A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Laboratory Experiments
3. Results and Discussion
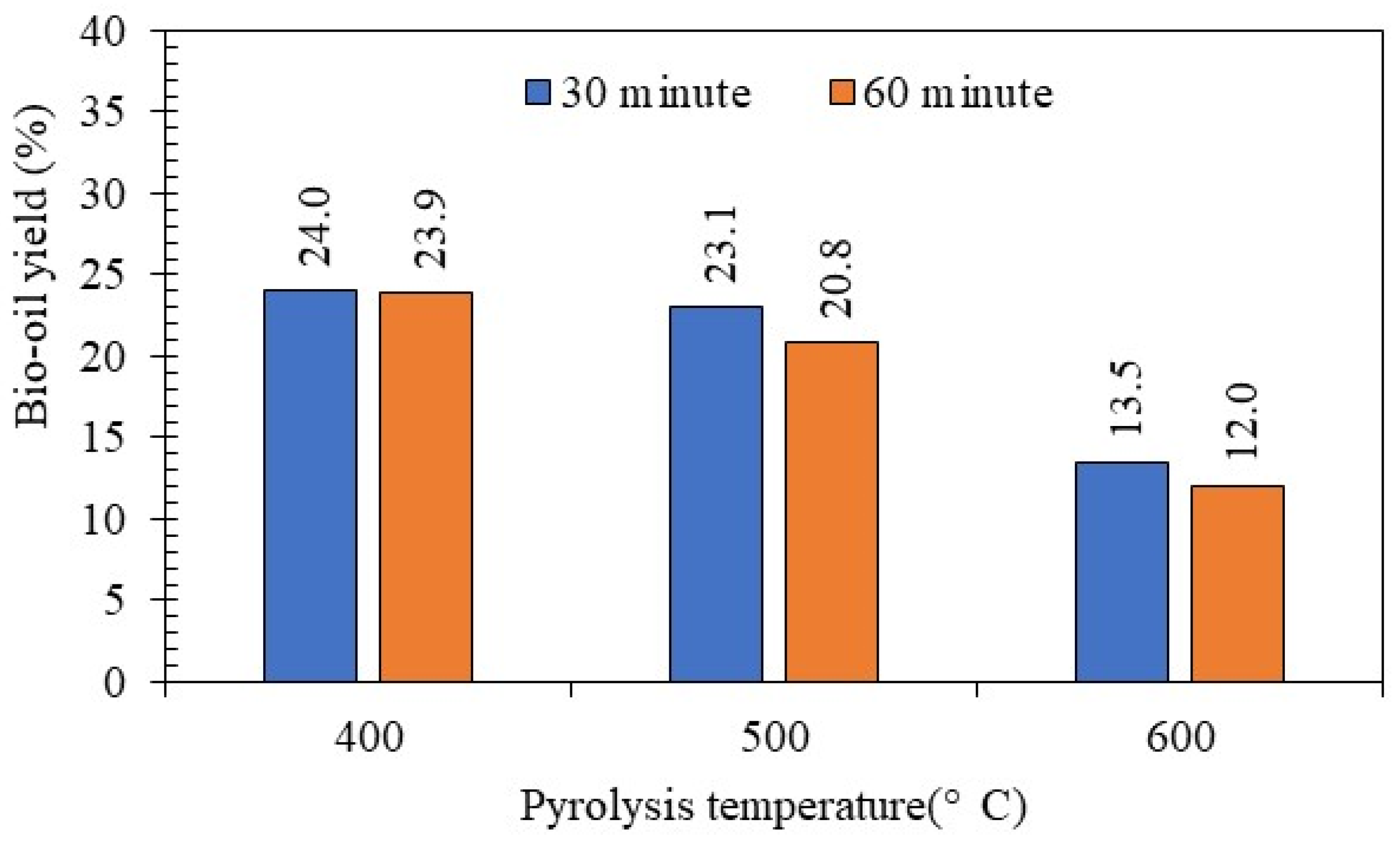
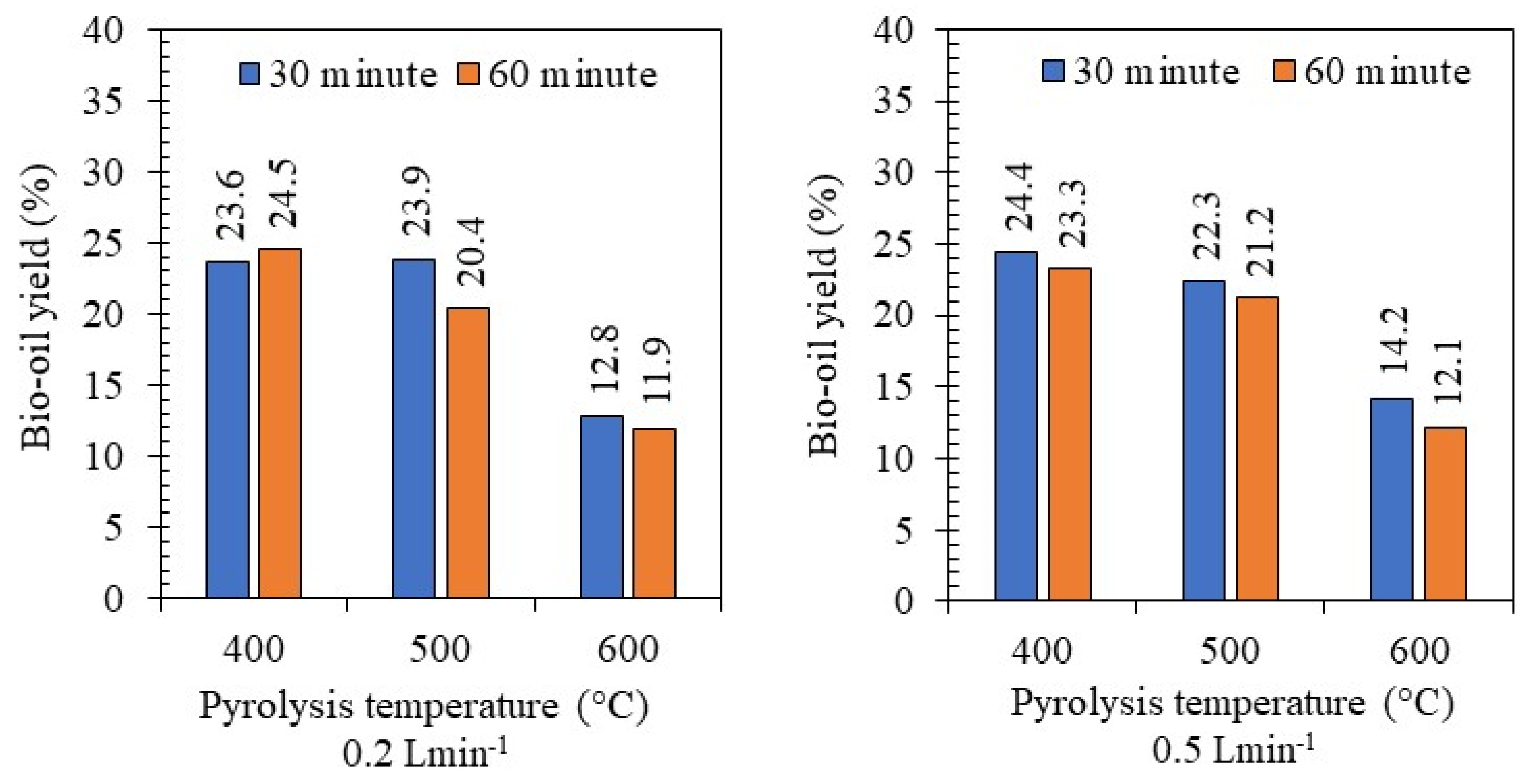
3.1. Pyrolysis Yield
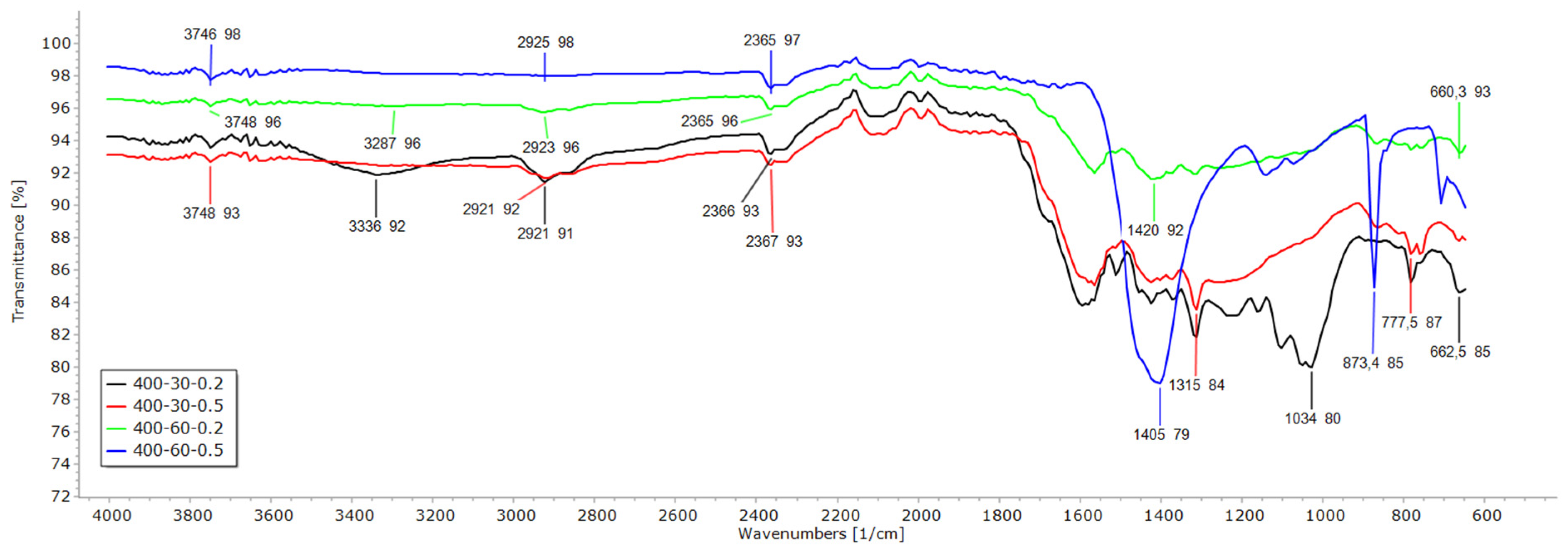
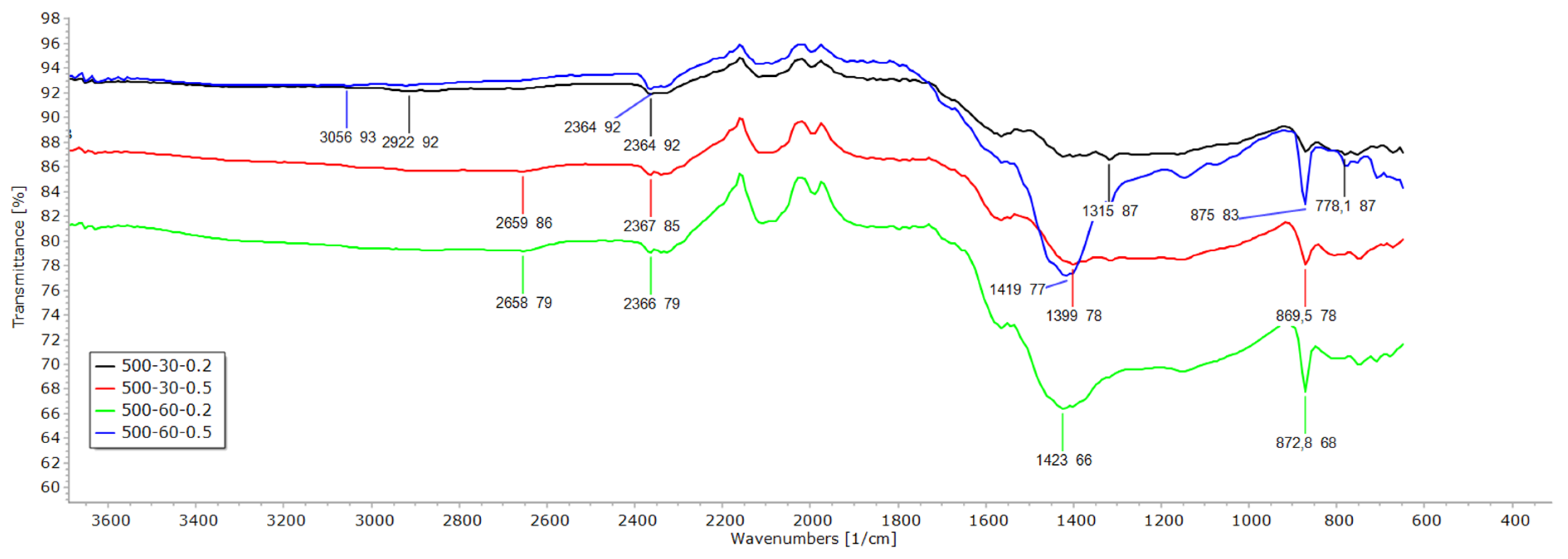
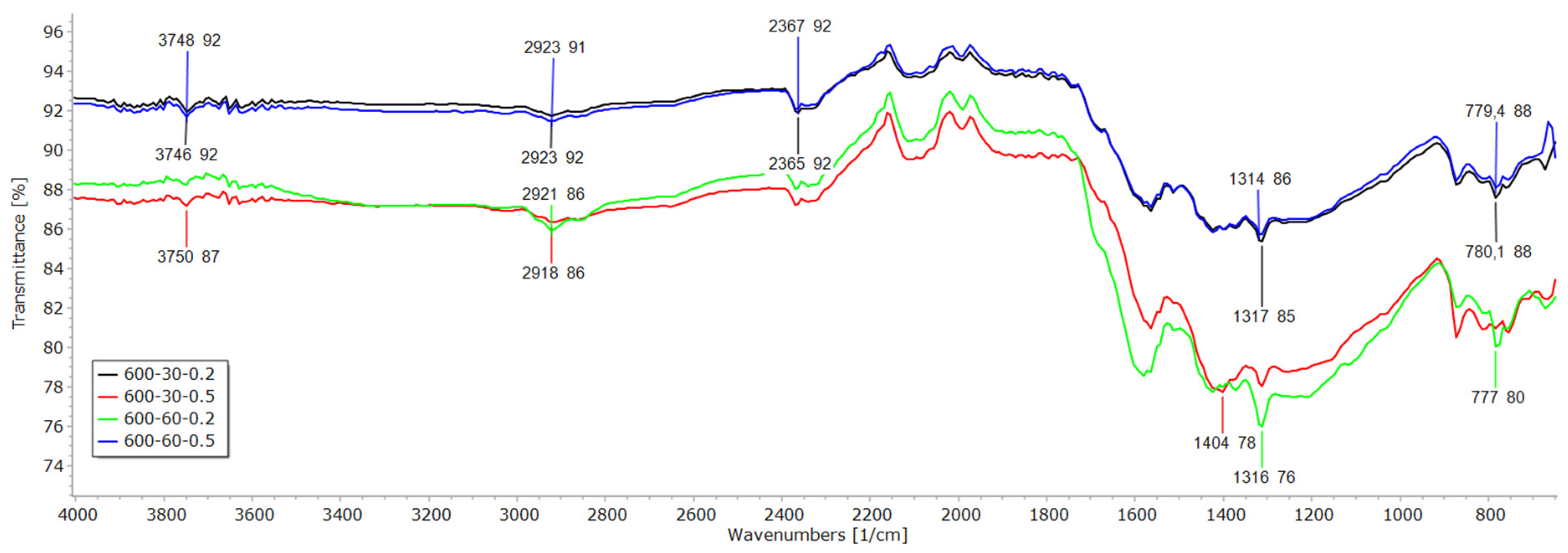
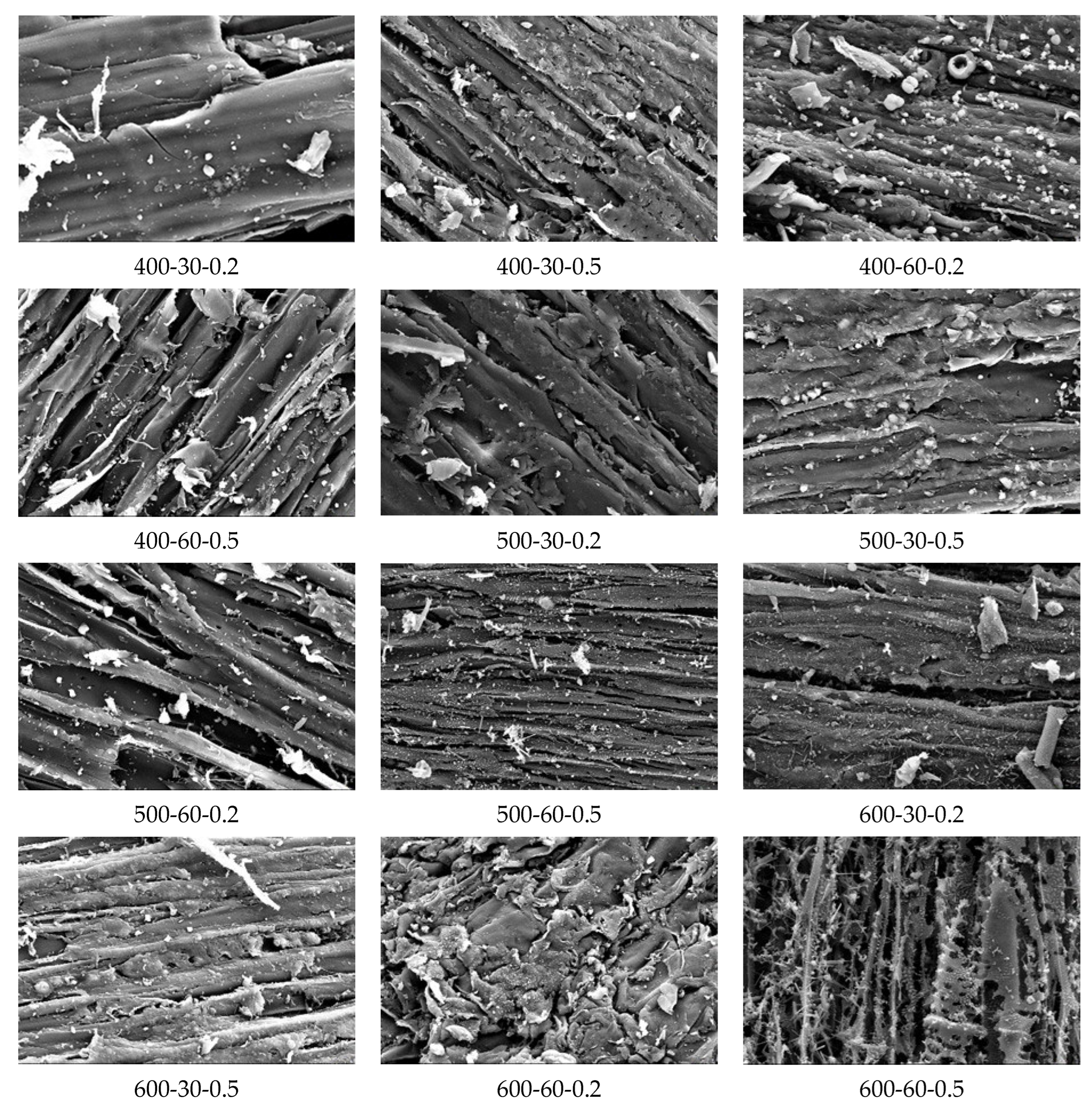
3.2. FTIR, EDX and SEM
3.3. Elemental Analyses and High Calorific Values (HCV)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bukhtiyarova, M.; Lunkenbein, T.; Keahler, T.; Schleogl, R. Methanol synthesis from industrial CO2 sources: A contribution to chemical energy conversion. Catal. Lett. 2017, 147, 416–427. [Google Scholar] [CrossRef]
- Usanmaz Bozhuyuk, A.; Gurbuz, R.; AlptekİN, H.; Kayci, H. The Use of Different Waste Mulch Materials Against Weeds Which are Problems in Tomato (Solanum lycopersicum L.) Cultivation. Selcuk J. Agric. Food Sci. 2022, 36, 226–232. [Google Scholar] [CrossRef]
- Alptekin, H.; Ozkan, A.; Gurbuz, R.; Kulak, M. Management of Weeds in Maize by Sequential or Individual Applications of Pre- and Post-Emergence Herbicides. Agriculture 2023, 13, 421. [Google Scholar] [CrossRef]
- Gürbüz, R.A.H. The efficiency of some post-emergence herbicides for controlling problematic weeds of lawn areas. Proc. Appl. Bot. Genet. Breed. 2022, 183, 159–168. [Google Scholar] [CrossRef]
- Selvarajoo, A. Biomass char gasification with carbon dioxide as an alternative energy. IOP Conf. Ser. Earth Environ. Sci. 2020, 489, 012033. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N. Application of Gaussian smoothing technique in evaluation of biomass pyrolysis kinetics in Macro-TGA. Energy Procedia 2017, 138, 778–783. [Google Scholar] [CrossRef]
- Önür, M.E.; Ekinci, K.; Civan, M.; Bilgili, M.E.; Yurdakul, S. Quality Properties and Torrefaction Characteristics of Pellets: Rose Oil Distillation Solid Waste and Red Pine Sawdust. Sustainability 2023, 15, 10971. [Google Scholar] [CrossRef]
