Biosorption of Hexavalent Chromium by Freshwater Microalgae Craticula subminuscula from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Absorption: Cultivation and Toxicity Test
2.2. Adsorption Experiments
2.2.1. Single-Factor Experiments
2.2.2. Optimization Adsorption Experiments
2.2.3. Kinetic Study and Isotherm Studies
2.2.4. Characterization of the Biomass
2.3. Desorption Studies
2.4. Data Analysis
3. Results and Discussion
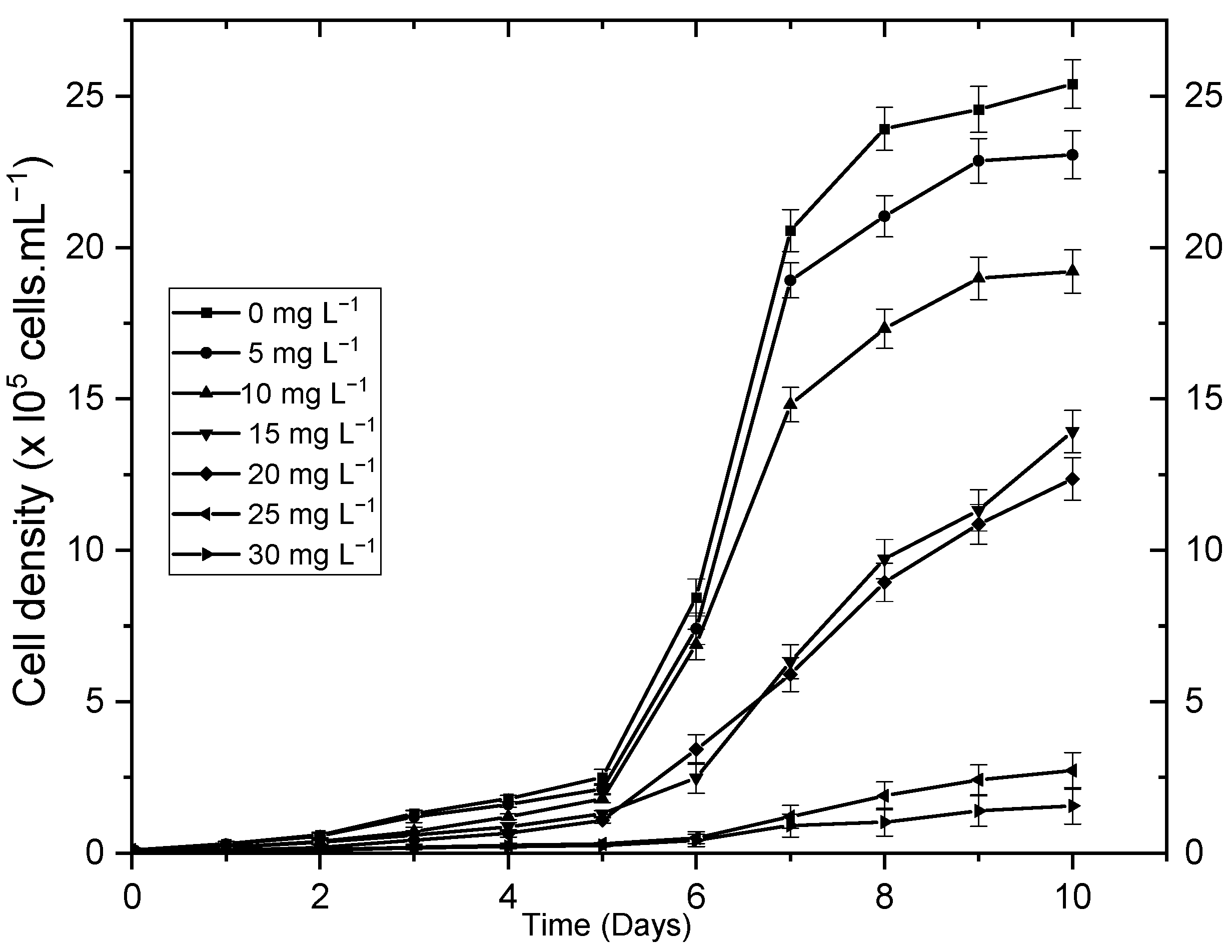
3.1. Absorption and Microalgae Tolerance to Cr(VI)
3.2. Adsorption Experiments
3.2.1. Single Factor Experiments
- a.
- Effect of initial pH
- b.
