Thermodynamic Analysis of Gas Turbine Systems Fueled by a CH4/H2 Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermodynamic Analysis
2.2. Toxic Compounds Emission Modelling
3. Results and Discussion
3.1. Gas Turbine Operation Analysis
3.1.1. Simple Gas Turbine System
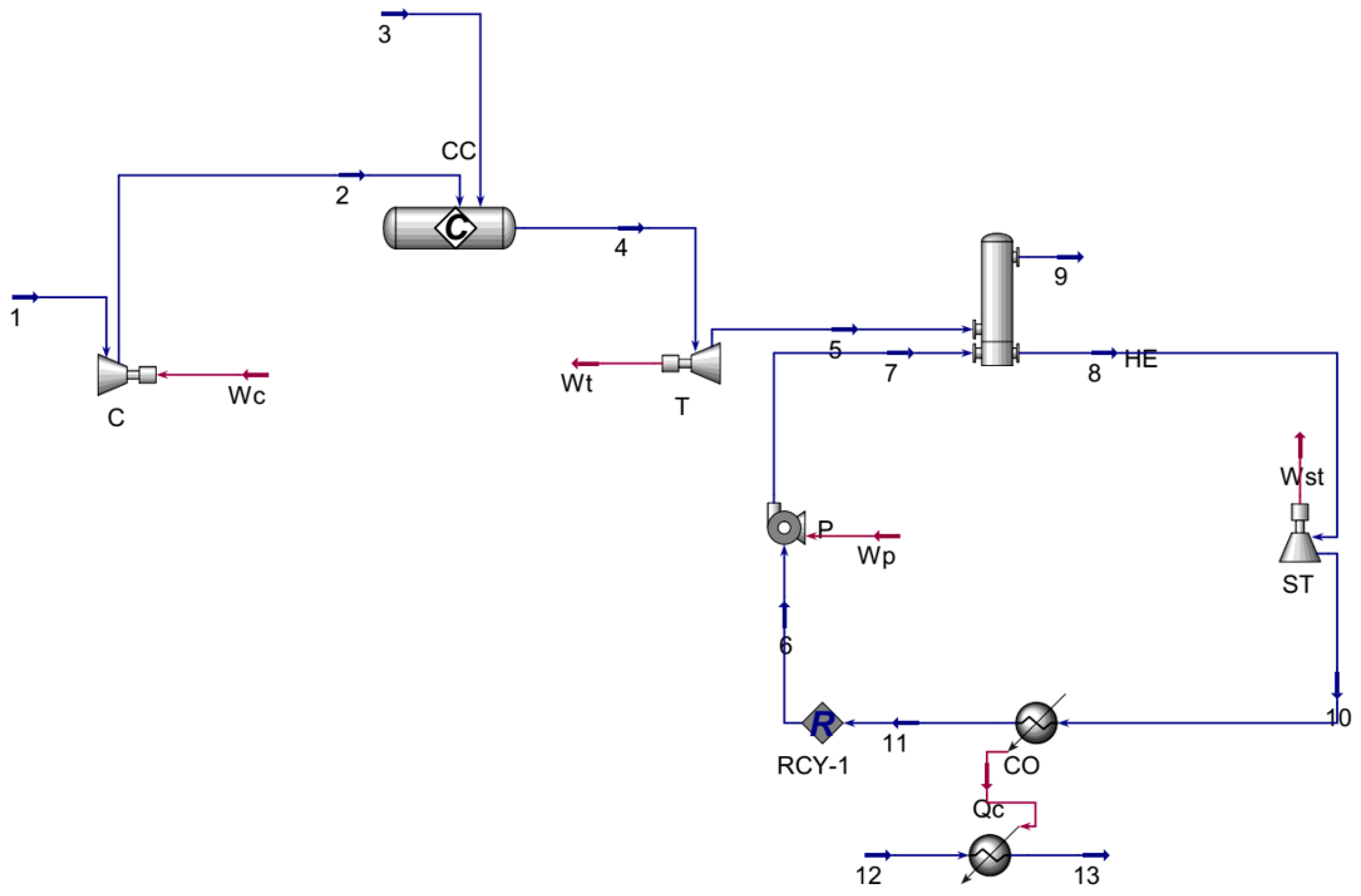
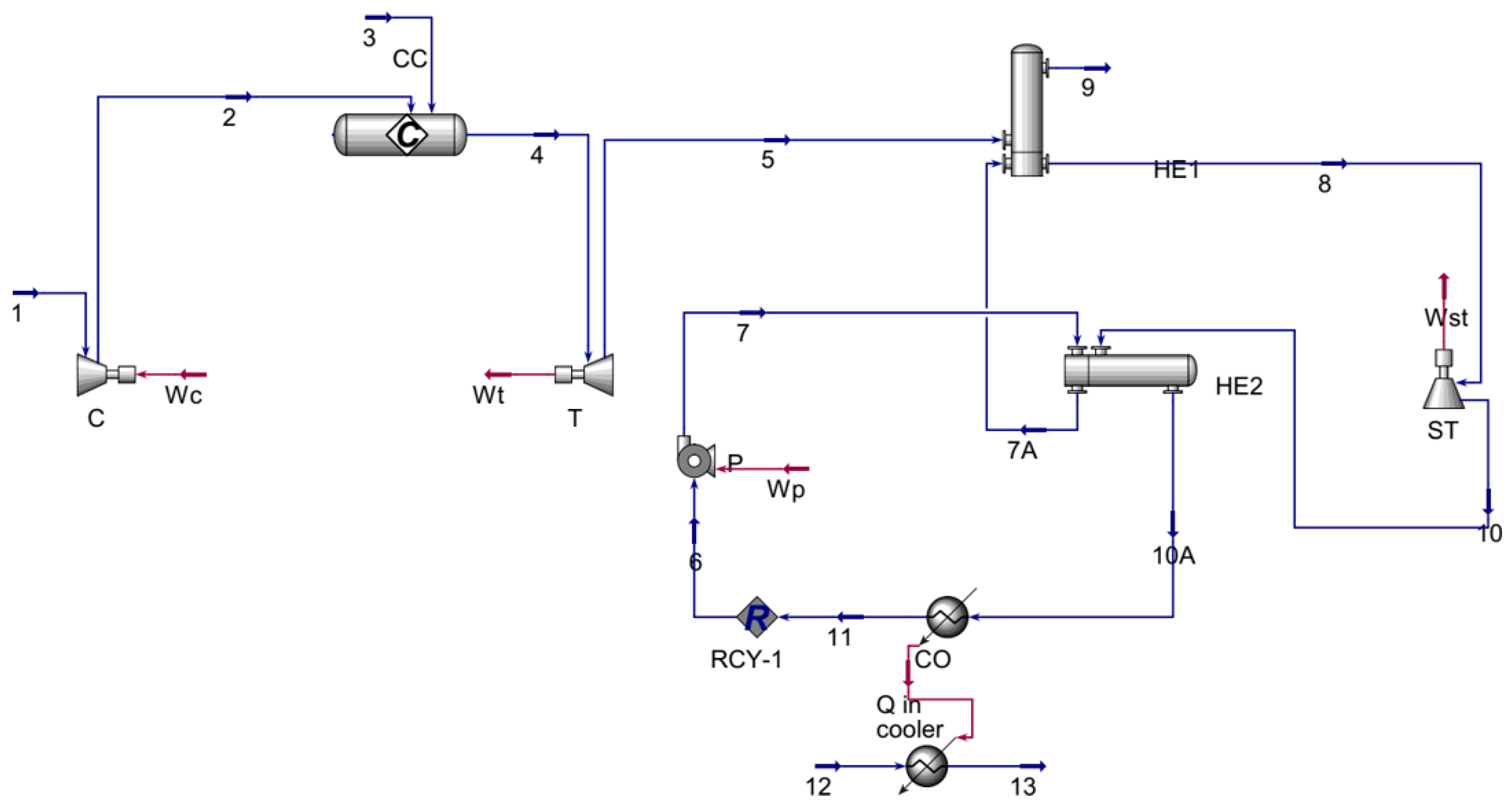
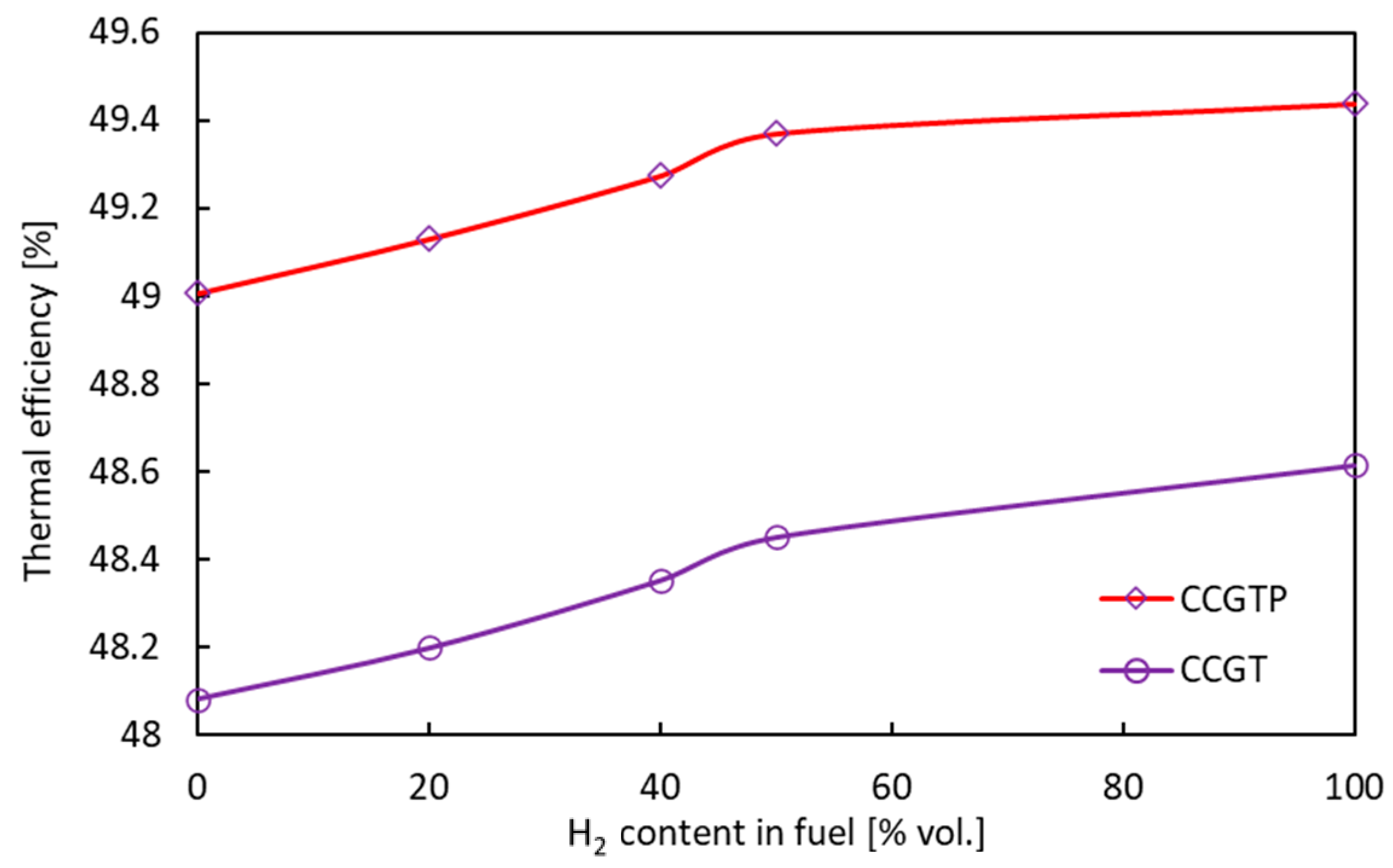
3.1.2. Combined Cycle Gas Turbine CCGT
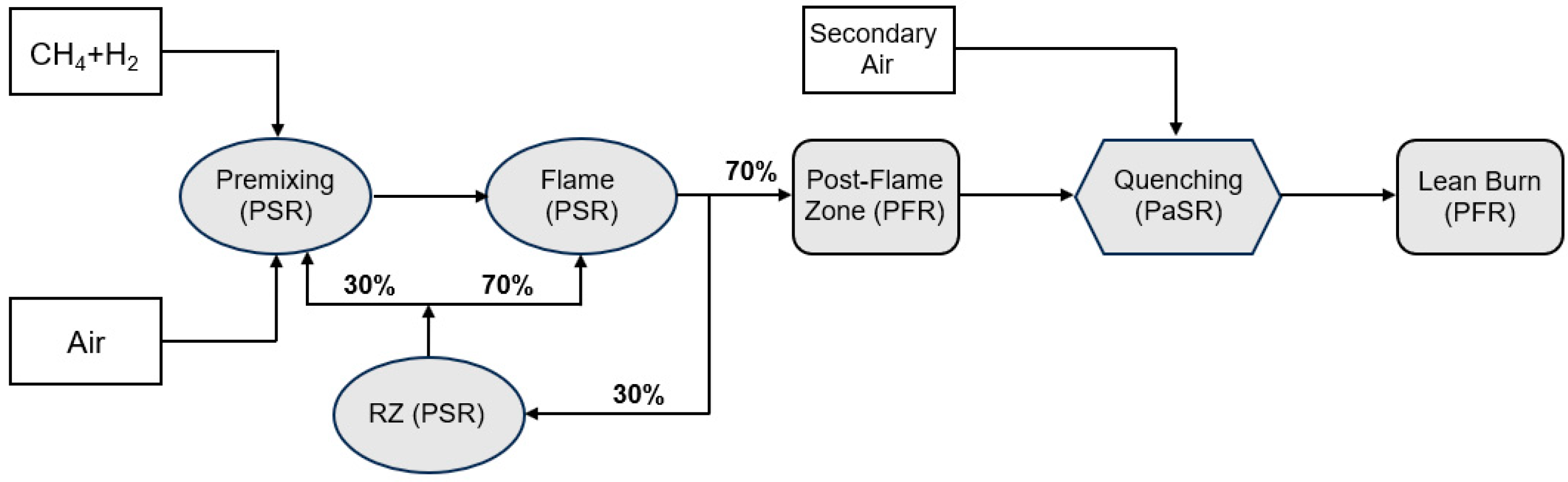
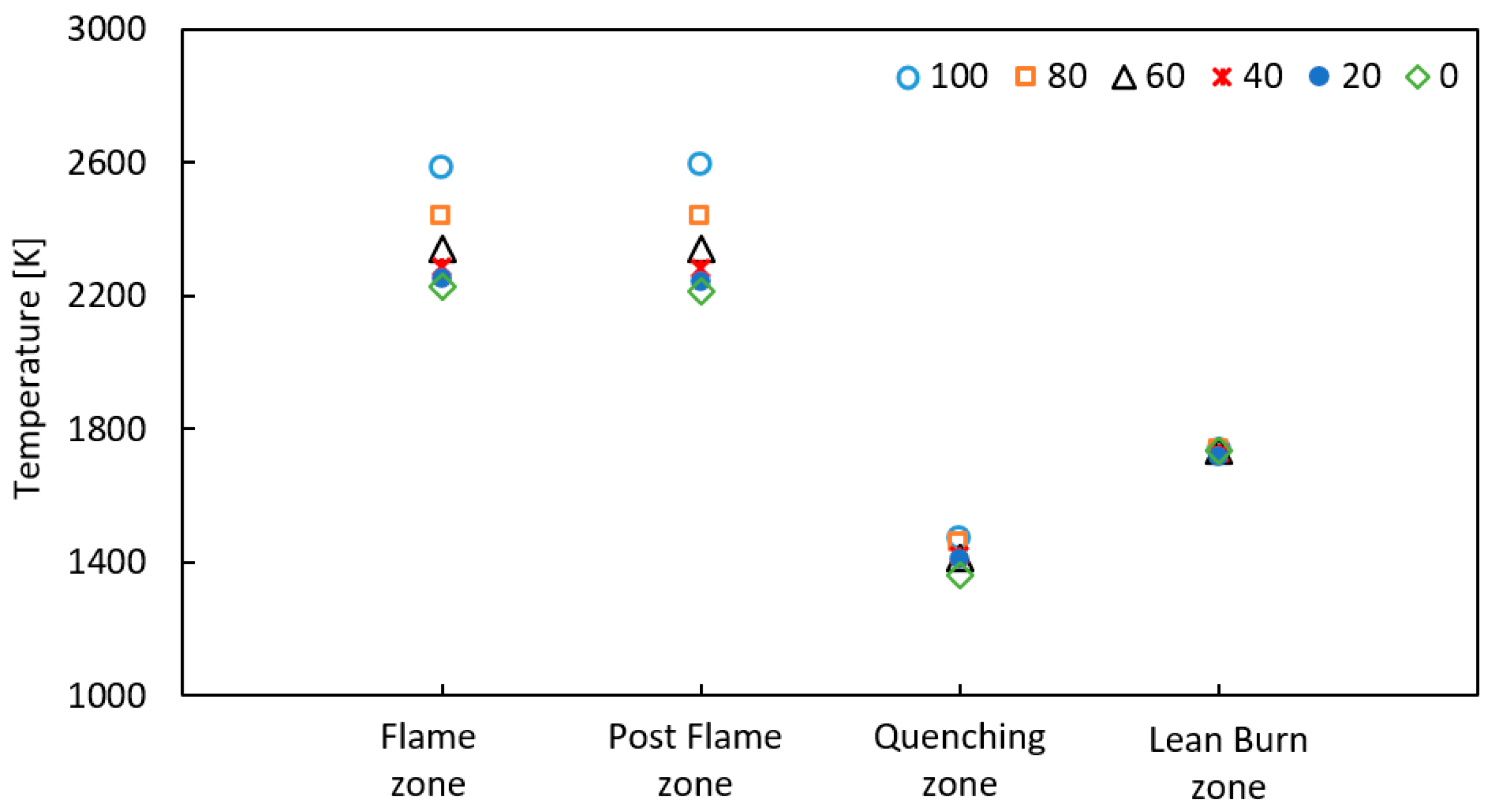
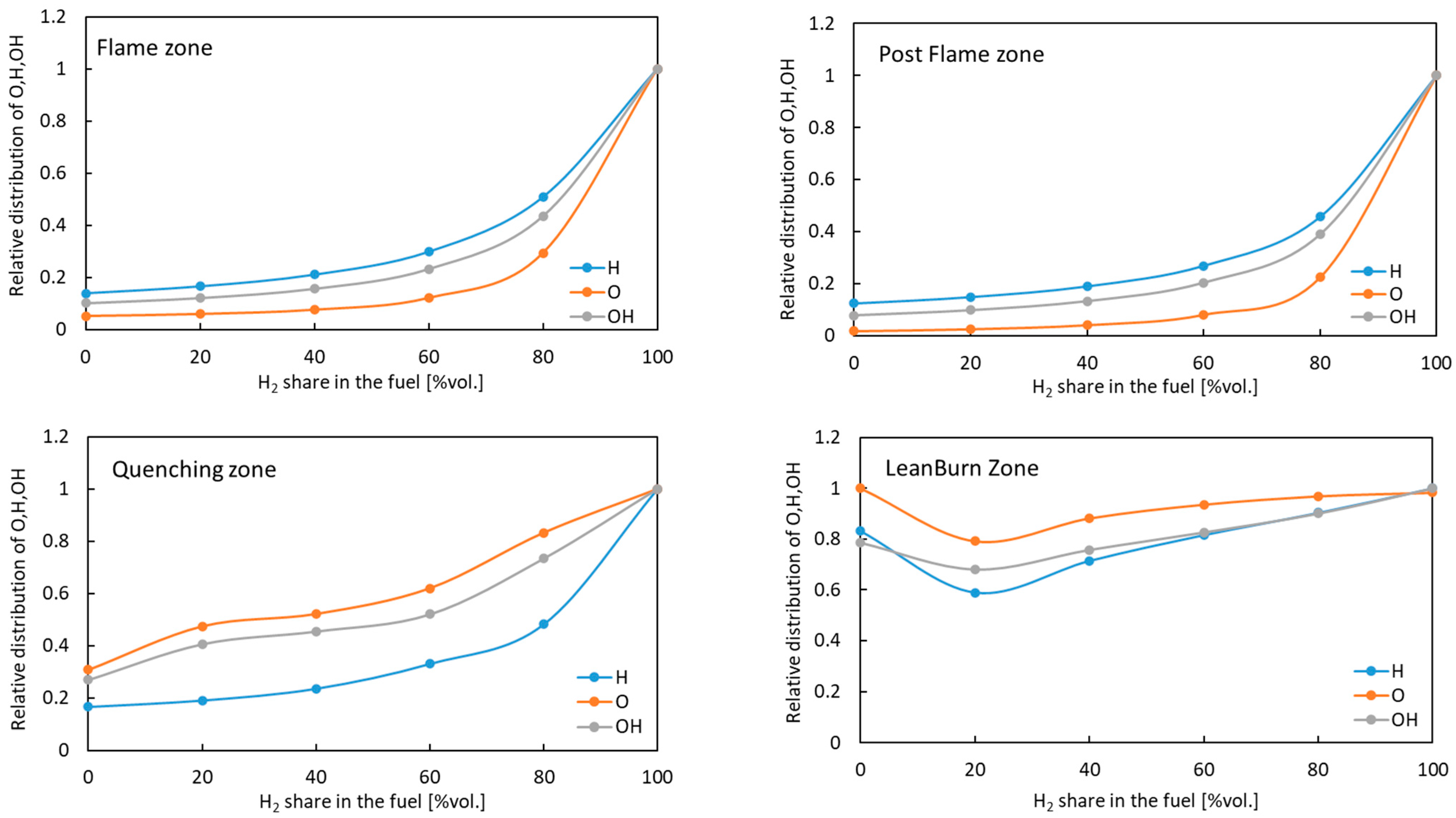
3.2. Numerical Results from the Chemical Reaction Network
4. Conclusions
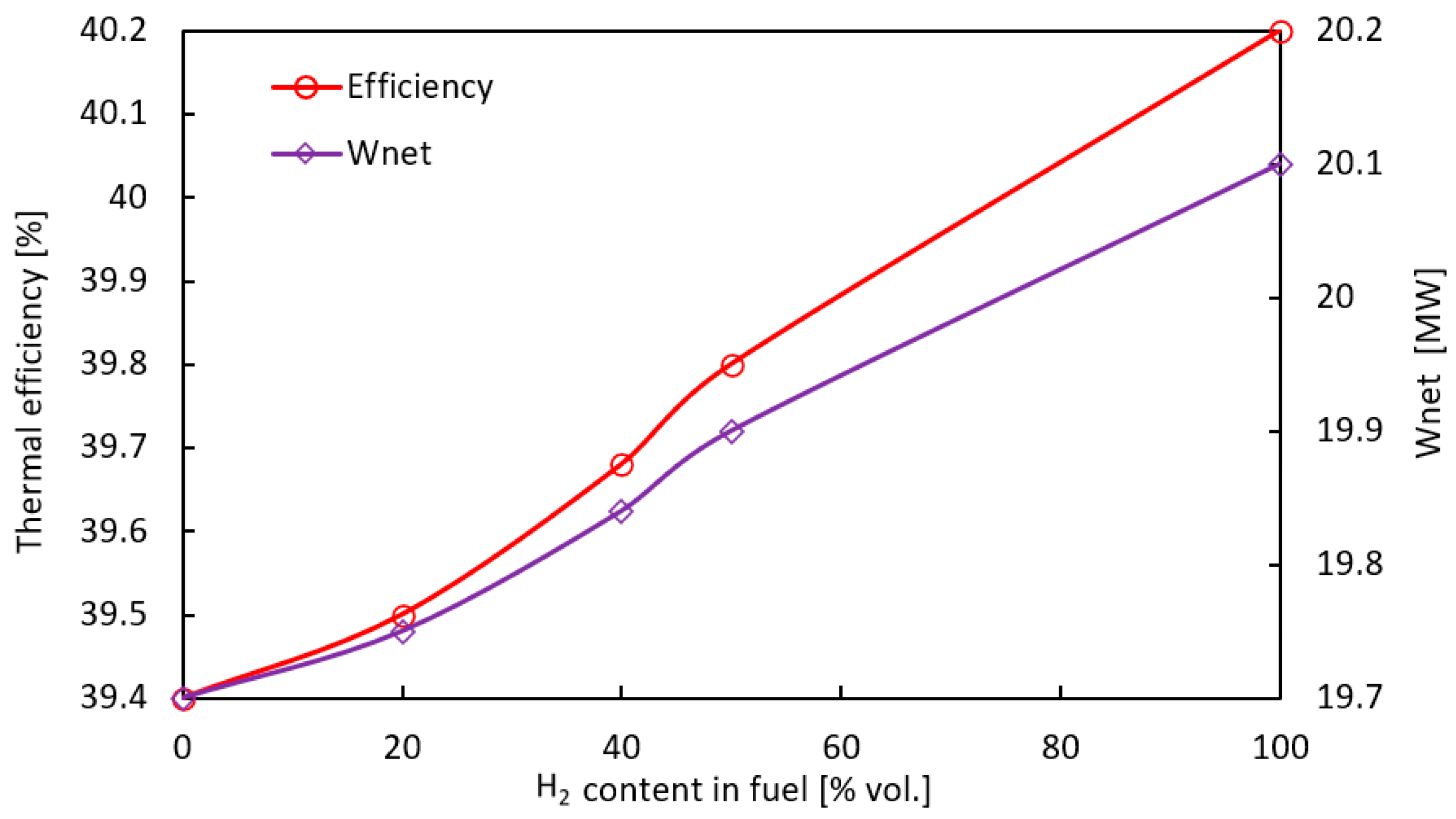
- Substitution of hydrocarbon fuels with hydrogen contributed to increased efficiency and generated power in both GT and CCGT thermodynamic cycles. The main reason is an increase in the enthalpy of the flue gas due to an increase in the share of water vapor in the flue gas. This allows efficiency gains of 0.8% and 0.53% for the TG and CCGT systems, respectively. The lower growth for the combined system is due to lower power output of the steam turbine as a result of the reduction of the exhaust gas mass flux by about 9% when changing the fuel from CH4 to H2.
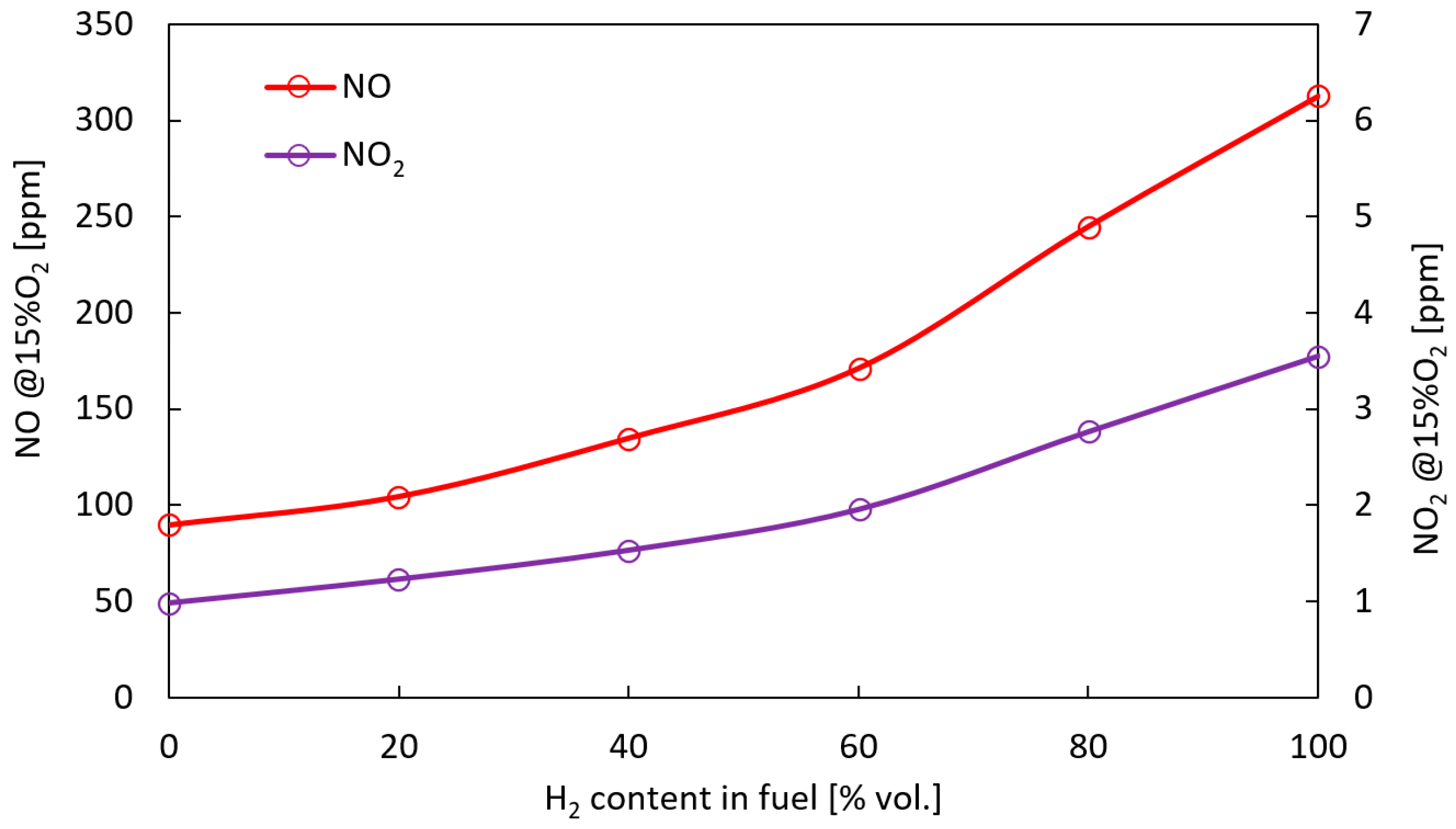
- The results indicate that the addition of H2 increases NO and NO2 emission. Emissions more than three times higher were recorded for pure hydrogen than for methane. The significant increase of NOx formation was the result of the reactions taking place in the in