Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review
Abstract
:1. Introduction
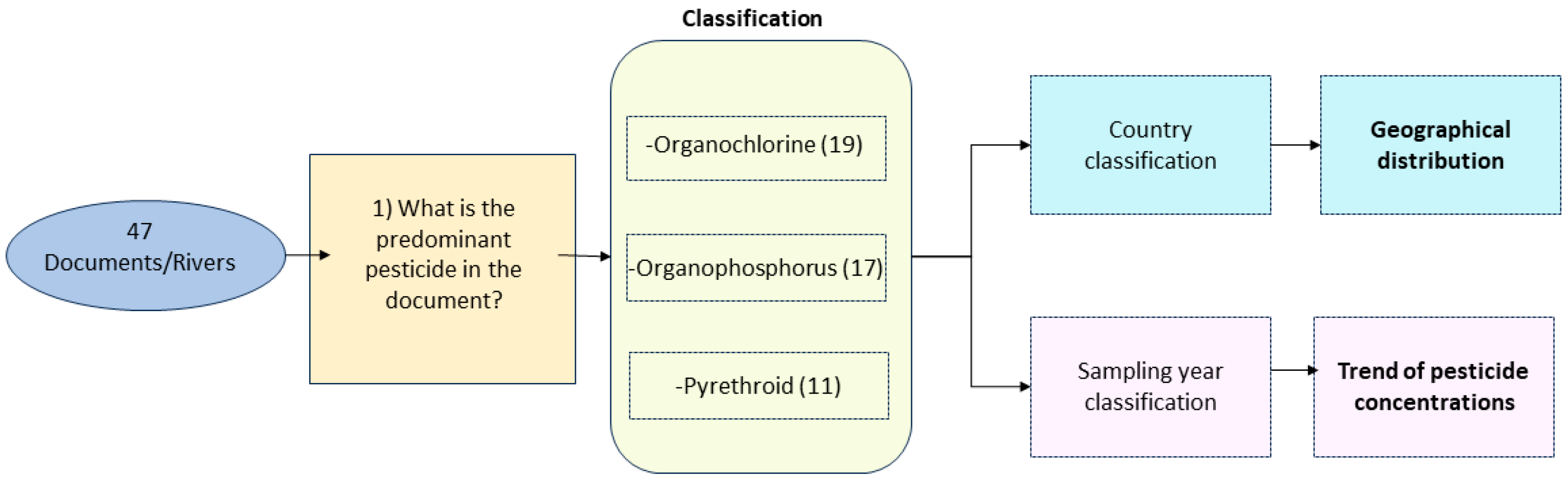
2. Research Methodology
2.1. Research Design
- ₋
- What are the main pesticides detected in river waters worldwide?
- ₋
- What are the concentrations of these pesticides? Do they comply with the specifications regulated by institutions?
- ₋
- What are the environmental and human health implications of pesticide exposure?
2.2. Data Collection
2.3. Inclusion and Exclusion Criteria (Shortlisting)
- Type of document: journal article, book, book chapter, review, report;
- Language: English;
- Year: 2007–2024;
- Peer-reviewed article;
- Methodology: quantitative analysis;
- Type of pesticide: organochlorine, organophosphorus, and pyrethroid compounds.
- Type of document: proceedings, thesis;
- Accessibility: the document is not accessible or cannot be downloaded;
- Language: non-English;
- Year: before 2007;
- Non-peer-reviewed;
- Methodology: qualitative analysis;
- Other types of pesticides.
2.4. Data Evaluation
- (1)
- Documents were excluded if pesticide concentrations were reported in groundwater systems;
- (2)