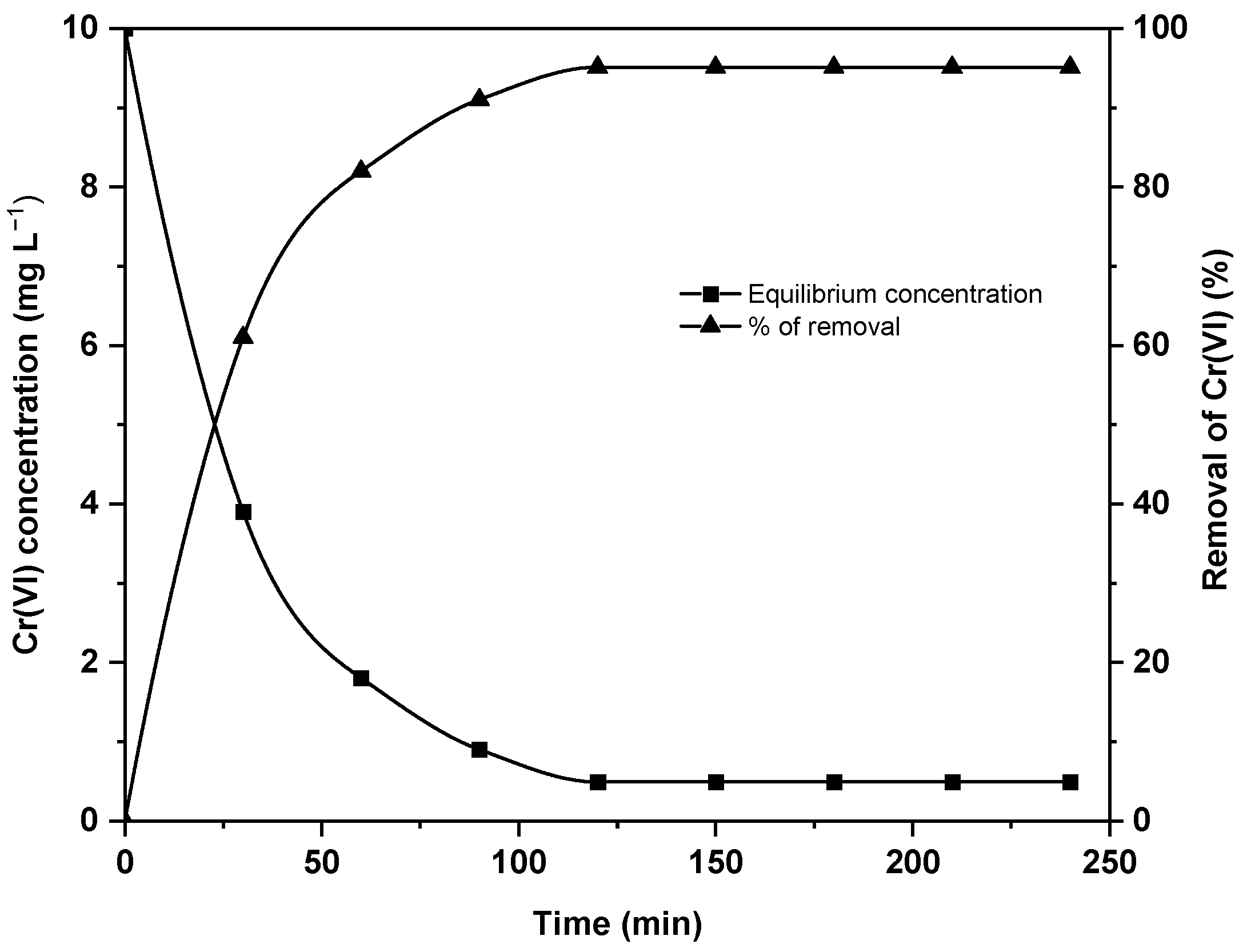
- Effect of contact time
- c.
- Effect of biosorbent dosage
- d.
- Effect of initial Cr(VI) concentration
- e.
- Effect of temperature
3.2.2. Optimization of Cr(VI) Removal Conditions for C. subminuscula
3.3. Kinetic and Isotherm Studies
3.4. Thermodynamic Interpretation
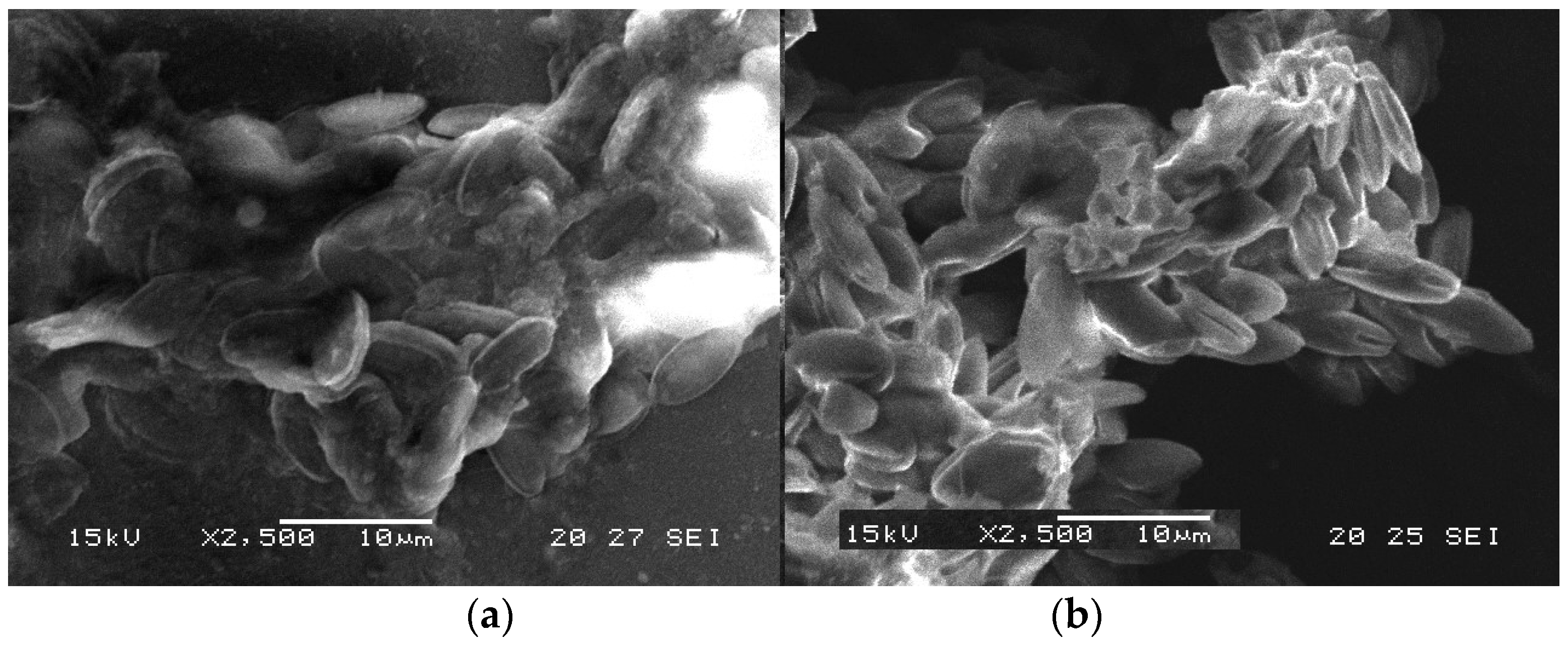
3.5. Characterization of the Biomass
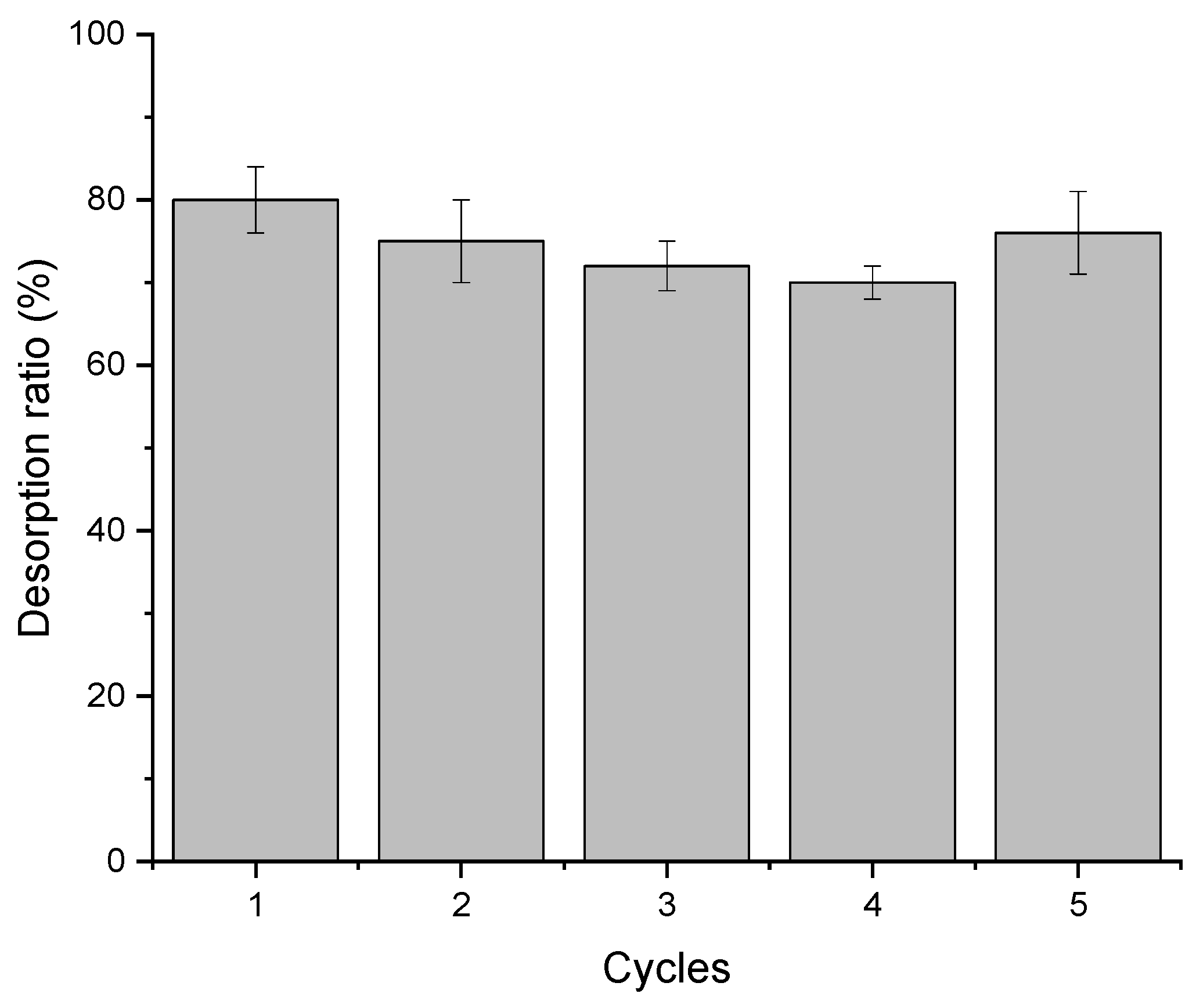
3.6. Desorption Studies
3.7. Post-Treatment of Biosorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 1–12, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Bihuang, X.; Chao, S.; Zhe, X.; Xuchun, L.; Xiaolin, Z.; Jiajia, C.; Bingcai, P. One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 2017, 308, 791–797. [Google Scholar]
- Ren, G.; Wang, X.; Huang, P.; Zhong, B.; Zhang, Z.; Yang, L.; Yang, X. Chromium (VI) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci. Total Environ. 2017, 607–608, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.A. Impacts of Chromium from Tannery Effluent and Evaluation of Alternative Treatment Options. J. Environ. Prot. 2010, 1, 53–58. [Google Scholar] [CrossRef]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Singh, D.; Garg, V.K. A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes’ carbons. J. Hazard. Mater. 2009, 171, 83–92. [Google Scholar] [CrossRef]
- Cabatingan, L.K.; Agapay, R.C.; Rakels, J.L.L.; Ottens, M.; van der Wielen, L.A.M. Potential of Biosorption for the Recovery of Chromate in Industrial Wastewaters. Ind. Eng. Chem. Res. 2001, 40, 2302–2309. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- Peña-Castro, J.M.; Martínez-Jerónimo, F.; Esparza-García, F.; Cañizares-Villanueva, R.O. Heavy metals removal by the mi-croalga Scenedesmus incrassatulus in continuous cultures. Bioresour. Technol. 2004, 94, 219–222. [Google Scholar] [CrossRef]
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as Eco-friendly Tools for the Removal of Heavy Metals from Wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 53–76. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Chen, P.-W.; Hsu, C.-Y.; Lee, L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Unnikannan, P.; Sruthi, P. Phycoremediation of Tannery Wastewater Using Microalgae Scenedesmus Species. Int. J. Phytoremediation 2015, 17, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Napan, K. Influence of heavy metals from flue gas integration with algal production on biodiesel production. In Graduate Research Symposium; Utah State University: Logan, UT, USA, 2014; p. 80. Available online: https://digitalcommons.usu.edu/grs/80 (accessed on 6 October 2023).
- Zhou, G.-J.; Ying, G.-G.; Liu, S.; Zhou, L.-J.; Chen, Z.-F.; Peng, F.-Q. Simultaneous removal of inorganic and organic com-pounds in wastewater by freshwater green microalgae. Environ. Sci. Process. Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Jácome-Pilco, C.R.; Cristiani-Urbina, E.; Flores-Cotera, L.B.; Velasco-García, R.; Ponce-Noyola, T.; Cañizares-Villanueva, R.O. Continuous Cr(VI) removal by Scenedesmus incrassatulus in an airlift photobioreactor. Bioresour. Technol. 2009, 100, 2388–2391. [Google Scholar] [CrossRef] [PubMed]