- Bilgili, M.E. Exploitable Potential of Biomass Energy in Electrical Energy Production in the Mediterranean Region of Turkey. J. Agric. Sci. 2022, 28, 666–676. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Oxon, UK, 2015. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Elad, Y. Biochar Impact on Development and Productivity of Pepper and Tomato Grown in Fertigated Soilless Media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Zeng, G.M.; Wang, X.; Hu, X.J.; Gu, Y.L.; Yang, Z.Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cao, J.Q.; Wang, J. Pyrolytic Characteristics of Pine Wood in a Slowly Heating and Gas Sweeping Fixed-Bed Reactor. J. Anal. Appl. Pyrolysis 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, S. Urban Air Pollution and Avenue Trees Benefits, Interactions and Future Prospects; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 1–306. [Google Scholar]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of Biochars to Evaluate Recalcitrance and Agronomic Performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero, C.M.; Arce, G.L.A.F.; Avila, I. Optimization of Slow Pyrolysis Process Parameters Using a Fixed Bed Reactor for Biochar Yield from Rice Husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Kavindi, G.A.G.; Lei, Z. Development of Activated Hydrochar from Paddy Straw for Nutrient Adsorption and Crop Water Management. Water Resour. Manag. 2019, 1, 67–77. [Google Scholar]
- Garg, R.; Anand, N.; Kumar, D. Pyrolysis of Babool Seeds (Acacia nilotica) in a Fixed Bed Reactor and Bio-Oil Characterization. Renew. Energy 2016, 96, 167–177. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Primaz, C.T.; Silva, M.G.P.; Machado, M.E.; Cardoso, C.A.; Jacques, R.A.; Caramao, E.B. Production and Chromatographic Characterization of Bio-Oil from the Pyrolysis of Mango Seed Waste. Ind. Crops Prod. 2016, 83, 529–536. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Sun, J.K.; Lian, F.; Liu, Z.Q.; Zhu, L.Y.; Song, Z.G. Biochars Derived from Various Crop Straws: Characterization and Cd(II) Removal Potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.X.; Wang, H.T.; Lu, W.J.; Zhou, Z.Y.; Zhang, Y.B.; Ren, L. Influence of Pyrolysis Temperature on Characteristics and Heavy Metal Adsorptive Performance of Biochar Derived from Municipal Sewage Sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Klemencova, K.; Grycova, B.; Lestinsky, P. Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties. Sustainability 2022, 14, 6193. [Google Scholar] [CrossRef]
