety of wastes, such as demolition rubble and industrial wastes were often used for land leveling, while dredge sediments from the Odra river, composts and sewage sludge were commonly used for soil enrichment in organic matter, despite the fact that they often contained relatively high concentrations of heavy metals [8,9,10,31,32,33].
It should be emphasized that in all the sites under study, the soil surface was completely covered with plants, and the lawn sward in none of the locations showed any symptoms indicating the effects of unfavorable habitat factors. The lawns had a highly diversified botanical composition, which was undoubtedly caused by the spontaneous succession of various resistant plant species commonly found in urban conditions. It is worth mentioning that high biodiversity is one of the important factors contributing to the sustainability of urban ecosystems. In total, 58 plant species were found, including 10 grass species and 48 other plant species, which were almost exclusively dicotyledons (Table 2).

Table 2.
Species composition of swards, with special focus on the percentage of ryegrass in various groups of lawns.
Taking into account all the sites, perennial ryegrass constituted the largest share of all the species, although in individual sites, its share varied and accounted for between 2 to 54% (mean value: 21%) of the lawn sward biomass. The second grass species in terms of its share, with a contribution comparable to that of ryegrass (20%), was meadow bluegrass (Poa pratensis, L.). Also relatively abundant were several other grass species, in particular red fescue (Festuca rubra, L.), common cocksfoot (Dactylis glomerata, L.) and couch grass (Elymus regens, L.). The total share of grasses in most of the sites was over 50% of the sward biomass. In most of the lawns, the share of dicotyledonous plants was comparable to that of grasses, and particularly frequent were dandelion (Taraxacum officinale, L.), yarrow (Achillea millefolium, L.), narrowleaf plantain (Plantago lanceolata, L.), common plantain (Plantago major, L.), white clover (Trifolium repens, L.), red clover (Trifolium pretense, L.) and common chickweed (Stellaria media, L.) (Table 2).
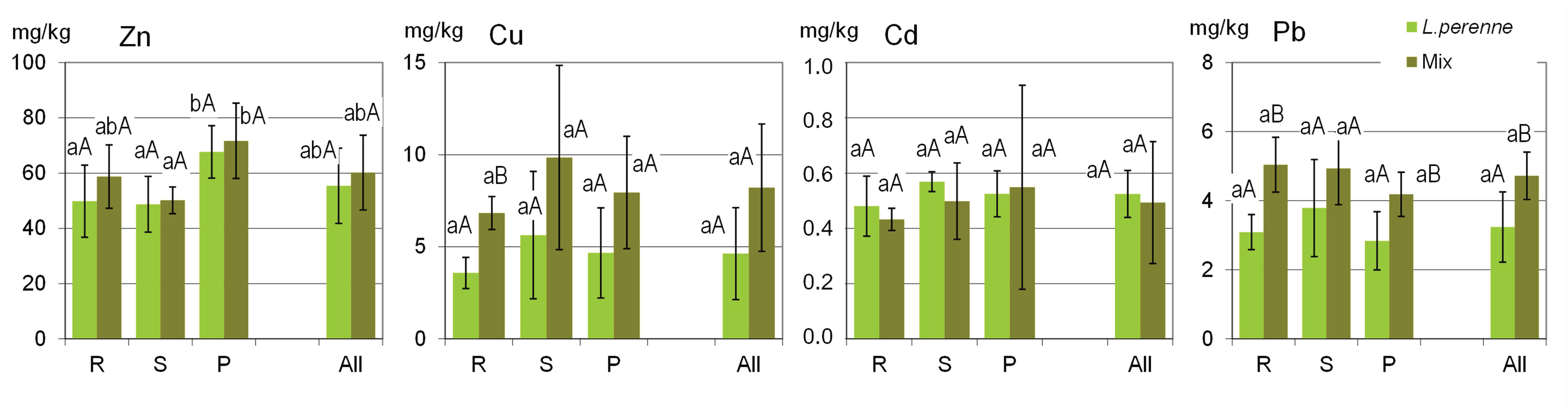
The variation in metal concentrations in the plant biomass was much smaller than in the case of soils (Figure 3), which confirms that plants have physiological mechanisms regulating the uptake of these elements from soils. The ranges in the metal concentrations in the plant biomass were generally typical of those commonly occurring in grasses and other non-accumulating plants [38,39].
