A Review on the Effective Utilization of Organic Phase Change Materials for Energy Efficiency in Buildings
Abstract
:1. Introduction
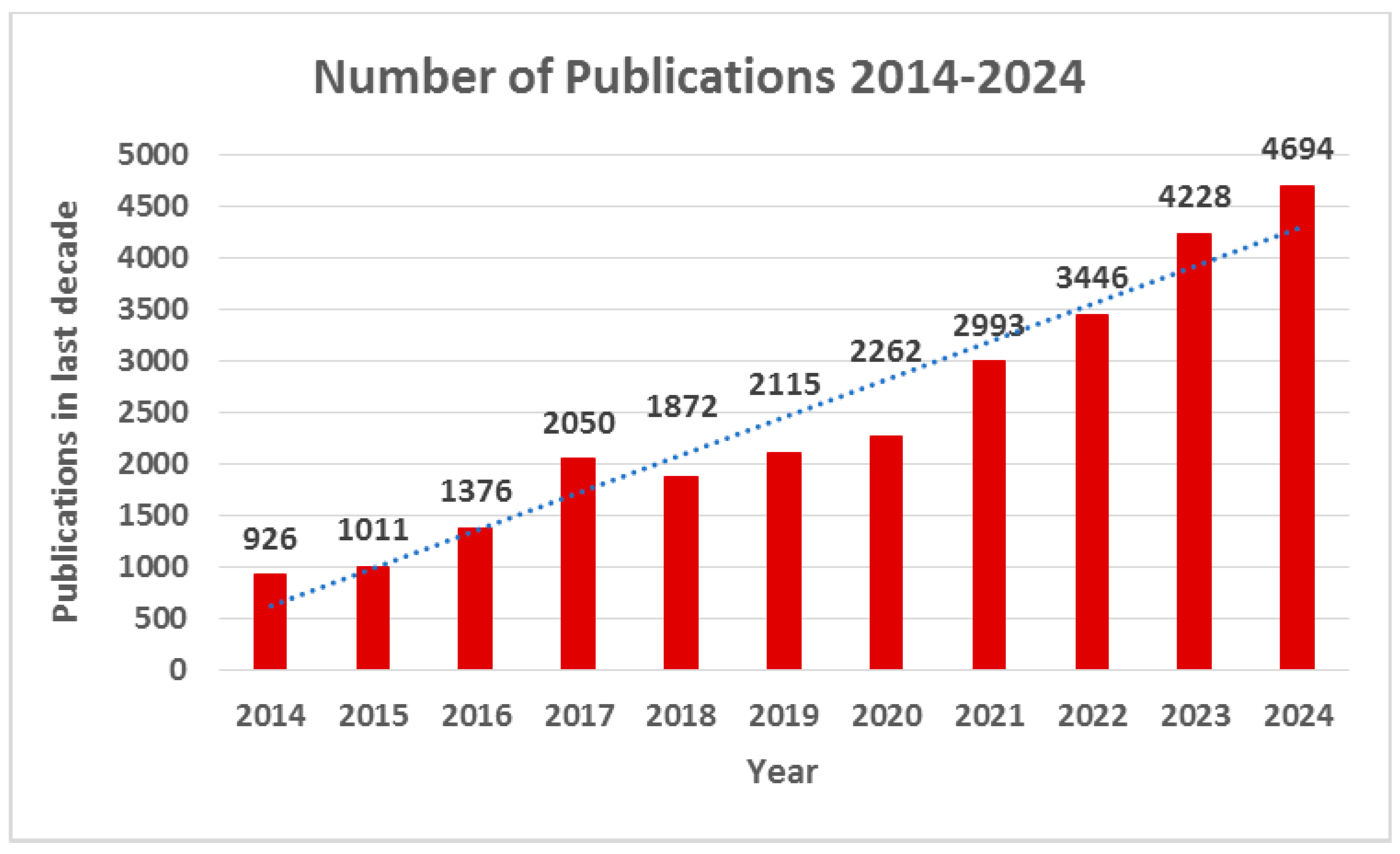
1.1. Annual Trend in the Number of Publications
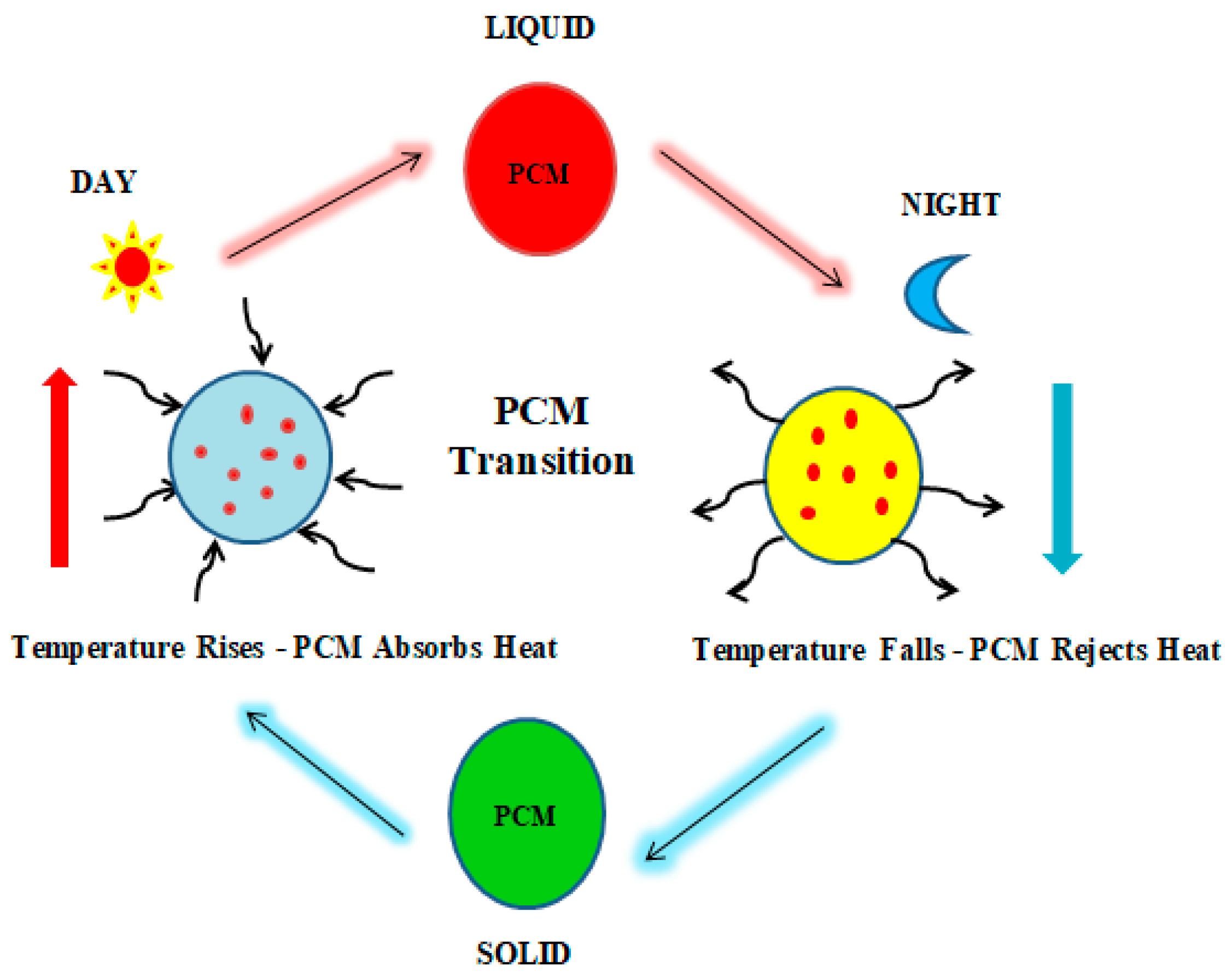
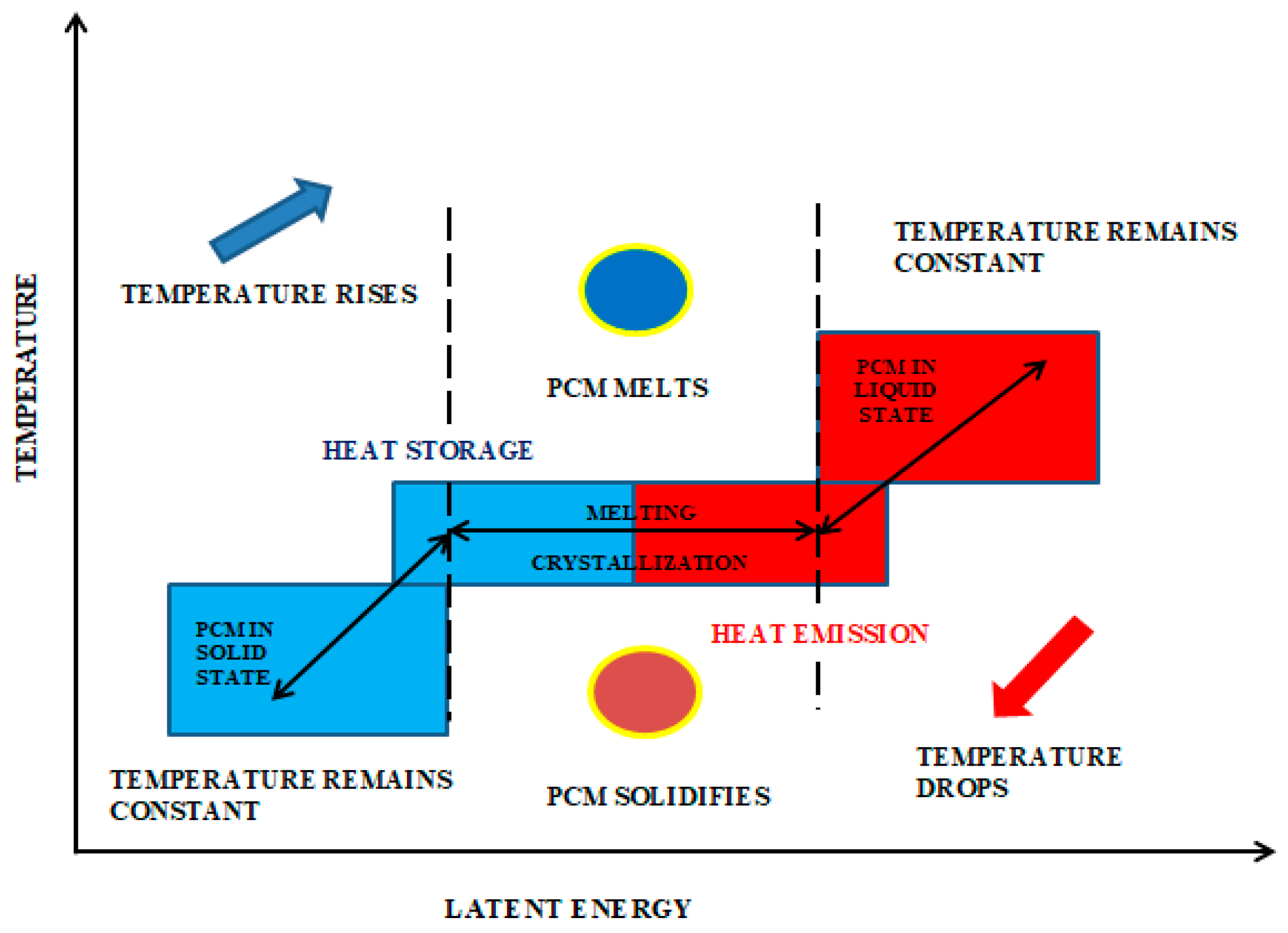
1.2. Principle of PCM
1.3. Classification of PCMs
2. Organic PCMs and Their Applications
3. Inorganic PCM and Their Applications
4. Eutectic PCM and Their Applications
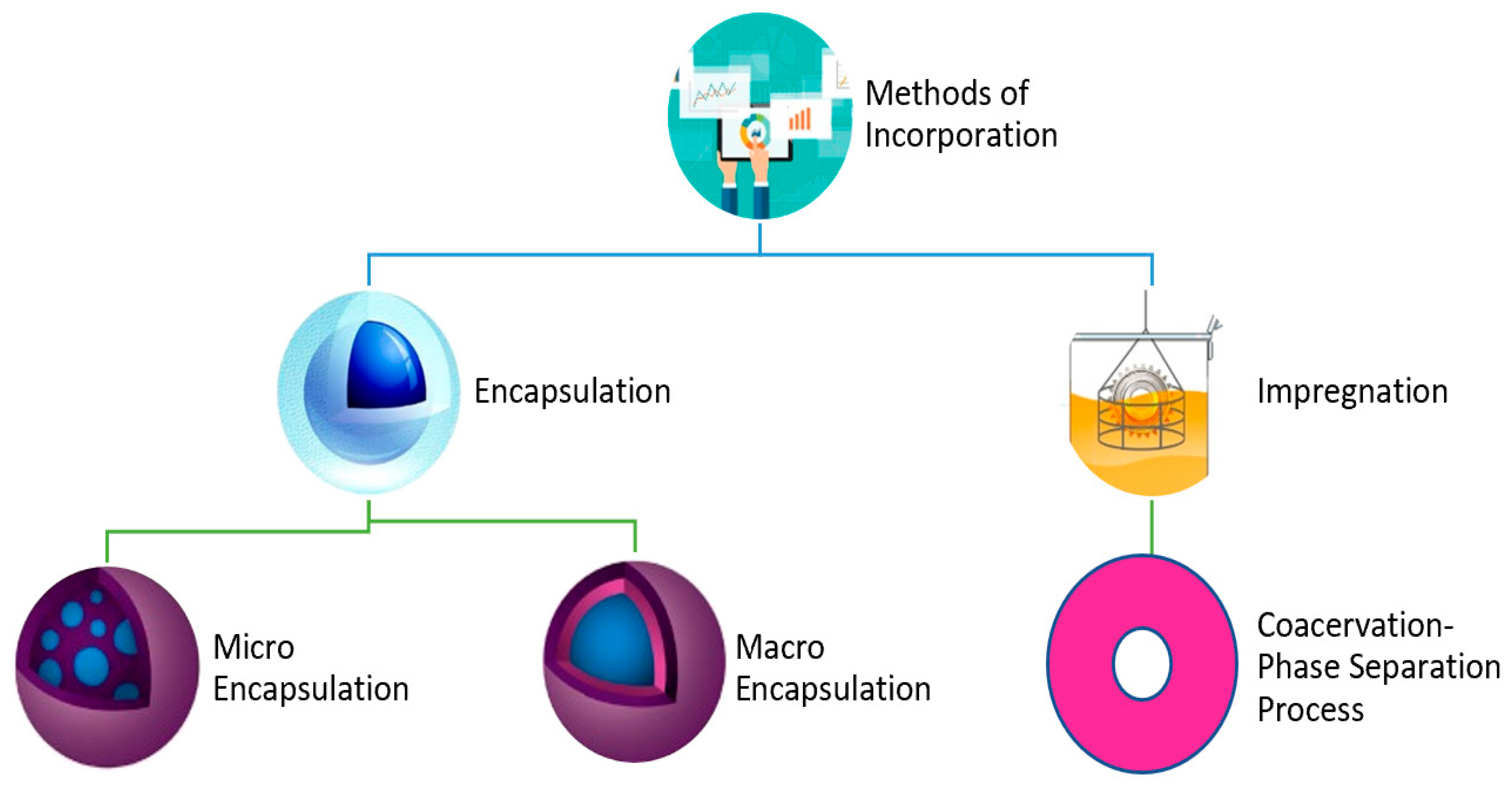
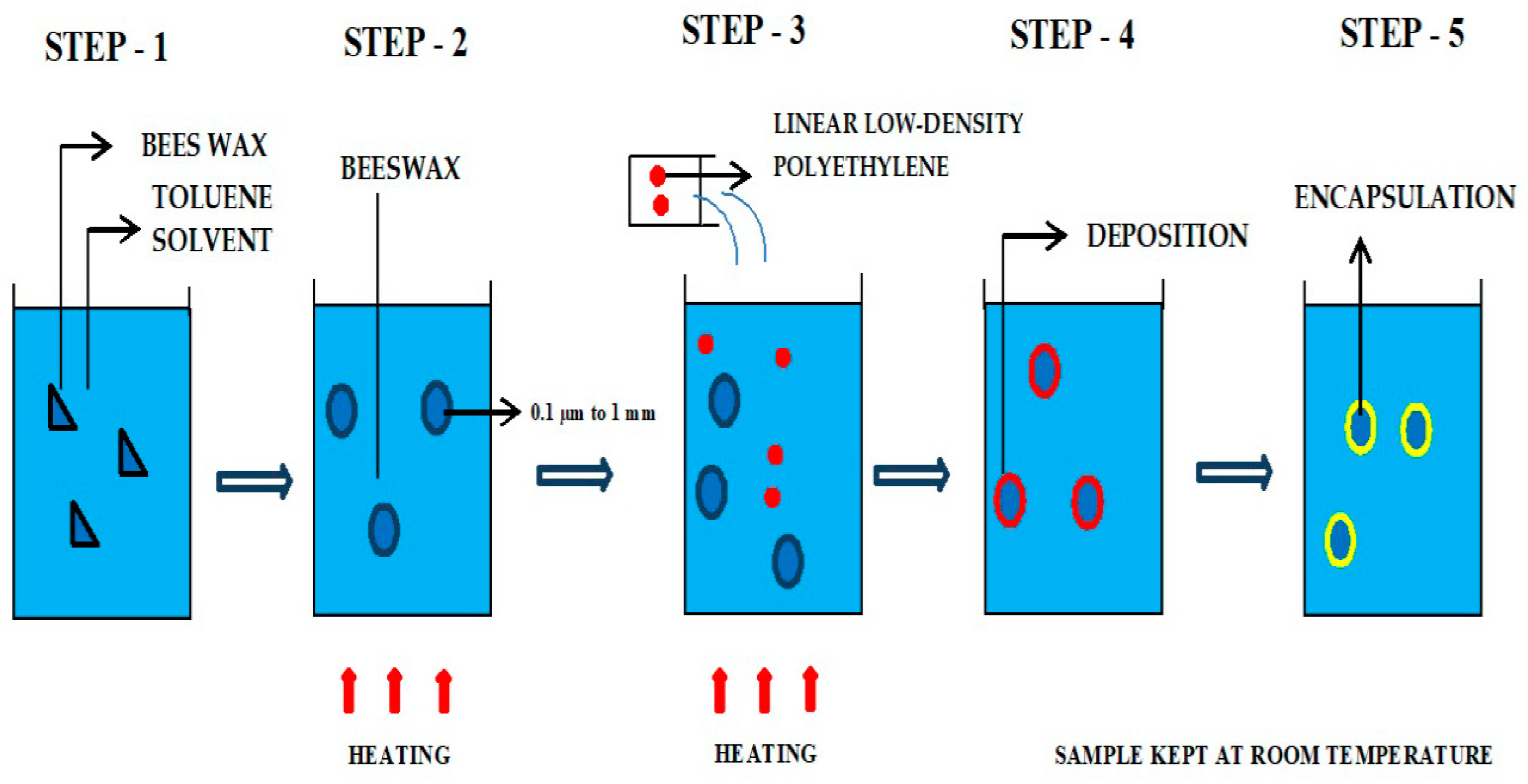
5. Incorporation Techniques
5.1. Techniques of Encapsulation
5.2. Technique of Coacervation
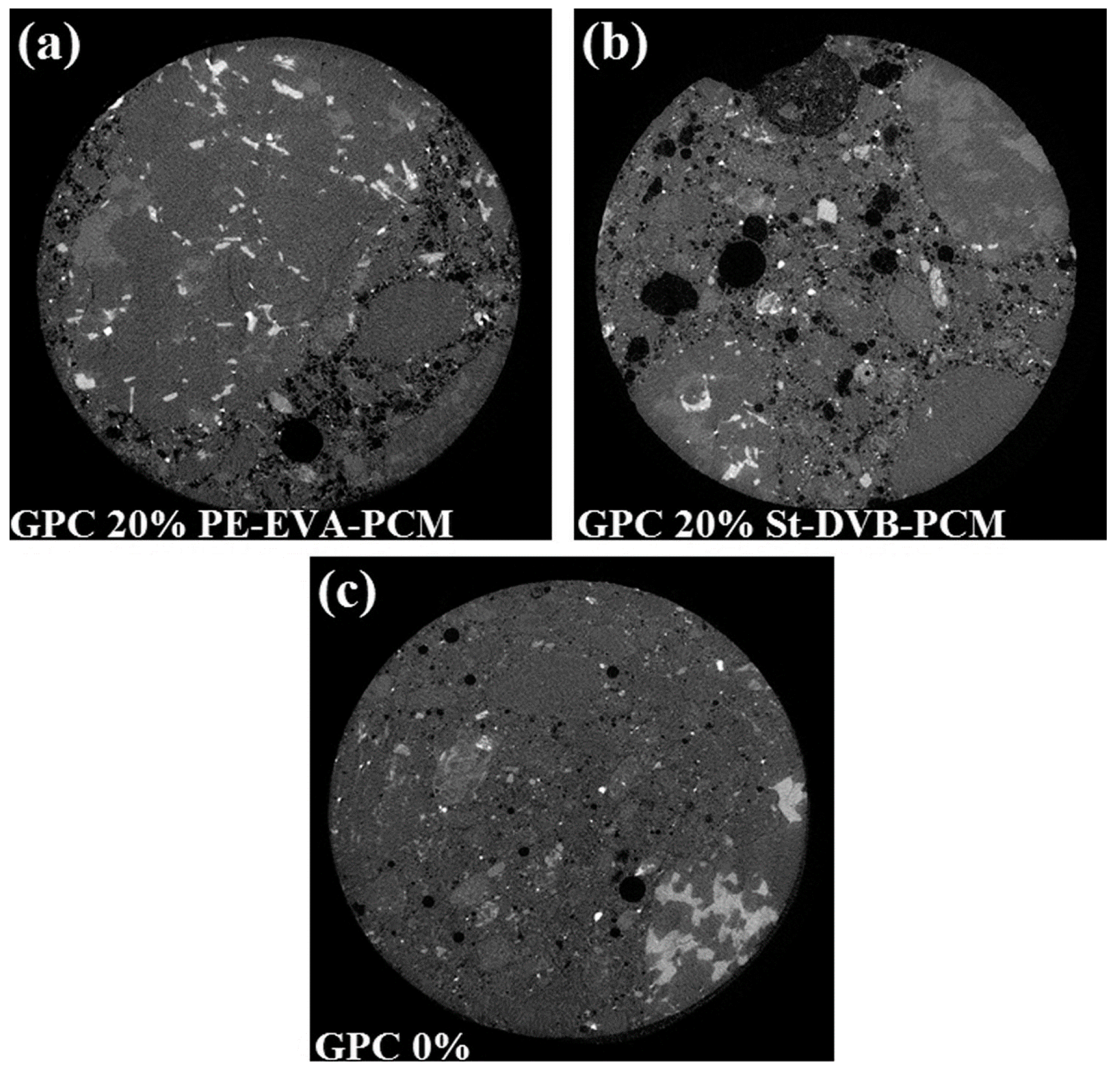
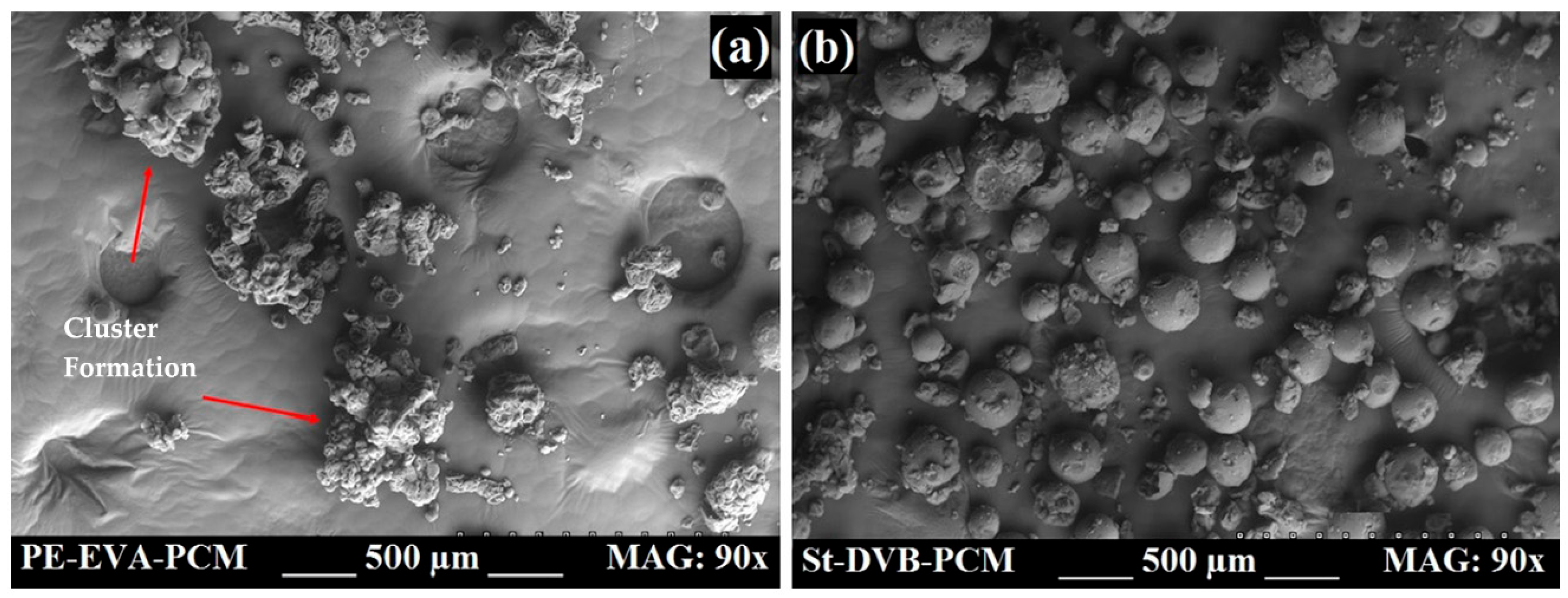