- Wystalska, K.; Kwarciak-Kozłowska, A. The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials 2021, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Katuwal, S.; Ashworth, A.J.; Rafsan, N.-A.-S.; Kolar, P. Characterization of Poultry Litter Biochar and Activated Biochar as a Soil Amendment for Valorization. Biomass 2022, 2, 209–223. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies 2021, 14, 1092. [Google Scholar] [CrossRef]
- Gorshkov, A.; Berezikov, N.; Kaltaev, A.; Yankovsky, S.; Slyusarsky, K.; Tabakaev, R.; Larionov, K. Analysis of the Physicochemical Characteristics of Biochar Obtained by Slow Pyrolysis of Nut Shells in a Nitrogen Atmosphere. Energies 2021, 14, 8075. [Google Scholar] [CrossRef]
- Yang, X.D.; Wan, Y.S.; Zheng, Y.L.; He, F.; Yu, Z.B.; Huang, J.; Chen, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Zhu, Y.G.; Reid, B.J.; Sun, G.X. The Role of Biochar Properties in Influencing the Sorption and Desorption of Pb(II), Cd(II) and As(III) in Aqueous Solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 10815–10837. [Google Scholar]
- Temel, S.; Keskin, B.; Güner, Z.; Atalay, A.İ. Determination of yield and quality characteristics of common reed (Phragmites australis (Cav.) Trin. Ex Steud) HARVESTED AT DIFFERENT GROWTH STAGES. Turk. J. Field Crops 2023, 28, 70–78. [Google Scholar] [CrossRef]
- Keskin, B.; Temel, S.; Tohumcu, Y.S.A. The Effects of Different Sowing Times on Seed Yield and Some Yield Components of Mountain Spinach Grown in Arid Conditions. J. Inst. Sci. Technol. 2023, 13, 1394–1404. [Google Scholar] [CrossRef]
- Altikat, A. Determination of Pyrolyse Yields of Dağ Ispanağı (Atriplex nitens Sch.) and Modeling with Artificial Neural Networks. Master’s Thesis, Iğdır University, Iğdır, Turkey, 2022. [Google Scholar]
- EN 60-529. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj2-PTCxO2DAxWbQfUHHcurCf4QFnoECBcQAQ&url=https%3A%2F%2Fwebstore.iec.ch%2Fpreview%2Finfo_iec60529%257Bed2.1%257Db.pdf&usg=AOvVaw2EEto4pivCD_I3ZTXK_SR0&opi=89978449 (accessed on 21 November 2023).
- EN 61010-1. Available online: https://webstore.iec.ch/publication/4279 (accessed on 21 November 2023).
- Altikat, A.; Alma, M.H.; Altikat, S. A comparative study of deep learning neural network architectures and sensitivity analyses for the prediction of color changes in biochar. Int. J. Energy Res. 2022, 46, 20960–20974. [Google Scholar] [CrossRef]