quenching zone, where the highest values of free radicals (O, H, and OH) were obtained. Increasing the share of H2 in the fuel raises the amount of radicals, resulting in a growth in the net reaction rate for NOx formation by several times compared to methane fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A/F | air to fuel ratio |
| A/Q | air to energy coefficient |
| C | compressor |
| CC | combustion chamber |
| CCGT | combined cycle gas turbine |
| CCGTP | preheated combined cycle gas turbine |
| cp | specific heat at constant pressure |
| CRN | chemical reactor network |
| GT | gas turbine |
| GTs | gas turbine systems |
| HRSG | heat recovery steam generator |
| NG | natural gas |
| PaSR | partially stirred reactor |
| PFR | plug flow reactor |
| PSR | perfectly stirred reactors |
| Qin | input power |
| RES | renewable energy sources |
| RQL | rich-quench-lean combustor |
| RZ | recirculation zone |
| STF | stoichiometric flame temperature |
| TIT | turbine inlet temperature |
| TOT | turbine outlet temperature |
| WC | compressor power |
| Wnet | gas turbine system power |
| Wp | pump work |
| WST | steam turbine power |
| WT | turbine power |
| φ | equivalence ratio |
| η | gas turbine efficiency |
References
- UN. Kyoto Protocol to the United Nations Framework Convention on Climate Change; UN: New York, NY, USA, 1998. [Google Scholar]
- United Nations. Summary of the Paris Agreement. United Nations Framework Convention on Climate Change; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Saravanamuttoo, H.; Roger, G.F.C.; Cohen, H.; Straznicky, P.V. Gas Turbine Theory, 6th ed.; Pearson Education Ltd.: London, UK, 2009. [Google Scholar]