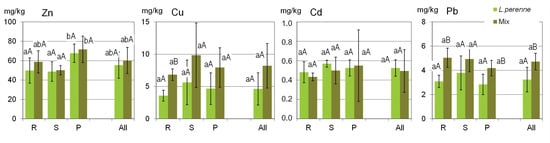
Figure 3.
Ranges of metal concentrations in the biomass of ryegrass (L. perenne) and in the biomass of other components of the lawn sward (Mix) analyzed from all the sites (All) and from the various groups of lawns (R—residential; S—street; P—parks). Columns show the mean values, and the error bars represent the values of the standard deviation SD. Different lowercase and capital letters indicate significant differences (p < 0.05) between the groups of sites (R, S, P) and between L. perenne and Mix, respectively.
The mean concentrations of Zn, Cu, Cd and Pb in the ryegrass biomass were 55, 4.6, 0.52 and 3.2 mg/kg, respectively. The concentrations of individual metals in the biomass of ryegrass and the mixture of other sward plants did not differ significantly between various groups of lawns (Figure 3). What is worth mentioning is the fact that the mean concentrations of Cu and Pb in the ryegrass biomass were apparently lower than those in the mixed biomass of other sward components, although statistically significant differences (p < 0.05) were confirmed only in the R group (lawns in the residential areas). The remaining two metals, i.e., Zn and Cd, were accumulated in ryegrass in similar concentrations as in the mixture of the other sward components.
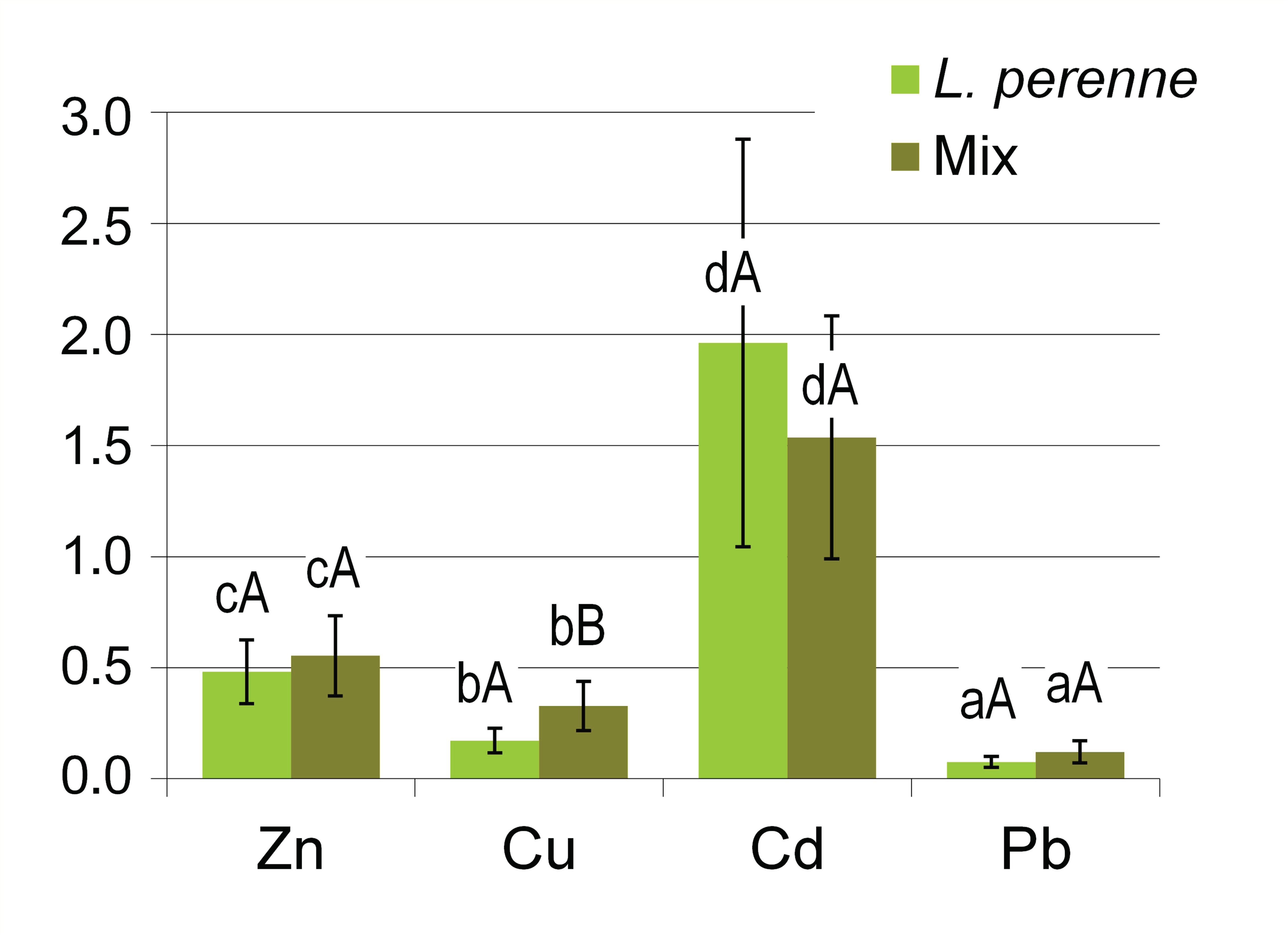