- Altikat, A.; Alma, M.H. Prediction carbonization yields and the sensitivity analyses using deep learning neural networks and support vector machines. Int. J. Environ. Sci. Technol. 2023, 20, 5071–5080. [Google Scholar] [CrossRef]
- Altikat, A.; Alma, M.H. Application of new hybrid models based on artificial neural networks for modeling pyrolysis yields of Atriplex nitens S. Int. J. Energy Res. 2022, 46, 4445–4461. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of Agricultural Biomass Residues: Comparative Study of Corn Cob, Wheat Straw, Rice Straw and Rice Husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Schroeder, P.; Nascimento, B.P.; Romeiro-ga, M.K.; Cunha, M.C. Chemical and Physical Analysis of The Liquid Fractions from Soursop Seed Cake Obtained Using Slow Pyrolysis Conditions. J. Anal. Appl. Pyrolysis 2017, 124, 161–174. [Google Scholar] [CrossRef]
- Moreira, R.; Reis, O.R.; Vaz, J.M.; Penteado, J.C.; Spinace, E.V. Production of Biochar, Bio-Oil and Synthesis Gas from Cashew Nut Shell by Slow Pyrolysis. Waste Biomass Valorization 2017, 8, 217–224. [Google Scholar] [CrossRef]
- Mena, L.E.; Pecora, A.A.B.; Beraldo, A.L. Slow Pyrolysis of Bamboo Biomass: Analysis of Biochar Properties. Chem. Eng. Trans. 2014, 37, 115–120. [Google Scholar]
- Yorgun, S.; Yıldız, D. Slow Pyrolysis of Paulownia Wood: Effects of Pyrolysis Parameters on Product Yields and Bio-Oil Characterization. J. Anal. Appl. Pyrolysis 2015, 114, 68–78. [Google Scholar] [CrossRef]
- Aysu, T.; Kucuk, M.M. Biomass Pyrolysis in a Fixed-Bed Reactor: Effects of Pyrolysis Parameters on Product Yields and Characterization of Products. Energy 2014, 64, 1002–1025. [Google Scholar] [CrossRef]
- Azduwin, K.; Ridzuan, M.J.; Hafis, S.M.; Amran, T. Slow Pyrolysis of Imperata Cylindrica in a Fixed Bed Reactor. Int. J. Environ. Sci. Technol. 2012, 1, 176–180. [Google Scholar]
- Chukwuneke, J.L.; Sinebe, J.E.; Ugwuegbu, D.C.; Agulonu, C.C. Production by Pyrolysis and Analysis of Bio-Oil from Mahogany Wood (Swietenia macrophylla). Br. J. Appl. Sci. Technol. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Morali, U.; Sensoz, S. Pyrolysis of Hornbeam Shell (Carpinus betulus L.) in a Fixed Bed Reactor: Characterization of Bio-Oil and Bio-Char. Fuel 2015, 150, 672–678. [Google Scholar] [CrossRef]