- Documents were excluded if all reported pesticide concentrations were below 1 ng L−1.
2.5. Selection
- (1)
- Only documents published within the last ten years (2014–2024) were selected;
- (2)
- To prevent duplication of rivers, the document reporting the highest pesticide levels was selected.
Classification
2.6. Physicochemical Properties of Pesticides
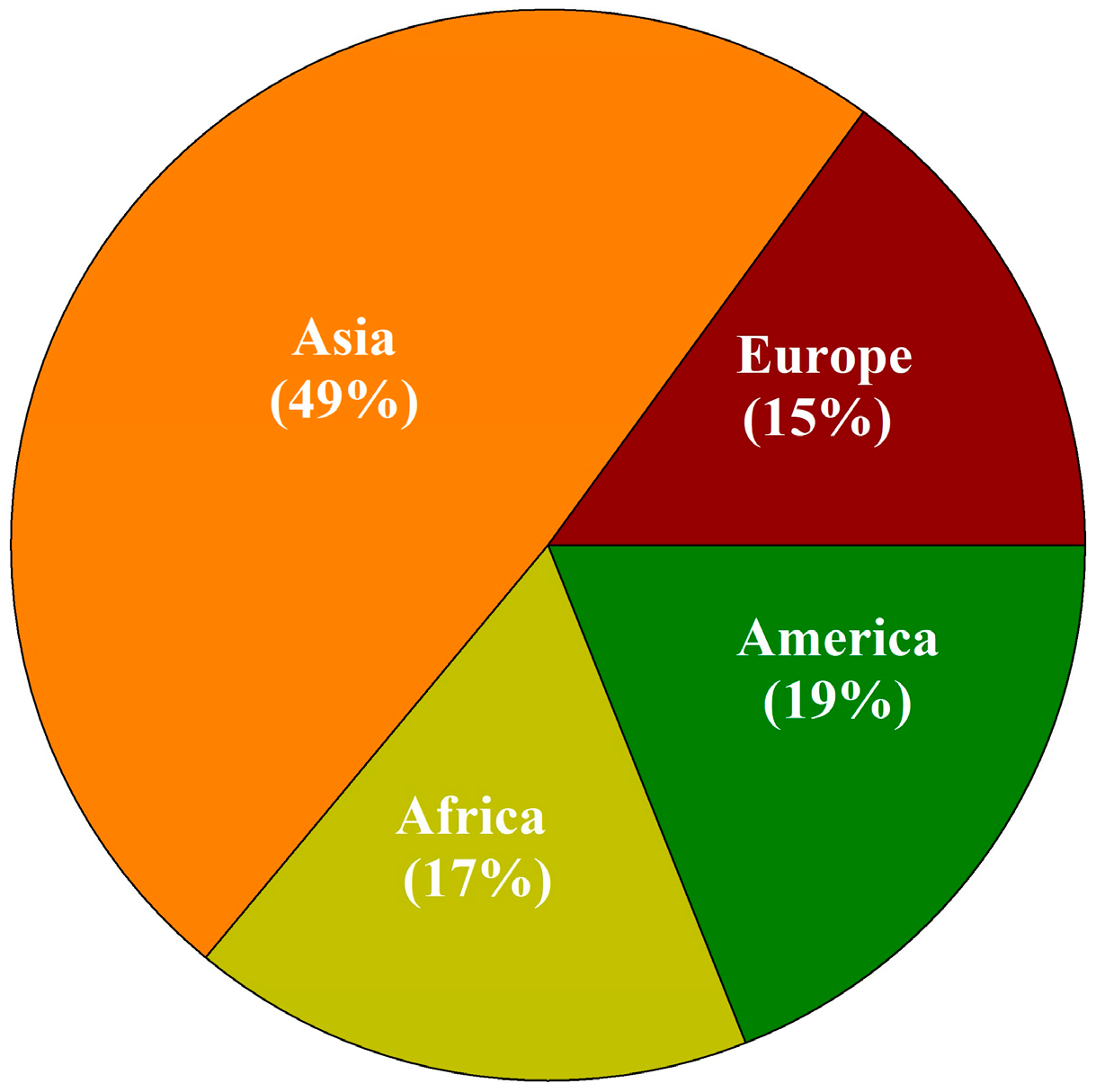
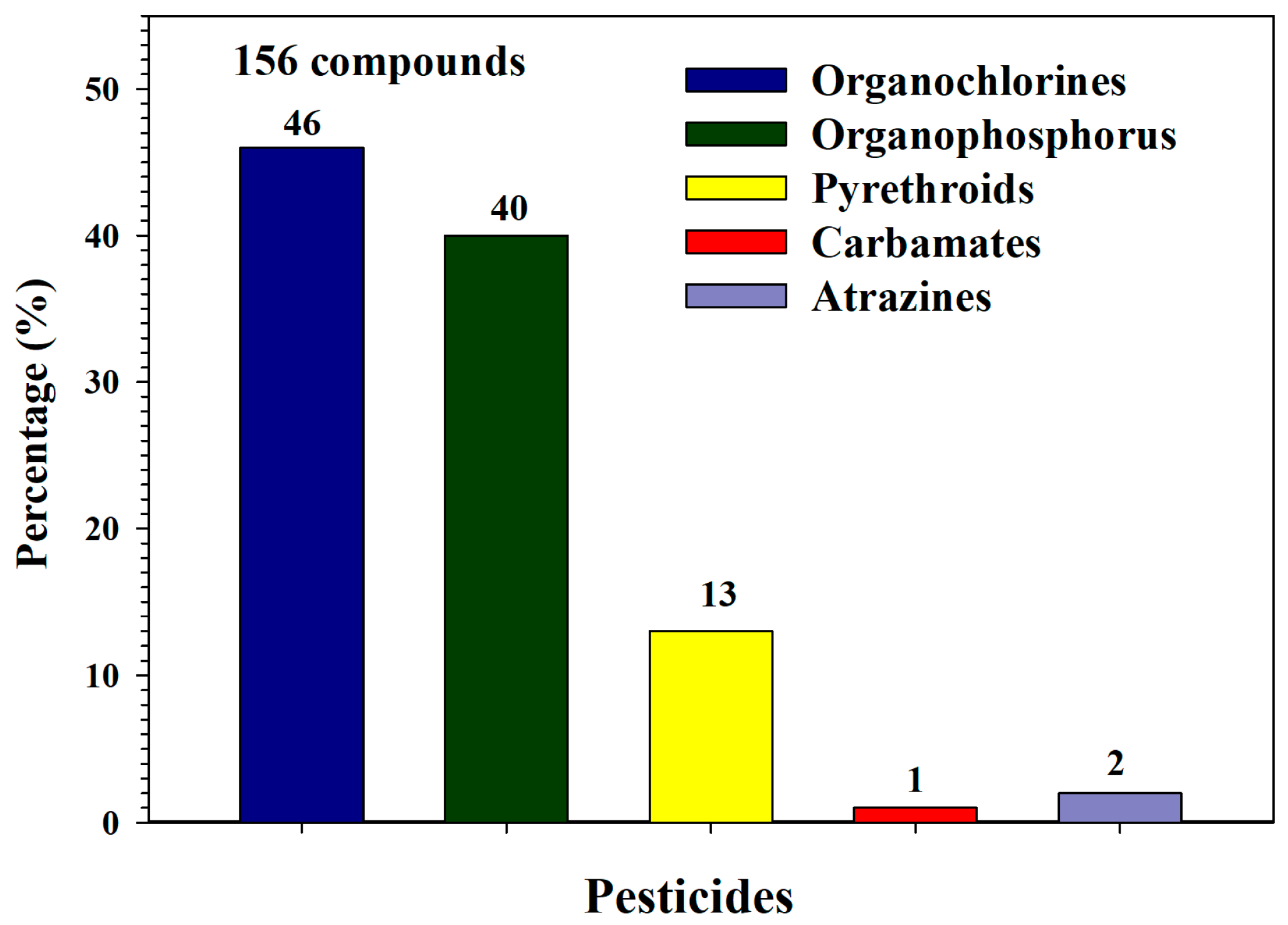
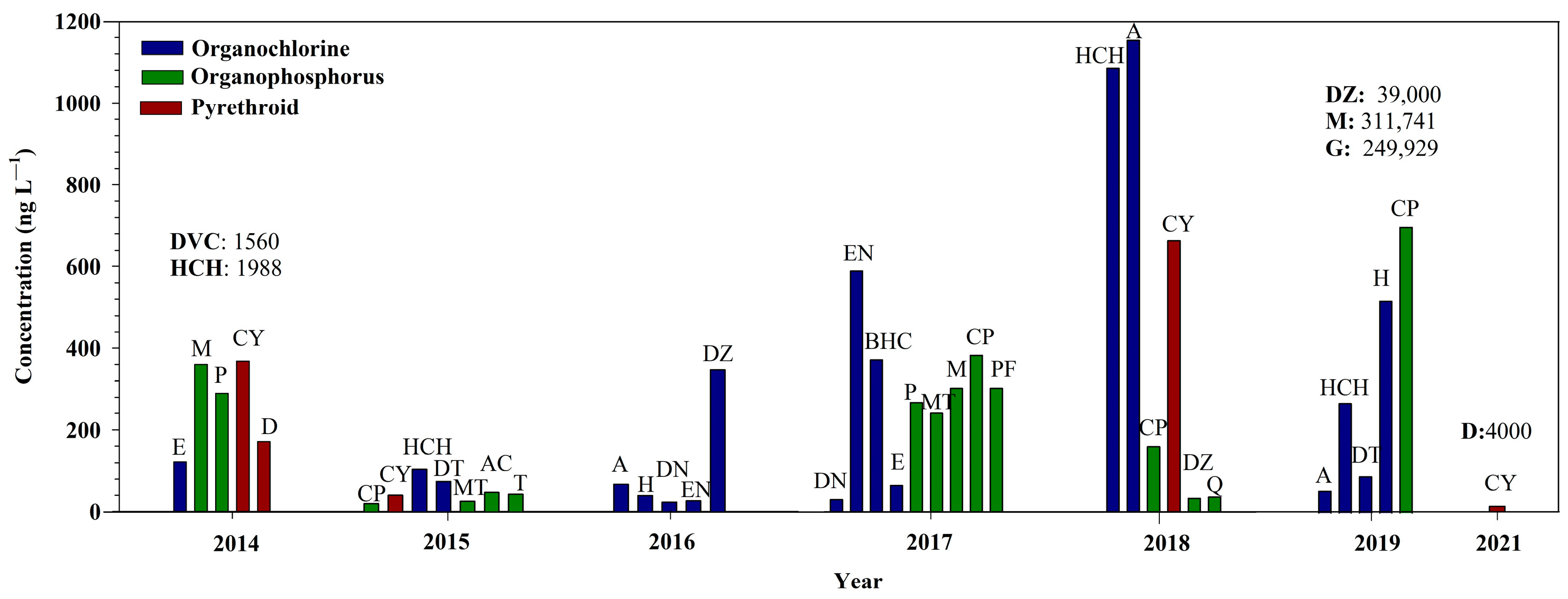
3. Results
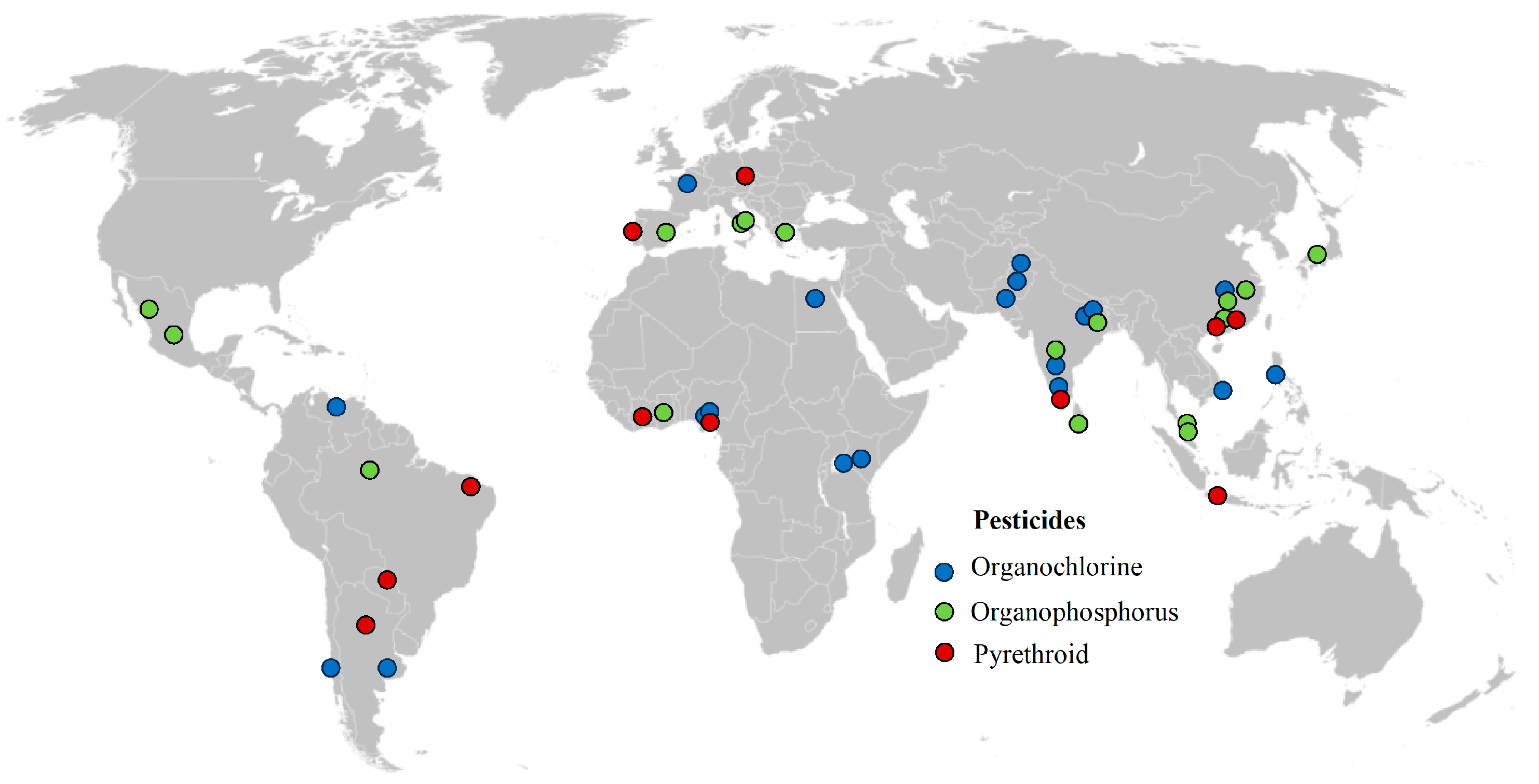
3.1. Organochlorine Pesticides
3.2. Organophosphorus Pesticides
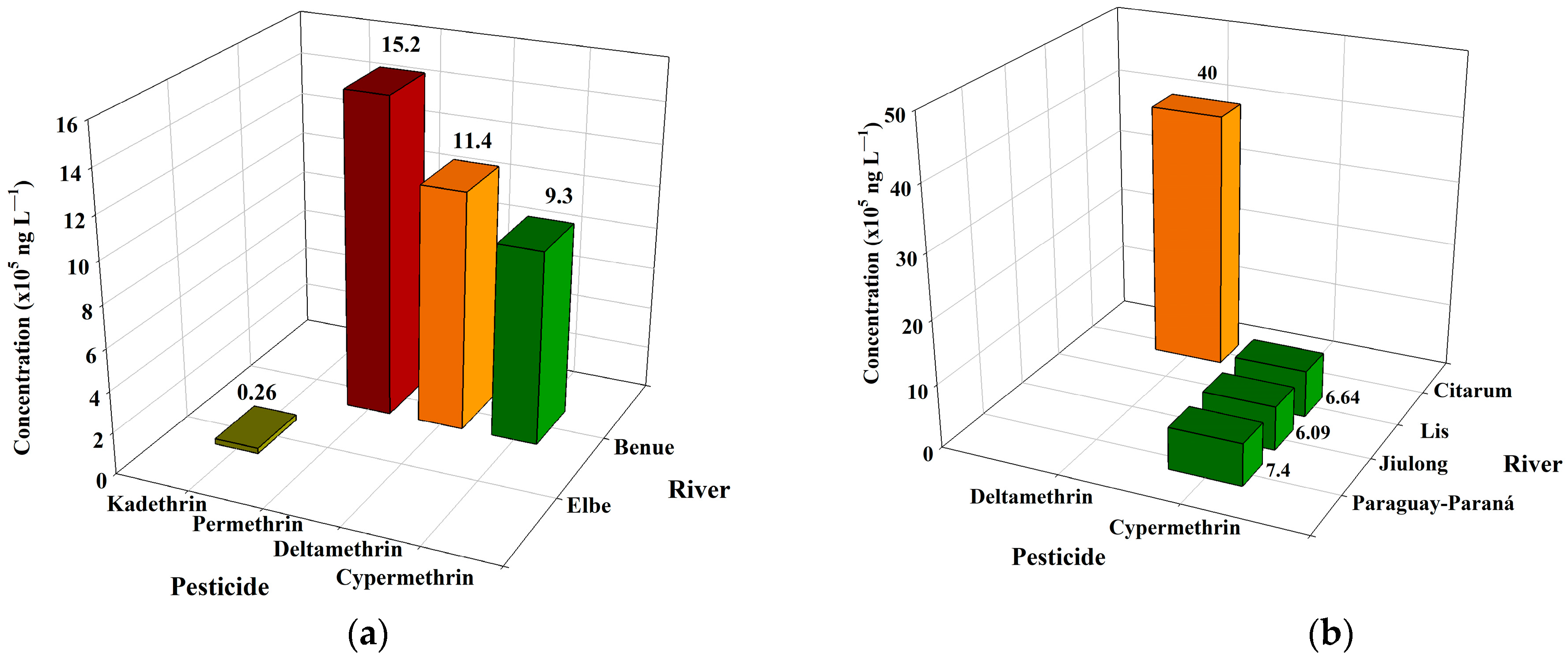
3.3. Pyrethroid Pesticides
4. Discussion
4.1. Food Contamination
4.2. Human Health Risks
4.3. Impact on the Ecosystems
5. Future Research Directions
- i.
- Source identification: Implement studies to determine the sources of pesticide contamination in water bodies to prevent their spread. These efforts should be supported by global collaboration, incorporating scientific research, regulatory frameworks, and active community engagement;
- ii.
- Monitoring programs: Establish monitoring programs to assess pesticide levels in major rivers, taking their geographical distribution into account;
- iii.