- Travieso, L.; Cañizares, R.O.; Borja, R.; Benítez, F.; Domínguez, A.R.; Dupeyrón, R.; Valiente, V. Heavy metal removal by microalgae. Bull. Environ. Contam. Toxicol. 1999, 62, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.M.; Hess, D.; McNeil, B.T.; Guy, T.; Quinn, J.C. Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol. Environ. Saf. 2017, 139, 367–376. [Google Scholar] [CrossRef]
- Magro, C.D.; Deon, M.C.; Rossi, A.D.; Reinehr, C.O.; Hemkemeier, M.; Colla, L.M. Chromium (VI) biosorption and removal of chemical oxygen demand by Spirulina platensis from wastewater-supplemented culture medium. J. Environ. Sci. Health Part A 2012, 47, 1818–1824. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Konwarh, R.; Pramanik, S.; Kalita, D.; Mahanta, C.L.; Karak, N. Ultrasonication—A complementary ‘green chemistry’ tool to biocatalysis: A laboratory-scale study of lycopene extraction. Ultrason. Sonochem. 2012, 19, 292–299. [Google Scholar] [CrossRef]
- Konwarh, R.; Misra, M.; Mohanty, A.K.; Karak, N. Diameter-tuning of electrospun cellulose acetate fibers: A Box-Behnken design (BBD) study. Carbohydr. Polym. 2013, 92, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Hossain, S.M.Z.; Mohammed, M.E.; Irfan, M.F.; Haq, B.; Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Experimental study and parameters optimization of microalgae based heavy metals removal process using a hybrid response surface methodology-crow search algorithm. Sci. Rep. 2020, 10, 15068. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide C1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Sbihi, K.; Cherifi, O. Toxicity and biosorption of chromium from aqueous solutions by the diatom Planothidium lanceolatum (Brébisson) Lange-Bertalot. AJSIR 2012, 3, 27–38. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Lagergreen, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Für Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Maleki, A.; Naghizadeh, M.; Hayati, B.; Joo, S.W. Adsorption of hexavalent chromium by metal organic frameworks from aqueous solution. J. Ind. Eng. Chem. 2015, 28, 211–216. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications. Biotechnol. Progr. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.F.; Kong, F.; Song, O.; Ren, N.Q.; Ren, H.Y. Simultaneous chromium removal and lipid accumulation by microalgae under acidic and low temperature conditions for promising biodiesel production. Bioresour. Technol. 2023, 370, 128515. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Canizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal. Behav. 2012, 7, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Juarez, A.B.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Vesentini, N.; Conforti, V.; Gualtieri, P. In vivo microspectroscopy monitoring of chromium effects on the photosynthetic and photoreceptive apparatus of Eudorina unicocca and Chlorella kessleri. J. Environ. Monit. 2008, 10, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Murthy, Z.; Jha, B. Biosorption of hexavalent chromium by chemically modified seaweed, Cystoseira indica. Chem. Eng. J. 2008, 137, 480–488. [Google Scholar] [CrossRef]