- Lozza, G. Turbine a Gas e Cicli Combinati; Societ à Editrice Esculapio: Bologna, Italy, 2006. [Google Scholar]
- Han, J.C.; Dutta, S.; Ekkad, S. Gas Turbine Heat Transfer and Cooling Technology; Taylor & Francis: Abingdon, UK, 2012. [Google Scholar]
- Bathie, W.W. Fundamentals of Gas Turbines, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Horlock, J.H. Advanced Gas Turbine Cycles; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Rogers, G.F.C.; Mayhew, Y.R. Engineering Thermodynamics Work and Heat Transfer, 3rd ed.; Longmans, Green: London, UK, 1980. [Google Scholar]
- Desideri, U. Fundamentals of Gas Turbine Cycles: Thermodynamics, Efficiency and Specific Power. In Modern Gas Turbine Systems: High Efficiency, Low Emission, Fuel Flexible Power Generation; Woodhead Publishing: Sawston, UK, 2013; pp. 44–85. [Google Scholar] [CrossRef]
- Maslennikov, V.M.; Shterenberg, V.J. Advanced Gas Turbine System Utilizing Partial Oxidation Technology for Ecologically Clean Power Generation. Int. J. Low-Carbon Technol. 2011, 6, 55–63. [Google Scholar] [CrossRef]
- Nishimura, M. Hydrogen Gas Turbine Offers Promise of Clean Electricity. Nat. J. 2023. Available online: https://www.nature.com/articles/d42473-022-00211-0?fbclid=IwAR2UVeGGeV9LvKHcs_TTsMrJV5JN0yWV_aSX2q-0-i59T_yb_NMO7qRAKIk (accessed on 3 November 2023).
