The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Analysis
2.2.1. Physicochemical Properties
2.2.2. Enzymes in Soil
2.3. Pollution Assessment Methodology
2.3.1. Contamination Factor (CF)
2.3.2. Enrichment Factor (EF)
2.3.3. The Nemerow Pollution Index (PN)
2.3.4. Pollution Load Index (PLI)
2.4. Plant Analysis
2.4.1. Translocation Factor (TF) and Bioaccumulation Factor (BCF)
2.4.2. Catalase in Plants
2.4.3. Chlorophyll Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Soil Properties
3.2. Heavy Metals in Soil
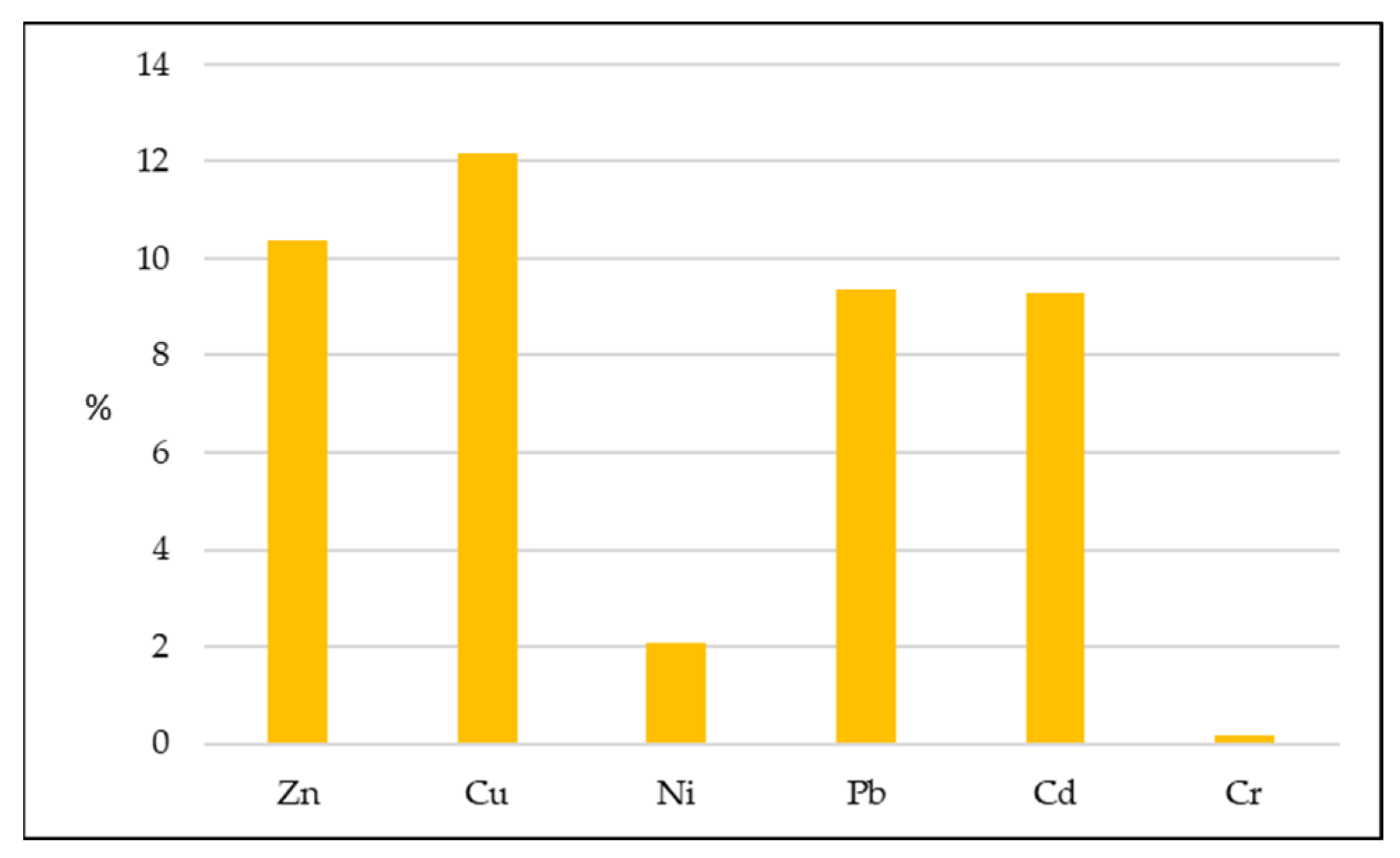
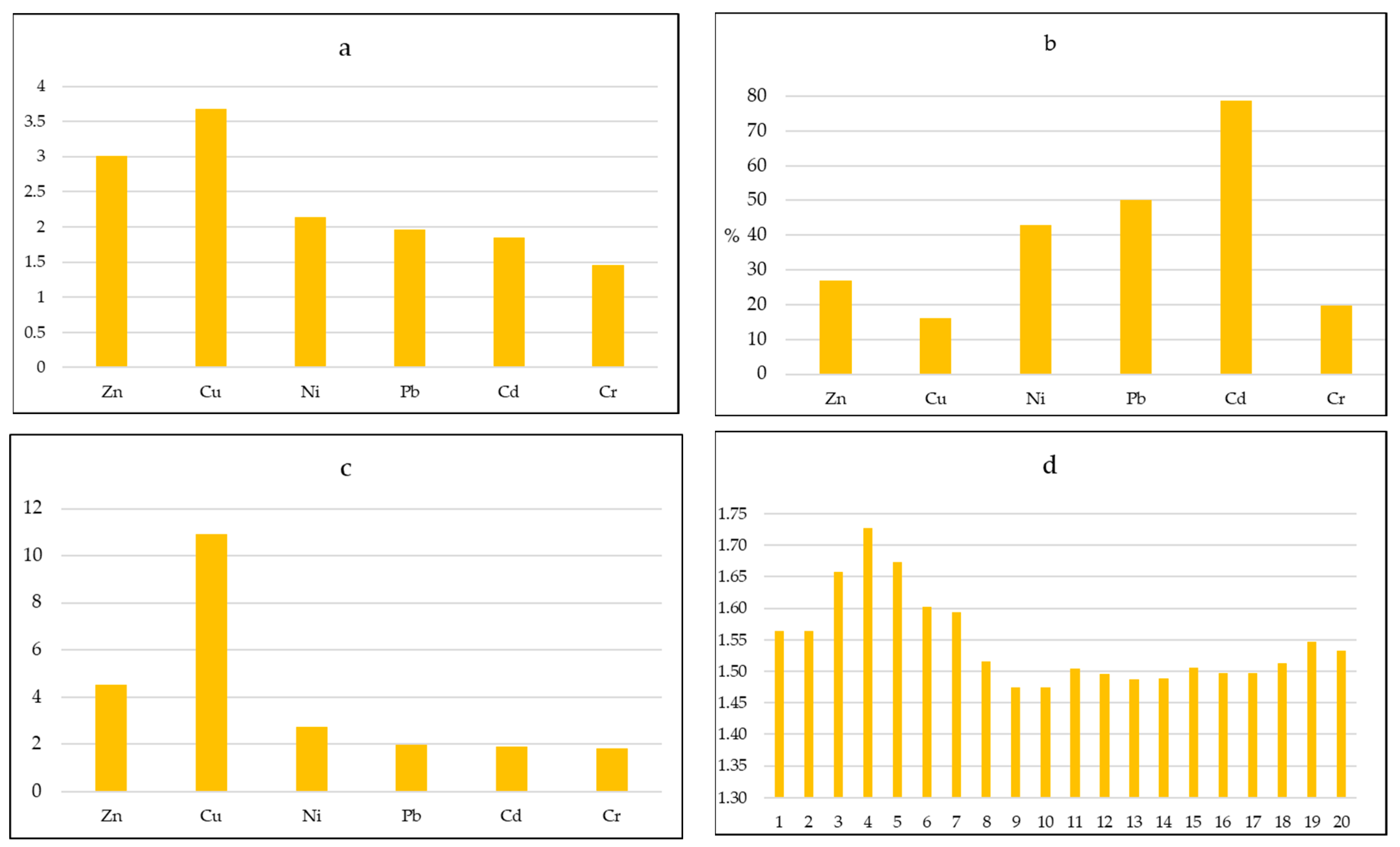
3.3. Evaluation of Heavy Metal Pollution
3.4. The Activity of Enzymes in Soil
3.5. Heavy Metals in Plants
3.6. The Activity of Catalase in Plants
3.7. Chlorophyll Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.-L.; Zhang, G.-L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutrit. 2015, 61, 30–46. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Jankowski, K.; Ciepiela, G.A.; Jankowska, J.; Szulc, W.; Kolczarek, R.; Sosnowski, J.; Wiśniewska-Kadzajan, B.; Malinowska, E.; Radzka, E.; Czeluściński, W.; et al. Content of lead and cadmium in aboveground plant organs of grasses growing on the areas adjacent to route of big traffic. Environ. Sci. Pollut. Res. 2015, 22, 978–987. [Google Scholar] [CrossRef]
- Wieczorek, K.; Turek, A.; Szczesio, M.; Wolf, W. Comprehensive evaluation of metal pollution in urban soils of post-industrial city-Łódź, Poland. Molecules 2020, 25, 4350. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Norman, M.; Burman, L. Road traffic emission factors for heavy metals. Atmos. Environ. 2009, 43, 4681–4688. [Google Scholar] [CrossRef]
- Duong, T.T.T.; Lee, B.K. Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. J. Environ. Manag. 2011, 92, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, A.; Kollaros, G. Heavy metal contamination of soil due to road traffic. Am. J. Eng. Res. 2016, 5, 354–363. [Google Scholar]
- Skorbiłowicz, M.; Skorbiłowicz, E.; Rogowska, W. Heavy metal concentration in roadside soils on the Białystok-Budzisko route in northeastern Poland. Minerals 2021, 11, 1290. [Google Scholar] [CrossRef]
- Tytła, M. Identification of the chemical forms of heavy metals in municipal sewage sludge as a critical element of ecological risk assessment in terms of its agricultural or natural use. Int. J. Environ. Res. Public Heatlh 2020, 17, 4640. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Senila, L.R. Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chem. Cent. J. 2012, 6, 119. [Google Scholar] [CrossRef]