- Ucar, S.; Karagöz, S. The Slow Pyrolysis of Pomegranate Seeds: The Effect of Temperature on The Product Yields and Bio-Oil Properties. J. Anal. Appl. Pyrolysis 2009, 84, 151–156. [Google Scholar] [CrossRef]
- Ucar, S.; Ozkan, A.R. Characterization of Products from the Pyrolysis of Rapeseed Oil Cake. Bioresour. Technol. 2008, 99, 8771–8776. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.P.S. A Slow Pyrolysis of Cotton Stalk (Gossypium arboretum) Waste for Bio-Oil Production. J. Pharm. Chem. Biol. Sci. 2015, 3, 143–149. [Google Scholar]
- Smith, J. Impact of Pyrolysis Time and Temperature on Biochar Properties. J. Biochar Stud. 2020, 5, 123–134. [Google Scholar]
- Jones, D.; Lee, H. Enhancing Carbon Content in Biochar: Effects of Pyrolysis Conditions. Biochar Res. 2019, 7, 45–52. [Google Scholar]
- Patel, S.; Kim, D. Role of Gas Flow Rate in Biochar Production. Int. J. Pyrolysis 2021, 12, 234–245. [Google Scholar]
- Lee, J.; Chang, S. Oxygenated Functional Groups in Biochar: Their Role in Enhancing Energy Content. Bioenergy Res. 2019, 11, 601–610. [Google Scholar]
- Garcia, L. Biochar Characterization at Different Pyrolysis Temperatures. J. Environ. Manag. 2018, 10, 112–119. [Google Scholar]
- Nguyen Huy, B.; Lanh, N.V.; Hung, B.N. The Composition of Syngas and Biochar Produced by Gasifier from Viet Nam Rice Husk. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 2258–2263. [Google Scholar] [CrossRef][Green Version]
- Martin, G.; Davis, E. Analyzing Biochar Composition: The Role of FTIR. J. Biochar Environ. Sci. 2021, 13, 77–85. [Google Scholar]
- Nguyen, T.; Wang, Y. Optimizing Biochar Production for Energy Content. Adv. Biochar Stud. 2022, 14, 202–210. [Google Scholar]
- Morsy, O.; Hourfar, F.; Zhu, Q.; Almansoori, A.; Elkamel, A. A Superstructure Mixed-Integer Nonlinear Programming Optimization for the Optimal Processing Pathway Selection of Sludge-to-Energy Technologies. Sustainability 2023, 15, 4023. [Google Scholar] [CrossRef]
- Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability 2023, 15, 1643. [Google Scholar] [CrossRef]
