Development and Characterization of Sustainable Coatings on Cellulose Fabric and Nonwoven for Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Preparation of the Textile Materials
2.1.2. Preparation of Sustainable Coatings
- erythritol slows down bacterial growth in biofilms.
- gelatin has numerous functional groups that enable further surface functionalization to achieve active targeting of diseased cells.
- collagen can create a moist healing environment that enables wound healing, and it is able to inhibit or inactivate the MMPs while providing the enzymes with additional collagen sources so that the body’s own collagen can be utilized for new tissue growth.
- propolis is a natural broad-spectrum antibiotic, which, as such, is ideal against biofilms in chronic wounds.
- alginate fibers have the task of maintaining a physiologically moist microenvironment, minimizing bacterial infection at the wound site, and facilitating wound healing.
2.2. Determination of the Morphology of the Materials
2.3. Activity of Hydrogen Ions
2.4. Drop Test Method
2.5. Determination of the Crease Recovery Angle
3. Results and Discussion
3.1. Results of the Morphology of the Materials
3.2. Results of the Activity of Hydrogen Ions
3.3. Results of the Drop Test Method
3.4. Results of the Crease Recovery Angle
3.5. Results of the Determination of Thickness and Mass per Unit Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Namazi, H. Polymers in our daily life. Bioimpacts 2017, 7, 73–74. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Thakur, V.K.; Njuguna, J. Bio-Based Sustainable Polymers and Materials: From Processing to Biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Brunšek, R.; Kopitar, D.; Schwarz, I.; Marasović, P. Biodegradation Properties of Cellulose Fibers and PLA Biopolymer. Polymers 2023, 15, 3532. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hong, P.Z.; Liao, M.N.; Kong, S.Z.; Huang, N.; Ou, C.Y.; Li, S.D. Preparation and Characterization of Chitosan-Agarose Composite Films. Materials 2016, 9, 816. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falco, I.; Randazzo, W.; Sanchez, G.; Lopez-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocolloid. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef]
- Lei, J.; Zhou, L.; Tang, Y.J.; Luo, Y.; Duan, T.; Zhu, W.K. High-strength konjac glucomannan/silver nanowires composite films with antibacterial properties. Materials 2017, 10, 524. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Paul, U.C.; Picone, P.; Di Carlo, M.; Athanassiou, A.; Heredia-Guerrero, J.A. Multifunctional bioplastics inspired by wood composition: Effect of hydrolyzed lignin addition to xylan–cellulose matrices. Biomacromolecules 2020, 21, 910–920. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligoz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Li, S.B.; Yi, J.J.; Yu, X.M.; Wang, Z.Y.; Wang, L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Ojagh, S.M.; Fooladi, A.A.I.; Shabanpour, B.; Gharahei, M. Effects of probiotic cells on the mechanical and antibacterial properties of sodium-caseinate films. App. Food Biotechnol. 2018, 5, 155–162. [Google Scholar]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Aziz, S.G.G.; Almasi, H. Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded nanoliposomes. Food Bioprocess Technol. 2018, 11, 1552–1565. [Google Scholar] [CrossRef]
- Echeverria, I.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite-clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.T.; da Cunha, K.F.; Fonseca, L.M.; Antunes, M.D.; El Halal, S.L.M.; Fiorentini, A.M.; Zavareze, E.D.; Dias, A.R.G. Action of ginger essential oil (Zingiber officinale) encapsulated in proteins ultrafine fibers on the antimicrobial control in situ. Int. J. Biol. Macromol. 2018, 118, 107–115. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. antibacterial and barrier properties of gelatin coated by electrospun polycaprolactone ultrathin fibers containing black pepper oleoresin of interest in active food biopackaging applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yilmaz, B.A.; Sargin, I. Effect of different animal fat and plant oil additives on physicochemical, mechanical, antimicrobial and antioxidant properties of chitosan films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Variyar, P.S. Development of guar gum based active packaging films using grape pomace. J. Food Sci. Tech. 2018, 55, 1982–1992. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics, 1st ed.; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.-J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar]