- Dehghanimadvar, M.; Shirmohammadi, R.; Sadeghzadeh, M.; Aslani, A.; Ghasempour, R. Hydrogen Production Technologies: Attractiveness and Future Perspective. Int. J. Energy Res. 2020, 44, 8233–8254. [Google Scholar] [CrossRef]
- Therkelsen, P.; Werts, T.; McDonell, V.; Samuelsen, S. Analysis of NOx Formation in a Hydrogen-Fueled Gas Turbine Engine. J. Eng. Gas Turbine Power 2009, 131, 031507. [Google Scholar] [CrossRef]
- Reale, F.; Sannino, R. Numerical Modeling of Energy Systems Based on Micro Gas Turbine: A Review. Energies 2022, 15, 900. [Google Scholar] [CrossRef]
- Liu, X.; Bertsch, M.; Subash, A.A.; Yu, S.; Szasz, R.Z.; Li, Z.; Petersson, P.; Bai, X.S.; Aldén, M.; Lörstad, D. Investigation of Turbulent Premixed Methane/Air and Hydrogen-Enriched Methane/Air Flames in a Laboratory-Scale Gas Turbine Model Combustor. Int. J. Hydrogen Energy 2021, 46, 13377–13388. [Google Scholar] [CrossRef]
- Yoshirnura, T.; McDonell, V.; Samuelsen, S. Evaluation of Hydrogen Addition to Natural Gas on the Stability and Emissions Behavior of a Model Gas Turbine Combustor. Proc. ASME Turbo Expo 2008, 2, 573–581. [Google Scholar] [CrossRef]
- Ditaranto, M.; Li, H.; Hu, Y. Evaluation of a Pre-Combustion Capture Cycle Based on Hydrogen Fired Gas Turbine with Exhaust Gas Recirculation (EGR). Energy Procedia 2014, 63, 1972–1975. [Google Scholar] [CrossRef]
- Dutka, M.; Ditaranto, M.; Løvås, T. NOx Emissions and Turbulent Flow Field in a Partially Premixed Bluff Body Burner with CH4 and H2 Fuels. Int. J. Hydrogen Energy 2016, 41, 12397–12410. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 2nd ed.; McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Todd, D.; Battista, R. Demonstrated Applicability of Hydrogen Fuel for Gas Turbines. Eng. Environ. Sci. 2001, 4, 741–748. [Google Scholar]