- Kumar, N.V.; Sawargaonkar, G.L.; Rani, C.S.; Singh, A.; Prakash, T.R.; Triveni, S.; Kamdi, P.J.; Pasumarthi, R.; Karthik, R.; Venkatesh, B. Comparative Analysis of Pigeonpea Stalk Biochar Characteristics and Energy Use under Different Biochar Production Methods. Sustainability 2023, 15, 14394. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Taweekun, J.; Ja’afar, F.; Saifullah Abu Bakar, M.; Azad, A.K.; Roy, H.; et al. Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability 2023, 15, 7628. [Google Scholar] [CrossRef]
- Mishra, R.; Mohanty, K. Bio-oil and biochar production using thermal and catalytic pyrolysis of low-value waste neem seeds over low-cost catalysts: Effects of operating conditions on product yields and studies of physicochemical characteristics of bio-oil and biochar. Biochar 2021, 3, 641–656. [Google Scholar] [CrossRef]
- Varma, A.; Mondal, P. Pyrolysis of pine needles: Effects of process parameters on products yield and analysis of products. J. Therm. Anal. Calorim. 2018, 131, 2057–2072. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Wang, H. Effects of Heating Rate, Particle Size, Aerobic Atmosphere, and Flow Rate on the Pyrolysis Characteristics of Huadian Oil Shale. Solid Fuel Chem. 2023, 57, 112–122. [Google Scholar] [CrossRef]
- Karadağ, E.; Bilge, S.; Donar, Y.O.; Sinağ, A. Catalytic pyrolysis of olive oil residue to produce synthesis gas: The effect of bulk and nano metal oxides. Turk. J. Chem. 2022, 46, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, S.; Sergiienko, R.; Ilkiv, B.; Nakamura, T.; Ohtsuka, M. Effect of Oxygen Flow Rate on Properties of Aluminum-Doped Indium-Saving Indium Tin Oxide (ITO) Thin Films Sputtered on Preheated Glass Substrates. Metals 2021, 11, 1604. [Google Scholar] [CrossRef]
- Zhu, T.; Jing, W.; Zhang, X.; Bian, W.; Han, Y.; Liu, T.; Hou, Y.; Ye, Z. Gas-phase elemental mercury removal by nano-ceramic material. Nanomater. Nanotechnol. 2020, 10. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.; Wong, K.; Bong, C.P.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Uroić Štefanko, A.; Leszczyńska, D. Impact of Biomass Source and Pyrolysis Parameters on Physicochemical Properties of Biochar Manufactured for Innovative Applications. Front. Energy Res. 2020, 8, 138. [Google Scholar] [CrossRef]
- Dooley, K.L. Slow Pyrolysis Biochar from Forestry Residue and Municipal and Farm Wastes: Characterization and Their Use in Greenhouses as a Soil Amendment. 2015. Available online: https://api.semanticscholar.org/CorpusID:138570074, (accessed on 21 November 2023).