- Sibi, G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. GEE 2016, 1, 172–177. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of hexavalent and trivalent chromium by palm flower (Borassus aethiopum). Chem. Eng. J. 2008, 141, 99–111. [Google Scholar] [CrossRef]
- Ferrari, M.; Cozza, R.; Marieschi, M.; Torelli, A. Role of Sulfate Transporters in Chromium Tolerance in Scenedesmus acutus M. (Sphaeropleales). Plants 2022, 11, 223. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent Chromium Removal from Water by Microalgal-Based Materials: Adsorption, Desorption and Recovery Studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef] [PubMed]
- Susanto, A.; Kartika, R.; Koesnarpadi, S. Lead Biosorption (Pb) and Cadmium (Cd) by Flavobacterium Sp Bacteria. Int. J. Sci. Technol. Res. 2019, 8, 3611–3615. [Google Scholar]
- Kunugi, M.; Sekiguchi, T.; Onizawa, H.; Jimbo, I. Utilization of Diatoms to Collect Metallic Ions. Proc. Schl. Eng. Tokai Univ. 2014, 39, 13–18. [Google Scholar]
- Rai, P.K.; Tripathi, B.D. Removal of heavy metals by the nuisance cyanobacteria Microcystis in continuous cultures: An eco-sustainable technology. Environ. Sci. 2007, 4, 53–59. [Google Scholar] [CrossRef]
- Deng, L.; Wang, H.; Deng, N. Photoreduction of chromium(VI) in the presence of algae, Chlorella vulgaris. J. Hazard. Mater. 2006, 138, 288–292. [Google Scholar] [CrossRef]
- Rodgher, S.; Espíndola, E.L.G.; Simões, F.C.F.; Tonietto, A.E. Cadmium and Chromium Toxicity to Pseudokirch-neriella sub-capitata and Microcystis aeruginosa, Braz. Arch. Biol. Technol. 2012, 55, 161–169. [Google Scholar] [CrossRef]
- Cherifi, O.; Sbihi, K.; Bertrand, M.; Cherifi, K. The siliceous microalga Navicula subminuscula (Manguin) as a biomaterial for removing metals from tannery effluents: A laboratory study. J. Mater. Environ. Sci. 2017, 8, 884–893. [Google Scholar]
- Wang, W.-X.; Dei, R.C.H. Influences of phosphate and silicate on Cr(VI) and Se(IV) accumulation in marine phytoplankton. Aquat. Toxicol. 2001, 52, 39–47. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Conforti, V.; Gualtieri, P. Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonas reinhardtii. Environ. Res. 2007, 105, 234–239. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Sathvika, T.; Manasi, V.; Rajesh, N. Rajesh, Adsorption of chromium supported with various column modelling studies through the synergistic influence of Aspergillus and cellulose. J. Environ. Chem. Eng. 2016, 4, 3193–3204. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Muthukkauppan, M.; Parthiban, P. A study on the physicochemical characteristics of tannery effluent collected from Chennai. Int. Res. J. Eng. Technol. 2018, 5, 24–28. [Google Scholar]
- Lissaneddine, A.; Aziz, K.; Ouazzani, N.; El Achaby, M.; Haydari, I.; Mandi, L.; Aziz, F. Continuous treatment of highly concentrated tannery wastewater using novel porous composite beads: Central composite design optimization study. J. Environ. Health Sci. Engineer. 2023, 21, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 2009, 163, 396–402. [Google Scholar] [CrossRef] [PubMed]