- Ahmed, S.A.; Zhou, S.; Tsegay, S.; Ahmad, N.; Zhu, Y. Effects of Hydrogen-Enriched Biogas on Combustion and Emission of a Dual-Fuel Diesel Engine. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–16. [Google Scholar] [CrossRef]
- Alnaeli, M.; Alnajideen, M.; Navaratne, R.; Shi, H.; Czyzewski, P.; Wang, P.; Eckart, S.; Alsaegh, A.; Alnasif, A.; Mashruk, S.; et al. High-Temperature Materials for Complex Components in Ammonia/Hydrogen Gas Turbines: A Critical Review. Energies 2023, 16, 6973. [Google Scholar] [CrossRef]
- Wright, M.L.; Lewis, A.C. Emissions of NOx from Blending of Hydrogen and Natural Gas in Space Heating Boilers. Elementa 2022, 10, 00114. [Google Scholar] [CrossRef]
- Boulahlib, M.S.; Medaerts, F.; Boukhalfa, M.A. Experimental Study of a Domestic Boiler Using Hydrogen Methane Blend and Fuel-Rich Staged Combustion. Int. J. Hydrogen Energy 2021, 46, 37628–37640. [Google Scholar] [CrossRef]
- Rohani, B.; Saqr, K.M. Effects of Hydrogen Addition on the Structure and Pollutant Emissions of a Turbulent Unconfined Swirling Flame☆. Int. Commun. Heat Mass Transf. 2012, 39, 681–688. [Google Scholar] [CrossRef]
- Kashir, B.; Tabejamaat, S.; Jalalatian, N. On Large Eddy Simulation of Blended CH4–H2 Swirling Inverse Diffusion Flames: The Impact of Hydrogen Concentration on Thermal and Emission Characteristics. Int. J. Hydrogen Energy 2015, 40, 15732–15748. [Google Scholar] [CrossRef]