- Czarnowska, K.; Milewska, A. The content of heavy metals in an indicator plant (Taraxacum officinale) in Warsaw. Pol. J. Environ. Stud. 2000, 9, 125–128. [Google Scholar]
- Petrova, S.; Yurukova, L.; Velcheva, I. Taraxacum officinale as a biomonitor of metals and toxic elements (Plovdiv, Bulgaria). Bulg. J. Agric. Sci. 2013, 19, 241–247. [Google Scholar]
- Kano, N.; Hori, T.; Zhang, H.; Miyamoto, M.; Anak, D.E.V.; Mishima, K. Study on the behavior and removal of cadmium and zinc using Taraxacum officinale and gazania under the application of biodegradable chelating agents. Appl. Sci. 2021, 11, 1557–1574. [Google Scholar] [CrossRef]
- Bomze, K.; Rutkowska, B.; Szulc, W. The content of trace metals in dandelion (Taraxacum officinale) depending on the distance from the transport route. Soil Sci. Ann. 2007, 58, 38–42. [Google Scholar]
- Kajka, J.; Rutkowska, B. Accumulation of selected heavy metals in soils and common dandelion (Taraxacum officinale) near a road with high traffic intensity. Soil Sci. Ann. 2018, 69, 11–16. [Google Scholar]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ. 2012, 35, 199–201. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Keeler, K.M.; Bunnell, D.B.; Diana, J.S.; Adams, J.V.; Mychek-Londer, J.G.; Warner, D.M.; Yule, D.L.; Vinson, M.R. Evaluating the importance of abiotic and biotic drivers on Bythotrephes biomass in Lakes Superior and Michigan. J. Great Lakes Res. 2015, 41, 150–160. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Valeo, C. Evaluating the effects of environmental stress on leaf chlorophyll content as an index for tree health. Earth Environ. Sci. 2022, 1006, 012007. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Zahab, A.A.; Osta, B.; Romanos, D.; Haddad, G. Heavy metals accumulation effects on the photo-synthetic performance of geophytes in Mediterranean reserve. J. King Saud Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Dick, R.P.; Kandeler, E. Enzymes in soils. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 448–456. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Zielińska, A.; Jaskulska, I.; Rydlewska, M.; Klunek, K.; Polkowska, M. The Effect of enzyme activity on carbon sequestration and the cycle of available macro- (P, K, Mg) and microelements (Zn, Cu) in Phaeozems. Agriculture 2023, 13, 172. [Google Scholar] [CrossRef]
- Chabot, M.; Morales, E.; Cummings, J.; Rios, N.; Giatpaiboon, S.; Mogul, R. Simple kinetics, assay, and trends for soil microbial catalases. Anal. Biochem. 2020, 610, 113901. [Google Scholar] [CrossRef]