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefinery 2020, 11, 2247–2267. [Google Scholar] [CrossRef]
- Volpe, R.; Messineo, A.; Millan, M.; Volpe, M.; Kandiyoti, R. Assessment of olive wastes as energy source: Pyrolysis, torrefaction and the key role of H loss in thermal breakdown. Energy 2015, 82, 119–127. [Google Scholar] [CrossRef]
- Yek, P.; Liew, R.K.; Osman, M.S.; Wong, C.; Lam, S. Microwave pyrolysis using self-generated pyrolysis gas as activating agent: An innovative single-step approach to convert waste palm shell into activated carbon. E3S Web Conf. 2017, 22, 00195. [Google Scholar] [CrossRef]
- Mariyam, S.; Alherbawi, M.; Rashid, N.; Al-Ansari, T.; McKay, G. Bio-Oil Production from Multi-Waste Biomass Co-Pyrolysis Using Analytical Py–GC/MS. Energies 2022, 15, 7409. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhang, Q.; Wang, X.; Wang, X.; Jin, P. Characteristics of Pyrolysis and Copyrolysis Products Sewage Sludge in Different Temperature Ranges. J. Chem. 2022, 2022, 7160782. [Google Scholar] [CrossRef]
- Li, J.; Shang, Y.; Wei, W.; Liu, Z.y.; Qiao, Y.; Qin, S.; Tian, Y. Comparative Study on Pyrolysis Kinetics Behavior and High-Temperature Fast Pyrolysis Product Analysis of Coastal Zone and Land Biomasses. ACS Omega 2022, 7, 9319–9331. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Rashid, N.; Zaini, N. Effect of Temperature on Calorific Value of Pyrolyzed Empty Fruit Bunch (EFB) Derived Biochar. J. Eng. Environ. Sci. Res. 2021, 3, 138. [Google Scholar] [CrossRef]

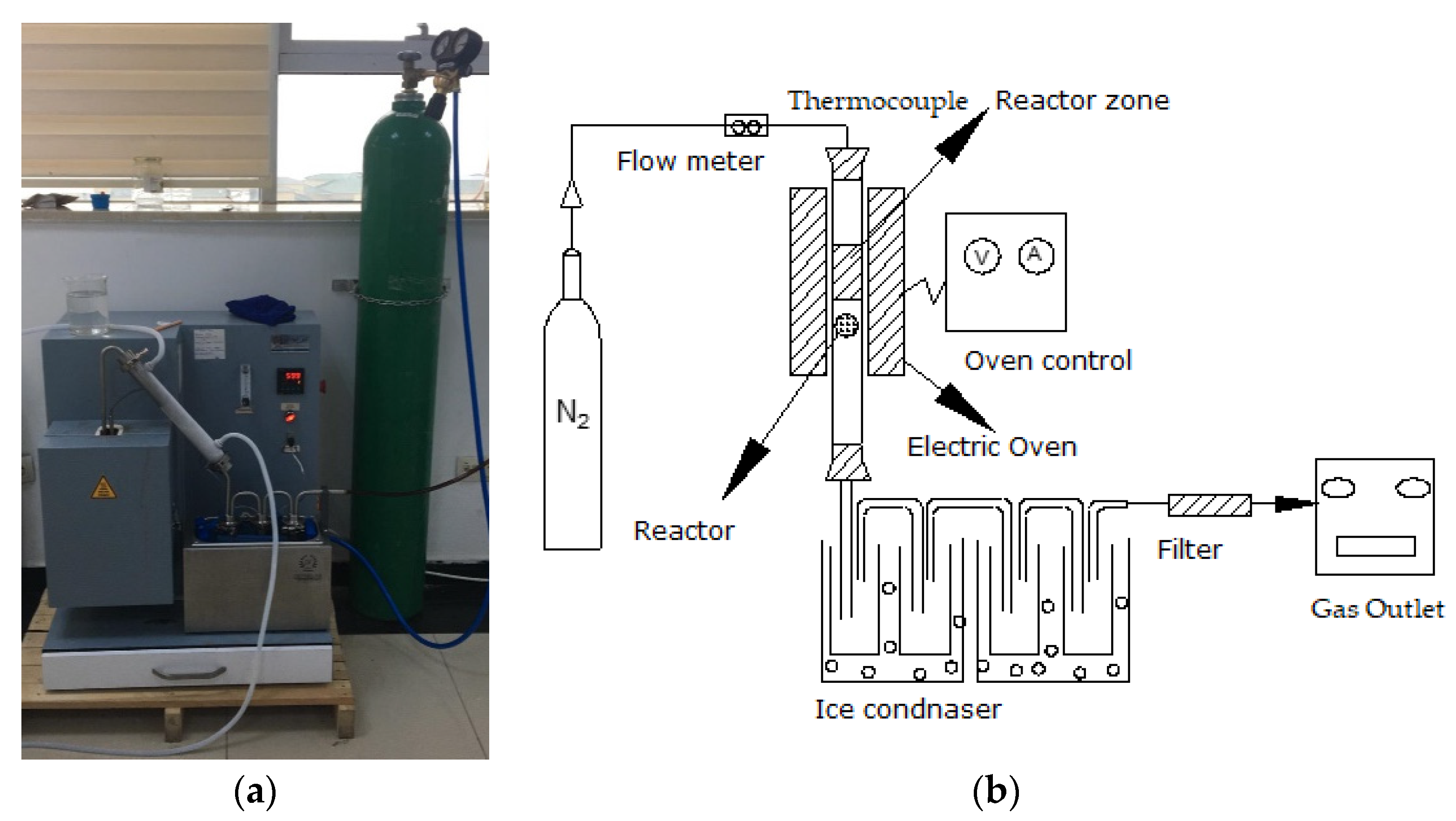








| Input Type | Scale Range | Accuracy |
|---|---|---|
| P1 100 resistance thermometer | −200–600 °C | ±%0.2 |
| J (Fe-CuNi) thermocouple | 0–600 °C | ±%0.2 |
| K (Nicr-Ni) thermocouple | 0–1200 °C | ±%0.2 |
| T(Cu-CuNi) thermocouple | 0–400 °C | ±%0.2 |
| S (Pt/0 Rh-Pt) | 0–100 °C | ±%0.2 |
| Environmental Characteristics | ||
| Ambient storage temperature | 0–50 °C/25–70 °C | |
| Relative humidity | Up to 80% at 31 °C, 50% at 40 °C | |
| Protection class According to EN 60-529 standard [35] | Front panel: IP65 Back panel: IP20 | |
| Electrical Characteristics | ||
| Power supply | 230 V AC +%10–%20, 50/60 Hz | |
| Power consumption | Maximum 7 V/A | |
| Connection | 2.5 mm socket terminals | |
| Line resistance | Maximum 100 ohm for thermocouple, max. 200 ohm for 3-wire Pt 100 | |
| Safety requirement | EN 61010-1 [36] | |
| Experimental Factors | Levels |
|---|---|
| Pyrolysis temperature (°C) | 400–500–600 |
| Holding time (min) | 30–60 |
| Gas flow rate (L min−1) | 0.2–0.5 |
| Sources of Variation | Pyrolysis Yields | ||
|---|---|---|---|
| Biochar | Bio-Oil | Synthesis Gas | |
| Pyrolysis Temperature | 0.000 ** | 0.000 ** | 0.000 ** |
| Holding time | 0.970 ns | 0.000 ** | 0.003 ** |
| Gas flow rate | 0.000 ** | 0.731 ns | 0.122 ns |
| Replication | 0.357 ns | 0.398 ns | 0.244 ns |
| Pyrolysis Temperature * Holding time | 0.000 ** | 0.005 ** | 0.067 ns |
| Pyrolysis Temperature * Gas flow rate | 0.119 ns | 0.113 ns | 0.393 ns |
| Holding time * Gas flow rate | 0.256 ns | 0.498 ns | 0.440 ns |
| Pyrolysis Temperature * Holding time * Gas flow rate | 0.330 ns | 0.002 ** | 0.035 * |