- Taimoor, A.A.; Muhammad, A.; Saleem, W.; Zain-ul-abdein, M. Humidified Exhaust Recirculation for Efficient Combined Cycle Gas Turbines. Energy 2016, 106, 356–366. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Q.; Wang, M.; Feng, X. Thermodynamic Performance Comparison between ORC and Kalina Cycles for Multi-Stream Waste Heat Recovery. Energy Convers. Manag. 2017, 143, 482–492. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Zonouz, M.J. Analysis of an Integrated Cryogenic Air Separation Unit, Oxy-Combustion Carbon Dioxide Power Cycle and Liquefied Natural Gas Regasification Process by Exergoeconomic Method. Energy Convers. Manag. 2017, 139, 245–259. [Google Scholar] [CrossRef]
- Alobaid, F.; Starkloff, R.; Pfeiffer, S.; Karner, K.; Epple, B.; Kim, H.G. A Comparative Study of Different Dynamic Process Simulation Codes for Combined Cycle Power Plants—Part B: Start-up Procedure. Fuel 2015, 153, 707–716. [Google Scholar] [CrossRef]
- Alobaid, F.; Starkloff, R.; Pfeiffer, S.; Karner, K.; Epple, B.; Kim, H.G. A Comparative Study of Different Dynamic Process Simulation Codes for Combined Cycle Power Plants—Part A: Part Loads and off-Design Operation. Fuel 2015, 153, 692–706. [Google Scholar] [CrossRef]
- Lm6000 Fact Sheet Product Specifications. Available online: https://www.ge.com/content/dam/gepower/global/en_US/documents/gas/gas-turbines/aero-products-specs/lm6000-fact-sheet-product-specifications.pdf (accessed on 21 December 2023).