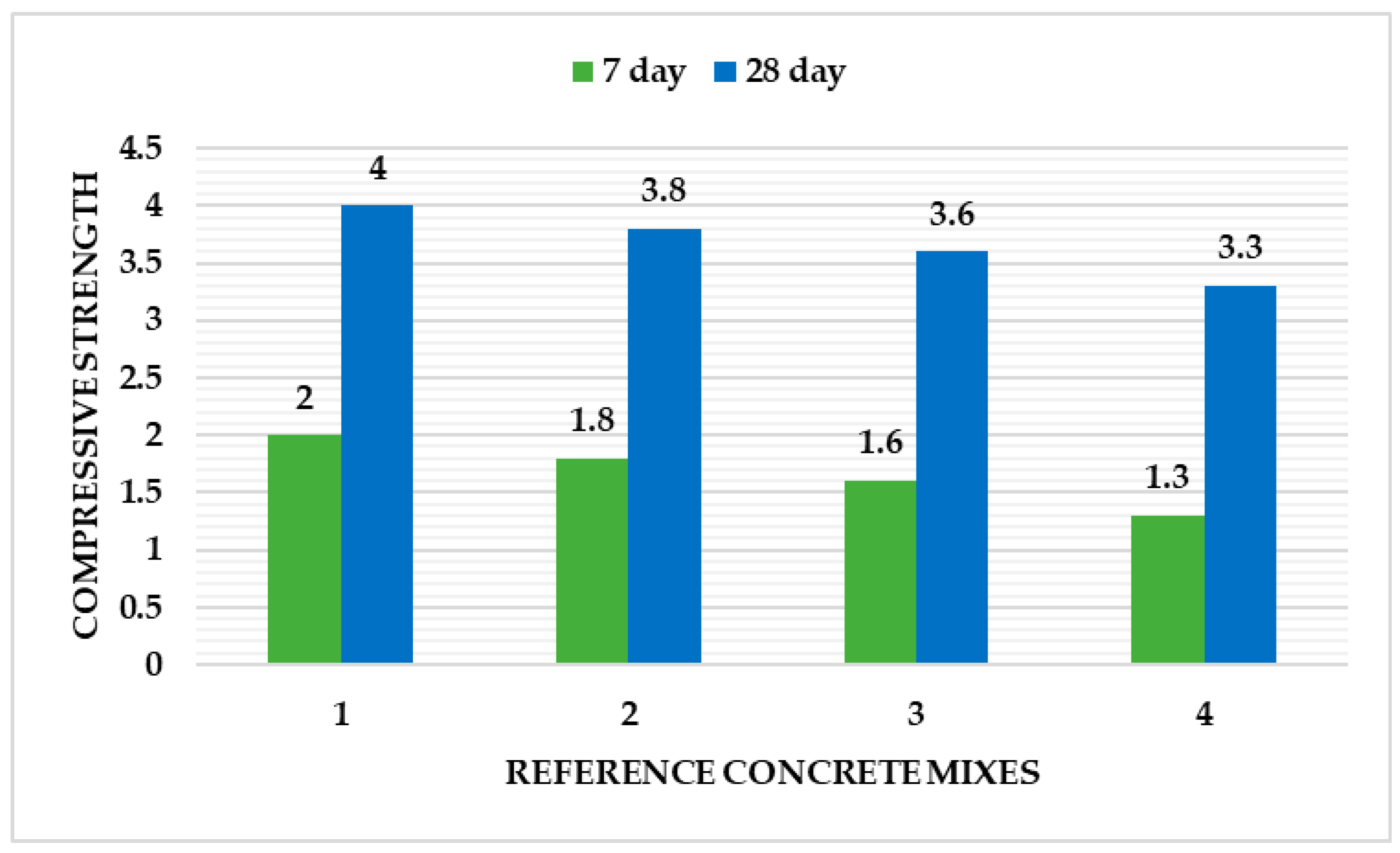
6. Testing—Mechanical Properties
6.1. Shrinkage, Cracking, Leaking
6.2. Durability
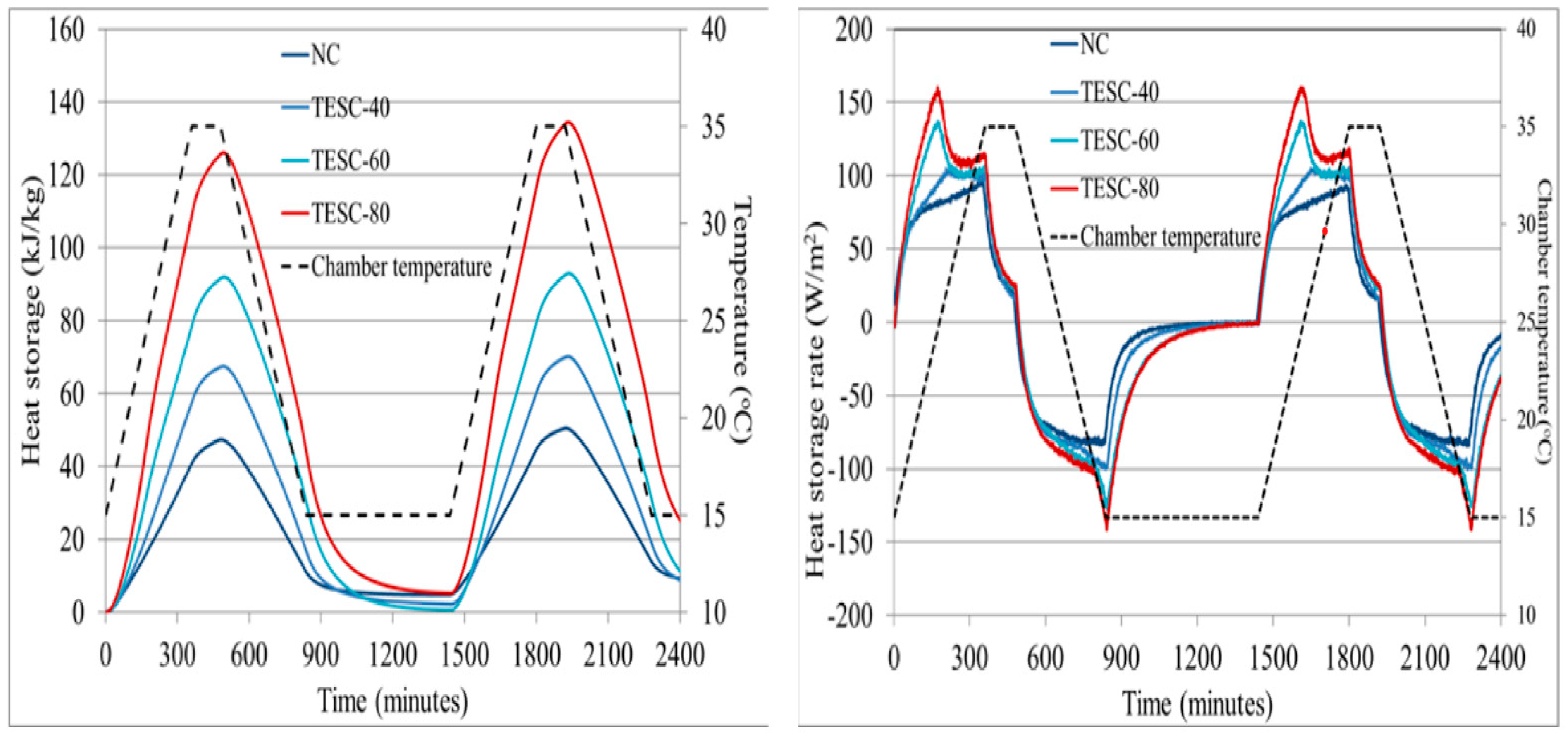
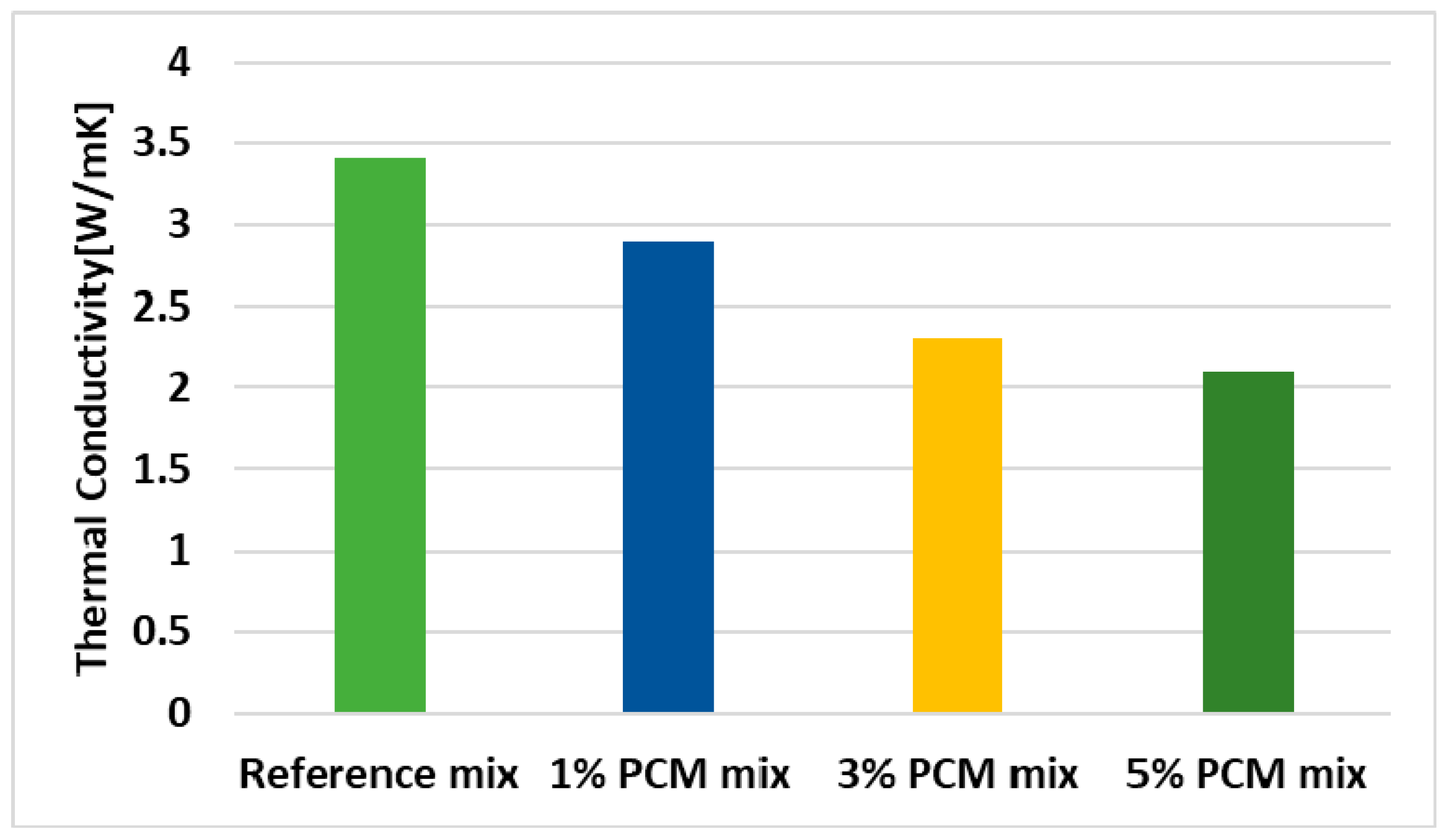
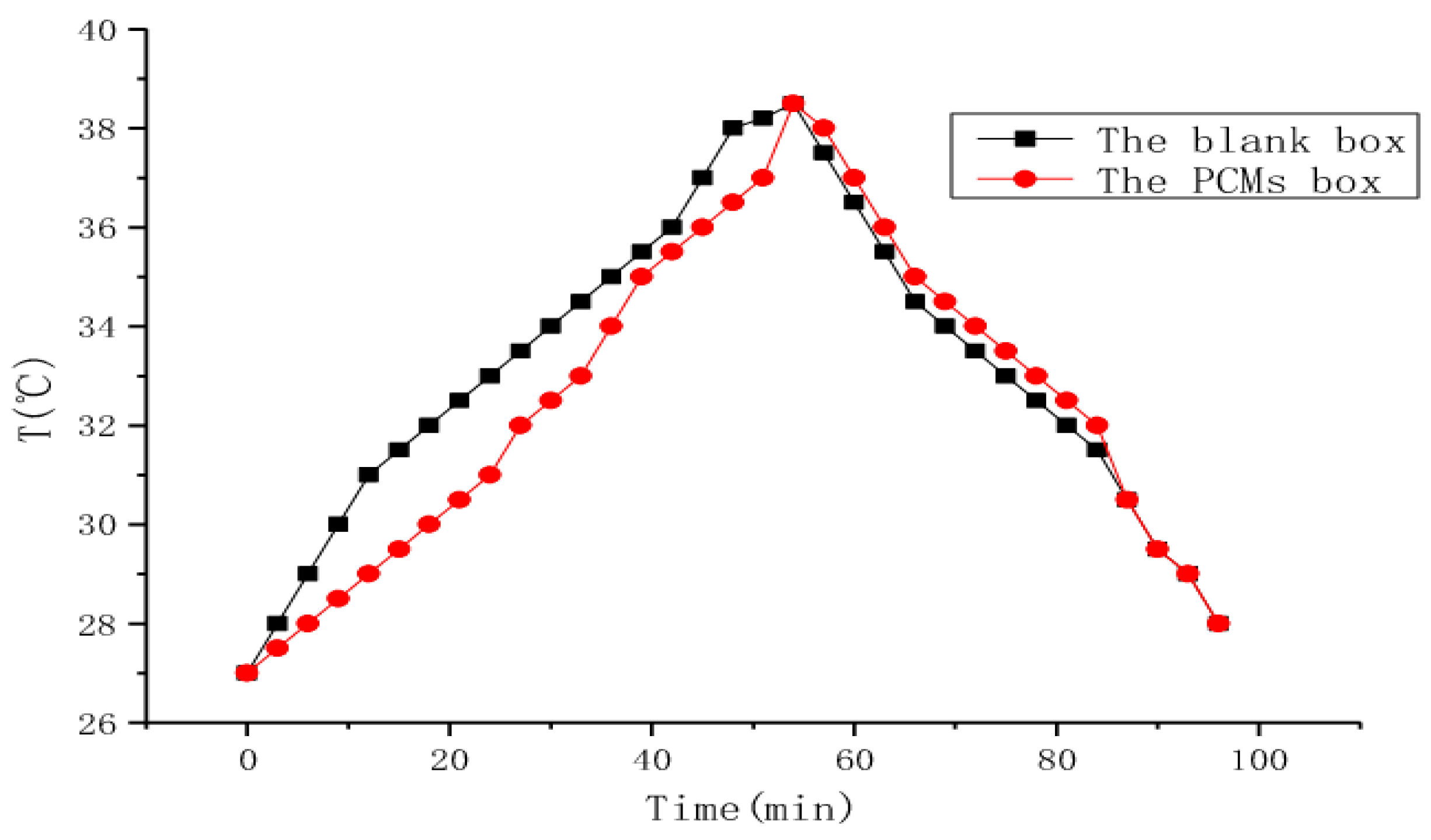
6.3. Thermal Conductivity and Thermal Diffusivity
- Th = temperature of the hot plate surface.
- TC = temperature of cold plate surface;
- E = heat flux transducer W/m2.
- ρ = density of the material (in kg/m3);
- cp = specific heat capacity at constant pressure (in J/(kg·K)).
7. Real-Time Applications
8. Challenges and Limitations of PCMs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| 1. | PCM | Phase change materials |
| 2. | MEPCM | Micro-/macroencapsulated phase change materials |