- PN-ISO 10381-5; Soil Quality—Sampling—Part 5: Guidance on the Procedure for the Investigation of Urban and Industrial Sites with Regard to Soil Contamination. Polish Standards Committee: Warsaw, Poland, 2009.
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; ISRIC, FAO: Wageningen, The Netherlands, 2002. [Google Scholar]
- PN-ISO 14235; Chemical and Agricultural Analysis—Soil Quality—Determining the Content of Organic Carbon by Oxidation of Potassium Dichromate (VI) in the Environment of Sulphuric Acid (VI). Polish Standards Committee: Warsaw, Poland, 2003.
- Haluschak, P.; Laboratory Methods of Soil Analysis. Canada Manitoba Soil Survey. 2006; pp. 3–133. Available online: https://www.gov.mb.ca/agriculture/soil/soil-survey/pubs/laboratory_methods_of_soil_analysis.pdf (accessed on 14 December 2023).
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Obrador, A.; Alvarez, J.M.; Lopez-Valdivia, L.M.; Gonzalez, D.; Novillo, J.; Rico, M.I. Relationships of soil properties with Mn and Zn distribution in acidic soils and their uptake by a barley crop. Geoderma 2007, 137, 432–443. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M. Heavy metals in soils along unpaved roads in south west Cameroon: Contamination levels and health risks. Ambio 2016, 45, 374–386. [Google Scholar] [CrossRef]
- Zonta, R.; Zaggia, L.; Argrse, E. Heavy metal and grain size distributions in estuarine shallow water sediments of the Cona Marsh (Venice Lagoon. Italy). Sci. Total Environ. 1994, 151, 19–28. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of selected element concentrations and enrichment ratiosin background and anthropogenically impacted roadside areas. Arch. Environ. Contam. Toxicol. 2000, 38, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Martínez-Guijarro, R.; Paches, M.; Romero, I.; Aguado, D. Enrichment and contamination level of trace metals in the Mediterranean marine sediments of Spain. Sci. Total Environ. 2019, 693, 133566. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Arnon, D.I.; Allen, M.B.; Whatley, F.R. Photosynthesis by isolated chloroplasts IV. General concept and comparison of three photochemical reactions. Biochim. Biophys. Acta 1956, 20, 449–461. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accommodation, and implication to soil surveys. In Soil Spatial Variability, 1st ed.; Nielsen, D.R., Bouma, J., Eds.; Pudoc: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- Statistica. Data Analysis Software System, Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019; Available online: https://www.tibco.com/products/data-science (accessed on 28 September 2021).