- Suen, D.W.-S.; Chan, E.M.-H.; Lau, Y.-Y.; Lee, R.H.-P.; Tsang, P.W.-K.; Ouyang, S.; Tsang, C.-W. Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning. Sustainability 2023, 15, 11638. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Macan, J.; Emira, P. Modification of polyurethane-coated fabrics by sol–gel thin films. J. Appl. Polym. Sci. 2014, 131, 39914. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Liu, D.; Sun, L.L.; Li, B.F.; Hou, H. Physicochemical and Biocompatibility Properties of Type I Collagen from the Skin of Nile Tilapia (Oreochromis niloticus) for Biomedical Applications. Mar. Drugs 2019, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.L.; Marian, E. Sodium Alginate-Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Coll. Certif. Wound Spec. 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef]
- Greatrex-White, S.; Moxey, H. Wound assessment tools and nurses’ needs: An evaluation study. Int. Wound J. 2015, 12, 293–301. [Google Scholar] [CrossRef]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A.R. Erythritol as sweetener-wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef]
- Janus, M.M.; Volgenant, C.M.C.; Brandt, B.W.; Buijs, M.J.; Keijser, B.J.F.; Crielaard, W.; Zaura, E.; Krom, B.P. Effect of erythritol on microbial ecology of in vitro gingivitis biofilms. J. Oral Microbiol. 2017, 9, 1337477. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.; Manjusha, A.; Ahmed, A.; Arnold, R.D. Chapter 15—Animal-Based Materials in the Formulation of Nanocarriers for Anticancer Therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Kesharwani, P., Paknikar, K.M., Gajbhiye, V., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–341. [Google Scholar]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 1, pp. 330–352. [Google Scholar]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Mbamalu, V.C. Glycerin and the Market. Master’s Thesis, The University of Tennessee at Chattanooga, Chattanooga, TN, USA, May 2013. [Google Scholar]
- Glicerin. Available online: https://radin.hr/hr/kemikalije/organske-kemikalije/glicerin (accessed on 26 December 2023).
- Stuermer, E.K. Wound biofilm: A bacterial success story. J. Wound Manag. 2023, 24, 6–13. [Google Scholar]
- Hodge, J.G.; Zamierowski, D.S.; Robinson, J.L.; Mellott, A.J. Evaluating polymeric biomaterials to improve next generation wound dressing design. Biomater. Res. 2022, 26, 50. [Google Scholar] [CrossRef]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.-M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. 3), 7–15. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 11827:2012; Textiles—Composition Testing—Identification of Fibres. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 3801:1977; Textiles—Woven Fabrics—Determination of Mass per Unit Length and Mass per Unit Area. International Organization for Standardization: Geneva, Switzerland, 1977.
- ISO 5084:1996; Textiles—Determination of Thickness of Textiles and Textile Products. International Organization for Standardization: Geneva, Switzerland, 1996.
- AATCC. Test Method 81-2006, pH of the Water-Extract from Wet Processed Textiles. In AATCC Technical Manual/2010; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2010. [Google Scholar]
- AATCC. Test Method 79-2007, Absorbency of Textiles. In AATCC Technical Manual/2010; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2010. [Google Scholar]
- EN ISO 2313-1:2021; Textiles—Determination of the Recovery from Creasing of a Folded Specimen of Fabric by Measuring the Angle of Recovery—Part 1: Method of the Horizontally Folded Specimen. International Organization for Standardization: Geneva, Switzerland, 2021.