| 3. | k | Thermal conductivity |
| 4. | T | Temperature |
| 5. | ρ | Density (kg/m3) |
| 6. | TES | Thermal energy storage |
| 7. | ΔHF | Heat of fusion |
| 8. | LLDPE | Linear low-density polyethylene |
| 9. | PMMA | Polymethyl methacrylate |
| 10. | PETA | Pentaerythritol tetraacrylate |
References
- Waterman, A.T. Positive ionisation of certain hot salts, together with some observations on the electrical properties of molybdenite at high temperatures. Philos. Mag. 1917, 33, 225. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, J.H.; Seo, J.; Jeong, S.; Kim, S. Application of PCM thermal energy storage system to reduce building energy con-sumption. J. Therm. Anal. Calorim. 2013, 111, 279–288. [Google Scholar] [CrossRef]
- Haily, E.; Ousaleh, H.A.; Zari, N.; Faik, A.; Bouhfid, R.; Qaiss, A. Use of a form-stable phase change material to improve thermal properties of phosphate sludge-based geopolymer mortar. Constr. Build. Mater. 2023, 386, 131570. [Google Scholar] [CrossRef]
- Zhan, H.; Mahyuddin, N.; Sulaiman, R.; Khayatian, F. Phase change material (PCM) integrations into buildings in hot climates with simulation access for energy performance and thermal comfort: A review. Constr. Build. Mater. 2023, 397, 132312. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2016, 73, 983–999. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Irani, F.; Ranjbar, Z.; Moradian, S.; Jannesari, A. Microencapsulation of n -heptadecane phase change material with starch shell. Prog. Org. Coat. 2017, 113, 31–38. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ogawa, M.; Katayama, R.; Yonemura, K.; Nakao, S.-I. A facile microencapsulation of phase change materials within silicone-based shells by using glass capillary devices. Colloids Surf. A Physicochem. Eng. Asp. 2018, 567, 297–303. [Google Scholar] [CrossRef]
- Rashid, F.L.; Al-Obaidi, M.A.; Dulaimi, A.; Bernardo, L.F.A.; Eleiwi, M.A.; Mahood, H.B.; Hashim, A. A Review of Recent Improvements, Developments, Effects, and Challenges on Using Phase-Change Materials in Concrete for Thermal Energy Storage and Release. J. Compos. Sci. 2023, 7, 352. [Google Scholar] [CrossRef]
- Analyze Search Results. Available online: http://surl.li/ogwwqb (accessed on 13 July 2024).