- González Bermúdez, Y.; Rodríguez Rico, I.L.; Guibal, E.; Calero de Hoces, M.; Martín-Lara, M.Á. Biosorption of hexavalent chromium from aqueous solution by Sargassum muticum brown alga. Application of statistical design for process optimization. Chem. Eng. J. 2012, 183, 68–76. [Google Scholar] [CrossRef]
- Rezaei, H. Biosorption of chromium by using Spirulina sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Jaafari, J.; Barzanouni, H.; Mazloomi, S.; Amir, N.; Abadi Farahani, S.K.; Soleimani, P.; Haghighat, G.A. Effective adsorptive removal of reactive dyes by magnetic chitosan nanoparticles: Kinetic, isothermal studies and response surface methodology. Int. J. Biol. Macromol. 2020, 164, 344–355. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of copper ions from aqueous solution using Chlorella pyrenoidosa: Optimization, equilibrium and kinetics studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bio-remediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Mishra, B.B.; Devi, N. Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J. Clean. Prod. 2019, 209, 617–629. [Google Scholar] [CrossRef]
- Ayele, A.; Suresh, A.; Benor, S.; Konwarh, R. Optimization of chromium(VI) removal by indigenous microalga (Chlamydomonas sp.)-based biosorbent using response surface methodology. Water Environ. Res. 2021, 93, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Shokri Khoubestani, R.; Mirghaffari, N.; Farhadian, O. Removal of three and hexavalent chromium from aqueous solutions using a microalgae biomass-derived biosorbent. Environ. Prog. Sustain. Energy 2015, 34, 949–956. [Google Scholar] [CrossRef]
- Husien, S.; Labena, A.; El-Belely, E.F.; Mahmoud, H.M.; Hamouda, A.S. Absorption of hexavalent chromium by green micro algae Chlorella sorokiniana: Live planktonic cells. Water Pract. Technol. 2019, 14, 515–529. [Google Scholar] [CrossRef]
- Kafil, M.; Berninger, F.; Koutra, E.; Kornaros, M. Utilization of the microalga Scenedesmus quadricauda for hexavalent chromium bioremediation and biodiesel production. Bioresour. Technol. 2022, 346, 126665. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Khan, Z.H.; Ying, H.; Chen, D.-Y. Utilization of Citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: In-sights and comparison between modified and raw biochar. Sci. Total Environ. 2021, 771, 144955. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Qaswar, M.; Ali, S.; Khan, Z.H.; Ying, H.; Chen, D.-Y.; Núñez-Delgado, A. Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 105104. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Liu, L.; Lin, X.L.; Luo, J.; Yang, J.; Luo, X.; Liao, H. Cheng, Biosorption of copper ions through microalgae from pig-gery digestate: Optimization, kinetic, isotherm and mechanism. J. Clean. Prod. 2021, 319, 128724. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Qaswar, M.; Ali, S.; Ying, H.; Liu, Z.; Mahmood, M.; Chen, D.-Y. Fabri-cation, characterization and U(VI) sorption properties of a novel biochar derived from Tribulus terrestris via two different approaches. Sci. Total Environ. 2021, 780, 146617. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Núñez-Delgado, A.; Mehmood, S.; Ali, S.; Qaswar, M.; Shakoor, A.; Chen, D.-Y. Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism. Environ. Res. 2021, 201, 111518. [Google Scholar] [CrossRef]