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds 2007, 3, 52–56. [Google Scholar]
- Rezić, I.; Somogyi Škoc, M.; Majdak, M.; Jurić, S.; Stracenski, K.S.; Vinceković, M. Functionalization of Polymer Surface with Antimicrobial Microcapsules. Polymers 2022, 14, 1961. [Google Scholar] [CrossRef]

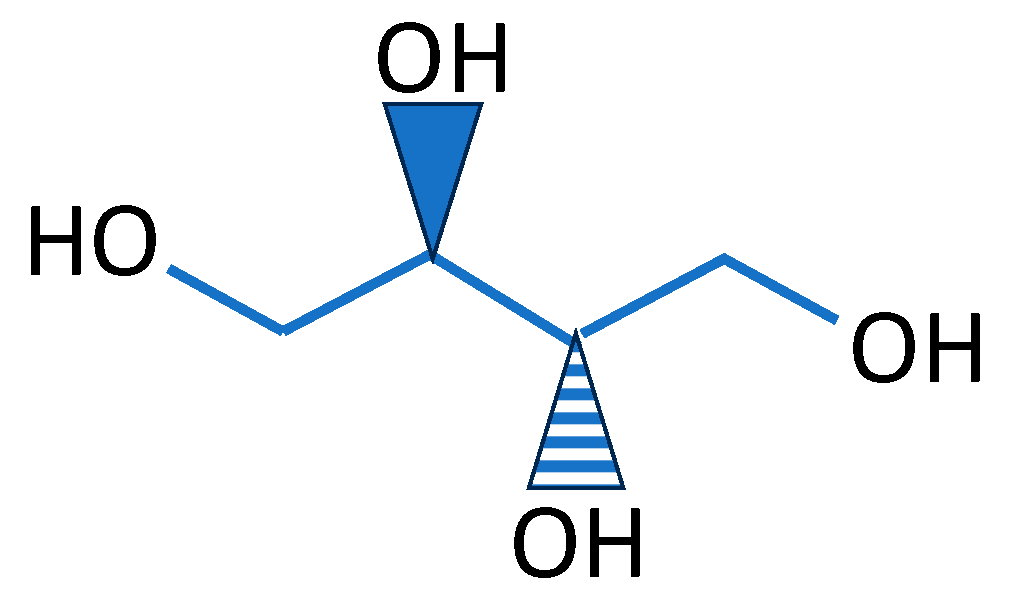

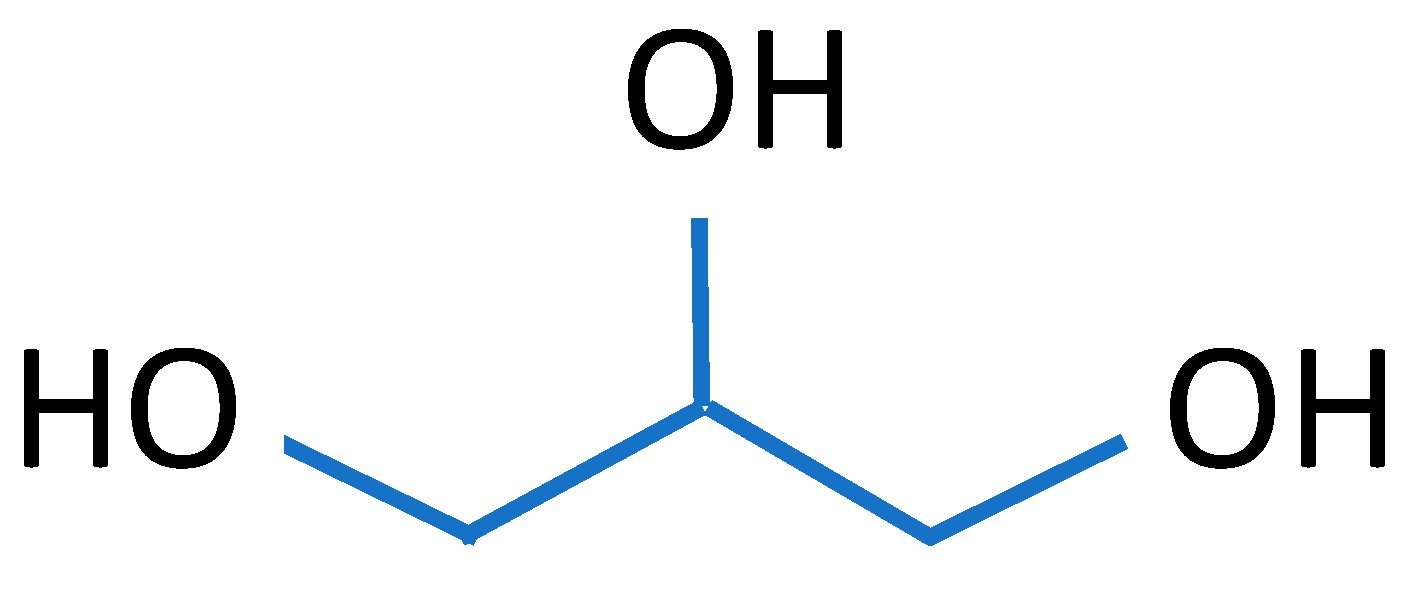


| Source of Biopolymer | Biopolymer | Characteristics | Application |
|---|---|---|---|
| polysaccharides | agarose [6] | extremely hydrophilic, high porosity, and very stable under alkaline conditions | in the production of capsules, tablets, tablet liquid, other drug coating materials, food, research fields, etc. |
| alginates [7] | renewable, nontoxic, control swelling properties, low cost, biocompatibility, good mechanical properties, chemical stability, good water barrier properties, good mechanical properties, stiffness | food, biomedical, pharmaceutical, drug delivery systems (DDSs), traditional wound dressings, dental impression material, in some formulations, preventing gastric reflux, etc. | |
| cellulose [8] | renewable, nontoxic, low energy consumption, high surface area, good oxygen, hydrocarbon barrier properties, high mechanical strength, high water vapor permeability, low cost, low density, high specificity, biocompatibility, odorless, tasteless, low cytotoxicity, chemical stability | developing paper and textiles, biomedical fields—medical implants; tissue engineering; wound healing; dressing; bone regeneration; cartilage renewal; orthodontic applications; drug delivery; etc. | |
| glucomannan [9] | great capacity to capture water and hydrate, forming a viscous gel | food additive (emulsifier and thickener) with the E number E425 | |
| hemicellulose [10] | highly hydrophilic, soluble in alkali, and easily hydrolyzed by acids | functional food and pharmaceutical fields, etc. | |
| chitosan [11] | renewable, nontoxic, increased absorption properties, high antimicrobial activity, high biocompatibility, low production cost, good gas/aroma/UV/oil barrier properties, wettability, antioxidant, water-insoluble, good film-forming ability, good optical properties, transparent, flexible | biodegradable antimicrobial food packaging, bioprinting, temperature-sensitive hydrogels, wound management, winemaking and fungal source chitosan, natural biocontrol and elicitor, agricultural and horticultural use, etc. | |