- Lu, S.; Shen, T.; Xing, J.; Song, Q.; Shao, J.; Zhang, J.; Xin, C. Preparation and characterization of cross-linked polyurethane shell microencapsulated phase change materials by interfacial polymerization. Mater. Lett. 2017, 211, 36–39. [Google Scholar] [CrossRef]
- Feczkó, T.; Trif, L.; Németh, B.; Horák, D. Silica coated (polygly cidyl methacrylate ethylene dimethacrylate) beads containing organic PCM. Thermochim. Acta 2016, 641, 24–28. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Wang, Y.; Han, N.; Wang, J.; Wang, N.; Zhang, X. Chitosan composite microencapsulated comb-like polymeric phase change material via coacervation microen-capsulation. Carbohydr. Polym. 2018, 200, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Dulac, J.; Petrichenko, K.; Graham, P. Towards Zero-Emission Efficient and Resilient Buildings; Global Status Report; Global Alliance for Buildings and Construction (GABC): Kongens Lyngby, Denmark, 2016. [Google Scholar]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Kong, X.; Pang, B.; Chen, Y.; Zheng, S.; Sun, L. A Review of PCM Energy Storage Technology Used in Buildings for the Global Warming Solution. In Energy Solutions to Combat Global Warming; Zhang, X., Dincer, I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 611–644. ISBN 978-3-319-26950-4. [Google Scholar]
- Kalnaes, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future re-search opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Janarthanan, B.; Sagadevan, S. Thermal energy storage using phase change materials and their applications: A review. Int. J. ChemTech Res. 2015, 8, 205–256. [Google Scholar]
- Cui, Y.Q.; Riffat, S. Review on Phase Change Materials for Building Applications. Appl. Mech. Mater. 2011, 71–78, 1958–1962. [Google Scholar] [CrossRef]
- Madessa, H.B. A Review of the Performance of Buildings Integrated with Phase Change Material: Opportunities for Application in Cold Climate. Energy Procedia 2014, 62, 318–328. [Google Scholar] [CrossRef]
- Nkwetta, D.N.; Haghighat, F. Thermal energy storage with phase change material—A state-of-the art review. Sustain. Cities Soc. 2014, 10, 87–100. [Google Scholar] [CrossRef]
- Telkes, M. Thermal storage for solar heating and cooling. In Proceedings of the Workshop on Solar Energy Storage Subsystems for the Heating and Cooling of Buildings, Charlottesville, VA, USA, 16–18 April 1975; pp. 17–23. [Google Scholar]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.D.; Kousksou, T. Conductivity particles dispersed organic and inorganic phase change materials for solar energy storage–an energy-based comparative evaluation. Energy Procedia 2012, 14, 643–648. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Novel low melting point quaternary eutectic system for solar thermal energy storage. Appl. Energy 2013, 102, 1422–1429. [Google Scholar] [CrossRef]
- Trisnadewi, T.; Putra, N. Phase change material (PCM) with shaped stabilized method for thermal energy storage: A review. AIP Conf. Proc. 2020, 2255, 030065. [Google Scholar]
- Jelle, B.P.; Kalnæs, S.E. Phase change materials for application in energy-efficient buildings. In Cost-Effective Energy Efficient Building Retrofitting; Woodhead Publishing: Sawston, UK, 2017; pp. 57–118. [Google Scholar]
- Su, J.; Wang, L.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly (mela-mine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.; Youngblood, J.P. Synthesis and Characterization of Microencapsulated Phase Change Materials with Poly(urea−urethane) Shells Containing Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Munusamy, Y.; Shanmugam, S.; Shi-Ying, K. Development of form stable Poly(methyl methacrylate) (PMMA) coated thermal phase change material for solar water heater applications. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012008. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Yang, Y.; Zhao, L.; Song, G.; Tang, G. Fabrication and characterization of microcapsulated phase change materials with an additional function of thermochromic performance. Sol. Energy 2016, 139, 591–598. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization, and thermal properties of PMMA/n-heptadecane micro-capsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Sudhakar, K.; Reddy, N.N.; Jayaramudu, T.; Jayaramudu, J.; Reddy, A.B.; Manjula, B.; Sadiku, E.R. Aerogels and foamed nanostructured polymer blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 75–99. [Google Scholar]
- Dincer, I. (Ed.) Comprehensive Energy Systems; Elsevier: Amsterdam, NX, Netherlands, 2018. [Google Scholar]
- Safari, A.; Saidur, R.; Sulaiman, F.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Rahman, A.; Dickinson, M.E.; Farid, M.M. Microencapsulation of a PCM through membrane emulsification and nano compression-based determination of microcapsule strength. Mater. Renew. Sustain. Energy 2012, 1, 4. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nano encapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Preparation and characteristics of microencapsulated palmitic acid with TiO2 shell as shape-stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2014, 123, 183–188. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Yu, Y.-H.; Jia, L.; Mannan, M.S.; Chen, Y.; Cheng, Z. Nano-encapsulated PCM via Pickering Emulsification. Sci. Rep. 2015, 5, 13357. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wei, W.-L.; Hsu, K.-Y.; Ho, W.-H. Thermal stability of epoxy-silica hybrid materials by thermogravimetric analysis. Thermochim. Acta 2003, 412, 139–147. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Tang, F.; Liu, L.; Alva, G.; Jia, Y.; Fang, G. Synthesis and properties of microencapsulated octadecane with silica shell as shape–stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 160, 1–6. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Fabrication and performances of mi-croencapsulated palmitic acid with enhanced thermal properties. Energy Fuels 2015, 29, 1010–1018. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Synthesis and characterization of microencapsulated paraffin with titanium dioxide shell as shape-stabilized thermal energy storage materials in buildings. Energy Build. 2014, 72, 31–37. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Huang, X.; Gao, H.; Wang, G. Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity. Appl. Energy 2018, 211, 1203–1215. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Casini, M. Smart Buildings-Advanced Materials and Nanotechnology to Improve Energy-Efficiency and Environment Performance; Woodhead Publishing: Sawston, UK, 2016; pp. 107–125. [Google Scholar]
- Mert, M.S.; Mert, H.H.; Sert, M. Microencapsulated oleic–capric acid/hexadecane mixture as phase change material for thermal energy storage. J. Therm. Anal. Calorim. 2019, 136, 1551–1561. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Effect of carbon nano tube interfacial geometry on thermal transport in solid-liquid phase change materials. Appl. Energy 2015, 154, 271–276. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Rodrigues, F. Literature review on the use of phase change materials in glazing and shading solutions. Renew. Sustain. Energy Rev. 2016, 53, 515–535. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Kamali, S. Review of free cooling system using phase change material for building. Energy Build. 2014, 80, 131–136. [Google Scholar] [CrossRef]
- Zhai, X.Q.; Wang, X.L.; Wang, T.; Wang, R.Z. A review on phase change cold storage in the air-conditioning system: Materials and applications. Renew. Sustain. Energy Rev. 2013, 22, 108–120. [Google Scholar] [CrossRef]
- Riffat, S.; Mempouo, B.; Fang, W. Phase change material developments: A review. Int. J. Ambient. Energy 2015, 36, 102–115. [Google Scholar] [CrossRef]
- Thenge, R.R.; Chandak, M.P.; Adhao, V.S. Biosphere a novel drug delivery system. Int. J. Pharm. Technol. 2020, 16, 5. [Google Scholar]
- Hunger, H.; Emtrop, A.G.; Mandilaras, I.; Browers, H.J.H.; Founti, M. The behaviour of self-compacting concrete containing mi-cro-encapsulated Phase Change Materials. Cem. Concr. Compos. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Fan, T.-L.; Chen, M.-M.; Zhao, F.-Q. The Preparation of Phase Change Energy Storage Ceramsite from Waste Autoclaved Aerated Concrete. Procedia Environ. Sci. 2016, 31, 227–231. [Google Scholar] [CrossRef]