- Aziz, F.; Ouazzani, N.; Mandi, L.; Mamoun, M.; Uheida, A. Composite nanofibers of polyacrylonitrile/natural clay for decontamination of water containing Pb(II), Cu(II), Zn(II) and pesticides. Sep. Sci. Technol. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Lissaneddine, A.; Pons, M.N.; Aziz, F.; Ouazzani, N.; Mandi, L.; Mousset, E. Electrosorption of phenolic compounds from olive mill wastewater: Mass transport consideration under a transient regime through an alginate-activated carbon fixed-bed electrode. J. Hazard. Mater. 2022, 430, 128480. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; El Achaby, M.; Mamouni, R.; Saffaj, N.; Aziz, F. A novel hydrogel beads based copper-doped Cerastoderma edule shells@Alginate biocomposite for highly fungicide sorption from aqueous medium. Chemosphere 2023, 311, 136932. [Google Scholar] [CrossRef]
- Haydari, I.; Aziz, K.; Kaya, S.; Daştan, T.; Ouazzani, N.; Mandi, L.; Aziz, F. Green synthesis of reduced graphene oxide and their use on column adsorption of phenol from olive mill wastewater. Process. Saf. Environ. Prot. 2023, 170, 1079–1091. [Google Scholar] [CrossRef]
- Elhamji, S.; Haydari, I.; Sbihi, K.; Aziz, K.; Elleuch, J.; Kurniawan, A.T.; Chen, Z.; Yap, P.S.; Aziz, F. Uncovering applicability of Navicula permitis algae in removing phenolic compounds: A promising solution for olive mill wastewater treatment. J. Water Process. Eng. 2023, 56, 104313. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data. 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Wagner, H.; Liu, Z.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics. 2010, 3, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.M.; Kansiz, P.; Heraud, J.; Beardall, B.; Wood, D. McNaughton, fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga chaetoceros muellerii (bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by Fourier-Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Marchand, J.; Blanckaert, V.; Lukomska, E.; Ulmann, L.; Wielgosz-Collin, G.; Rabesaotra, V.; Moreau, B.; Bougaran, G.; Mimouni, V.; et al. Nitrogen and phosphorus limitations induce carbon partitioning and membrane lipid remodelling in the marine diatom Phaeodactylum tricornutum. Eur. J. Phycol. 2019, 54, 342–358. [Google Scholar] [CrossRef]
- Hamad, H.; AbdElhafez, S.; Elsenety, M.; Sorour, M.K.; Amin, N.; Abdelwahab, O.; El-Ashtoukhy, E.-S. Fabrication and characterization of functionalized lignin-based adsorbent prepared from black liquor in the paper industry for superior removal of toxic dye. Fuel 2022, 323, 124288. [Google Scholar] [CrossRef]
- Ali, R.; Elsagan, Z.; AbdElhafez, S. Lignin from Agro-Industrial Waste to an Efficient Magnetic Adsorbent for Hazardous Crystal Violet Removal. Molecules 2022, 27, 1831. [Google Scholar] [CrossRef]
- Scarsini, M. The Transition Kinetic Toward Nitrogen Deprivation in the Marine Diatom Phaeodactylum tricornutum: A Multidisciplinary Approach. Ph.D. Thesis, Le Mans University, Le Mans, France, 2021. Available online: https://www.theses.fr/s206840 (accessed on 6 October 2023).