| starch [12] | renewable, nontoxic, low cost, abundance, transparent, colorless, flavorless, tasteless, good lipid/oxygen/UV barrier properties, great film-forming ability, low water vapor permeability | food, processing industry, pharmaceutical, biomedical—tissue engineering of bone; bone fixation; carrier for the controlled release of drugs and hormones; as hydrogels; etc. | |
| proteins | casein [13] | an amorphous white solid, tasteless and odorless | ointments, paper, paints, glues, textiles, varnishes, food, beverages, pharmaceuticals, health and personal care products, agriculture, etc. |
| collagen [14] | renewable, nontoxic, excellent film formation ability, biocompatibility, antioxidant properties, good moisture/oxygen barrier properties, ensure structural integrity | orthopaedic, sports medicine, dental, wound care, cardiovascular, general, plastic, and reconstructive surgery, etc. | |
| whey proteins [15] | antioxidant, antihypertensive, antitumor, hypolipidemic, antiviral, antibacterial, chelating agent | beverages, breads, snack items, bakery, confectionary products, and other nutritional food products in the treatment of cancer, HIV, hepatitis B, cardiovascular disease, osteoporosis, antimicrobial agents, etc. | |
| soya proteins [16] | highly soluble, dispersible, and dissolves rapidly and steadily | food, beverage, pharmaceutical, health and personal care products, agriculture, animal feed, poultry, biodegradable foams, edible films, packaging materials, biomedical materials, etc. | |
| zein [17] | renewable, nontoxic, good oxygen/gas barrier properties, high thermal resistance, high tensile strength, hydrophobic properties, high antimicrobial potential, good antioxidant properties, form adhesive film, high toughness, low water vapor permeability | pharmaceuticals, coatings, fibers, films, plastics, adhesives, inks, etc. | |
| gelatin [18] | renewable, nontoxic, low-cost, abundant, excellent film-forming ability, biocompatible, flexible, transparent, excellent water/UV/aroma/oxygen barrier properties, low water vapor permeability | foaming, emulsifying, and wetting agents in food, pharmaceutical, medical, and technical applications | |