- Polish Soil Classification (Systematyka Gleb Polski); Soil Science Society of Poland, Commission on Soil Genesis, Classification and Cartography, Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze: Wrocław/Warszawa, Poland, 2019; 235p.
- USDA. Keys to Soil Taxonomy, 10th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2006; pp. 1–332. [Google Scholar]
- Różański, S.; Dąbkowska-Naskręt, H. Spatial and profile distribution of mercury in urbanozems of Bydgoszcz city. Environ. Prot. Nat. Resour. 2011, 49, 193–201. [Google Scholar]
- Bartkowiak, A. The accumulation of selected heavy metals in soils in the vicinityof a busy road. Soil Sci. Annu. 2020, 71, 118–124. [Google Scholar] [CrossRef]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; FAO Soils Bulletin 39; FAO: Rome, Italy, 1988. [Google Scholar]
- Acosta, J.A.; Jansen, B.; Kalibitz, K.; Faz, A.; Martinez-Martinez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Kandeler, E. Biochemical characterization of urban soil profiles from Stuttgart, Germany. Soil Biol. Biochem. 2005, 37, 1373–1385. [Google Scholar] [CrossRef]
- Xia, X.; Chen, X.; Liu, R.; Liu, H. Heavy metals in urban soils with various types of land use in Beijing, China. J. Hazard. Mater. 2011, 186, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Wydro, U.; Jabłońska-Trypuć, A.; Hawrylik, E.; Butarewicz, A.; Rodziewicz, J.; Janczukowicz, W.; Wołejko, E. Heavy metals behavior in soil/plant system after sewage sludge application. Energies 2021, 14, 1584. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment dated 1 September 2016 on assessment procedures for the land surface pollution (Journal of Laws, Item 1359, 5 September 2016). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001395/O/D20161395.pdf (accessed on 14 December 2023).
- Guo, G.; Wu, F.; Xie, F.; Zhang, R. Spatial distribution and pollution assessment of heavy metals in urban soils from southwest. China J. Environ. Sci. 2012, 24, 410–418. [Google Scholar] [CrossRef]
- Czech, T.; Baran, A.; Wieczorek, J. Content of heavy metals in soil and plants from an area Borzęcin municipality (Malopolska province). Ecol. Eng. 2014, 37, 89–98. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lemanowicz, J.; Lamparski, R. Assessment of selected heavy metals and enzyme activity in soils within the zone of influence of various tree species. Sci. Rap. 2020, 10, 14077–14086. [Google Scholar] [CrossRef]
- Christoforidis, A.; Stamatis, N. Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 2009, 151, 257–263. [Google Scholar] [CrossRef]
- Popoola, O.E.; Bamgbose, O.; Okonkwo, O.J.; Arowolo, T.A.; Popoola, A.O.; Awofolu, O.R. Heavy metals content in classroom dust of some public primary schools in Metropolitan Lagos, Nigeria. Res. J. Environ. Earth Sci. 2012, 4, 460–465. [Google Scholar]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Alloway, B.J. Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Commun. Soil Sci. Plant Anal. 2008, 39, 538–550. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; ISBN 978-1-4200-9368-1. [Google Scholar]