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2012, 12, 165–178. [Google Scholar] [CrossRef]
- Shamshad, K.; Naushad, M.; Jibra, I.; Chinna, B.; Gaurav, S. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]








| Independent Variables | Range and Levels | ||
|---|---|---|---|
| −1 | 0 | −2 | |
| A (biosorbent dose, mg L−1) | 5 | 10 | 15 |
| B (Treatment time, min) | 90 | 120 | 150 |
| C (pH) | 0.5 | 1 | 2 |
| Concentration of Cr(VI) Ion Exposed (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | |
| Percentage of Cr(VI) biosorption | 0 | 99.94 ± 0.71 | 96.48 ± 0.64 | 58.89 ± 0.87 | 23.44 ± 0.42 | 10.73 ± 0.31 | 8.21 ± 0.28 |
| Treatment | Factors and Their Levels | Observed Cr(VI) Removal (%) | Predicted Cr(VI) Removal (%) | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Biosorbent Dosage (mg L−1) | Treatment Time (min) | pH | |||
| 1 | 10 (0) | 90 (−1) | 0.5 (−1) | 83.71 | 84.57 |
| 2 | 5 (−1) | 150 (+1) | 1 (0) | 84.53 | 84.56 |
| 3 | 10 (0) | 90 (−1) | 2 (+1) | 74.81 | 74.92 |
| 4 | 15 (+1) | 150 (+1) | 1 (0) | 88.01 | 88.95 |
| 5 | 10 (0) | 120 (0) | 1 (0) | 95.31 | 95.30 |
| 6 | 5 (−1) | 90 (−1) | 1 (0) | 84.45 | 83.51 |
| 7 | 15 (+1) | 120 (0) | 2 (+1) | 77.05 | 76.96 |
| 8 | 15 (+1) | 120 (0) | 0.5 (−1) | 87.01 | 86.19 |
| 9 | 10 (0) | 120 (0) | 1 (0) | 95.32 | 95.30 |
| 10 | 15 (+1) | 90 (−1) | 1 (0) | 85.65 | 85.62 |
| 11 | 10 (0) | 150 (+1) | 2 (+1) | 77.21 | 76.67 |
| 12 | 5 (−1) | 120 (0) | 0.5 (−1) | 83.05 | 83.44 |
| 13 | 10 (0) | 120 (0) | 1 (0) | 95.27 | 95.30 |
| 14 | 5 (−1) | 120 (0) | 2 (+1) | 72.2 | 72.72 |
| 15 | 10 (0) | 150 (+1) | 0.5 (−1) | 87.41 | 86.98 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 729.99 | 9 | 81.11 | 99.13 | <0.0001 | significant |
| A-biosorbent dosage | 23.24 | 1 | 23.24 | 28.40 | 0.0031 | |
| B-treatment time | 8.22 | 1 | 8.22 | 10.05 | 0.0248 | |
| C-pH | 199.10 | 1 | 199.10 | 243.33 | <0.0001 | |
| AB | 1.30 | 1 | 1.30 | 1.59 | 0.2632 | |
| AC | 0.5900 | 1 | 0.5900 | 0.7211 | 0.4346 | |
| BC | 0.1128 | 1 | 0.1128 | 0.1378 | 0.7257 | |
| A2 | 103.67 | 1 | 103.67 | 126.70 | <0.0001 | |
| B2 | 69.59 | 1 | 69.59 | 85.04 | 0.0003 | |
| C2 | 254.40 | 1 | 254.40 | 310.91 | <0.0001 | |
| Residual | 4.09 | 5 | 0.8182 | |||
| Lack of Fit | 4.09 | 3 | 1.36 | 1947.54 | 0.11 | nonsignificant |
| Pure Error | 0.0014 | 2 | 0.0007 | |||
| Cor Total | 734.08 | 14 |
| Biosorbent Dosage (mg L−1) | Treatment Time (min) | pH | Predicted Value of Cr(VI) Removal (%) | Actual Value of Cr(VI) Removal (%) |
|---|---|---|---|---|
| 10.913 | 129.47 | 1.09 | 95.30 a | 95.32 a |
| Microalgae Species | Heavy Metal | Conditions | Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| Chlorella vulgaris | Cr(VI) | 25 °C, pH = 2, biomass 1 g L−1, [Cr(VI)] = 147 mg L−1,time 240 min | 43.00 | [41] |
| Scenedesmus quadricauda | Cr(VI) | 25 °C, pH = 1, biomass 2 g L−1, [Cr(VI)] = 100 mg L−1,time 120 min | 47.6 | [67] |
| Planothidium lanceolatum | Cr(VI) | 20 °C, pH = 1, biomass 0.4 g L−1, [Cr(VI)] = 10 mg L−1,time 30 min. | 87.00 | [26] |
| Chlamydomonas sp. | Cr(VI | [Cr(VI)] = 152 mg g−1, time 30 min. pH = 4, biomass 1.5 g L−1 | 91.31 | [66] |
| Scenedesmus sp. | Cr(VI) | 30 °C, pH = 1, [Cr(VI)] = 10 mg L−1, time 120 min | 92.89 | [65] |