- Technological advancements: Develop new or modified materials, technologies, and processes capable of reducing or eliminating pesticides in water bodies. These advancements should be accessible, sustainable, and cost-effective.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction, Analytical and Advanced Methods for Determination of Pesticides in Environment and Foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The Benefits of Pesticides to Mankind and the Environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide Productivity and Food Security. A Review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Damalas, C. Understanding Benefits and Risks of Pesticide Use. Sci. Res. Essays 2009, 4, 945–949. [Google Scholar]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Md Meftaul, I.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the Urban Environment: A Potential Threat That Knocks at the Door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators—Global, Regional and Country Trends, 1990–2020; FAO Analytical Briefs, no. 46, ed.; FAO: Rome, Italy, 2022; ISBN 978-92-5-136614-1. [Google Scholar]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. ISBN 978-3-319-27455-3. [Google Scholar]
- Chawla, P.; Kaushik, R.; Shiva Swaraj, V.J.; Kumar, N. Organophosphorus Pesticides Residues in Food and Their Colorimetric Detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 292–307. [Google Scholar] [CrossRef]
- Glinski, D.A.; Purucker, S.T.; Van Meter, R.J.; Black, M.C.; Henderson, W.M. Analysis of Pesticides in Surface Water, Stemflow, and Throughfall in an Agricultural Area in South Georgia, USA. Chemosphere 2018, 209, 496–507. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mahmood, H.Z.; Damalas, C.A. Pesticide Use and Risk Perceptions among Farmers in the Cotton Belt of Punjab, Pakistan. Crop Prot. 2015, 67, 184–190. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Ahmadipour, A.; Rahimi, H.-R.; Abdollahi, M. Adverse Effects of Organophosphorus Pesticides on the Liver: A Brief Summary of Four Decades of Research. Arch. Ind. Hyg. Tox. 2017, 68, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, M.; Mishra, S.R.; et al. Sustainable Remediation Technologies for Removal of Pesticides as Organic Micro-Pollutants from Water Environments: A Review. Appl. Surf. Sci. Adv. 2024, 19, 100558. [Google Scholar] [CrossRef]
- Chaudhari, Y.S.; Kumar, P.; Soni, S.; Gacem, A.; Kumar, V.; Singh, S.; Yadav, V.K.; Dawane, V.; Piplode, S.; Jeon, B.H.; et al. An Inclusive Outlook on the Fate and Persistence of Pesticides in the Environment and Integrated Eco-Technologies for Their Degradation. Toxicol. Appl. Pharmacol. 2023, 466, 116449. [Google Scholar] [CrossRef]
- Ngin, P.; Haglund, P.; Proum, S.; Fick, J. Pesticide Screening of Surface Water and Soil along the Mekong River in Cambodia. Sci. Total Environ. 2024, 912, 169312. [Google Scholar] [CrossRef]
- Tröger, R.; Ren, H.; Yin, D.; Postigo, C.; Nguyen, P.D.; Baduel, C.; Golovko, O.; Been, F.; Joerss, H.; Boleda, M.R.; et al. What’s in the Water?—Target and Suspect Screening of Contaminants of Emerging Concern in Raw Water and Drinking Water from Europe and Asia. Water Res. 2021, 198, 117099. [Google Scholar] [CrossRef]
- Rapp-Wright, H.; Regan, F.; White, B.; Barron, L.P. A Year-Long Study of the Occurrence and Risk of over 140 Contaminants of Emerging Concern in Wastewater Influent, Effluent and Receiving Waters in the Republic of Ireland. Sci. Total Environ. 2023, 860, 160379. [Google Scholar] [CrossRef]
- Stehle, S.; Bline, A.; Bub, S.; Petschick, L.L.; Wolfram, J.; Schulz, R. Aquatic Pesticide Exposure in the U.S. as a Result of Non-Agricultural Uses. Environ. Int. 2019, 133, 105234. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current Status of Persistent Organic Pesticides Residues in Air, Water, and Soil, and Their Possible Effect on Neighboring Countries: A Comprehensive Review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Rohani, M.F. Pesticides Toxicity in Fish: Histopathological and Hemato-Biochemical Aspects—A Review. Emerg. Contam. 2023, 9, 100234. [Google Scholar] [CrossRef]
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. In Green Chemistry: An Inclusive Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. [Google Scholar] [CrossRef]
- Md Anawar, H.; Chowdhury, R. Remediation of Polluted River Water by Biological, Chemical, Ecological and Engineering Processes. Sustainability 2020, 12, 7017. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Sharma, D.K.; Sarkar, M.; Mishra, B.K.; Puri, V.; Priyadarshini, I.; Thong, P.H.; Ngo, P.T.T.; Nhu, V.-H. Water Pollution Examination through Quality Analysis of Different Rivers: A Case Study in India. Environ. Dev. Sustain. 2022, 24, 7471–7492. [Google Scholar] [CrossRef]
- Padowski, J.C.; Gorelick, S.M. Corrigendum: Global Analysis of Urban Surface Water Supply Vulnerability (2014 Environ. Res. Lett. 9 104004). Environ. Res. Lett. 2014, 9, 119501. [Google Scholar] [CrossRef]
- Brovini, E.M.; Cardoso, S.J.; Quadra, G.R.; Vilas-Boas, J.A.; Paranaíba, J.R.; Pereira, R.d.O.; Mendonça, R.F. Glyphosate Concentrations in Global Freshwaters: Are Aquatic Organisms at Risk? Environ. Sci. Poll. Res. 2021, 28, 60635–60648. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: New York, NY, USA, 2020; pp. 29–42. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides Impacts on Human Health and the Environment with Their Mechanisms of Action and Possible Countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An Overview on the Potential Hazards of Pyrethroid Insecticides in Fish, with Special Emphasis on Cypermethrin Toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Fang, C.; Lou, X.; Zhang, X.; Li, S.; Tang, Y.; Shi, Y.; Huang, D. Simultaneous Determination of Seven Pyrethroid Pesticide Residues in Aquatic Products by Gas Chromatography. Fishes 2024, 9, 79. [Google Scholar] [CrossRef]
- Savoca, D.; Vazzana, M.; Arizza, V.; Maccotta, A.; Orecchio, S.; Longo, F.; Giudice, V.; D’Oca, G.; Messina, S.; Marrone, F.; et al. Contamination Profiles of Selected Pollutants in Procambarus Clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications. J. Xenobiot. 2024, 14, 893–906. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A. Bioaccumulation, Biodistribution, Toxicology and Biomonitoring of Organofluorine Compounds in Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef]
- Matsushita, T.; Morimoto, A.; Kuriyama, T.; Matsumoto, E.; Matsui, Y.; Shirasaki, N.; Kondo, T.; Takanashi, H.; Kameya, T. Removals of Pesticides and Pesticide Transformation Products during Drinking Water Treatment Processes and Their Impact on Mutagen Formation Potential after Chlorination. Water Res. 2018, 138, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, H.R.; Roubík, H. Water Purification Using Activated Carbon Prepared from Agriculture Waste—Overview of a Recent Development. Biomass. Convers. Biorefin. 2023, 13, 15577–15590. [Google Scholar] [CrossRef]
- Elmorsi, R.R.; Abou-El-Sherbini, K.S.; Shehab El-Dein, W.A.; Lotfy, H.R. Activated Eco-Waste of Posidonia Oceanica Rhizome as a Potential Adsorbent of Methylene Blue from Saline Water. Biomass. Convers. Biorefin. 2024, 14, 2529–2542. [Google Scholar] [CrossRef]
- Saeed, M.F.; Shaheen, M.; Ahmad, I.; Zakir, A.; Nadeem, M.; Chishti, A.A.; Shahid, M.; Bakhsh, K.; Damalas, C.A. Pesticide Exposure in the Local Community of Vehari District in Pakistan: An Assessment of Knowledge and Residues in Human Blood. Sci. Total Environ. 2017, 587–588, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef]
- Aparicio, V.; De Gerónimo, E. Pesticide Pollution in Argentine Drinking Water: A Call to Ensure Safe Access. Environ. Chall. 2024, 14, 100808. [Google Scholar] [CrossRef]
- Marete, G.M.; Lalah, J.O.; Mputhia, J.; Wekesa, V.W. Pesticide Usage Practices as Sources of Occupational Exposure and Health Impacts on Horticultural Farmers in Meru County, Kenya. Heliyon 2021, 7, e06118. [Google Scholar] [CrossRef]
- Tariq, M.I.; Afzal, S.; Hussain, I.; Sultana, N. Pesticides Exposure in Pakistan: A Review. Environ. Int. 2007, 33, 1107–1122. [Google Scholar] [CrossRef]
- Bianchi, M.; Paravani, E.V.; Acosta, M.G.; Odetti, L.M.; Simoniello, M.F.; Poletta, G.L. Pesticide-Induced Alterations in Zebrafish (Danio rerio) Behavior, Histology, DNA Damage and MRNA Expression: An Integrated Approach. Comp. Biochem. Phys. C 2024, 280, 109895. [Google Scholar] [CrossRef] [PubMed]
- Crupkin, A.C.; Iturburu, F.G.; Crupkin, M.; Menone, M.L. Myofibrilar Functional Dysregulation in Fish: A New Biomarker of Damage to Pesticides. Ecotoxicol. Environ. Saf. 2018, 158, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rubira, R.J.G.; Batista, V.R.G.; Correia, R.R.; Pazin, W.M.; Maximino, M.D.; Ruiz, G.C.M.; Teixeira, G.R.; Job, A.E. Biological Responses to Imazapic and Methyl Parathion Pesticides in Bioinspired Lipid Membranes and Tilapia Fish. J. Hazard. Mater. 2023, 458, 131943. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.S.; Bruce, K.H.R.; da Costa, J.C.; Pereira, D.; da Silva, G.S.; Val, A.L. Effects of Climate Change and Mixtures of Pesticides on the Amazonian Fish Colossoma Macropomum. Sci. Total Environ. 2024, 922, 171379. [Google Scholar] [CrossRef] [PubMed]