- Szatanik-Kloc, A. Effect of pH and selected heavy metals in soils on their content in plants. Acta Agrophys. 2004, 4, 177–183. [Google Scholar]
- Mafuyai, G.M.; Kamoh, N.M.; Kangpe, N.S.; Ayuba, S.M.; Eneji, I.S. Heavy metals contamination in roadside dust along major traffic roads in Jos Metropolitan area, Nigeria. J. Environ. Earth Sci. 2015, 5, 48–57. [Google Scholar]
- Wang, C.; Ye, Z.; Wang, W.; Jin, M. Traffic-related heavy metal contamination in urban areas and correlation with traffic activity in China. Transp. Res. Rec. 2016, 2571, 80–89. [Google Scholar] [CrossRef]
- Ali, S.M.; Malik, R.N. Spatial distribution of metals in top soils of Islamabad City, Pakistan. Environ. Monit. Assess. 2011, 172, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Atiemo, M.S.; Ofosu, G.F.; Mensah, H.K.; Tutu, A.O.; Linda Palm, N.D.M.; Blankson, S.A. Contamination Assessment of Heavy Metals in Road Dust from Selected Roads in Accra, Ghana. Res. J. Environ. Earth Sci. 2011, 3, 473–480. [Google Scholar]
- Magno, J.L.; Budianta, W. Spatial distribution and pattern of heavy metals in urban soils of Yogyakarta, Indonesia. Earth Environ. Sci. 2022, 1071, 012032. [Google Scholar] [CrossRef]
- Dec, D. Assessment of the microbiological activity in agricultural and urban soils. Soil Sci. Annu. 2014, 65, 156–160. [Google Scholar] [CrossRef]
- Hok, L.; de MoraesSá, J.C.; Reyes, M.; Boulakia, S.; Tivet, F.; Leng, V.; Kong, R.; Briedis, C.; da Cruz Hartman, D.; Ferreira, L.A.; et al. Enzymes and C pools as indicators of C build up in short-term conservation agriculture in a savanna ecosystem in Cambodia. Soil Tillage Res. 2018, 177, 125–133. [Google Scholar] [CrossRef]
- Ma, W.; Li, G.; Wu, J.; Xu, G.; Wu, J. Response of soil labile organic carbon fractions and carbon-cycle enzyme activities to vegetation degradation in a wet meadow on the Qinghai–Tibet Plateau. Geoderma 2020, 377, 114565. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ahn, M.-Y. Organo-Mineral–Enzyme Interaction and Soil Enzyme Activity. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 271–292. [Google Scholar] [CrossRef]
- Chaperon, S.; Sauvé, S. Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biol. Biochem. 2007, 39, 2329–2338. [Google Scholar] [CrossRef]
- Fotovat, A.; Naidu, R. Ion exchange resin and MINTEQA2 speciation of Zn and Cu in alkaline sodic and acidic soil extracts. Aust. J. Soil Res. 1997, 35, 711–726. [Google Scholar] [CrossRef]
- Zajęcka, E.; Świercz, A. Biomonitoring of the urban environment of Kielce and Olsztyn (Poland) based on studies of total and bioavailable lead content in soils and common dandelion (Taraxacum officinale agg.). Minerals 2021, 11, 52. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Mayerova, M.; Petrova, S.; Madaras, M.; Lipavsky, J.; Simon, T.; Vanek, T. Non-enhanced phytoextraction of cadmium, zinc, and lead by high-yielding crops. Environ. Sci. Pollut. Res. 2017, 24, 14706–14716. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Malandrino, M.; Fabbri, D. Bioremediation methods for the recovery of lead-contaminated soils: A review. Appl. Sci. 2020, 10, 3528. [Google Scholar] [CrossRef]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar] [CrossRef]