Similar relationships can also be observed when considering the values of the bioaccumulation factor (BAF) that were determined separately for ryegrass and the mixture of other plants. It should be noted here that there is no consensus in the literature on the definition of bioavailability indices of elements in soils [40]. The BAF factor was calculated here as the ratio of the concentration of a given element in the aboveground plant biomass to its total concentration in the soil [40,41,42]. The BAF values differed markedly between the elements, while the differences between ryegrass and the Mix group of plants were much less pronounced (Figure 4). These results confirm the well-known patterns of phytoavailability of various heavy metals. The BAF values clearly increased in the order Pb < Cu < Zn < Cd, which confirms that lead belongs to those heavy metals that are very poorly taken up by plants, while zinc and cadmium are highly phytoavailable [26,38]. In particular, the BAF values determined for cadmium were higher that 1.0 in most of the groups of plants under study. Unlike what was found for Cu and Pb, cadmium bioaccumulation by perennial ryegrass was slightly (statistically insignificantly) higher compared with the mixture of other plants (Mix) growing on lawns under the same conditions.
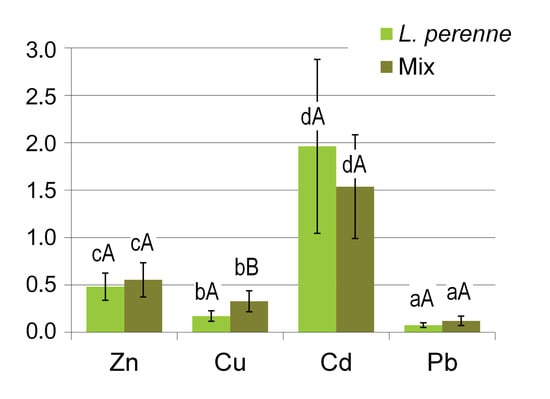
Figure 4.
Bioaccumulation factors (BAFs) for various metals: a comparison of perennial ryegrass (L. perenne) with a mixture of other components of the lawn sward (Mix). Columns show the mean values, and the error bars represent the values of the standard deviation SD. Different lowercase and capital letters indicate significant differences (p < 0.05) between various metals and between L. perenne and Mix, respectively.