| Craticula subminuscula | Cr(VI) | 25 °C, pH = 1.09, biomass 10.915 mg L−1, [Cr(VI)] = 10 mg L−1,time 129.47 min. | 95.30 | This study |
| Parachlorella kessleri | Cr(VI) | Time196h Cr(VI)] = 30 mg L−1, 23 °C | 96.1 | [64] |
| Scenedesmus quadricauda | Cr(VI) | Biomass 0.8 g L−1, [Cr(VI)] = 5 mg L−1, 25 °C, time 8 Days | 98.1 | [69] |
| Chlorella sorokiniana | Cr(VI) | [Cr(VI)] = 100 mg L−1, time 1day. pH = 8, T° 40 °C, biomass 20 mL/100 mL | 99.67 | [68] |
| Kinetics Model | Coefficients | Value |
|---|---|---|
| qe experimental in mg g−1 | 277.57 | |
| Pseudo first order | K1 in min−1 | 0.00011 |
| qe calculated in mg g−1 | 142.14 | |
| R2 | 0.80 | |
| Pseudo second order | k2 in g·mg−1·min−1 | 0.0004 |
| qe calculated in mg g−1 | 289.017 | |
| R2 | 0.9955 |
| Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|
| qmax in mg g−1 | KL in L·mg−1 | RL | R2 | KF in mg g−1 | R2 | |
| 295.85 | 7.81 | 0.0042 | 0.999 | 281.88 | 0.0155 | 0.907 |
| ∆G in kJ·mol−1 | ∆H in kJ·mol−1 | ∆S in J·mol−1·K−1 | R2 | ||||
|---|---|---|---|---|---|---|---|
| Temperature in K | |||||||
| 288 | 293 | 298 | 303 | 308 | |||
| −40.52 | −41.22 | −41.92 | −42.63 | −43.33 | 140.7 | 0.99 | |
| Wavenumber (cm−1) | Band Assignment & Functional Groups |
|---|---|
| 3800–3000 | √O-H of water, √N-H of amide, √C-O of carbohydrates |
| 3028 | √C-H of C=CH- chains of lipids |
| 2950 | √asCH3 of methyl groups |
| 2928 | √asCH2 of methylene |
| 2854 | √CH2 and √CH3 of methyl and methylene groups |
| 1745 | √C=O ester of lipids and fatty acids |
| 1654 | √C=O of proteins (Amide I) |
| 1547 | δN-H and √C-N of proteins (Amide II) |
| 1450 | δasCH2 and δasCH3 of methyl and methylene groups |
| 1396 | δCH2 and δCH3 from proteins and C-O from carboxylic groups |
| 1232 | √asP=O from phosphodiester of nucleic acids and phospholipids |
| 1200–1000 | √C-O-C from polysaccharides |
| 1075 & 950 | Siloxane, silicate frustules |
| 940 | P-O-P of polyphosphates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbihi, K.; Elhamji, S.; Lghoul, S.; Aziz, K.; El Maallem, A.; Mabrouki, J.; El-Sheekh, M.; Aziz, F. Biosorption of Hexavalent Chromium by Freshwater Microalgae Craticula subminuscula from Aqueous Solutions. Sustainability 2024, 16, 918. https://doi.org/10.3390/su16020918
Sbihi K, Elhamji S, Lghoul S, Aziz K, El Maallem A, Mabrouki J, El-Sheekh M, Aziz F. Biosorption of Hexavalent Chromium by Freshwater Microalgae Craticula subminuscula from Aqueous Solutions. Sustainability. 2024; 16(2):918. https://doi.org/10.3390/su16020918
Chicago/Turabian StyleSbihi, Karim, Sara Elhamji, Siham Lghoul, Khalid Aziz, Abdelali El Maallem, Jamal Mabrouki, Mostafa El-Sheekh, and Faissal Aziz. 2024. "Biosorption of Hexavalent Chromium by Freshwater Microalgae Craticula subminuscula from Aqueous Solutions" Sustainability 16, no. 2: 918. https://doi.org/10.3390/su16020918
APA StyleSbihi, K., Elhamji, S., Lghoul, S., Aziz, K., El Maallem, A., Mabrouki, J., El-Sheekh, M., & Aziz, F. (2024). Biosorption of Hexavalent Chromium by Freshwater Microalgae Craticula subminuscula from Aqueous Solutions. Sustainability, 16(2), 918. https://doi.org/10.3390/su16020918