- Rahmanian, M.; Habib, K.; Younes, R.D.; MirHasan, R.S. Effects of heavy metal resistant soil microbes inoculation and soil Cd concentration on growth and metal uptake of millet, couch grass and alfalfa. Afr. J. Microbiol. Res. 2011, 5, 403–410. [Google Scholar]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef]
- Bretzel, F.; Benvenuti, S.; Pistelli, L. Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ. Sci. Pollut. Res. 2014, 21, 2325–2333. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, Y.; Li, X. Effects of EDTA on mechanism of lead accumulation in typha orientalis Presl. Bull. Environ. Contam. Toxicol. 2009, 83, 553–557. [Google Scholar] [CrossRef] [PubMed]
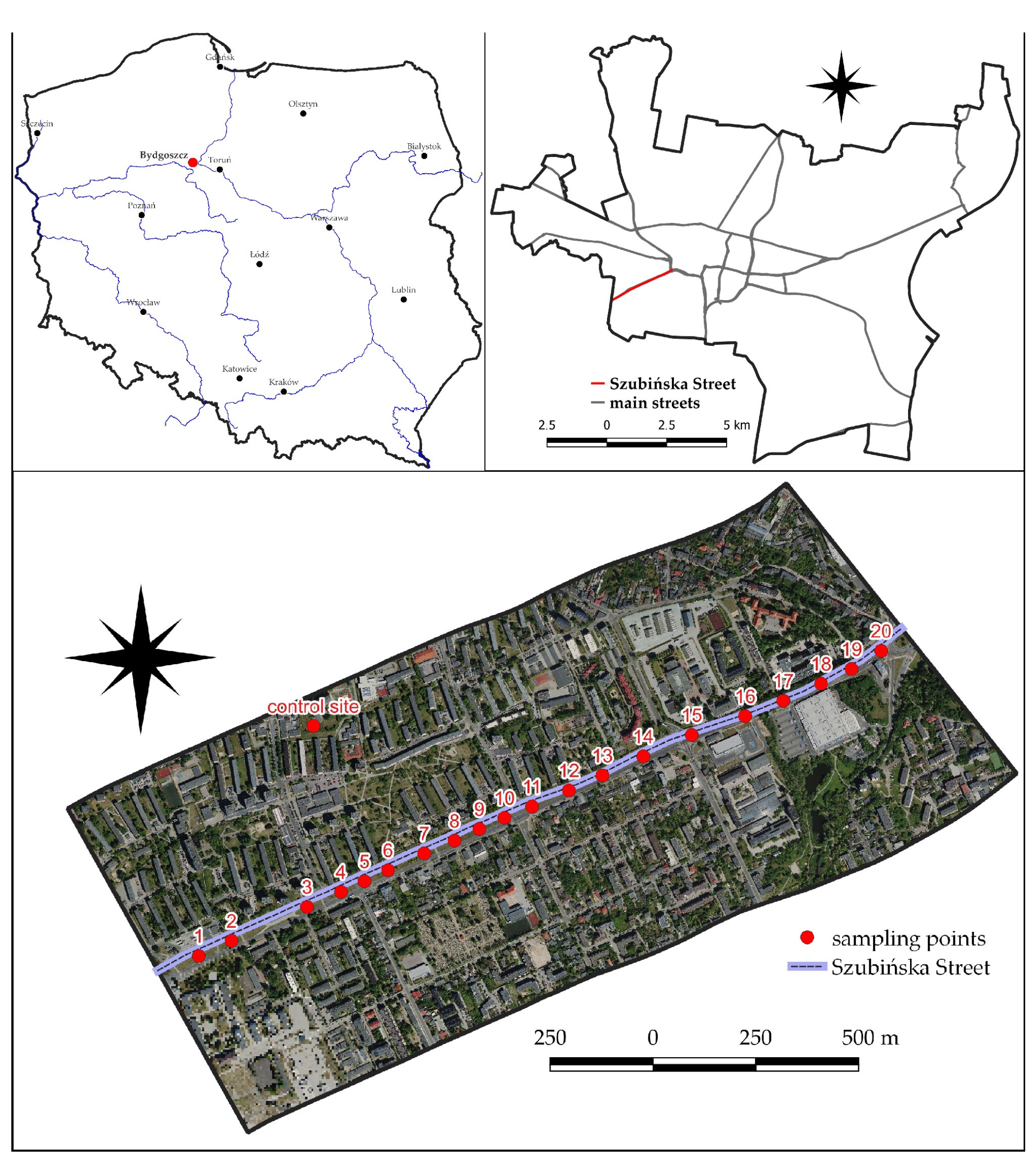


| Sampling Point | Coordinates | Sampling Point | Coordinates | Sampling Point | Coordinates | |||
| X | Y | X | Y | X | Y | |||
| 1 | 430,495.94 | 583,743.90 | 8 | 431,118.06 | 584,023.88 | 15 | 431,695.07 | 584,280.75 |
| 2 | 430,575.70 | 583,780.75 | 9 | 431,178.57 | 584,052.48 | 16 | 431,825.16 | 584,326.41 |
| 3 | 430,759.42 | 583,863.26 | 10 | 431,239.62 | 584,079.43 | 17 | 431,917.85 | 584,363.81 |
| 4 | 430,841.93 | 583,899.56 | 11 | 431,306.73 | 584,106.94 | 18 | 432,009.71 | 584,405.07 |
| 5 | 430,899.14 | 583,925.42 | 12 | 431,396.39 | 584,145.99 | 19 | 432,083.97 | 584,440.82 |
| 6 | 430,956.34 | 583,952.37 | 13 | 431,477.80 | 584,182.29 | 20 | 432,156.02 | 584,484.83 |
| 7 | 431,044.35 | 583,993.07 | 14 | 431,577.36 | 584,228.50 | C | 430,774.82 | 584,302.76 |
| Selected physical and chemical properties | |||||
| Sand (%) | Silt (%) | Clay (%) | pH KCl | EC1:5 (µS cm−1) | Corg (g kg−1) |
| 77.51 | 20.38 | 2.11 | 7.69 | 555 | 12.70 |
| The total content of heavy metals (mg·kg−1) | |||||
| Zn | Cu | Ni | Pb | Cd | Cr |
| 56.31 | 14.67 | 4.80 | 13.90 | 0.05 | 10.58 |
| The available forms of heavy metals (mg·kg−1) | |||||
| Zn | Cu | Ni | Pb | Cd | Cr |
| 10.01 | 4.22 | 0.11 | 0.19 | 0.05 | 0.04 |
| The activity of enzymes | |||||
| DEHs | CAT | ||||
| 0.943 | 0.869 | ||||
| Fractions (%) | pH KCl | EC1:5 (µS cm−1) | Corg (g kg−1) | |||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| Min | 55.93 | 11.71 | 0.99 | 7.30 | 550 | 7.20 |
| Max | 96.41 | 40.16 | 3.90 | 7.76 | 885 | 33.30 |
| Mean | 80.10 | 19.56 | 1.62 | 7.57 | 630 | 15.35 |
| Median | 81.39 | 17.78 | 1,37 | 7.58 | 605 | 13.55 |
| SD | 7.55 | 6.39 | 0.61 | 0.13 | 87.32 | 6.34 |
| CV | 9.43 | 32.66 | 37.65 | 1.68 | 13.89 | 41.30 |
| Skewness | −1.17 | 1.60 | 2.70 | −0.51 | 1.61 | 1.11 |
| Kurtosis | 4.33 | 3.57 | 9.44 | −0.05 | 2.33 | 1.29 |
| Total Content | Available Forms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||||||
| Zn | Cu | Ni | Pb | Cd | Cr | Zn | Cu | Ni | Pb | Cd | Cr | |