- Hrynko, I.; Kaczyński, P.; Łozowicka, B. A Global Study of Pesticides in Bees: QuEChERS as a Sample Preparation Methodology for Their Analysis—Critical Review and Perspective. Sci. Total Environ. 2021, 792, 148385. [Google Scholar] [CrossRef]
- Braak, N.; Neve, R.; Jones, A.K.; Gibbs, M.; Breuker, C.J. The Effects of Insecticides on Butterflies—A Review. Environ. Pollut. 2018, 242, 507–518. [Google Scholar] [CrossRef]
- Pandey, S.P.; Mohanty, B. Disruption of the Hypothalamic-Pituitary-Thyroid Axis on Co-Exposures to Dithiocarbamate and Neonicotinoid Pesticides: Study in a Wildlife Bird, Amandava Amandava. Neurotoxicology 2017, 60, 16–22. [Google Scholar] [CrossRef]
- Xiao, K.; Lu, Z.; Wang, J.; Cai, M. 52 Organic Pesticides in Feathers of Three Species of Migratory Birds Overwintering in the Tibetan Plateau. Ecol. Indic. 2023, 149, 110164. [Google Scholar] [CrossRef]
- Chen, L.; Diao, J.; Zhang, W.; Zhang, L.; Wang, Z.; Li, Y.; Deng, Y.; Zhou, Z. Effects of Beta-Cypermethrin and Myclobutanil on Some Enzymes and Changes of Biomarkers between Internal Tissues and Saliva in Reptiles (Eremias argus). Chemosphere 2019, 216, 69–74. [Google Scholar] [CrossRef]
- Odetti, L.M.; González, E.C.L.; Siroski, P.A.; Simoniello, M.F.; Poletta, G.L. How the Exposure to Environmentally Relevant Pesticide Formulations Affects the Expression of Stress Response Genes and Its Relation to Oxidative Damage and Genotoxicity in Caiman Latirostris. Environ. Toxicol. Pharmacol. 2023, 97, 104014. [Google Scholar] [CrossRef]
- Tavalieri, Y.E.; Galoppo, G.H.; Canesini, G.; Luque, E.H.; Muñoz-de-Toro, M.M. Effects of Agricultural Pesticides on the Reproductive System of Aquatic Wildlife Species, with Crocodilians as Sentinel Species. Mol. Cell. Endocrinol. 2020, 518, 110918. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil. Ecology 2020, 147, 103356. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Arunachalam, V.; Magu, S.P. Impact of Azadirachtin, an Insecticidal Allelochemical from Neem on Soil Microflora, Enzyme and Respiratory Activities. Bioresour. Technol. 2007, 98, 3154–3158. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fantke, P. Toward Harmonizing Global Pesticide Regulations for Surface Freshwaters in Support of Protecting Human Health. J. Environ. Manag. 2022, 301, 113909. [Google Scholar] [CrossRef]
- Leoci, R.; Ruberti, M.; Leoci, R.; Ruberti, M. Pesticides: An Overview of the Current Health Problems of Their Use. J. Geosci. Environ. Prot. 2021, 09, 1–20. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Guo, L.; Xu, X.; Liu, L.; Xu, L.; Song, S.; Xu, C.; Kuang, H. Advances in Immunoassays for Organophosphorus and Pyrethroid Pesticides. TrAC Trends Anal. Chem. 2020, 131, 116022. [Google Scholar] [CrossRef]
- da Silva Sousa, J.; do Nascimento, H.O.; de Oliveira Gomes, H.; do Nascimento, R.F. Pesticide Residues in Groundwater and Surface Water: Recent Advances in Solid-Phase Extraction and Solid-Phase Microextraction Sample Preparation Methods for Multiclass Analysis by Gas Chromatography-Mass Spectrometry. Microchem. J. 2021, 168, 106359. [Google Scholar] [CrossRef]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Melchor Martínez, E.M.; Sosa-Hernández, J.E.; Bilal, M.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Highly Hazardous Pesticides and Related Pollutants: Toxicological, Regulatory, and Analytical Aspects. Sci. Total Environ. 2022, 807, 151879. [Google Scholar] [CrossRef]
- Leyva Morales, J.B.; Valdez Torres, J.B.; Bastidas Bastidas, P.d.J.; Angulo Escalante, M.Á.; Sarmiento Sánchez, J.I.; Barraza Lobo, A.L.; Olmeda Rubio, C.; Chaidez Quiroz, C. Monitoring of pesticides residues in northwestern Mexico rivers. Acta Univ. 2017, 27, 45–54. [Google Scholar] [CrossRef]
- Lourençato, L.; Favaretto, N.; Hansel, F.; De, A.; Scheer, A.; Luz, L.; Cláudio, L.; Souza, P.; Dieckow, J.; Buch, A. Effects on Water Quality of Pesticide Use in Farmland Under Intensive Soil Management in Southern Brazil. Int. J. Plant Soil Sci. 2015, 5, 155–166. [Google Scholar] [CrossRef]
- United Nations. Stockholm Convention on Persistent Organic Pollutants. In Proceedings of the Persistent Organic Pollutants Review Committee, 18th Meeting, Roma, Italy, 26–30 September 2022; Assessment of alternatives to DDT: Geneva, Switzerland, 2012. [Google Scholar]
- The Ministry of Health Drinking Water Quality. 3 Chemical and Physical determinands: Part 2.3 Pesticides. In Guidelines for Drinking-Water Quality Management for New Zealand; Ministry of Health, New Zealand Government: Wellington, New Zealand, 2019; pp. 1–1634. [Google Scholar]
- Taiwo, A.M. A Review of Environmental and Health Effects of Organochlorine Pesticide Residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Liu, Q. Sources, Concentrations and Risk Factors of Organochlorine Pesticides in Soil, Water and Sediment in the Yellow River Estuary. Mar. Pollut. Bull. 2015, 100, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Qi, S.; Zhang, J.; Wu, C.; Xing, X. Organochlorine Pesticides in Soil, Water and Sediment along the Jinjiang River Mainstream to Quanzhou Bay, Southeast China. Ecotoxicol. Environ. Saf. 2013, 89, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Kaczyński, P.; Wolejko, E.; Piekutin, J.; Sagitov, A.; Toleubayev, K.; Isenova, G.; Abzeitova, E. Evaluation of Organochlorine Pesticide Residues in Soil and Plants from East Europe and Central Asia. Desalination Water Treat. 2016, 57, 1310–1321. [Google Scholar] [CrossRef]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global Footprints of Organochlorine Pesticides: A Pan-Global Survey. Environ. Geochem. Health 2022, 44, 149–177. [Google Scholar] [CrossRef]
- Jackovitz, A.M.; Hebert, R.M. Chapter 27: Wildlife Toxicity Assessment for Hexachlorocyclohexane (HCH). In Wildlife Toxicity Assessments for Chemicals of Military Concern, 1st, ed.; Williams, M.A., Reddy, G., Quinn, M.J., Jr., Johnson, M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 473–497. [Google Scholar] [CrossRef]
- Kaushik, P.; Kaushik, G. An Assessment of Structure and Toxicity Correlation in Organochlorine Pesticides. J. Hazard. Mater. 2007, 143, 102–111. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Removal of Organochlorine Pesticides (OCPs) from Aqueous Solutions Using Hydrogen Peroxide, Ultrasonic Waves, and a Hybrid Process. Sep. Purif. Technol. 2018, 192, 457–464. [Google Scholar] [CrossRef]
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification; WHO, 2019th ed.; WHO: Geneve, Switzerland, 2020; Volume 1. [Google Scholar]
- Dahshan, H.; Megahed, A.; Abd-Elall, A.; Abd-El-Kader, M.A.-G.; Nabawy, E.; Elbana, M. Monitoring of Pesticides Water Pollution-The Egyptian River Nile. J. Environ. Health Sci. Eng. 2016, 14, 15. [Google Scholar] [CrossRef]
- Shah, Z.U.; Parveen, S. Pesticides Pollution and Risk Assessment of River Ganga: A Review. Heliyon 2021, 7, e07726. [Google Scholar] [CrossRef]
- Ogbeide, O.; Tongo, I.; Ezemonye, L. Risk Assessment of Agricultural Pesticides in Water, Sediment, and Fish from Owan River, Edo State, Nigeria. Environ. Monit. Assess. 2015, 187, 654. [Google Scholar] [CrossRef]
- Net, S.; Dumoulin, D.; El-Osmani, R.; Rabodonirina, S.; Ouddane, B. Case Study of PAHs, Me-PAHs, PCBs, Phthalates and Pesticides Contamination in the Somme River Water, France. Int. J. Environ. Res. 2014, 8, 1159–1170. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Y.; Qiao, X.; Guo, R.; Liu, C.; Wang, X.; Zhao, X. Risk Assessment of Organochlorine Pesticides in Drinking Water Source of the Yangtze River. Ecotoxicol. Environ. Saf. 2019, 182, 109390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Nguyen, B.T.; Tran, H.T.T.; Mai, H.; Duong, T.T.; Bach, Q.-V. Seasonal, Spatial Variation, and Potential Sources of Organochlorine Pesticides in Water and Sediment in the Lower Reaches of the Dong Nai River System in Vietnam. Arch. Environ. Contam. Toxicol. 2019, 77, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chaudhry, M.N.; Ahmad, S.R.; Nazir, R.; Zhao, Z.; Javed, R.; Alghamdi, H.A.; Mahmood, A. Ecological and Human Health Hazards; Integrated Risk Assessment of Organochlorine Pesticides (OCPs) from the Chenab River, Pakistan. Sci. Total Environ. 2023, 882, 163504. [Google Scholar] [CrossRef]
- Navarrete, I.A.; Tee, K.A.M.; Unson, J.R.S.; Hallare, A. V Organochlorine Pesticide Residues in Surface Water and Groundwater along Pampanga River, Philippines. Environ. Monit. Assess. 2018, 190, 289. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Ferrer, J.; Villouta, M.V. First Report on Organochlorine Pesticides in Water in a Highly Productive Agro-Industrial Basin of the Central Valley, Chile. Chemosphere 2017, 174, 148–156. [Google Scholar] [CrossRef]
- Ali, U.; Bajwa, A.; Iqbal Chaudhry, M.J.; Mahmood, A.; Syed, J.H.; Li, J.; Zhang, G.; Jones, K.C.; Malik, R.N. Significance of Black Carbon in the Sediment–Water Partitioning of Organochlorine Pesticides (OCPs) in the Indus River, Pakistan. Ecotoxicol. Environ. Saf. 2016, 126, 177–185. [Google Scholar] [CrossRef]
- Ndunda, E.N.; Madadi, V.O.; Wandiga, S.O. Organochlorine Pesticide Residues in Sediment and Water from Nairobi River, Kenya: Levels, Distribution, and Ecological Risk Assessment. Environ. Sci. Pollut. Res. 2018, 25, 34510–34518. [Google Scholar] [CrossRef]