- Aydin, H. Exergetic Sustainability Analysis of LM6000 Gas Turbine Power Plant with Steam Cycle. Energy 2013, 57, 766–774. [Google Scholar] [CrossRef]
- Barigozzi, G.; Perdichizzi, A.; Gritti, C.; Guaiatelli, I. Techno-Economic Analysis of Gas Turbine Inlet Air Cooling for Combined Cycle Power Plant for Different Climatic Conditions. Appl. Therm. Eng. 2015, 82, 57–67. [Google Scholar] [CrossRef]
- Bhargava, R.K.; Bianchi, M.; De Pascale, A.; Negri Di Montenegro, G.; Peretto, A. Gas Turbine Based Power Cycles-A State-of-the-Art Review. In Challenges of Power Engineering and Environment; Springer: Berlin/Heidelberg, Germany, 2007; pp. 309–331. [Google Scholar]
- Guteša Božo, M.; Mashruk, S.; Zitouni, S.; Valera-Medina, A. Humidified Ammonia/Hydrogen RQL Combustion in a Trigeneration Gas Turbine Cycle. Energy Convers. Manag. 2021, 227, 113625. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Syred, N.; Bowen, P. Central Recirculation Zone Visualization in Confined Swirl Combustors for Terrestrial Energy. J. Propuls. Power 2013, 29, 195–204. [Google Scholar] [CrossRef]
- Mashruk, S. Nitric Oxide Formation Analysis Using Chemical Reactor Modelling and Laser Induced Fluorescence Measurements on Industrial Swirl Flames. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2022. [Google Scholar]
- Directive 2010/75/EU of the European Parliament and of the Council on Industrial Emissions (Integrated Pollution Prevention and Control). 2010. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:334:0017:0119:en:PDF (accessed on 21 December 2023).