| Min | 104.8 | 40.55 | 7.90 | 28.02 | 0.46 | 34.52 | 10.63 | 1.92 | 0.09 | 1.34 | 0.01 | 0.03 |
| Max | 345.4 | 242.9 | 16.91 | 91.14 | 0.47 | 55.15 | 29.59 | 13.16 | 0.70 | 6.22 | 0.11 | 0.05 |
| Mean | 169.6 | 52.59 | 10.25 | 52.62 | 0.39 | 24.15 | 16.53 | 5.22 | 0.21 | 2.37 | 0.03 | 0.04 |
| Median | 146.9 | 38.28 | 9.66 | 20.63 | 0.41 | 22.80 | 14.83 | 4.90 | 0.17 | 2.07 | 0.03 | 0.04 |
| SD | 120.3 | 50.69 | 1.98 | 17.17 | 0.16 | 5.03 | 5.55 | 2.27 | 0.13 | 1.15 | 0.02 | 0.01 |
| CV | 70.90 | 96.38 | 19.36 | 32.62 | 42.02 | 20.83 | 33.55 | 43.42 | 63.15 | 48.59 | 59.09 | 59.09 |
| Skewness | 1.74 | 3.06 | 2.01 | 2.77 | −0.45 | 2.06 | 0.87 | 1.99 | 2.87 | 2.27 | 1.54 | −0.02 |
| Kurtosis | 2.72 | 9.69 | 5.34 | 9.20 | −1.13 | 4.13 | −0.24 | 6.64 | 9.61 | 5.54 | 2.63 | −0.74 |
| Variables | Equation | r | R2 | |
|---|---|---|---|---|
| Dependent | Independent | |||
| T Zn | T Cu | y = 130.1737 + 0.6849x | 0.515 | 0.265 |
| T Zn | T Ni | y = −0.3054 + 16.6608x | 0.556 | 0.309 |
| T Zn | Clay | y = 69.931 + 59.5285x | 0.548 | 0.301 |
| T Zn | Corg | y = 963226 + 4.57011x | 0.437 | 0.191 |
| T Ni | Clay | y = 6.9486 + 1.8879x | 0.521 | 0.272 |
| T Ni | Corg | y = 7.0132 + 0.1953x | 0.560 | 0.313 |
| T Cd | Clay | y = 0.5551 − 0.1252x | −0.585 | 0.342 |
| A Zn | T Zn | y = 8.2728 + 0.0495x | 0.585 | 0.342 |
| A Zn | Clay | y = 7.3106 + 5.6691x | 0.618 | 0.381 |
| A Zn | pH | y = 221.93 − 27.1486x | −0.617 | 0.380 |
| A Zn | EC | y = −4.3792 + 0.0334x | 0.516 | 0.227 |
| A Zn | Corg | y = 3.5741 + 0.6469x | 0.732 | 0.536 |
| A Cu | Clay | y = 0.3444 + 3.0061x | 0.825 | 0.681 |
| A Cu | EC | y = −5.9796 + 0.0179x | 0.698 | 0.487 |
| A Cu | Corg | y = 0.9859 + 0.2758x | 0.786 | 0.618 |
| A Cu | T Zn | y = 1.3954 + 0.023x | 0.685 | 0.469 |
| A Cu | T Ni | y = −2.4267 + 0.7639x | 0.760 | 0.578 |
| A Cu | A Zn | y = 1.1562 + 0.2462x | 0.621 | 0.385 |
| Zn A-P | A Zn | y = 42.4903 + 1.4812x | 0.448 | 0.333 |
| Cu A-P | A Pb | y = 14.7701 + 40.5993x | 0.577 | 0.201 |
| Chl a | Ni A-P | y = 1.8199 − 0.0602x | −0.994 | 0.988 |
| Chl b | Ni A-P | y = 0.9193 − 0.0311x | −0.991 | 0.983 |
| Chl a | Cr A-P | y = 1.3511 − 0.0249x | −0.924 | 0.852 |
| Chl b | Cr A-P | y = 0.7763 − 0.0127x | −0.907 | 0.823 |
| Cu R | A Pb | y = 19.7261 + 35.459x | 0.604 | 0.365 |
| Cr R | A Pb | y = 5.7472 + 2.776x | 0.524 | 0.275 |
| CAT | Clay | y = 0.3529 + 0.07x | 0.539 | 0.291 |
| CAT | Corg | y = 0.352 + 0.0075x | 0.596 | 0.356 |
| CAT | A Zn | y = 0.3191 + 0.0089x | 0.631 | 0.399 |
| CAT | A Cu | y = 0.3505 + 0.222x | 0.623 | 0.388 |
| CAT | A Pb | y = 0.3523 + 0.4851x | 0.689 | 0.476 |
| DEHs | Clay | y = 0.3982 + 0.0913x | 0.614 | 0.373 |
| DEHs | Corg | y = 0.3342 + 0.0138x | 0.959 | 0.920 |
| DEHs | A Zn | y = 0.3691 + 0.0107x | 0.659 | 0.434 |
| DEHs | A Cu | y = 0.4042 + 0.0272x | 0.663 | 0.439 |
| CAT A-P | Zn R | y = 0.8292 + 0.0024x | 0.837 | 0.701 |
| CAT A-P | Cu R | y = 0.9051 + 0.0038x | 0.704 | 0.495 |
| CAT R | Zn R | y = 0.9994 + 0.0017x | 0.830 | 0.689 |