- Ogola, J.O.; Olale, K.; Mogwasi, R.; Mainya, O. Organochlorine Pesticide Residues in Water and Sediments in River Kibos-Nyamasaria in Kisumu County: An Inlet River of Lake Victoria, Kenya. Sci. Afr. 2024, 23, e02094. [Google Scholar] [CrossRef]
- Gandla, V.K.; Chiluka, M.; Gupta, H.; Sinha, S.N.; Chakraborty, P. Sediment-Water Partitioning and Risk Assessment of Organochlorine Pesticides along the Urban, Peri-Urban and Rural Transects of Krishna River Basin, Peninsular India. Sci. Total Environ. 2023, 874, 162360. [Google Scholar] [CrossRef]
- Muhammed, H.A.; Yahaya, A.; Abdullahi, S.S.; Jagaba, A.H.; Birniwa, A.H. Mitigating Water Contamination by Controlling Anthropogenic Activities of Organochlorine Pesticides (OCPs) for Surface Water Quality Assurance. Case Stud. Chem. Environ. Eng. 2023, 8, 100474. [Google Scholar] [CrossRef]
- Chakraborty, P.; Khuman, S.N.; Selvaraj, S.; Sampath, S.; Devi, N.L.; Bang, J.J.; Katsoyiannis, A. Polychlorinated Biphenyls and Organochlorine Pesticides in River Brahmaputra from the Outer Himalayan Range and River Hooghly Emptying into the Bay of Bengal: Occurrence, Sources and Ecotoxicological Risk Assessment. Environ. Pollut. 2016, 219, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Rex, K.R.; Chakraborty, P. Legacy and New Chlorinated Persistent Organic Pollutants in the Rivers of South India: Occurrences, Sources, Variations before and after the Outbreak of the COVID-19 Pandemic. J. Hazard. Mater. 2022, 437, 129262. [Google Scholar] [CrossRef] [PubMed]
- Taufeeq, A.; Baqar, M.; Sharif, F.; Mumtaz, M.; Ullah, S.; Aslam, S.; Qadir, A.; Majid, M.; Jun, H. Assessment of Organochlorine Pesticides and Health Risk in Tobacco Farming Associated with River Barandu of Pakistan. Environ. Sci. Pollut. Res. 2021, 28, 38774–38791. [Google Scholar] [CrossRef]
- Silva-Barni, M.F.; Smedes, F.; Fillmann, G.; Miglioranza, K.S.B. Passive Sampling of Pesticides and Polychlorinated Biphenyls along the Quequén Grande River Watershed, Argentina. Environ. Toxicol. Chem. 2019, 38, 340–349. [Google Scholar] [CrossRef]
- Cárdenas-Izaguirre, S.F.; Márquez-Romance, A.M.; Guevara-Pérez, E.; Pérez-Pacheco, S.A. An Approach to Models for Transport and Transformation of Organochlorine Pesticides in Rivers. Environ. Qual. Manag. 2022, 31, 369–391. [Google Scholar] [CrossRef]
- WHO (Ed.) 8. Chemical Aspects. In Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneve, Switzerland, 2017; Volume 1, pp. 155–201. ISBN 978-92-4-154995-0. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine Pesticides, Their Toxic Effects on Living Organisms and Their Fate in the Environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A Treatise on Organophosphate Pesticide Pollution: Current Strategies and Advancements in Their Environmental Degradation and Elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Gochev, V.; Velkova, Z. Electrochemical Biosensors for Direct Determination of Organophosphorus Pesticides: A Review. Curr. Anal. Chem. 2016, 12, 37–42. [Google Scholar] [CrossRef]
- Poomagal, S.; Sujatha, R.; Kumar, P.S.; Vo, D.V.N. A Fuzzy Cognitive Map Approach to Predict the Hazardous Effects of Malathion to Environment (Air, Water and Soil). Chemosphere 2021, 263, 127926. [Google Scholar] [CrossRef]
- Xiong, S.; Deng, Y.; Zhou, Y.; Gong, D.; Xu, Y.; Yang, L.; Chen, H.; Chen, L.; Song, T.; Luo, A.; et al. Current Progress in Biosensors for Organophosphorus Pesticide Based on Enzyme Functionalized Nanostructures: A Review. Anal. Meth. 2018, 10, 5468–5479. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Ding, Y.; Liu, B.; Xiao, W. A Systems-Level Approach for Investigating Organophosphorus Pesticide Toxicity. Ecotoxicol. Environ. Saf. 2018, 149, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; Chidya, R.; Jadoon, W.; Sakugawa, H. Temporal Trends in Organophosphorus Pesticides Use and Concentrations in River Water in Japan, and Risk Assessment. J. Environ. Sci. 2019, 79, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, Y.; Liu, S.; Zhang, J.; Zhang, C.; Jiang, H.; Han, X.; He, L.; Wang, S.; Zhang, K. Colorimetric and Fluorescent Sensors for Detection of Nerve Agents and Organophosphorus Pesticides. Sens. Actuators B 2021, 344, 130278. [Google Scholar] [CrossRef]
- Ning, Y.; Li, K.; Zhao, Z.; Chen, D.; Li, Y.; Liu, Y.; Yang, Q.; Jiang, B. Simultaneous Electrochemical Degradation of Organophosphorus Pesticides and Recovery of Phosphorus: Synergistic Effect of Anodic Oxidation and Cathodic Precipitation. J. Taiwan Inst. Chem. Eng. 2021, 125, 267–275. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative Stress and Mitochondrial Dysfunction in Organophosphate Pesticide-Induced Neurotoxicity and Its Amelioration: A Review. Environ. Sci. Pollut. Res. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Sarlak, Z.; Khosravi-Darani, K.; Rouhi, M.; Garavand, F.; Mohammadi, R.; Sobhiyeh, M.R. Bioremediation of Organophosphorus Pesticides in Contaminated Foodstuffs Using Probiotics. Food Control 2021, 126, 108006. [Google Scholar] [CrossRef]
- Stenersen, J. Chemical Pesticides Mode of Action and Toxicology, 1st ed.; CRC Press: Washington, DC, USA, 2004; pp. 165–220. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations-Chlorpyrifos. In Specifications and Evaluations for Agricultural Pesticides; FAO: Roma, Italy, 2015. [Google Scholar]
- Pundir, C.S.; Malik, A. Preety Bio-Sensing of Organophosphorus Pesticides: A Review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Chidya, R.C.G.; Abdel-dayem, S.M.; Takeda, K.; Sakugawa, H. Spatio-Temporal Variations of Selected Pesticide Residues in the Kurose River in Higashi-Hiroshima City, Japan. J. Environ. Sci. Health B 2018, 53, 602–614. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, J.; Li, Z.; Chen, A.; Zhang, Q. The Occurrence and Risk Assessment of Five Organophosphorus Pesticides in River Water from Shangyu, China. Environ. Monit. Assess. 2016, 188, 614. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Yang, Y.; Tam, N.F.-Y.; Tao, R.; Dai, Y.-N. Pesticides in Three Rural Rivers in Guangzhou, China: Spatiotemporal Distribution and Ecological Risk. Environ. Sci. Pollut. Res. 2019, 26, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, V.; Martinez-Capel, F.; Masiá, A.; Picó, Y. Patterns of Presence and Concentration of Pesticides in Fish and Waters of the Júcar River (Eastern Spain). J. Hazard. Mater. 2014, 265, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Omar, T.F.T.; Aris, A.Z.; Lee, Y. Surface Water Organophosphorus Pesticides Concentration and Distribution in the Langat River, Selangor, Malaysia. Expo. Health 2016, 8, 497–511. [Google Scholar] [CrossRef]
- Mondal, R.; Mukherjee, A.; Biswas, S.; Kole, R.K. GC-MS/MS Determination and Ecological Risk Assessment of Pesticides in Aquatic System: A Case Study in Hooghly River Basin in West Bengal, India. Chemosphere 2018, 206, 217–230. [Google Scholar] [CrossRef]
- Papadakis, E.N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Makris, K.C.; Papadopoulou-Mourkidou, E. A Pesticide Monitoring Survey in Rivers and Lakes of Northern Greece and Its Human and Ecotoxicological Risk Assessment. Ecotoxicol. Environ. Saf. 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Nyantakyi, J.A.; Wiafe, S.; Akoto, O. Seasonal Changes in Pesticide Residues in Water and Sediments from River Tano, Ghana. J. Environ. Public Health 2022, 2022, 8997449. [Google Scholar] [CrossRef]
- Zainuddin, A.H.; Wee, S.Y.; Aris, A.Z. Occurrence and Potential Risk of Organophosphorus Pesticides in Urbanised Linggi River, Negeri Sembilan, Malaysia. Environ. Geochem. Health 2020, 42, 3703–3715. [Google Scholar] [CrossRef]
- Montuori, P.; Aurino, S.; Nardone, A.; Cirillo, T.; Triassi, M. Spatial Distribution and Partitioning of Organophosphates Pesticide in Water and Sediment from Sarno River and Estuary, Southern Italy. Environ. Sci. Pollut. Res. 2015, 22, 8629–8642. [Google Scholar] [CrossRef]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological Risk and Estimates of Organophosphate Pesticides Loads into the Central Mediterranean Sea from Volturno River, the River of the “Land of Fires” Area, Southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Y.; Zhai, Y.; Zhang, C.; Pan, Z.; Yue, W. Influence of Surface-Water Irrigation on the Distribution of Organophosphorus Pesticides in Soil-Water Systems, Jianghan Plain, Central China. J. Environ. Manag. 2021, 281, 111874. [Google Scholar] [CrossRef]
- Shipley, E.R.; Vlahos, P.; Chandrajith, R.; Wijerathna, P. Agrochemical Exposure in Sri Lankan Inland Water Systems. Environ. Adv. 2022, 7, 100150. [Google Scholar] [CrossRef]
- Lari, S.Z.; Khan, N.A.; Gandhi, K.N.; Meshram, T.S.; Thacker, N.P. Comparison of Pesticide Residues in Surface Water and Ground Water of Agriculture Intensive Areas. J. Environ. Health Sci. Eng. 2014, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; de Oliveira, R.; Silva de Souza Nunes, G.; Rizzi, C.; Villa, S.; De Caroli Vizioli, B.; Montagner, C.C.; Waichman, A.V. Ecological Risk Assessment of Pesticides in Urban Streams of the Brazilian Amazon. Chemosphere 2022, 291, 132821. [Google Scholar] [CrossRef] [PubMed]