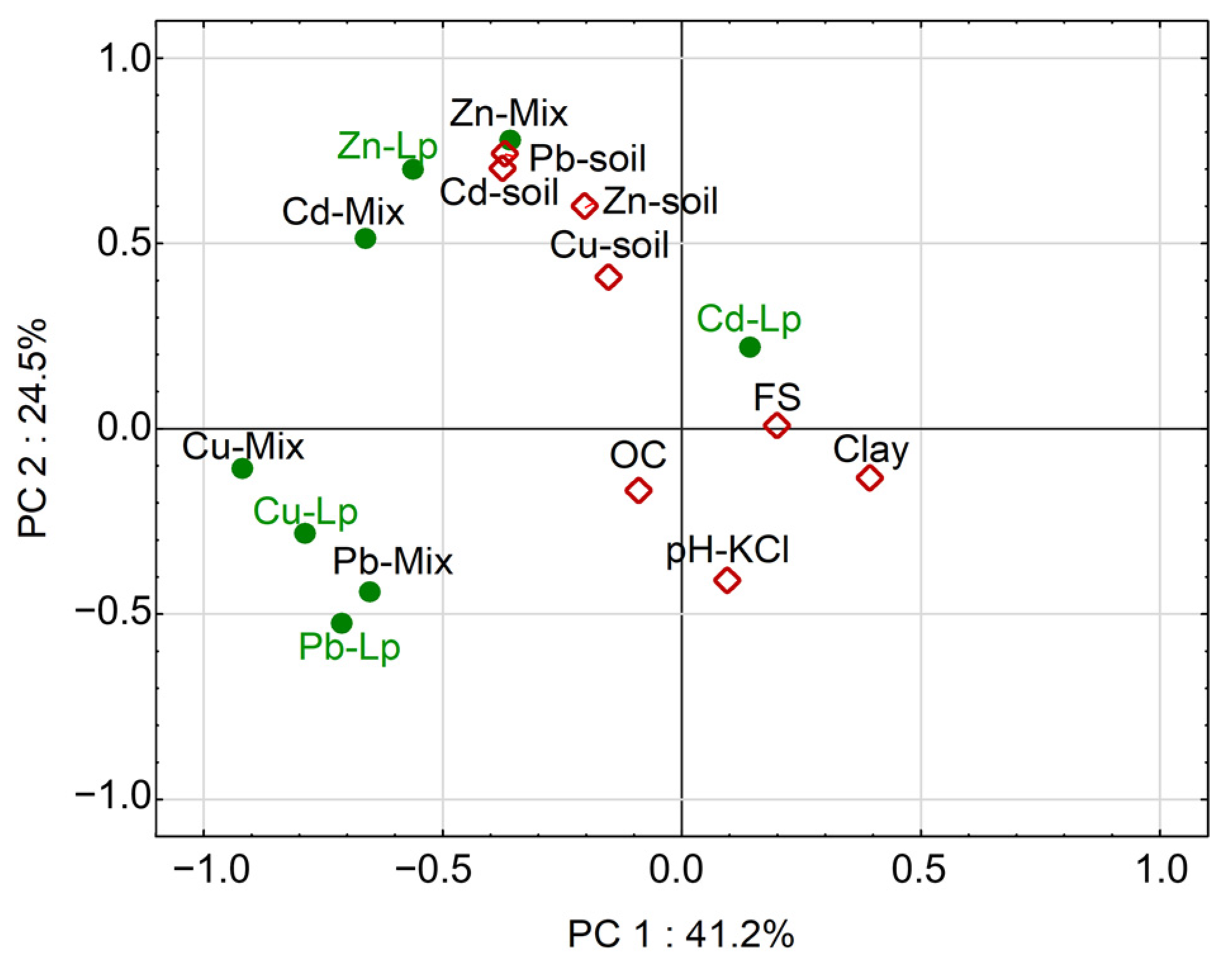
Statistical analysis of the factors that had a crucial impact on metal concentrations in the biomass and other sward components was carried out using the PCA method. The PCA graph (Figure 5) confirms that the total concentrations of all elements in the soil were strongly related to each other, so they are grouped in the chart in close proximity to each other. It should be stressed that they showed minimal dependence on those properties of soils that determine the natural concentrations of trace elements, i.e., FS, clay or OC. The lack of such relationships indicates that the higher concentrations of metals in soils under study were not natural, but rather anthropogenic.
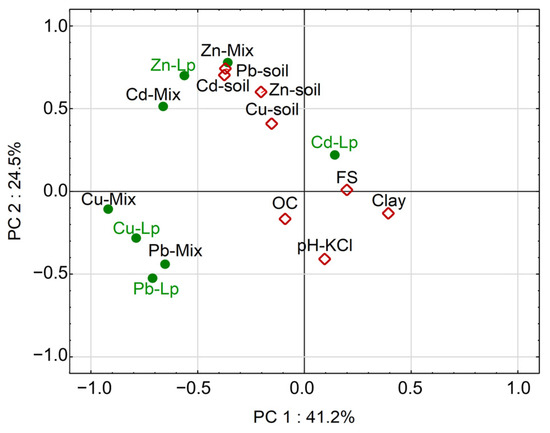
Figure 5.
PCA showing the relationships between the concentrations of metals in soils and plant material, BAF values and various soil factors. For explanations of abbreviations and symbols, see the text.