| Pyrolysis Temperature (°C) | Biochar Yield (%) |
|---|---|
| 400 | 37.14 a * |
| 500 | 32.44 b |
| 600 | 30.60 c |
| Gas flow rate (L/min-1) | Biochar yield (%) |
| 0.2 | 34.07 a |
| 0.5 | 32.72 b |
| Pyrolysis Temperature (°C) | Bio-Oil Yield (%) |
|---|---|
| 400 | 23.94 a * |
| 500 | 21.95 b |
| 600 | 12.75 c |
| Holding time (°C) | Bio-oil yield (%) |
| 30 | 20.19 a |
| 60 | 18.90 b |
| Pyrolysis Temperature (°C) | Synthesis Gas Yield (%) |
|---|---|
| 400 | 38.92 c * |
| 500 | 46.24 b |
| 600 | 56.64 a |
| Holding time (°C) | Synthesis gas yield (%) |
| 30 | 46.42 b |
| 60 | 48.12 a |
| Pyrolysis | Holding Time (°C) | Flow Rate | C | O | Na | Mg | Al | Si | P | Cl | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | (L °C−1) | ||||||||||||
| 400 | 30 | 0.2 | 36.34 | 30.91 | 9.22 | 1.12 | 0.25 | 0.35 | 1.66 | 1.11 | 15.82 | 2.32 | 0.88 |
| 400 | 30 | 0.5 | 48.83 | 38 | 2.54 | 0.87 | 0.41 | 0.52 | 2.05 | 1.38 | 3.57 | 0.79 | 1.03 |
| 400 | 60 | 0.2 | 55.13 | 20.23 | 4.73 | 1.09 | 0.54 | 0.81 | 3.85 | 1.2 | 7.89 | 2.97 | 1.56 |
| 400 | 60 | 0.5 | 57.39 | 30.62 | 2.26 | 0.76 | 0.34 | 0.41 | 1.84 | 0.2 | 1.65 | 3.28 | 1.26 |
| 500 | 30 | 0.2 | 54.41 | 22.74 | 2.64 | 2.09 | 0.32 | 0.58 | 1.73 | 1.3 | 4.19 | 8.65 | 1.34 |
| 500 | 30 | 0.5 | 57.63 | 15.97 | 4.16 | 0.97 | 0.72 | 1 | 4.82 | 1.05 | 6.35 | 4.81 | 2.51 |
| 500 | 60 | 0.2 | 62.31 | 26.03 | 2.34 | 0.92 | 0.3 | 0.52 | 2.41 | 0.22 | 2.49 | 1.63 | 0.85 |
| 500 | 60 | 0.5 | 64.05 | 25.36 | 2.5 | 0.66 | 0.47 | 0.36 | 1.87 | 0.24 | 2.08 | 1.35 | 1.06 |
| 600 | 30 | 0.2 | 62.31 | 26.03 | 2.34 | 0.92 | 0.3 | 0.52 | 2.41 | 0.22 | 2.49 | 1.63 | 0.85 |
| 600 | 30 | 0.5 | 67.72 | 20.36 | 2.07 | 0.72 | 0.49 | 0.52 | 1.84 | 0.38 | 2.93 | 1.84 | 1.13 |
| 600 | 60 | 0.2 | 66.78 | 15.37 | 3.34 | 0.87 | 0.5 | 0.68 | 3.48 | 1.18 | 4.78 | 1.87 | 1.15 |
| 600 | 60 | 0.5 | 66.61 | 20.49 | 3.51 | 0.58 | 0.45 | 0.37 | 1.32 | 0.84 | 3.05 | 0.88 | 1.9 |
| Feedstock | 45,97 | 41,71 | 1.96 | 0.87 | 0.54 | 0.84 | 4.12 | 0.47 | 1.89 | 0.42 | 1.21 | ||
| Pyrolysis Temperature (°C) | Holding Time (°C) | Gaz Flow Rate (L °C−1) | HCV (MJ kg−1) |
|---|---|---|---|
| 400 | 30 | 0.2 | 17.67 |
| 400 | 30 | 0.5 | 17.8 |
| 400 | 60 | 0.2 | 19.51 |
| 400 | 60 | 0.5 | 22.85 |
| 500 | 30 | 0.2 | 21.21 |
| 500 | 30 | 0.5 | 23.1 |
| 500 | 60 | 0.2 | 24.18 |
| 500 | 60 | 0.5 | 24.22 |
| 600 | 30 | 0.2 | 24.22 |
| 600 | 30 | 0.5 | 25.23 |
| 600 | 60 | 0.2 | 25.88 |
| 600 | 60 | 0.5 | 26.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. https://doi.org/10.3390/su16020937
Altıkat A, Alma MH, Altıkat A, Bilgili ME, Altıkat S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability. 2024; 16(2):937. https://doi.org/10.3390/su16020937
Chicago/Turabian StyleAltıkat, Alperay, Mehmet Hakkı Alma, Aysun Altıkat, Mehmet Emin Bilgili, and Sefa Altıkat. 2024. "A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach" Sustainability 16, no. 2: 937. https://doi.org/10.3390/su16020937
APA StyleAltıkat, A., Alma, M. H., Altıkat, A., Bilgili, M. E., & Altıkat, S. (2024). A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability, 16(2), 937. https://doi.org/10.3390/su16020937