| sugar alcohol | erythritol | low hygroscopicity, good crystallinity, refreshing sweet taste, stable at high temperatures, stable in a wide pH range | food, medicine, cosmetics, chemical industry, pharmaceuticals, etc. |
| glycerol | soluble, clear, nearly colorless, odorless, viscous, hygroscopic liquid with a high boiling point | processing aid in cosmetics, toiletries, personal care, pharmaceuticals, foodstuffs, etc. | |
| lipids and waxes | vegetable oil and animal fats [19] | More cost-effective, float on water but are not soluble in it; greasy; lubricating | petroleum and metal processing, chemical industry, pharmaceutical |
| waxes [20] | extremely hydrophobic | foods, cosmetics, pharmaceuticals, chemical engineering, paintings, etc. |
| Polymer | Erythritol C4H10O4 | Gelatin C6H12O6 | Collagen C57H91N19O16 | Glycerol C3H8O3 | Propolis C17H16O4 | Sodium Alginate Fibres |
|---|---|---|---|---|---|---|
| Molar mass [g mol−1] | 122.12 | 45,00 | 1302.5 | 92.09 | 281.31 | / |
| CAS number | 149-32-6 | 9000-70-8 | 9000-70-8 | 56-81-5 | / | / |
| Note | from corn | 100% beef gelatin | fish collagen | / | propolis extract in an aqueous solution with niacin and sage | sodium alginate fibers with sodium and calcium carbonate |
| Erythritol (1 g) + distilled water mixing on a magnetic stirrer with a triangular magnet | Gelatin (5 g) + distilled water mixing on a magnetic stirrer with a triangular magnet | Collagen (5 g) + distilled water mixing on a magnetic stirrer with a triangular magnet | |||
| ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| + propolis 10 mL | + alginate 10 mL | + propolis 10 mL | + alginate 10 mL | + propolis 10 mL | + alginate 10 mL |
| + glycerol 5 mL | + glycerol 5 mL | + glycerol 5 mL | + glycerol 5 mL | + glycerol 5 mL | + glycerol 5 mL |
| 20 ± 2 °C 15 min | |||||
| ↓ The samples were kept at room temperature for 24 h ↓ The samples were fixed at 40 °C for 60 min ↓ Materials morphology with Dino-Lite pH of the Water-Extract from Wet Processed Textiles Drop test method Determination of recovery by measuring the recovery angle Determination of thickness and mass per unit area, before and after treatment | |||||
| Code | Biopolymer | Active Compound | Note | |
|---|---|---|---|---|
| Nonwoven fabric | NN | / | / | untreated |
| NEP | Erythritol | propolis | ||
| NEA | sodium alginate | |||
| NGP | Gelatin | propolis | ||
| NGA | sodium alginate | |||
| NCP | Collagen | propolis | ||