| H2 [% vol.] | CH4 [% vol.] | LHVm [MJ/kg] | LHVv [MJ/m3] | SFT [K] | A/F [-] |
|---|---|---|---|---|---|
| 0 | 100 | 50.0 | 35.8 | 2224 | 17.1 |
| 20 | 80 | 52.2 | 30.8 | 2233 | 17.6 |
| 40 | 60 | 55.4 | 25.8 | 2247 | 18.4 |
| 60 | 40 | 61.1 | 20.8 | 2268 | 19.1 |
| 80 | 20 | 73.4 | 15.8 | 2304 | 22.8 |
| 100 | 0 | 120.0 | 10.8 | 2379 | 34.1 |
| Parameter | Unit | Value |
|---|---|---|
| Ambient temperature | K | 300 |
| Ambient pressure | MPa | 0.1 |
| Compression ratio | - | 28 |
| Fuel inlet temperature | K | 300 |
| Fuel inlet pressure | MPa | 3.0 |
| Compressor isentropic efficiency | - | 0.88 |
| Turbine isentropic efficiency | - | 0.86 |
| Steam inlet temperature | K | 803 |
| Flue gas outlet temperature | K | 503 |
| Combustion chamber efficiency | - | 0.99 |
| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| Mixing zone | |||
| Pressure | P0 | MPa | 28.3 |
| Temperature | T | K | 823 |
| Residence time in premixing zone | τp | s | 0.0005 |
| Recirculation zone | |||
| Pressure | P0 | MPa | 28.3 |
| Temperature in recirculation zone | TR | K | 1600 |
| Residence time in recirculation zone | τR | s | 0.0015 |
| Fraction of recirculating mass | mrec | wt.% | 30 |
| Flame zone | |||
| Pressure | P | MPa | 28.3 |
| Temperature | TF | K | 1600 |
| Residence time in flame zone | τF | s | 0.0011–0.0015 |
| Equivalence ratio in flame zone | φF | - | 1.8 |
| Post-flame zone | |||
| Pressure | P | MPa | 28.3 |
| Starting axial position | x0 | cm | 0 |
| Ending axial position | x1 | cm | 5 |
| Quenching zone | |||
| Pressure | P | MPa | 28.3 |
| Temperature | TQ | K | 1600 |
| Residence time in quenching zone | τQ | s | 0.0012 |
| Secondary air temperature | Ts | K | 823 |
| Lean-burn zone | |||
| Pressure | P | MPa | 28.3 |
| Starting axial position | x0 | cm | 0 |
| Ending axial position | x1 | cm | 5 |
| Equivalence ratio in lean-burn zone | φLB | - | 0.36–0.4 |
| H2 [% vol.] | CH4 [% vol.] | T5 (TOT) [K] | WC [MW] | WT [MW] | A/Q [kg/MJ] | Cp [kJ/kgK] | Adiabatic Index |
|---|---|---|---|---|---|---|---|
| 0 | 100 | 928 | 23.7 | 43.4 | 0.860 | 1.328 | 1.253 |
| 20 | 80 | 936 | 23.0 | 42.8 | 0.847 | 1.332 | 1.252 |
| 40 | 60 | 935 | 23.1 | 42.9 | 0.832 | 1.338 | 1.252 |
| 50 | 50 | 934 | 22.8 | 42.7 | 0.826 | 1.342 | 1.251 |
| 100 | 0 | 932 | 22.4 | 42.5 | 0.798 | 1.382 | 1.248 |
| H2 [% vol.] | CH4 [% vol.] | CCGT | CCGTP | ||
|---|---|---|---|---|---|
| WST [MW] | Wnet [MW] | WST [MW] | Wnet [MW] | ||
| 0 | 100 | 4.34 | 24.04 | 4.80 | 24.50 |
| 20 | 80 | 4.35 | 24.10 | 4.82 | 24.57 |
| 40 | 60 | 4.34 | 24.18 | 4.80 | 24.64 |
| 50 | 50 | 4.33 | 24.23 | 4.79 | 24.69 |
| 100 | 0 | 4.21 | 24.30 | 4.62 | 24.72 |
| H2 Content in Fuel [% vol.] | Flame Zone | Post-Flame Zone | Quenching Zone | Lean-Burn Zone | ||||
|---|---|---|---|---|---|---|---|---|
| O2 [%] | CO [%] | O2 [%] | CO [%] | O2 [%] | CO [%] | O2 [%] | CO [ppm] | |
| 0 | 0.03 | 9.45 | 0 | 10.01 | 13.11 | 2.66 | 11.33 | 2.9 |
| 20 | 0.02 | 8.99 | 0 | 9.38 | 13.01 | 2.09 | 11.29 | 2.0 |
| 40 | 0.02 | 8.26 | 0 | 8.49 | 13.14 | 1.92 | 11.21 | 2.0 |
| 60 | 0.01 | 7.04 | 0 | 7.14 | 13.07 | 1.73 | 11.19 | 1.8 |
| 80 | 0.01 | 4.80 | 0 | 4.83 | 13.14 | 1.07 | 11.31 | 1.2 |
| 100 | 0.00 | 0 | 0 | 0 | 13.26 | 0 | 11.51 | 0 |
| H2 Content in Fuel [% vol.] | Flame Zone | Post-Flame Zone | Quenching Zone | Lean-Burn Zone | ||||
|---|---|---|---|---|---|---|---|---|
| NO | NO2 | NO | NO2 | NO | NO2 | NO | NO2 | |
| 0 | 36.0 | 0.0 | 42.2 | 0.0 | 135.8 | 2.2 | 150.6 | 1.7 |
| 20 | 43.6 | 0.0 | 43.4 | 0.0 | 157.4 | 3.7 | 169.2 | 2.0 |
| 40 | 54.7 | 0.0 | 43.6 | 0.0 | 177.4 | 4.1 | 210.6 | 2.5 |
| 60 | 69.8 | 0.0 | 43.6 | 0.0 | 210.9 | 2.9 | 281.0 | 2.8 |
| 80 | 85.4 | 0.0 | 54.5 | 0.0 | 385.4 | 5.6 | 395.4 | 4.5 |
| 100 | 127.7 | 0.0 | 131.1 | 0.0 | 483.4 | 7.5 | 495.4 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, L.; Ślefarski, R.; Jankowski, R. Thermodynamic Analysis of Gas Turbine Systems Fueled by a CH4/H2 Mixture. Sustainability 2024, 16, 531. https://doi.org/10.3390/su16020531
Mustafa L, Ślefarski R, Jankowski R. Thermodynamic Analysis of Gas Turbine Systems Fueled by a CH4/H2 Mixture. Sustainability. 2024; 16(2):531. https://doi.org/10.3390/su16020531
Chicago/Turabian StyleMustafa, Laith, Rafał Ślefarski, and Radosław Jankowski. 2024. "Thermodynamic Analysis of Gas Turbine Systems Fueled by a CH4/H2 Mixture" Sustainability 16, no. 2: 531. https://doi.org/10.3390/su16020531
APA StyleMustafa, L., Ślefarski, R., & Jankowski, R. (2024). Thermodynamic Analysis of Gas Turbine Systems Fueled by a CH4/H2 Mixture. Sustainability, 16(2), 531. https://doi.org/10.3390/su16020531