- Silva-Madera, R.J.; Salazar-Flores, J.; Peregrina-Lucano, A.A.; Mendoza-Michel, J.; Ceja-Gálvez, H.R.; Rojas-Bravo, D.; Reyna-Villela, M.Z.; Torres-Sánchez, E.D. Pesticide Contamination in Drinking and Surface Water in the Cienega, Jalisco, Mexico. Water Air Soil Pollut. 2021, 232, 43. [Google Scholar] [CrossRef]
- Lockridge, O.; Verdier, L.; Schopfer, L.M. Half-Life of Chlorpyrifos Oxon and Other Organophosphorus Esters in Aqueous Solution. Chem. Biol. Interact. 2019, 311, 108788. [Google Scholar] [CrossRef]
- Burns, C.J.; Pastoor, T.P. Pyrethroid Epidemiology: A Quality-Based Review. Crit. Rev. Toxicol. 2018, 48, 297–311. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. The Estrogenic and Androgenic Potential of Pyrethroids in Vitro. Review. Toxicol. Vitr. 2016, 34, 321–332. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and Health Effects—An Update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid Pesticide Residues in the Global Environment: An Overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Ranatunga, M.; Kellar, C.; Pettigrove, V. Toxicological Impacts of Synthetic Pyrethroids on Non-Target Aquatic Organisms: A Review. Environ. Adv. 2023, 12, 100388. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Gónzalez-Párraga, P.; Meseguer, J.; Cuesta, A.; Esteban, M.A. Modulatory Effects of Deltamethrin-Exposure on the Immune Status, Metabolism and Oxidative Stress in Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 2014, 36, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, A.; Kumar, J. Pyrethroid Pesticides: An Overview on Classification, Toxicological Assessment and Monitoring. J. Hazard. Mater. Adv. 2023, 10, 100284. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Mediation of Oxidative Stress Toxicity Induced by Pyrethroid Pesticides in Fish. Comp. Biochem. Phys. C 2020, 234, 108758. [Google Scholar] [CrossRef] [PubMed]
- Corcellas, C.; Eljarrat, E.; Barceló, D. First Report of Pyrethroid Bioaccumulation in Wild River Fish: A Case Study in Iberian River Basins (Spain). Environ. Int. 2015, 75, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kadala, A.; Charreton, M.; Jakob, I.; Cens, T.; Rousset, M.; Chahine, M.; Le Conte, Y.; Charnet, P.; Collet, C. Pyrethroids Differentially Alter Voltage-Gated Sodium Channels from the Honeybee Central Olfactory Neurons. PLoS ONE 2014, 9, e112194. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Elucidation of Pyrethroid and DDT Receptor Sites in the Voltage-Gated Sodium Channel. Neurotoxicology 2017, 60, 171–177. [Google Scholar] [CrossRef]
- Gajendiran, A.; Abraham, J. An Overview of Pyrethroid Insecticides. Front. Biol. 2018, 13, 79–90. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-Degrading Microorganisms and Their Potential for the Bioremediation of Contaminated Soils: A Review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef]
- Shilpakar, O.; Karki, B. Cypermethrin Poisoning Manifesting with Prolonged Bradycardia: A Case Report. Toxicol. Rep. 2021, 8, 10–12. [Google Scholar] [CrossRef]
- Cantalamessa, F. Acute Toxicity of Two Pyrethroids, Permethrin, and Cypermethrin in Neonatal and Adult Rats. Arch. Toxicol. 1993, 67, 510–513. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, Insecticidal Activity, Resistance, Photodegradation and Toxicity of Pyrethroids (A Review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, E.M.; Williams, M.T.; Vorhees, C.V. Effects of Pyrethroids on Brain Development and Behavior: Deltamethrin. Neurotoxicol. Teratol. 2021, 87, 106983. [Google Scholar] [CrossRef]
- Fernández-Ramos, C.; Šatínský, D.; Solich, P. New Method for the Determination of Carbamate and Pyrethroid Insecticides in Water Samples Using On-Line SPE Fused Core Column Chromatography. Talanta 2014, 129, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, M.; Yusiasih, R.; Endah, E.S.; Koesmawati, T.A.; Ridwan, Y.S.; Rohman, O.; Wulan, D.R.; Amran, M.B.; Pitoi, M.M. Pyrethroid Residues in Indonesian River Citarum: A Simple Analytical Method Applied for an Ecological and Human Health Risk Assessment. Chemosphere 2023, 335, 139067. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Sousa, S.; Vera, J.; Bitencourt, L.; Vieira, J.; Jorge, S.; Silva, J.G.; Correia, M.; Domingues, V.F.; Delerue-Matos, C. Multi-Residue Analysis of Fifty Pesticides in River Waters and in Wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 66787–66803. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, K.; Hassan, M.; Xu, C.; Zhang, B.; Gin, K.Y.H.; He, Y. Occurrence, Distribution and Risk Assessment of Pesticides in a River-Reservoir System. Ecotoxicol. Environ. Saf. 2018, 166, 320–327. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, B.; Qiu, X.; Chen, M.; Ma, Z.; Yu, X. Distribution and Risk Assessment of 82 Pesticides in Jiulong River and Estuary in South China. Chemosphere 2016, 144, 1177–1192. [Google Scholar] [CrossRef]
- Akan, J.C.; Battah, N.; Waziri, M.; Mahmud, M. Organochlorine, Organophosphorus and Pyrethroid Pesticides Residues in Water and Sediment Samples from River Benue in Vinikilang, Yola, Adamawa State, Nigeria Using Gas Chromatography-Mass Spectrometry Equipped with Electron Capture Detector. Am. J. Environ. Prot. 2015, 3, 164–173. [Google Scholar] [CrossRef]
- Victor, K.; Marie, D.; Cyrile, Y.; Kouamé, D.; Sanogo, T. Water and Sediments Contamination by Pesticides in Sassandra River at Guessabo Area (Central-Western of Ivory Coast). Trends Appl. Sci. Res. 2023, 18, 94–102. [Google Scholar] [CrossRef]
- Etchegoyen, M.A.; Ronco, A.E.; Almada, P.; Abelando, M.; Marino, D.J. Occurrence and Fate of Pesticides in the Argentine Stretch of the Paraguay-Paraná Basin. Environ. Monit. Assess. 2017, 189, 63. [Google Scholar] [CrossRef]
- Arisekar, U.; Jeya Shakila, R.; Shalini, R.; Jeyasekaran, G. Pesticides Contamination in the Thamirabarani, a Perennial River in Peninsular India: The First Report on Ecotoxicological and Human Health Risk Assessment. Chemosphere 2021, 267, 129251. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, G.E.; Amé, M.V.; Monferrán, M.V.; Bonansea, R.I.; Hued, A.C. A Multi-Level Approach Using Gambusia Affinis as a Bioindicator of Environmental Pollution in the Middle-Lower Basin of Suquía River. Ecol. Indic. 2015, 48, 706–720. [Google Scholar] [CrossRef]
- Duaví, W.C.; Gama, A.F.; Damasceno, É.P.; Moreira, L.B.; Da Silva, V.P.A.; Nascimento, R.F.; Cavalcante, R.M. Are Pesticides Only a Problem from Rural Areas? The Case of a Highly Urbanised Tropical Mangrove (Fortaleza, CE, Brazil). Int. J. Environ. Anal. Chem. 2023, 103, 5868–5886. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, A.N. (Eds.) Natural Bioactive Products in Sustainable Agriculture; Springer Singapore: Singapore, 2020; ISBN 978-981-15-3023-4. [Google Scholar]
- Li, C.; Zhu, H.; Li, C.; Qian, H.; Yao, W.; Guo, Y. The Present Situation of Pesticide Residues in China and Their Removal and Transformation during Food Processing. Food Chem. 2021, 354, 129552. [Google Scholar] [CrossRef]
- FAO. Pesticides Use and Trade, 1990–2021; FAOSTAT Analytical Briefs Series No. 70., ed; Food and Agriculture Organization: Rome, Italy, 2023. [Google Scholar]
- Zhang, W. Global Pesticide Use: Profile, Trend, Cost/Benefit and More. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1. [Google Scholar]
- Chandra, R.; Sharpanabharathi, N.; Prusty, B.A.K.; Azeez, P.A.; Kurakalva, R.M. Organochlorine Pesticide Residues in Plants and Their Possible Ecotoxicological and Agri Food Impacts. Sci. Rep. 2021, 11, 17841. [Google Scholar] [CrossRef]
- Sharma, D.; Nagpal, A.; Pakade, Y.B.; Katnoria, J.K. Analytical Methods for Estimation of Organophosphorus Pesticide Residues in Fruits and Vegetables: A Review. Talanta 2010, 82, 1077–1089. [Google Scholar] [CrossRef]
- Pathak, S.; Solanki, H.; Renuka, A.; Kundu, R. Levels of Organochlorinated Pesticide Residues in Vegetables. Int. J. Veg. Sci. 2016, 22, 423–431. [Google Scholar] [CrossRef]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Occurrence of Organochlorine Pesticide Residues in Biological and Environmental Matrices in Africa: A Two-Decade Review. Heliyon 2020, 6, e03518. [Google Scholar] [CrossRef]
- Rai, S.; Singh, A.K.; Srivastava, A.; Yadav, S.; Siddiqui, M.H.; Mudiam, M.K.R. Comparative Evaluation of QuEChERS Method Coupled to DLLME Extraction for the Analysis of Multiresidue Pesticides in Vegetables and Fruits by Gas Chromatography-Mass Spectrometry. Food Anal. Meth. 2016, 9, 2656–2669. [Google Scholar] [CrossRef]
- Donkor, A.; Osei-Fosu, P.; Dubey, B.; Kingsford-Adaboh, R.; Ziwu, C.; Asante, I. Pesticide Residues in Fruits and Vegetables in Ghana: A Review. Environ. Sci. Pollut. Res. 2016, 23, 18966–18987. [Google Scholar] [CrossRef] [PubMed]
- Chourasiya, S.; Khillare, P.S.; Jyethi, D.S. Health Risk Assessment of Organochlorine Pesticide Exposure through Dietary Intake of Vegetables Grown in the Periurban Sites of Delhi, India. Environ. Sci. Pollut. Res. 2015, 22, 5793–5806. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Neveen, A.; Marwa, A.; Ahmad Basel, A.Y. Occurrence of Pesticide Residues in Fruits and Vegetables for the Eastern Mediterranean Region and Potential Impact on Public Health. Food Control 2021, 119, 107457. [Google Scholar] [CrossRef]
- Nuapia, Y.; Chimuka, L.; Cukrowska, E. Assessment of Organochlorine Pesticide Residues in Raw Food Samples from Open Markets in Two African Cities. Chemosphere 2016, 164, 480–487. [Google Scholar] [CrossRef]