- Narain, J.; Jin, W.; Ghandehari, M.; Wilke, E.; Shukla, N.; Berardi, U.; El-Korchi, T.; Van Dessel, S. Design and Application of Concrete Tiles Enhanced with Microencapsulated Phase-Change Material. J. Archit. Eng. 2016, 22, 05015003. [Google Scholar] [CrossRef]
- Cao, V.D.; Bui, T.Q.; Kjøniksen, A.-L. Thermal analysis of multi-layer walls containing geopolymer concrete and phase change materials for building applications. Energy 2019, 186, 115792. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Valentini, L.; Salvioni, D.; Magistri, M.; Pamies, R.; Kjøniksen, A.L. Mechanical properties and micro-scale changes of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change material. Cem. Concr. Res. 2017, 100, 341–349. [Google Scholar] [CrossRef]
- Pilehvar, S.; Szczotok, A.M.; Rodríguez, J.F.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A.L. Effect of freeze-thaw cycles on the mechanical behaviour of geopolymer concrete and portland cement concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 2019, 200, 94–103. [Google Scholar] [CrossRef]
- Whiffen, T.R.; Riffat, S.B. A review of PCM technology for thermal energy storage in the built environment: Part I. Int. J. Low-Carbon Technol. 2013, 8, 147–158. [Google Scholar] [CrossRef]
- Suhai, Q.A.; Kaur, E.P.; Goyal, E.R. Effect of phase change materials (PCM’s) on recycled aggregate concrete. Int. J. Eng. Res. Technol. 2020, 9, 939–944. [Google Scholar]
- Cunha, S.; Aguiar, J.; Ferreira, V.; Tadeu, A. Mortars based in different binders with incorporation of phase-change materials: Physical and mechanical properties. Eur. J. Environ. Civ. Eng. 2015, 19, 1216–1233. [Google Scholar] [CrossRef]
- Uppal, T. Pacific University Analyses of Zero Energy Building Build by PCM RUBITHERM 21 Material. Int. J. Eng. Res. 2017, 6, 498–501. [Google Scholar]
- Nicolalde, J.F.; Cabrera, M.; Martínez-Gómez, J.; Salazar, R.B.; Reyes, E. Selection of a PCM for a Vehicle’s Rooftop by Multicriteria Decision Methods and Simulation. Appl. Sci. 2021, 11, 6359. [Google Scholar] [CrossRef]
- Müller, L.; Rubio-Pérez, G.; Bach, A.; Muñoz-Rujas, N.; Aguilar, F.; Worlitschek, J. Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials 2020, 13, 4486. [Google Scholar] [CrossRef]
- Norvell, C.; Sailor, D.J.; Dusicka, P. The effect of microencapsulated phase-change material on the compressive strength of structural concrete. J. Green Build. 2013, 8, 116–124. [Google Scholar] [CrossRef]
- Roberz, F.; Loonen, R.C.G.M.; Hoes, P.; Hensen, J.L.M. Ultra-lightweight concrete: Energy and comfort performance evaluation in relation to buildings with low and high thermal mass. Energy Build. 2018, 138, 432–442. [Google Scholar] [CrossRef]
- Mohseni, E.; Tang, W.; Khayat, K.H.; Cui, H. Thermal performance and corrosion resistance of structural—Functional concrete made with inorganic PCM. Constr. Build. Mater. 2020, 249, 118768. [Google Scholar] [CrossRef]
- Sukontasukkul, P.; Sangpet, T.; Newlands, M.; Yoo, D.Y.; Tangchirapat, W.; Limkatanyu, S.; Chindaprasirt, P. Thermal storage properties of light weight concrete incorporating phase change materials with different fusion points in hybrid form for high temperature applications. Heliyon 2020, 6, 04863. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Chen, C.; Mahkamov, K.; Han, F.; Zhao, C.; Lin, J.; Jiang, L.; Li, Y. Selection of a phase change material and its thickness for application in walls of buildings for solar-assisted steam curing of precast concrete. Renew. Energy 2020, 150, 808–820. [Google Scholar] [CrossRef]
- Kim, H.G.; Qudoos, A.; Jeon, I.K.; Woo, B.H.; Ryou, J.S. Assessment of PCM/SiC—Based composite aggregate in concrete: Energy storage performance. Constr. Build. Mater. 2020, 258, 119637. [Google Scholar] [CrossRef]
- Pisello, A.L.; D’alessandro, A.; Fabiani, C.; Fiorelli, A.P.; Ubertini, F.; Cabeza, L.F.; Materazzi, A.L.; Cotana, F. Multifunctional Analysis of Innovative PCM-filled Concretes. Energy Procedia 2017, 111, 81–90. [Google Scholar] [CrossRef]
- Hichem, N.; Noureddine, S.; Nadia, S.; Djamila, D. Experimental and Numerical Study of a Usual Brick Filled with PCM to Improve the Thermal Inertia of Buildings. Energy Procedia 2013, 36, 766–775. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Thermal Energy Storage Enhancement of Lightweight Cement Mortars with the Application of Phase Change Materials. Procedia Eng. 2017, 180, 1170–1177. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.L. Micro-encapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Carmona, M.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A.-L. Physical and mechanical properties of fly ash and slag geopolymer concrete containing different types of micro-encapsulated phase change materials. Constr. Build. Mater. 2018, 173, 28–39. [Google Scholar] [CrossRef]
- Stropnik, R.; Koželj, R.; Zavrl, E.; Stritih, U. Improved thermal energy storage for nearly zero energy buildings with PCM integration. Sol. Energy 2019, 190, 420–426. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M.A. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef]
- Jangeldinov, B.; Memon, S.A.; Kim, J.; Kabdrakhmanova, M. Evaluating the Energy Efficiency of PCM-Integrated Lightweight Steel-Framed Building in Eight Different Cities of Warm Summer Humid Continental Climate. Adv. Mater. Sci. Eng. 2020, 2020, 4381495. [Google Scholar] [CrossRef]
- Mazo, J.; Delgado, M.; Marin, J.M.; Zalba, B. Modeling a radiant floor system with Phase Change Material (PCM) integrated into a building simulation tool: Analysis of a case study of a floor heating system coupled to a heat pump. Energy Build. 2012, 47, 458–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Gero, J.S.; D’Cruz, N.; Radford, A.D. Energy in context: A multi criteria model for building design. Build. Environ. 1983, 18, 99–107. [Google Scholar] [CrossRef]
- Omrany, H.; Ghaffarianhoseini, A.; Raahemifar, K.; Tookey, J. Application of passive wall systems for improving the energy efficiency in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 62, 1252–1269. [Google Scholar] [CrossRef]
- Abbassi, F.; Dimassi, N.; Dehmani, L. Energetic study of a Trombe wall system under different Tunisian building configurations. Energy Build. 2014, 80, 302–308. [Google Scholar] [CrossRef]
- Odunfa, K.M.; Ojo, T.O.; Odunfa, V.O.; Ohunakin, O.S. Energy Efficiency in Building: Case of Buildings at the University of Ibadan, Nigeria. J. Build. Constr. Plan. Res. 2015, 3, 18–26. [Google Scholar] [CrossRef]
- Raof, B.Y. The correlation between building shape and building energy performance. Int. J. Adv. Res. 2017, 5, 552–561. [Google Scholar] [CrossRef]
- Laaouatni, A.; Martaj, N.; Bennacer, R.; El Omari, M.; El Ganaoui, M. Phase change materials for improving the building thermal inertia. Energy Procedia 2017, 139, 744–749. [Google Scholar] [CrossRef]
- Hachem-Vermette, C. Multistory building envelope: Creative design and enhanced performance. Sol. Energy 2018, 159, 710–721. [Google Scholar] [CrossRef]
- Sozer, H. Improving energy efficiency through the design of the building envelope. Build. Environ. 2010, 45, 2581–2593. [Google Scholar] [CrossRef]
- Hemsath, T.L. Conceptual energy modeling for architecture, planning and design: Impact of using building performance sim-ulation in early design stages. In Proceedings of the BS 2013 13th Conference of International Building Performance Simulation Association, Chambery, France, 26–28 August 2013; pp. 376–384. [Google Scholar]
- Wei, Z.; Falzone, G.; Wang, B.; Thiele, A.; Puerta-Falla, G.; Pilon, L.; Neithalath, N.; Sant, G. The durability of cementitious composites containing microencapsulated phase change materials. Cem. Concr. Compos. 2018, 81, 66–76. [Google Scholar] [CrossRef]
- Genovese, A.; Amarasinghe, G.; Glewis, M.; Mainwaring, D.; Shanks, R.A. Crystallisation, melting, recrystallisation and polymorphism of n-eicosane for application as a phase change material. Thermochim. Acta 2006, 443, 235–244. [Google Scholar] [CrossRef]
- Schultmann, F.; Sunke, N. Sustainable management of construction projects. In Proceedings of the CIB World Building Congress Construction and Development, Cape Town, South Africa, 14–17 May 2007; pp. 2428–2440. [Google Scholar]
- Liu, Z.; Yu, Z.; Yang, T.; Qin, D.; Li, S.; Zhang, G.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, S.; Chen, L.; Hou, W. Preparation and characterization of polyurea microencapsulated phase change material by interfacial polycondensation method. Powder Technol. 2016, 292, 217–222. [Google Scholar] [CrossRef]