The relationships of metal concentrations in the aboveground biomass of plants and various soil factors were different for individual metals. Plant Cd and Zn showed an apparent relationship with soil contamination with these elements, which again confirms the high phytoavailability of those metals, in particular when they were present at higher concentrations in soils. In the case of Cu and Pb, unlike for Cd and Zn, it was not the concentrations of these elements in the soils, but several other factors, that mainly determined their concentrations in the aboveground biomass of ryegrass (Cu-Lp, Pb-Lp) and other plants (Cu-Mix, Pb-Mix) (Figure 5). Apparently, those factors were not statistically significantly related to soil properties. The principal component PC1, which had the greatest impact (41.2%) on the patterns of metal concentrations in the plant biomass, was not related to soil properties and should probably be attributed to plant physiology [38,42,43] or biological factors occurring in the rhizosphere [44,45,46], factors that were not analyzed in detail in this study.
In summary, it should be stated that our research hypothesis about the generally poor bioaccumulation of metals by L. perenne was confirmed, in particular in relation to copper and lead. It should be emphasized that in the soil conditions occurring in green areas with different levels of contamination, in particular under lawns, perennial ryegrass L. perenne accumulated significantly lower amounts of lead and copper than other components of the lawn sward. These observations may be an important prerequisite for applying ryegrass when greening areas contaminated with these elements. Such areas include, among others, the surroundings of copper smelters, formerly called “the protection zones” in southwestern Poland [14,28], where the main metals accumulated in soils are copper and lead, and where the concentrations of Zn and Cd are relatively low. The results of this study may also be applicable to the phytoremediation of other areas where soils contaminated with Cu and Pb occur, including urban areas [8,9,10,47,48,49] and various post-industrial areas, in particular those related to copper and lead metallurgy [14,38,50,51,52].
4. Conclusions
This research confirmed the local occurrence of increased metal concentrations in soils of Wrocław city, although in most of the sites under study, these concentrations did not exceed the values defined as safe by Polish law. The accumulation of metals in the aboveground parts of the ryegrass L. perenne and other plants forming the lawn sward depended very much on the type of metal, and the observed patterns confirmed the relationships known from the literature, namely the very high phytoavailability of cadmium and zinc, and the low and very low phytoavailability of lead. The fact that perennial ryegrass accumulated significantly smaller amounts of lead and copper compared with other components of the lawn sward indicates that it is a species that should be considered a candidate for the phytostabilization and sustainable development of areas contaminated with these elements. This may be of particular importance not only within cities where soil contamination with lead is relatively common, but also in other areas, such as those neighboring a copper smelter, where the main contaminants are copper and lead.
Author Contributions
Conceptualization, A.D. and A.K.; methodology, A.D. and A.K.; software, A.D.; validation, A.K.; formal analysis, D.P.; investigation, A.D., A.B., D.K. and D.P.; resources, A.D.; data curation, A.D. and A.B.; writing—original draft preparation, A.D., A.B., D.K. and D.P.; writing—review and editing, A.K.; visualization, A.D.; supervision, A.D. and A.K.; project administration, A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was co-financed by the Wrocław University of Environmental and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okeke, F.O.; Eziyi, I.O.; Udeh, C.A.; Ezema, E.C. City as Habitat: Assembling the fragile city. Civil Eng. J. 2020, 6, 1143–1154. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.; Li, Q.; Huang, D. Dramatic uneven urbanization of large cities throughout the world in recent decades. Nature Comm. 2020, 11, 5366. [Google Scholar] [CrossRef]
- Petrova, S.; Nikolov, B.; Velcheva, I.; Angelov, N.; Valcheva, E.; Katova, A.; Golubinova, I.; Marinov-Serafimov, P. Buffer green patches around urban road network as a tool for sustainable soil management. Land 2022, 11, 343. [Google Scholar] [CrossRef]
- Ignatieva, M.; Haase, D.; Dushkova, D.; Haase, A. Lawns in cities: From a globalised urban green space phenomenon to sustainable nature-based solutions. Land 2020, 9, 73. [Google Scholar] [CrossRef]
- Mathew, S. Role of turfgrass in urban landscapes. J. Plant Dev. Sci. 2021, 13, 247–255. [Google Scholar]
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.; Boyko, C. The ecosystem services of urban soils: A review. Geoderma 2021, 395, 115076. [Google Scholar] [CrossRef]
- Winkler, J.; Koda, E.; Červenková, J.; Děkanovský, I.; Nowysz, A.; Mazur, Ł.; Jakimiuk, A.; Vaverková, M.D. Green space in an extremely exposed part of the city center “Aorta of Warsaw”-Case study of the urban lawn. Urban Ecosyst. 2023, 26, 1225–1238. [Google Scholar] [CrossRef]
- Wang, M.; Liu, R.; Chen, W.; Peng, C.; Markert, B. Effects of urbanization on heavy metal accumulation in surface soils. J. Environ. Sci. 2018, 64, 328–334. [Google Scholar] [CrossRef]
- Silva, H.F.; Silva, N.F.; Oliveira, C.M.; Matos, M.J. Heavy metals contamination of urban soils—A decade study in the city of Lisbon, Portugal. Soil Syst. 2021, 5, 27. [Google Scholar] [CrossRef]
- Bakhmatova, K.A.; Matynyan, N.N.; Sheshukova, A.A. Anthropogenic soils of urban parks: A review. Eur. Soil Sci. 2022, 55, 64–80. [Google Scholar] [CrossRef]
- Tang, S.; Wang, C.; Song, J.; Ihenetu, S.C.; Li, G. Advances in Studies on Heavy Metals in Urban Soil: A Bibliometric Analysis. Sustainability 2024, 16, 860. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Srivastava, S., Srivastava, A., Suprasanna, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar] [CrossRef]
- Karczewska, A.; Mocek, A.; Goliński, P.; Mleczek, M. Phytoremediation of copper-contaminated soil. In Phytoremediation: Management of Environmental Contaminants; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer: Cham, Switzerland, 2015; Volume 2, pp. 143–170. [Google Scholar] [CrossRef]
- Bidar, G.; Garçon, G.; Pruvot, C.; Dewaele, D.; Cazier, F.; Douay, F.; Shirali, P. Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environ. Poll. 2007, 147, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, M.; Adamo, P.; Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 2004, 319, 13–25. [Google Scholar] [CrossRef]
- Alvarenga, P.; Gonçalves, A.P.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci. Total Environ 2008, 406, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sarathchandra, S.S.; Rengel, Z.; Solaiman, Z.M. Remediation of heavy metal-contaminated iron ore tailings by applying compost and growing perennial ryegrass (Lolium perenne L.). Chemosphere 2022, 288, 132573. [Google Scholar] [CrossRef]
- Masotla, M.K.L.; Melato, F.A.; Mokgalaka-Fleischmann, N.S. Extraction Potential of Lolium perenne L.(Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies. Minerals 2023, 13, 873. [Google Scholar] [CrossRef]
- Akdeniz, H.; Hosaflıoğlu, İ. Effects of nitrogen fertilization on some turfgrass characteristics of perennial ryegrass (Lolium perenne L.). J. Agric. Sci. Technol. B 2016, 6, 226–237. [Google Scholar] [CrossRef][Green Version]
- Braun, R.C.; Patton, A.J. Perennial ryegrass (Lolium perenne) culm and inflorescence density in lawns: Effects of nitrogen fertilization and scalping timing and height. Crop Sci. 2022, 62, 489–502. [Google Scholar] [CrossRef]
- Klimont, K.; Bulińska-Radomska, Z.; Osińska, A.; Bajor, P. Formation of species composition of plants introduced and spontaneously settled on a fertilized municipal waste dump. Biul. Inst. Hod. Aklimat. Roślin 2013, 270, 109–121. (In Polish) [Google Scholar] [CrossRef]
- Rabêlo, F.H.; Borgo, L.; Lavres, J. The use of forage grasses for the phytoremediation of heavy metals: Plant tolerance mechanisms, classifications, and new prospects. In Phytoremediation. Methods, Management and Assessment; Matichenkov, V., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; Chapter 3; pp. 59–103. [Google Scholar]
- Zhang, J.; Yang, N.; Geng, Y.; Zhou, J.; Lei, J. Effects of the combined pollution of cadmium, lead and zinc on the phytoextraction efficiency of ryegrass (Lolium perenne L.). RSC Adv. 2019, 9, 20603–20611. [Google Scholar] [CrossRef]
- Patra, D.K.; Acharya, S.; Pradhan, C.; Patra, H.K. Poaceae plants as potential phytoremediators of heavy metals and ecorestoration in contaminated mining sites. Environ. Technol. Innov. 2021, 21, 101293. [Google Scholar] [CrossRef]