| NCA | sodium alginate | |||
| Cotton fabric | FN | / | / | untreated |
| FEP | Erythritol | propolis | ||
| FEA | sodium alginate | |||
| FGP | Gelatin | propolis | ||
| FGA | sodium alginate | |||
| FCP | Collagen | propolis | ||
| FCA | sodium alginate | |||
| Code | Morphology of the Materials | Code | Morphology of the Materials |
|---|---|---|---|
| NN |  | FN | 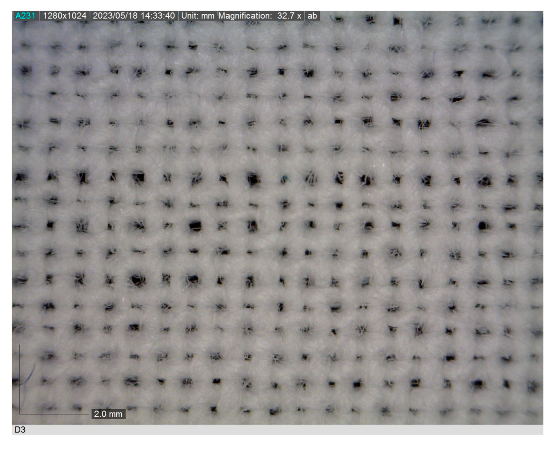 |
| NEP |  | FEP |  |
| NEA |  | FEA |  |
| NGP |  | FGP |  |
| NGA |  | FGA |  |
| NCP |  | FCP |  |
| NCA |  | FCA |  |
| Code | pH | Code | pH |
|---|---|---|---|
| NN | 7 | FN | 7 |
| NEP | 7 | FEP | 7 |
| NEA | 8 | FEA | 7 |
| NGP | 7 | FGP | 7 |
| NGA | 8 | FGA | 7 |
| NCP | 7 | FCP | 7 |
| NCA | 8 | FCA | 7 |
| Code | Time | A Drop of Methylene Blue Solution | Code | Time | A Drop of Methylene Blue Solution |
|---|---|---|---|---|---|
| NN | 1 s 6 cs |  | FN | 3 s 8 cs |  |
| NEP | 1 s 7 cs | 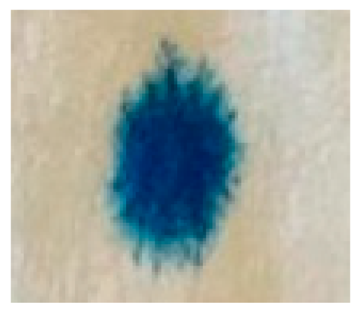 | FEP | 4 s 1 cs |  |
| NEA | 1 s 0 cs | 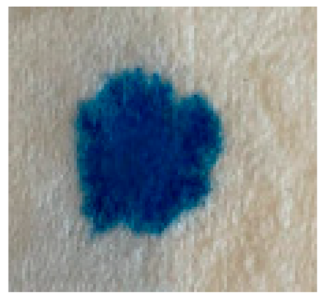 | FEA | 4 s 3 cs |  |
| NGP | 7 s 1 cs |  | FGP | 5 s 1 cs | 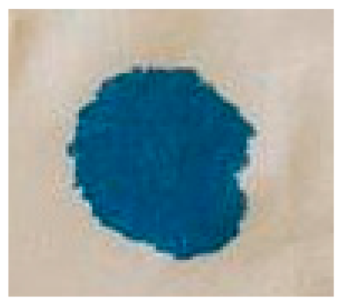 |
| NGA | 9 s 3 cs |  | FGA | 12 s 0 cs |  |
| NCP | 0 s 9 cs |  | FCP | 5 s 0 cs |  |
| NCA | 0 s 8 cs |  | FCA | 3 s 9 cs |  |
| The Angle of Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code/Direction of Production | Machine Direction (°) | Cross Machine Direction (°) | ||||||||||
| 1. | 2. | 3. | X (°) | σ | V [%] | 1. | 2. | 3. | X (°) | σ | V [%] | |
| NN | 101 | 94 | 98 | 97.7 | 2.87 | 2.94 | 107 | 96 | 93 | 98.7 | 6.02 | 6.10 |
| NEP | 93 | 98 | 94 | 95 | 2.16 | 2.27 | 91 | 94 | 97 | 94 | 2.45 | 2.61 |
| NEA | 65 | 74 | 79 | 72.7 | 5.79 | 7.96 | 87 | 84 | 91 | 87.4 | 2.87 | 3.28 |
| NGP | 92 | 96 | 98 | 95.4 | 2.49 | 2.61 | 94 | 98 | 101 | 97.7 | 2.87 | 2.94 |
| NGA | 91 | 89 | 94 | 91.4 | 2.05 | 2.24 | 93 | 98 | 90 | 93.7 | 3.30 | 3.52 |
| NCP | 94 | 89 | 90 | 91 | 2.16 | 2.37 | 98 | 102 | 107 | 102.4 | 3.68 | 3.59 |
| NCA | 93 | 103 | 98 | 98 | 4.08 | 4.16 | 110 | 114 | 105 | 109.7 | 3.68 | 3.35 |
| The Angle of Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code/Direction of Production | Machine Direction (°) | Cross Machine Direction (°) | ||||||||||
| 1. | 2. | 3. | X (°) | σ | V [%] | 1. | 2. | 3. | X (°) | σ | V [%] | |
| FN | 79 | 90 | 71 | 80 | 7.79 | 9.74 | 86 | 77 | 90 | 84.4 | 5.44 | 6.45 |