- Ng, D.Q.; Tseng, Y.L.; Shih, Y.F.; Lian, H.Y.; Yu, Y.H. Synthesis of novel phase change material microcapsule and its application. Polymer 2017, 133, 250–262. [Google Scholar] [CrossRef]
- Fu, Z.; Su, L.; Li, J.; Yang, R.; Zhang, Z.; Liu, M.; Li, J.; Li, B. Elastic silicone encapsulation of n-hexadecyl bromide by microfluidic approach as novel microencapsulated phase change materials. Thermochim. Acta 2014, 590, 24–29. [Google Scholar] [CrossRef]
- Lone, S.; Lee, H.M.; Kim, G.M.; Koh, W.-G.; Cheong, I.W. Facile and highly efficient microencapsulation of a phase change material using tubular microfluidics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 61–67. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Wang, T.; Tian, Z.; Liao, Z. A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite. Materials 2020, 13, 5368. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, P.; Bian, L.; He, H. Enhanced thermal conductivity of palmitic acid/mullite phase change composite with graphite powder for thermal energy storage. Renew. Energy 2019, 138, 833–841. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-mortar based construction materials for energy efficient buildings: A review on research trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Amaral, C.; Vicente, R.; Marques, P.; Barros-Timmons, A. Phase change materials and carbon nanostructures for thermal energy storage: A literature review. Renew. Sustain. Energy Rev. 2017, 79, 1212–1228. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Abokersh, M.H.; Osman, M.; El-Baz, O.; El-Morsi, M.; Sharaf, O. Review of the phase change material (PCM) usage for solar domestic water heating systems (SDWHS). Int. J. Energy Res. 2017, 42, 329–357. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review of current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Saffari, M.; de Gracia, A.; Ushak, S.; Cabeza, L.F. Passive cooling of buildings with phase change materials using whole-building energy simulation tools: A review. Renew. Sustain. Energy Rev. 2017, 80, 1239–1255. [Google Scholar] [CrossRef]
- Fokaides, P.A.; Kylili, A.; Kalogirou, S.A. Phase change materials (PCMs) integrated into transparent building elements: A review. Mater. Renew. Sustain. Energy 2015, 4, 6. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Passive thermal control in residential buildings using phase change materials. Renew. Sustain. Energy Rev. 2016, 55, 371–398. [Google Scholar] [CrossRef]
- Cao, L.; Su, D.; Tang, Y.; Fang, G.; Tang, F. Properties evaluation and applications of thermal energy storage materials in buildings. Renew. Sustain. Energy Rev. 2015, 48, 500–522. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khodadadi, J. Melting and convection of phase change materials in different shape containers: A review. Renew. Sustain. Energy Rev. 2015, 43, 449–477. [Google Scholar] [CrossRef]





| S. No. | Organic PCM Type | Melting Temperature (°C) | Thermal Conductivity | Specific Heat Capacity | Melting Heat (J/kg K) |
|---|---|---|---|---|---|
| W/(m.k) | (KJ/mol) | ||||
| 1 | 1-Dodecanol | 26 | 0.172 | 298.15 | 200 |
| 2 | Butyl stearate | 19 | 0.15 | 2.1 | 140 |
| 3 | Dimethyl sebacate | 21 | 0.15 | 2.1 | 120–135 |
| 4 | Erythritol palmitate | 21.9 | 0.73 | 2.7 | 201 |
| 5 | Glycerine | 18 | 0.285 | 2.43 | 198.7 |
| 6 | Heptadecane | 20.8–21.7 | 0.14 | 534.34 | 171–172 |
| 7 | Hexadecane | 18.1 | 0.14 | 501.6 | 236 |
| 8 | Lactic acid | 26 | 0.13 | 1800 | 184 |
| 9 | Lithium chloride ethanolate | 21 | 0.84 | 59.31 | 188 |
| 10 | Octadecyl 3-mencaptopropylate | 21 | 1 | 1100 | 143 |
| 11 | Octadecyl thioglycolate | 26 | 1 | - | 90 |
| 12 | Paraffin C16–C18 | 20–22 | 0.24 | 2.1 | 152 |
| 13 | Paraffin C17 | 21.7 | 0.24 | 2.1 | 213 |
| 14 | Paraffin C18 | 28 | 0.24 | 2.1 | 244 |
| 15 | Polyglycerol | 22 | - | 2.5 | 127.2 |
| 16 | Propyl palmitate | 19 | 3.38 | 3.6 | 186 |
| S. No. | Inorganic PCM | Melting Temperature (°C) | Thermal Conductivity | Specific Heat Capacity (KJ/mol) | Melting Heat (J/kg K) |
|---|---|---|---|---|---|
| W/(m.k) | |||||
| 1 | Na2HPO4·12H2O | 36–36.4 | 0.121 | 260.9 | 146.9–147 |
| 2 | Zn(NO3)2·6H2O | 35–36 | - | 286 | 265–281 |
| 3 | Na2CO3·10H2O | 32–36 | 0.6 | 112.3 | 246.5–247 |
| 4 | Na2SO4·10H2O | 31–32.4 | 0.547 | 181.18 | 251–254 |
| 5 | FeCl3·6H2O | 37 | - | 140 | 223 |
| 6 | CaBr2·6H2O | 34 | 0.382 | 75 | 115 |
| 7 | LiBr2·2H2O | 34 | - | 2.151 | 124 |
| 8 | Na2SO4·3H2O | 32 | - | 181.18 | 251 |
| 9 | CaCl2·6H2O | 30 | 0.382 | 172.92 | 192 |
| 10 | LiNO3·3H2O | 30 | 0.56 | 64 | 296 |
| 11 | CaCl2·12H2O | 29.8 | 0.54 | 3.28 | 174 |
| 12 | Mn(NO3)2·6H2O | 25.8 | - | 286 | 125.9 |
| 13 | FeBr3·6H2O | 21 | - | 140 | 105 |
| 14 | KF·4H2O | 18.5 | - | 66.55 | 231 |
| S. No. | Eutectic PCM Type | Melting Temperature (°C) | Melting Heat (J/kg K) |
|---|---|---|---|
| 1 | Oleic acid | 5.14 | 104 |
| 2 | Myristic acid | 17 | 131.7 |
| 3 | Capric acid | 19.83 | 154.1 |
| 4 | Lauric acid | 20.75 | 134 |
| 5 | Palmitic acid | 36.79 | 159 |
| S. No. | Methods of Incorporation |
|---|---|
| 1. | Direct incorporation |
| 2. | Immersion |
| 3. | Encapsulation 3.1 Microencapsulated PCMs 3.2 Macroencapsulated PCMs |
| 4. | Coacervation—phase separation process |
| Ref. No. | Building Element | PCM Type/Method | Country | Findings |
|---|---|---|---|---|
| [116] | Hollow bricks | Paraffin wax/microencapsulation | Algeria | Paraffin-based brick resulted in a 3.8-degree Celsius decrease in inner wall temperature. |
| [117] | Hollow bricks | Paraffin/direct impregnation | China | Heat flow has been reduced from 38.7 to 35.2 W/m2 to a new range of 19.2 to 26.1 W/m2. |
| [62] | Precast concrete wall | Paraffin wax/direct impregnation and steam curing | Beijing | The PCM (50 mm thickness) underwent a phase transformation at temperatures ranging from 37.4 to 43.5 °C. When the PCM was activated, temperature reduction was noted as 2.7 °C. |
| [59] | Concrete block | Paraffin/microencapsulation | India | A 30 cm × 30 cm concrete block with a 2 cm thickness reduced the maximum air temperature difference to 3 °C. |
| [118] | Window | Paraffin wax | Norway | PCM reduced infrared and UV radiation with good visibility. |
| [119] | Wall | Beeswax, graphene/ultrasonic method | Malaysia | The prepared sample’s thermal conductivity increased to 2.89 W/m-K, and the reduction in the rate of heat transfer of the sample (0.3% weight) increased by 12%. |
| [120] | Panel | Paraffin/heat and pressure impregnation | Thailand | A 100 mm burnt-clay panel was able to enhance the thermal insulation properties. |
| [121] | Panel | Paraffin/macroencapsulation | Australia | Hollow steel ball panels reduced the indoor temperature compared to standard concrete panels by 3–6%. |
| [122] | Tiles | Paraffin/direct impregnation | New York | The thermal storage ability of a 3.8 cm dense concrete tile with 13.5% PCM was equal to that of a 5.9 cm thick concrete tile. |
| [123] | Slab | Paraffin/macroencapsulation | Germany | Even distribution and large heat transfer surface. |
| [124] | PCM mortar | Microencapsulation | Portugal | The interiors’ temperature dropped. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaraj, D.; Senthilkumar, S.R.R.; Ramalingam, M.; Vanaraj, R.; Kim, S.-C.; Prabakaran, M.; Kim, I.-S. A Review on the Effective Utilization of Organic Phase Change Materials for Energy Efficiency in Buildings. Sustainability 2024, 16, 9317. https://doi.org/10.3390/su16219317
Kamaraj D, Senthilkumar SRR, Ramalingam M, Vanaraj R, Kim S-C, Prabakaran M, Kim I-S. A Review on the Effective Utilization of Organic Phase Change Materials for Energy Efficiency in Buildings. Sustainability. 2024; 16(21):9317. https://doi.org/10.3390/su16219317
Chicago/Turabian StyleKamaraj, Dhivya, Sellamuthu Ramachandran Rajagopal Senthilkumar, Malathy Ramalingam, Ramkumar Vanaraj, Seong-Cheol Kim, Mayakrishnan Prabakaran, and Ick-Soo Kim. 2024. "A Review on the Effective Utilization of Organic Phase Change Materials for Energy Efficiency in Buildings" Sustainability 16, no. 21: 9317. https://doi.org/10.3390/su16219317
APA StyleKamaraj, D., Senthilkumar, S. R. R., Ramalingam, M., Vanaraj, R., Kim, S.-C., Prabakaran, M., & Kim, I.-S. (2024). A Review on the Effective Utilization of Organic Phase Change Materials for Energy Efficiency in Buildings. Sustainability, 16(21), 9317. https://doi.org/10.3390/su16219317