| CAT R | Cu R | y = 1.0524 + 0.0027x | 0.708 | 0.502 |
| Minimum | Maximum | Mean | Median | SD * | CV | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|
| DEHs | 0.389 | 0.722 | 0.546 | 0.532 | 0.094 | 17.14 | 0.315 | −0.378 |
| CAT | 0.372 | 0.728 | 0.466 | 0.437 | 0.082 | 17.54 | 1.648 | 3.900 |
| Zn | Cu | Ni | Pb | Cd | Cr | CAT |
|---|---|---|---|---|---|---|
| Above-ground parts of plant | ||||||
| 22.56 | 8.43 | 5.69 | 7.51 | n.d. | 15.1 | 0.986 |
| Root | ||||||
| 31.22 | 7.99 | 6.12 | 4.38 | n.d. | 5.91 | 1.030 |
| Above-Ground Parts of Plant | Root | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||||||
| Zn | Cu | Ni | Pb | Cd | Cr | Zn | Cu | Ni | Pb | Cd | Cr | |
| Min | 22.56 | 18.03 | 5.71 | 14.10 | n.d. | 5.63 | 66.32 | 21.64 | 4.04 | 2.50 | n.d. | 5.13 |
| Max | 119.6 | 47.28 | 22.91 | 22.20 | n.d. | 44.01 | 93.54 | 42.71 | 61.41 | 23.10 | n.d. | 7.88 |
| Mean | 69.16 | 25.12 | 13.55 | 18.08 | n.d. | 24.82 | 78.75 | 29.06 | 10.06 | 10.77 | n.d. | 6.51 |
| Median | 63.97 | 23.50 | 23.77 | 18.05 | n.d. | 13.87 | 78.28 | 27.37 | 5.63 | 9.60 | n.d. | 17.26 |
| SD | 16.01 | 7.26 | 6.65 | 2.41 | n.d. | 15.67 | 11.13 | 4.97 | 12.31 | 5.13 | n.d. | 1.12 |
| CV | 23.15 | 28.89 | 49.07 | 13.33 | n.d. | 63.13 | 14.13 | 17.09 | 122.33 | 47.66 | n.d. | 17.26 |
| Skewness | 1.98 | 1.98 | 0.00 | 0.27 | n.d. | 0.22 | 0.28 | 1.13 | 3.90 | 0.67 | n.d. | 0.04 |
| Kurtosis | 3.89 | 3.70 | −2.04 | −0.38 | n.d. | −1.97 | −0.71 | 1.23 | 16.24 | 0.35 | n.d. | 1.06 |
| Minimum | Maximum | Mean | Median | SD * | CV | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|
| CATA–P | 1.011 | 1.045 | 1.018 | 0.014 | 0.082 | 17.54 | 1.806 | 3.853 |
| CATR | 1.127 | 1.161 | 1.134 | 1.130 | 0.008 | 0.738 | 1.843 | 4.036 |
| Minimum | Maximum | Mean | Median | SD * | CV | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|
| Chl a | 0.541 | 1.495 | 1.022 | 1.275 | 0.410 | 40.08 | 0.043 | −2.134 |
| Chl b | 0.321 | 0.742 | 0.505 | 0.638 | 0.209 | 41.38 | 0.010 | −2.115 |
| Chl a/Chl b | 1.923 | 2.515 | 2.041 | 2.001 | 0.121 | 5.92 | 3.240 | 12.58 |
| Chl a + Chl b | 0.802 | 2.226 | 1.495 | 1.455 | 0.615 | 41.17 | 0.035 | 2.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowiak, A.; Lemanowicz, J.; Rydlewska, M.; Sowiński, P. The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion. Sustainability 2024, 16, 812. https://doi.org/10.3390/su16020812
Bartkowiak A, Lemanowicz J, Rydlewska M, Sowiński P. The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion. Sustainability. 2024; 16(2):812. https://doi.org/10.3390/su16020812
Chicago/Turabian StyleBartkowiak, Agata, Joanna Lemanowicz, Magdalena Rydlewska, and Paweł Sowiński. 2024. "The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion" Sustainability 16, no. 2: 812. https://doi.org/10.3390/su16020812
APA StyleBartkowiak, A., Lemanowicz, J., Rydlewska, M., & Sowiński, P. (2024). The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion. Sustainability, 16(2), 812. https://doi.org/10.3390/su16020812