| FEP | 59 | 69 | 69 | 65.6 | 4.71 | 7.18 | 82 | 84 | 90 | 85.4 | 3.40 | 3.98 |
| FEA | 55 | 59 | 53 | 55.6 | 2.49 | 4.48 | 63 | 61 | 62 | 62 | 0.82 | 1.32 |
| FGP | 69 | 64 | 67 | 66.7 | 2.05 | 3.07 | 73 | 75 | 76 | 74.7 | 1.25 | 1.67 |
| FGA | 51 | 56 | 60 | 55.7 | 3.68 | 6.61 | 74 | 71 | 69 | 71.4 | 2.05 | 2.87 |
| FCP | 75 | 71 | 73 | 73 | 1.63 | 2.23 | 69 | 86 | 84 | 79.7 | 7.59 | 9.52 |
| FCA | 63 | 62 | 69 | 64.7 | 3.09 | 4.78 | 74 | 72 | 76 | 74 | 1.63 | 2.20 |
| Code/n | 1 | 2 | 3 | x | σ | V [%] |
|---|---|---|---|---|---|---|
| NN | 0.34 | 0.34 | 0.34 | 0.34 | 0 | 0 |
| NEP | 0.34 | 0.34 | 0.34 | 0.34 | 0 | 0 |
| NEA | 0.34 | 0.34 | 0.34 | 0.34 | 0 | 0 |
| NGP | 0.22 | 0.25 | 0.22 | 0.23 | 0.014 | 6.13 |
| NGA | 0.34 | 0.24 | 0.20 | 0.26 | 0.06 | 22.65 |
| NCP | 0.29 | 0.24 | 0.30 | 0.27 | 0.03 | 9.70 |
| NCA | 0.31 | 0.29 | 0.30 | 0.30 | 0.008 | 2.74 |
| Code/n | 1 | 2 | 3 | x | σ | V [%] |
|---|---|---|---|---|---|---|
| FN | 0.33 | 0.33 | 0.33 | 0.33 | 0 | 0 |
| FEP | 0.33 | 0.32 | 0.31 | 0.32 | 0.008 | 2.56 |
| FEA | 0.34 | 0.34 | 0.33 | 0.34 | 0.005 | 1.38 |
| FGP | 0.33 | 0.32 | 0.32 | 0.32 | 0.005 | 1.47 |
| FGA | 0.31 | 0.31 | 0.32 | 0.32 | 0.005 | 1.47 |
| FCP | 0.32 | 0.32 | 0.32 | 0.32 | 0 | 0 |
| FCA | 0.33 | 0.33 | 0.34 | 0.33 | 0.005 | 1.43 |
| Code | Mass per Unit Area (g m−2) | Code | Mass per Unit Area (g m−2) |
|---|---|---|---|
| FN | 39.15 | NN | 11.7 |
| FEP | 45.96 | NEP | 18.27 |
| FEA | 44.96 | NEA | 15.64 |
| FGP | 47.87 | NGP | 20.66 |
| FGA | 47.21 | NGA | 26.37 |
| FCP | 45.22 | NCP | 21.08 |
| FCA | 46.73 | NCA | 24.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somogyi Škoc, M.; Stevelić, N.; Rezić, I. Development and Characterization of Sustainable Coatings on Cellulose Fabric and Nonwoven for Medical Applications. Sustainability 2024, 16, 857. https://doi.org/10.3390/su16020857
Somogyi Škoc M, Stevelić N, Rezić I. Development and Characterization of Sustainable Coatings on Cellulose Fabric and Nonwoven for Medical Applications. Sustainability. 2024; 16(2):857. https://doi.org/10.3390/su16020857
Chicago/Turabian StyleSomogyi Škoc, Maja, Nina Stevelić, and Iva Rezić. 2024. "Development and Characterization of Sustainable Coatings on Cellulose Fabric and Nonwoven for Medical Applications" Sustainability 16, no. 2: 857. https://doi.org/10.3390/su16020857