- Mahajan, M.R.; Nangare, S.N.; Patil, P.O. Nanosize Design of Carbon Dots, Graphene Quantum Dots, and Metal–Organic Frameworks Based Sensors for Detection of Chlorpyrifos in Food and Water: A Review. Microchem. J. 2023, 193, 109056. [Google Scholar] [CrossRef]
- Khatun, P.; Islam, A.; Sachi, S.; Islam, M.Z.; Islam, P. Pesticides in Vegetable Production in Bangladesh: A Systemic Review of Contamination Levels and Associated Health Risks in the Last Decade. Toxicol. Rep. 2023, 11, 199–211. [Google Scholar] [CrossRef]
- Ibitomi, M.O.; Mohammed, F. Determination of Pesticide Residues in Fruits and Vegetables in Kaduna Metropolis, Nigeria. Int. J. Environ. Sci. Toxicol. Res. 2016, 4, 185–189. [Google Scholar]
- Chung, S.W.C. How Effective Are Common Household Preparations on Removing Pesticide Residues from Fruit and Vegetables? A Review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Bakirci, G.T.; Yaman Acay, D.B.; Bakirci, F.; Ötleş, S. Pesticide Residues in Fruits and Vegetables from the Aegean Region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- El-Sheikh, E.-S.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.-B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef]
- Szpyrka, E.; Kurdziel, A.; Matyaszek, A.; Podbielska, M.; Rupar, J.; Słowik-Borowiec, M. Evaluation of Pesticide Residues in Fruits and Vegetables from the Region of South-Eastern Poland. Food Control 2015, 48, 137–142. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Anand, P.; Riddhi, L. Rapid Determination of Pesticide Residues in Fruits and Vegetables, Using Ultra-High-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry. Food Chem. 2015, 168, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Mebdoua, S.; Lazali, M.; Ounane, S.M.; Tellah, S.; Nabi, F.; Ounane, G. Evaluation of Pesticide Residues in Fruits and Vegetables from Algeria. Food Addit. Contam. B 2017, 10, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fatunsin, O.T.; Oyeyiola, A.O.; Moshood, M.O.; Akanbi, L.M.; Fadahunsi, D.E. Dietary Risk Assessment of Organophosphate and Carbamate Pesticide Residues in Commonly Eaten Food Crops. Sci. Afr. 2020, 8, e00442. [Google Scholar] [CrossRef]
- Jallow, M.; Awadh, D.; Albaho, M.; Devi, V.; Ahmad, N. Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef]
- Tuck, S.; Furey, A.; Crooks, S.; Danaher, M. A Review of Methodology for the Analysis of Pyrethrin and Pyrethroid Residues in Food of Animal Origin. Food Addit. Contam. A 2018, 35, 911–940. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity Analysis of Endocrine Disrupting Pesticides on Non-Target Organisms: A Critical Analysis on Toxicity Mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mohan, K.; Ganesan, A.R.; Govarthanan, M.; Yusoff, A.R.M.; Gu, F.L. Persistence, Toxicological Effect and Ecological Issues of Endosulfan—A Review. J. Hazard. Mater. 2021, 416, 125779. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, L.; Liu, L.; Yang, Y.; Zhao, Q. Comparative in Vitro and in Silico Study on the Estrogenic Effects of 2,2-Bis(4-Chlorophenyl)Ethanol, 4,4′-Dichlorobenzophenone and DDT Analogs. Sci. Total Environ. 2023, 876, 162734. [Google Scholar] [CrossRef]
- da Silva, A.H., Jr.; de Oliveira, C.R.S.; Leal, T.W.; Mapossa, A.B.; Fiates, J.; Ulson de Souza, A.A.; Ulson de Souza, S.M.d.A.G.; da Silva, A. Organochlorine Pesticides Remediation Techniques: Technological Perspective and Opportunities. J. Hazard. Mat. Lett. 2024, 5, 100098. [Google Scholar] [CrossRef]
- Songa, E.A.; Okonkwo, J.O. Recent Approaches to Improving Selectivity and Sensitivity of Enzyme-Based Biosensors for Organophosphorus Pesticides: A Review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, Monitoring and Biodegradation of Organophosphate Pesticides: A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Adigun, A.A.; Wrench, N.; Levin, E.D.; Seidler, F.J.; Slotkin, T.A. Neonatal Parathion Exposure and Interactions with a High-Fat Diet in Adulthood: Adenylyl Cyclase-Mediated Cell Signaling in Heart, Liver and Cerebellum. Brain. Res. Bull. 2010, 81, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M. Neurotoxic Effects of Organophosphorus Pesticides and Possible Association with Neurodegenerative Diseases in Man: A Review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus Pesticides: Impacts, Detection and Removal Strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Lerro, C.C.; Koutros, S.; Andreotti, G.; Friesen, M.C.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Sandler, D.P.; Lubin, J.H.; Ma, X.; et al. Organophosphate Insecticide Use and Cancer Incidence among Spouses of Pesticide Applicators in the Agricultural Health Study. Occup Environ. Med. 2015, 72, 736. [Google Scholar] [CrossRef]
- Silva, M.H. Effects of Low-Dose Chlorpyrifos on Neurobehavior and Potential Mechanisms: A Review of Studies in Rodents, Zebrafish, and Caenorhabditis Elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Fu, H.; Tan, P.; Wang, R.; Li, S.; Liu, H.; Yang, Y.; Wu, Z. Advances in Organophosphorus Pesticides Pollution: Current Status and Challenges in Ecotoxicological, Sustainable Agriculture, and Degradation Strategies. J. Hazard. Mater. 2022, 424, 127494. [Google Scholar] [CrossRef]
- Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. Prenatal Exposure to Pyrethroid Insecticides and Birth Outcomes in Rural Northern China. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 264–270. [Google Scholar] [CrossRef]
- Işıldar, G.Y.; Günal, A.Ç.; Şahin, D.; Memmi, B.K.; Dinçel, A.S. How Potential Endocrine Disruptor Deltamethrin Effects Antioxidant Enzyme Levels and Total Antioxidant Status on Model Organisms. Turk. J. Biochem. 2020, 45, 415–421. [Google Scholar] [CrossRef]
- Muggelberg, L.L.; Huff Hartz, K.E.; Nutile, S.A.; Harwood, A.D.; Heim, J.R.; Derby, A.P.; Weston, D.P.; Lydy, M.J. Do Pyrethroid-Resistant Hyalella Azteca Have Greater Bioaccumulation Potential Compared to Non-Resistant Populations? Implications for Bioaccumulation in Fish. Environ. Pollut. 2017, 220, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.B.; Blaha, L. Pyrethroids and Developmental Neurotoxicity—A Critical Review of Epidemiological Studies and Supporting Mechanistic Evidence. Environ. Res. 2022, 214, 113935. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid Pesticides as Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. The Neurotoxicity of Organochlorine and Pyrethroid Pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, P.; Wielgomas, B.; Radwan, M.; Karwacka, A.; Kałużny, P.; Piskunowicz, M.; Dziewirska, E.; Hanke, W. Exposure to Pyrethroid Pesticides and Ovarian Reserve. Environ. Int. 2020, 144, 106028. [Google Scholar] [CrossRef]
- Marettova, E.; Maretta, M.; Legáth, J. Effect of Pyrethroids on Female Genital System. Review. Anim. Reprod. Sci. 2017, 184, 132–138. [Google Scholar] [CrossRef]
- Lucero, B.; Muñoz-Quezada, M.T. Neurobehavioral, Neuromotor, and Neurocognitive Effects in Agricultural Workers and Their Children Exposed to Pyrethroid Pesticides: A Review. Front. Hum. Neurosci. 2021, 15, 648171. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Schäfer, R.B.; van den Brink, P.J.; Liess, M. Ecological Impacts of Toxic Chemicals (Open Access). In Ecological Impacts of Toxic Chemicals; Sánchez-Bayo, F., van den Brink, P.J., Mann, R.M., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011; pp. 111–137. [Google Scholar]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The Mobility and Degradation of Pesticides in Soils and the Pollution of Groundwater Resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- C, F.C.; Kamalesh, T.; Senthil Kumar, P.; Rangasamy, G. An Insights of Organochlorine Pesticides Categories, Properties, Eco-Toxicity and New Developments in Bioremediation Process. Environ. Pollut. 2023, 333, 122114. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]

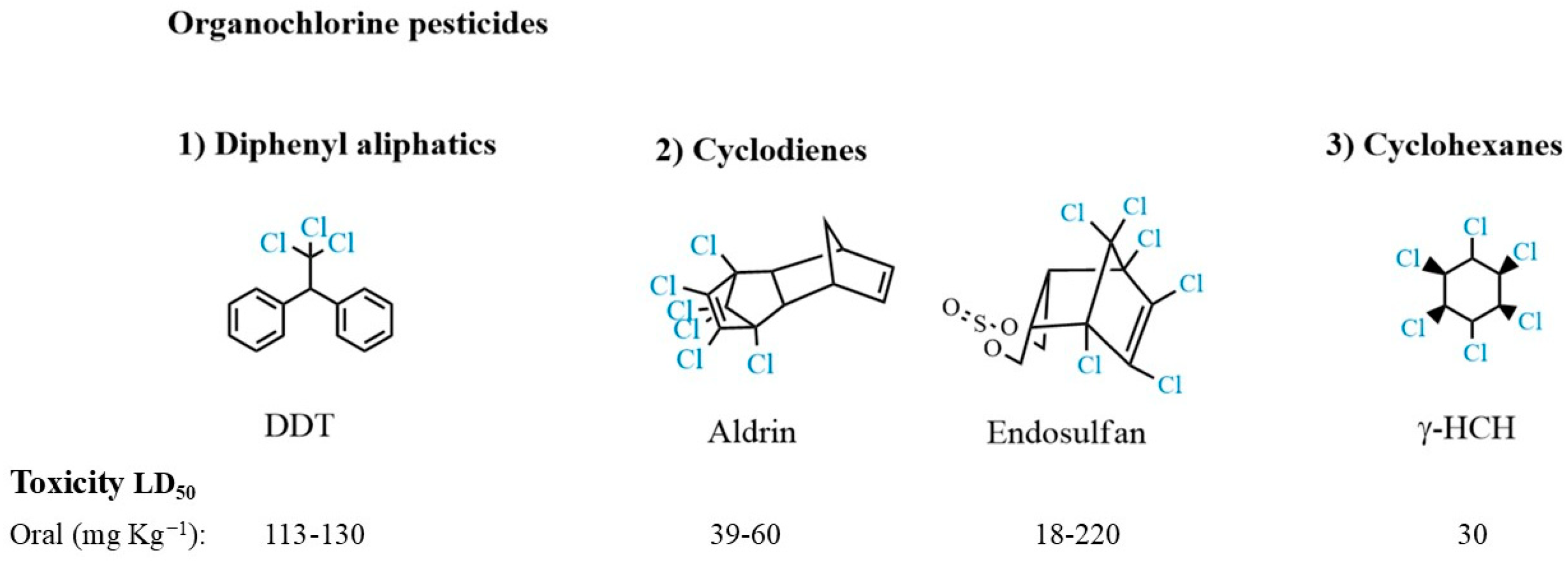


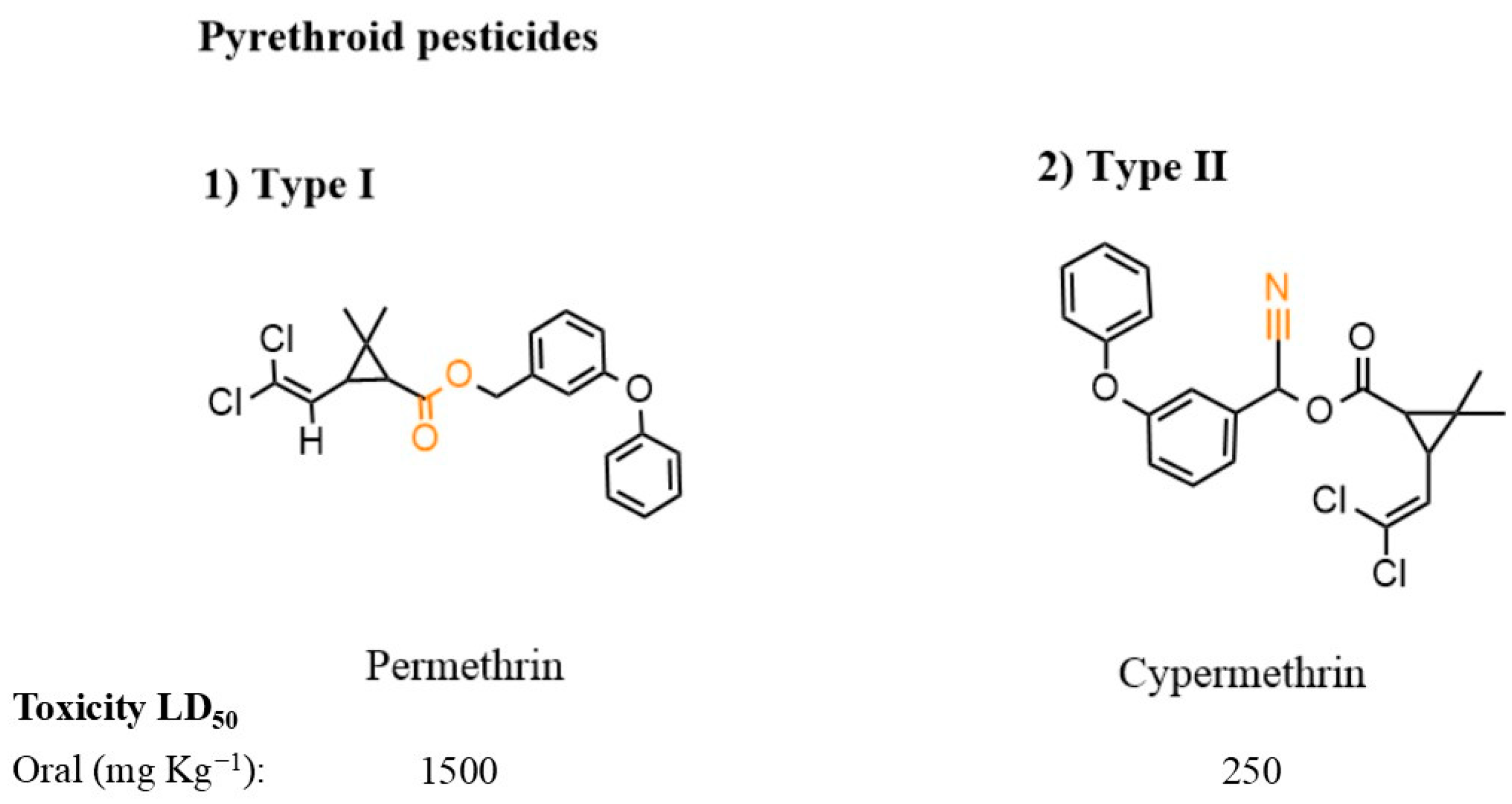
| Substance Group | Pesticides | DT50w * (days) | Sw ** (mg L−1) | Koc *** (mL g−1) | Guideline Value (ng L−1) |
|---|---|---|---|---|---|
| Organochlorine | Aldrin | 3830 | 0.027 | 17,500 | 30 |
| Hexachlorocyclohexane | 292–438 | 10 | 12,589 | 2000 | |
| Endosulfan | 20 | 0.32 | 11,500 | NMa (HBV = 20,000) | |
| Organophosphorus | Chlorpyrifos | 29.6 | 1.05 | 8151 | 300,000 |
| Malathion | 6 | 148 | 1800 | NMa (HBV = 900,000) | |
| Diazinon | 50 | 60 | 609 | B | |
| Pyrethroid | Cypermethrin | 1–35 | 0.004 | 20,800 | B |
| Deltamethrin | 17 | 0.002 | 10 × 106 | B | |
| Permethrin | 1 | 0.2 | 100,000 | E |
| River/Country | Substance Group | Pesticides | Concentration (ng L−1) | Season/Month/Year | Agricultural Product | Reference |
|---|---|---|---|---|---|---|
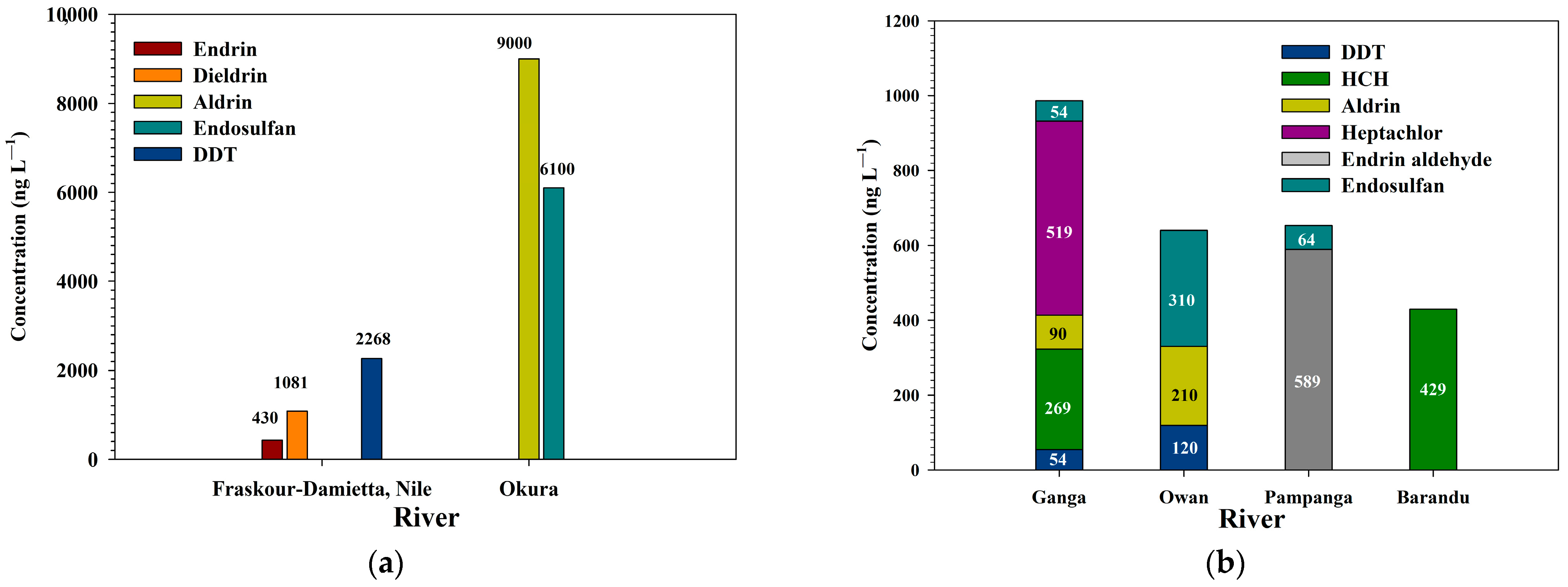
| 1. Fraskour–Damietta, Nile River, Egypt | Organochlorine | Endrin | 430 | January, 2009 | Cotton | [76] |
| Dieldrin | 1081 | |||||
| p,p-DDD | 1209 | |||||
| p,p-DDT | 2268 | |||||
| Organophosphorus | Chlorpyrifos | 578 | ||||
| Triazophos | 1488 | |||||
| 2. Ganga River, India | Organochlorine | DDT | 54 | Summer, 2019 | NM | [77] |
| HCH | 269 | |||||
| Aldrin | 90 | |||||
| Heptachlor | 519 | |||||