- Gołda, S.; Korzeniowska, J. Comparison of phytoremediation potential of three grass species in soil contaminated with cadmium. Environ. Prot. Nat. Res. 2016, 27, 8–14. [Google Scholar] [CrossRef]
- Rabêlo, F.H.S.; Vangronsveld, J.; Baker, A.J.; van Der Ent, A.; Alleoni, L.R.F. Are grasses really useful for the phytoremediation of potentially toxic trace elements? A review. Front. Plant Sci. 2021, 12, 778275. [Google Scholar] [CrossRef]
- Cuske, M.; Karczewska, A.; Gałka, B.; Dradrach, A. Some adverse effects of soil amendment with organic materials—The case of soils polluted by copper industry phytostabilized with red fescue. Int. J. Phytoremediation 2016, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, J.; Stanislawska-Glubiak, E. The phytoremediation potential of local wild grass versus cultivated grass species for zinc-contaminated soil. Agronomy 2023, 13, 160. [Google Scholar] [CrossRef]
- Pinna, M.V.; Diquattro, S.; Garau, M.; Grottola, C.M.; Giudicianni, P.; Roggero, P.P.; Castaldi, P.; Garau, G. Combining biochar and grass-legume mixture to improve the phytoremediation of soils contaminated with potentially toxic elements (PTEs). Heliyon 2024, 10, e26478. [Google Scholar] [CrossRef]
- Hołtra, A.; Zamorska-Wojdyła, D. The input of trace elements from the motor transport into urban soils of Wrocław, Poland. Sci. Total Environ. 2018, 631, 1163–1174. [Google Scholar] [CrossRef]
- Dradrach, A.; Karczewska, A. Mercury in soils of municipal lawns in Wrocław, Poland. Fresenius Environ. Bull. 2013, 22, 968–972. [Google Scholar]
- Gmochowska, W.; Pietranik, A.; Tyszka, R.; Ettler, V.; Mihaljevič, M.; Długosz, M.; Walenczak, K. Sources of pollution and distribution of Pb, Cd and Hg in Wrocław soils: Insight from chemical and Pb isotope composition. Geochemistry 2019, 79, 434–445. [Google Scholar] [CrossRef]
- Tan, K. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- da Silva, Y.J.A.B.; do Nascimento, C.W.A.; Biondi, C.M. Comparison of USEPA digestion methods to heavy metals in soil samples. Environ. Monit. Assess. 2014, 186, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister for the Environment of 1 September 2016 on the method how to carry out the assessment of soil contamination. Pol. J. Laws 2016 2016, 1395. (In Polish)
- Karczewska, A.; Kabała, C. Environmental risk assessment as a new basis for evaluation of soil contamination in Polish law. Soil Sci. Ann. 2017, 68, 67. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Furini, A. (Ed.) Plants and Heavy Metals; Springer Briefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes. Ecol. Ind. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Ramírez, A.; García, G.; Werner, O.; Navarro-Pedreño, J.; Ros, R.M. Implications of the phytoremediation of heavy metal contamination of soils and wild plants in the industrial area of Haina, Dominican Republic. Sustainability 2021, 13, 1403. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Bubak, A. Mobility, bioaccumulation in plants, and risk assessment of metals in soils. Sci. Total Environ. 2023, 882, 163574. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Poll. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Seneviratne, M.; Seneviratne, G.; Madawala, H.; Vithanage, M. Role of Rhizospheric Microbes in Heavy Metal Uptake by Plants. In Agro-Environmental Sustainability. Managing Environmental Pollution; Singh, J., Seneviratne, G., Eds.; Springer: Cham, Switzerland, 2017; Volume 2, pp. 147–163. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mat. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Ren, Y.; Luo, X.S.; Zhu, Y.G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef]
- Baldi, A.; Cecchi, S.; Grassi, C.; Zanchi, C.A.; Orlandini, S.; Napoli, M. Lead bioaccumulation and translocation in herbaceous plants grown in urban and peri-urban soil and the potential human health risk. Agronomy 2021, 11, 2444. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, P.; Yang, C.; Liu, J.; Si, W.; Zhang, S. Remediation effect of walnut shell biochar on Cu and Pb co-contaminated soils in different utilization types. J. Environ. Manag. 2024, 362, 121322. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef]
- Minkina, T.M.; Linnik, V.G.; Nevidomskaya, D.G.; Bauer, T.V.; Mandzhieva, S.S.; Khoroshavin, V.Y. Forms of Cu (II), Zn (II), and Pb (II) compounds in technogenically transformed soils adjacent to the Karabashmed copper smelter. J. Soils Sed. 2018, 18, 2217–2228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).