| Endosulfan | 54 | |||||
| 3. Owan River, Nigeria | Organochlorine | α-BHC | 380 | June, 2013 | Rubber, cocoa, plantain, maize | [78] |
| Aldrin | 210 | |||||
| DDT | 120 | |||||
| Ʃ-Endosulfan | 310 | |||||
| Carbamate | Carbofuran | 30 | ||||
| Triazine | Atrazine | 150 | ||||
| 4. Somme River, France | Organochlorine | Ʃ-HCH | 93 | April, 2014 | NM | [79] |
| 5. Yangtze River, China | Organochlorine | Ʃ-HCH | 0.4 | November, 2016 | NM | [80] |
| 6. Dong Nai River, Vietnam | Organochlorine | Aldrin | 68 | Rainy season, 2016 | Rice, maize, sorghum | [81] |
| Heptachlor | 40 | |||||
| Dieldrin | 24 | |||||
| Endrin | 27 | |||||
| 7. Chenab River, Pakistan | Organochlorine | α-HCH | 12 | April–June, 2018 | Wheat, rice | [82] |
| Aldrin | 0.6 | |||||
| Ʃ-Endosulfan | 6 | |||||
| 8. Pampanga River, Philippines | Organochlorine | Dieldrin | 30 * | Dry season, (February), 2017 | Rice | [83] |
| Endrin aldehyde | 589 * | |||||
| δ-BHC | 372 * | |||||
| Ʃ-Endosulfan | 64 * | |||||
| 9. Nuble River, Chile | Organochlorine | Ʃ-HCH | 18 | Wet season, (April–August), 2014 | Blueberries | [84] |
| Heptachlor | 1 | |||||
| Aldrin | 2 | |||||
| α-endosulfan | 0.3 | |||||
| 10. Indus River, Pakistan | Organochlorine | Ʃ-HCH | 60 | December, 2013 | Wheat, cotton, rice, sugarcane | [85] |
| Ʃ-DDTs | 105 | |||||
| 11. Nairobi River, Kenya | Organochlorine | β-HCH | 19 | Dry and rainy seasons (February–July), 2009 | NM | [86] |
| Heptachlor | 40 | |||||
| Aldrin | 15 | |||||
| Endrin | 7 | |||||
| 12. Kibos-Nyamasaria River, Kenya | Organochlorine | Dieldrin | 103 | Wet season | Tomatoes, maize, cassava | [87] |
| Ʃ-Endosulfan | 102 | |||||
| Ʃ-HCH | 96 | |||||
| Ʃ-DDTs | 162 | |||||
| 13. Krishna River, India | Organochlorine | Ʃ-HCH | 12 | September, 2019 | Cotton, paddy | [88] |
| Ʃ-Endosulfan | 5 | |||||
| Aldrin | 4 | |||||
| 14. Okura River, Nigeria | Organochlorine | Aldrin | 9000 | NM | Banana | [89] |
| Endosulfan | 6100 | |||||
| 15. Brahmaputra River, India | Organochlorine | Ʃ-HCH | 8 | June, 2012 | Potato | [90] |
| Ʃ-Endosulfan | 3 | |||||
| 16. Periyar River, India | Organochlorine | Ʃ-HCH | 15 | August–September, 2019 | Rice | [91] |
| Ʃ-Endosulfan | 2 | |||||
| 17. Barandu River, Pakistan | Organochlorine | Ʃ-HCH | 429 | March, 2018 | Tobacco | [92] |
| Aldrin | 2 | |||||
| Ʃ-Endosulfan | 9 | |||||
| 18. Quequén-Grande River, Argentina | Organochlorine | Ʃ-Endosulfan | 3 | July 2014–July 2015 | Soybean, potatoes | [93] |
| 19. Tucututemo River, Venezuela | Organochlorine | Aldrin | 21 * | Dry and rainy seasons (April–October), 2013–2016 | NM | [94] |
| Dieldrin | 18 * |
| River/Country | Substance Group | Pesticides | Concentration (ng L−1) | Season/Month/Year | Agricultural Product | Reference |
|---|---|---|---|---|---|---|
| 1. Kurose River, Japan | Organophosphorus | Diazinon | 348 | March 2016–February 2017 | Rice, beans, wheat, potatoes | [110] |
| 2. Shangyu Region, China | Organophosphorus | Dichlorvos | 1560 | August, 2014 | Waxberry, grape | [111] |
| Malathion | 360 | |||||
| Parathion | 290 | |||||
| 3. Guangzhou River, China | Organophosphorus | Chlorpyrifos | 19 | Wet season (July), 2012 | Chinese chive, banana | [112] |
| Malathion | 4 | |||||
| Dimethoate | 58 | |||||
| Pyrethroid | Cypermethrin | 5 | ||||
| 4. Júcar River, Spain | Organophosphorus | Chlorfenvinphos | 97 * | October, 2010 | Barley, garlic, onion, oat, potato, tomato, wheat, oranges | [113] |
| Chlorpyrifos | 36 * | |||||
| Diazinon | 9 * | |||||
| Malathion | 9 * | |||||
| Triazine | Atrazine | 8 * | ||||
| 5. Langat River, Malaysia | Organophosphorus | Quinalphos | 18 | September, 2015 | Cane orchard, oil palm plantation | [114] |
| Diazinon | 9 | |||||
| Chlorpyrifos | 20 | |||||
| 6. Hooghly River, India | Organophosphorus | Methyl-parathion | 45 | May–June, 2014–2015 | Cabbage, cauliflower, chili, oilseeds, lentil | [115] |
| Monocrotophos | 9 | |||||
| Organochlorine | Ʃ-HCH | 1988 | ||||
| Ʃ-endosulfan | 122 | |||||
| 7. Aliakmonas River, Greece | Organophosphorus | Chlorpyrifos ethyl | 33 | February, 2001 | Rice, corn, sugar beets, cotton | [116] |
| Parathion methyl | 149 | |||||
| 8. Tano River, Ghana | Organophosphorus | Parathion | 268 | Rainy season (October), 2017 | Cocoa, maize | [117] |
| Methamidophos | 241 | |||||
| Malathion | 303 | |||||
| Chlorpyrifos | 383 | |||||
| Profenofos | 303 | |||||
| Organochlorine | δ-HCH | 59 | ||||
| γ-HCH | 2 | |||||
| Pyrethroid | Permethrin | 19 | ||||
| Deltamethrin | 12 | |||||
| 9. Linggi River, Malaysia | Organophosphorus | Chlorpyrifos | 28 | July, 2018 | Palm oil plantation | [118] |
| Diazinon | 33 | |||||
| Quinalphos | 36 | |||||
| 10. Sarno River, Italy | Organophosphorus | Diazinon | 2 * | 2018 | Tomato | [119] |
| Dimethoate | 6 * | |||||
| Malathion | 5 * | |||||
| Chlorpyrifos | 12 * | |||||
| Dichlorvos | 2 * | |||||
| 11. Volturno River, Italy | Organophosphorus | Diazinon | 1 | 2017–2018 | Cereals, potatoes, vineyards, olive | [120] |
| Dimethoate | 2 | |||||
| Malathion | 1 | |||||
| Chlorpyrifos | 5 | |||||
| 12. Han River, China | Organophosphorus | Methamidophos | 39 | June–September, 2015 | NM | [121] |
| Dichlorvos | 20 | |||||
| Omethoate | 48 | |||||
| Diazinon | 48 | |||||
| Malathion | 7 | |||||
| Parathion | 16 | |||||
| 13. Mahaweli River, Sri Lanka | Organophosphorus | Diazinon | 390,000 * | August, 2019 | Rice | [122] |
| 14. Tamazula River, Mexico | Organophosphorus | Diazinon | 30 | June 2008–July 2009 | Tomatoes, bell peppers, cucumber, eggplant | [63] |
| Chlorpyrifos | 30 | |||||
| Malathion | 6 | |||||
| Organochlorine | Endosulfan | 36 | ||||
| Aldrin | 23 | |||||
| 15. Godavari River, India | Organophosphorus | Chlorpyrifos | 410 | September 2011–July 2012 | Cotton, chili, brinjal, tur, tomato, wheat, lemon, orange, jowar | [123] |
| 16. Amazon River, Brazil | Organophosphorus | Malathion | 535 * | November–December, 2019 | NM | [124] |
| Chlorpyrifos | 700 * | |||||
| 17. Lerma River, Mexico | Organophosphorus | Malathion | 311,760 | July–September, 2019 | Maize, wheat | [125] |
| Glyphosate | 252,000 |
| River/Country | Substance Group | Pesticides | Concentration (ng L−1) | Season/Month/Year | Agricultural Product | Reference |
|---|---|---|---|---|---|---|
| 1. Lis River, Portugal | Pyrethroid | Cypermethrin | 664 | February 2018–May 2019 | NM | [146] |
| Organochlorine | Aldrin | 1153 | ||||
| γ-HCH | 1085 | |||||
| Organophosphorus | Chlorpyrifos | 159 | ||||
| 2. Dongjiang River, China | Pyrethroid | Cypermethrin | 41.4 | Wet season (July), 2015; dry season (November), 2015 | Orange | [147] |
| Deltamethrin | 12.5 | |||||
| Organochlorine | Ʃ-HCHs | 104.6 | ||||
| Ʃ-DDTs | 75 | |||||
| Organophosphorus | Metamidophos | 26 | ||||
| Dichlorvos | 4.4 | |||||
| Acephate | 74.9 | |||||
| Chlorpyrifos | 16 | |||||
| Triazophos | 43.1 | |||||
| 3. Jiulong River, China | Pyrethroid | Cypermethrin | 609 * | Wet season (July), 2009; dry season (December), 2009 | Pomelo, banana | [148] |
| Organophosphorus | Triazophos | 1055 * | ||||
| 4. Benue River, Nigeria | Pyrethroid | Cypermethrin | 930,000 | NM | Cocoa, cotton, rice | [149] |
| Permethrin | 1,520,000 | |||||
| Deltamethrin | 1,140,000 | |||||
| Organochlorine | Aldrin | 3,750,000 | ||||
| Dieldrin | 5,240,000 | |||||
| Dichlorvos | 1,060,000 | |||||
| Organophosphorus | Diazinon | 1,170,000 | ||||
| Chlorpyrifos | 910,000 | |||||
| 5. Sassandra River, Ivory Coast | Pyrethroid | Cypermethrin | 13 | Rainy season (April), 2021–October 2021 | NM | [150] |
| Deltamethrin | 5.5 | |||||
| 6. Elbe River, Czech Republic | Pyrethroid | Kadethrin | 26,000 | NM | NM | [144] |
| 7. Paraguay-Paraná River, Argentina | Pyrethroid | Cypermethrin | 740 | October 2010–July 2012 | Rice | [151] |
| Organochlorine | Ʃ-Endosulfan | 120 | ||||
| Organophosphorus | Chlorpyrifos | 110 | ||||
| 8. Thamirabarani River, India | Pyrethroid | Cypermethrin | 77 * | March–July | Cashew, tea, cotton, paddy, rubber | [152] |
| Organochlorine | Endosulfan | 1776 * | ||||
| Aldrin | 98 * | |||||
| Endrin | 246 * | |||||
| 9. Citarum River, Indonesia | Pyrethroid | Deltamethrin | 4000 * | August, 2021 | NM | [145] |
| 10. Suquía River, Argentina | Pyrethroid | α-Cypermethrin | 30.4 * | March–August, 2010 | Soybean, corn | [153] |
| Organochlorine | Endosulfan-sulfate | 4.1 * | ||||
| 11. Ceará River, Brazil | Pyrethroid | Cypermethrin | 368 | July, 2014 | NM | [154] |
| Deltamethrin | 171 | |||||
| Permethrin | 47 | |||||
| Organophosphorus | Malathion | 226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Benítez, A.; Guevara-Lara, A.; Domínguez-Crespo, M.A.; Andraca-Adame, J.A.; Torres-Huerta, A.M. Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review. Sustainability 2024, 16, 8066. https://doi.org/10.3390/su16188066
López-Benítez A, Guevara-Lara A, Domínguez-Crespo MA, Andraca-Adame JA, Torres-Huerta AM. Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review. Sustainability. 2024; 16(18):8066. https://doi.org/10.3390/su16188066
Chicago/Turabian StyleLópez-Benítez, Acela, Alfredo Guevara-Lara, Miguel A. Domínguez-Crespo, José A. Andraca-Adame, and Aidé M. Torres-Huerta. 2024. "Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review" Sustainability 16, no. 18: 8066. https://doi.org/10.3390/su16188066







