Abstract
Organic farming encourages soil management practices that can improve soil health and fertility by increasing soil organic matter inputs and system sustainability. This study evaluated the effect of three years of continuous organic farming and intercropping orchard treatments on soil microbial diversity, microbial enumeration, respiration, soil fertility and fruit yields. Organic management resulted in higher soil organic matter content, Olsen P, and water holding capacity, but did not affect soil pH, electrical conductivity (EC), K, or Na levels. Growth parameters measured on all fruit trees were not significantly different among treatments. The enumeration of bacteria was significantly higher in organic plots when compared to conventionally managed plots. Soil respiration and substrate-induced respiration were significantly higher in the organic diverse plots in comparison to both conventional systems. The genomic analysis of prokaryotes (16S rRNA) and eukaryotes/fungi (ITS) revealed a significantly higher number of taxa, Shannon H index, and Equitability index in the organic systems for both prokaryotes and eukaryotes, in comparison to conventional farming, all of which are indicators of system sustainability. The relative abundance of Operational Taxonomic Units (OTUs) previously reported as diazotrophs, denitrifiers, or involved in the sulfur cycle, as well as Arbuscular Mychorrizae Fungi (AMF)/glomeromycotan, were highest in the organically managed soils than in the conventional plots. A multivariate correlation network clustering revealed that the microbial communities within the organic and conventional soils had strong dissimilarities regarding soil microbial niches. Our work provides evidence that organic management can be used for increasing soil microbial diversity and soil health, leading to higher levels of sustainability in fruit orchard systems.
1. Introduction
Long-term cropping systems studies have made a significant contribution to our understanding of organic agriculture, especially regarding the impact of organic farming soil management on nutrient and carbon cycling [1], yields and pest management [2]. In this regard, the recent literature has emphasized the need to use a systems approach to understand organic farming’s environmental, human and economic aspects compared to conventional farming systems [3,4,5,6].
A number of related studies have also looked at the relationship between soil properties and microbial communities. A meta-analysis of organic farming systems comparisons from both field and greenhouse trials ranging from 3 years to 100 years, in four climatic zones, found that the organic systems had increased microbial biomass carbon by up to 41%, total nitrogen by 51%, and showed increased activities of protease, urease and dehydrogenases as compared to conventional farming systems [7]. Interestingly, the microbial metabolic quotient (qCO2) was unaffected by the type of farming system for the same meta-analysis. These authors attribute the observed results to greater rates of organic matter inputs in organic farms, including manures, composts and legume cover crops. Several other studies, both from temperate and tropical regions, have highlighted that organic systems tend to be higher in soil organic carbon content [8,9,10,11], have higher microbial biomass [12,13,14] and soil respiration rates [13,15,16].
In addition to documenting changes in soil quality, many studies are using a range of methods to quantify and identify soil microbial taxa and, when possible, their function. These methods have drastically changed over the past 20 years from culture techniques to “next-generation DNA sequencing” (NGS) [17], dramatically increasing in sophistication and ability to quantify the presence and abundance of different taxa. Drawbacks of NGS include artifacts introduced by different DNA extraction protocols, bias on PCR steps, sequencing errors [18], limited DNA libraries and difficulty of linking taxonomic assignation to function [19]. However, the reduced cost allows for more samples to be tested, and for a better representation of soil bacteria [20] and fungal [21] diversity.
Identifying “keystone species” by NGS diversity data is advantageous in understanding soil ecological systems [18]. Some studies have compared the ratios of dominant phyla within the bacterial and fungal kingdoms, as indicators of nutrient availability or other aspects of soil health, while others look at the presence or absence of plant disease or disease antagonists [22]. For example, the ratio between the number of proteobacteria and acidobacteria was suggested to be indicative of the nutritional status of the soil and would be highest in organic soils with high inputs [19]. On occasion, the soil nutrient levels in organic and conventional systems were similar, but with different chemical speciation, different bioavailability and recalcitrance. Geisseler and Scow [23] found in a review of 64 long-term trials that the effect of mineral fertilizers (as compared to non-fertilized controls) led to a 15% increase in microbial biomass and 12.8% increase in soil organic carbon levels; these results appeared to be pH-dependent. Nitrogen inputs did not significantly affect soil microbial biomass, but the change in soil pH from NH4+ addition may have inhibitory effects on the soil microbiota. However, this review did not include high organic matter treatments in its analysis which may have affected its conclusions. Organic farming and its associated higher organic matter inputs also leads to an increase in water soil holding capacity [24] and soil porosity [11], which may also be associated with soil microbial abundance and diversity. Although the available literature showed the use of a wide range of different methods for assessing the effect of organic farming on increasing soil microbial diversity, they have come to similar conclusions.
Although different farming systems in desert climates have been compared [22,25], and AMF have been documented to be abundant and diverse in these organic farming systems [26,27], there is still a knowledge gap for the following: (a) the effect of organic and conventional soil nutrient management on soil microbial diversity for perennial fruit crops in arid climates; and (b) the effect of diverse intercropped systems on soil microbial diversity and soil health. High organic matter inputs and intercropping (or crop rotation) are common in organic farming systems, and their effect on soil microbes are often integrated as the ‘organic farming’ effect. The rhizodeposition of different carbon compounds and their effect on stimulating soil microbial biomass and changing the soil microbial community composition is known to be plant-specific [28].
Our general objective was to test the effect of three cumulative years of organic farming on plant growth, soil fertility levels, and soil microbial diversity and activity. In addition, the effect of crop diversity (citrus vs. citrus/mango/banana) within both systems (conventional and organic) was of interest. We hypothesized that both components of organic farming systems in this experiment (organic matter inputs and crop diversity) will stimulate soil microbial biomass, activity and diversity, and, therefore, contribute to better soil health and long-term cropping system sustainability.
2. Materials and Methods
2.1. Site Description and Experimental Design
Experiments were conducted in a fan-cooled greenhouse at the Sultan Qaboos University Agriculture Experiment Station, Muscat, Oman at 23°36′00.3″ N, 58°10′02.3″ E. The greenhouse (dimensions 9 m × 39 m) is covered with rigid polycarbonate, with a height of 3.5 m at the side, and 5.5 m at the roof, allowing for tall crops like fruit trees to be grown. Though greenhouse fruit production is not common in Oman, this production system allowed for year-round temperature control (range of approximately 22 to 32 °C) and isolated the plots from external contaminants such as pesticides.
The original greenhouse soil was coarse gravel with small boulders. Before planting, a 30 cm topsoil (mix of 2 parts sand and 1 part silt loam) layer was brought in to create a suitable growth medium. Initial soil fertility was low, and to our knowledge, no prior soil amendments or fertilizers were used in the brought-in soil substrate. Annual sesbania (Sesbania sesban) was seeded in October 2017 to enrich the soil and add organic matter. Though Rhizobium sp. was not added, the roots were nodulated. In March 2018, the cover crop was pulled and added as mulch to the organic plots into which trees had been previously planted (December 2017). The data included in this paper were collected in 2000, three years after the experiment was established. The experiment is ongoing, with plans to publish additional data after 6 and 9 years.
Fruit tree seedlings, previously grown in mixed media in pots for 1 to 2 years, were transplanted into the plots with 2 kg of local compost added to each hole. A drip irrigation system was installed, and subsequent fertility inputs were documented (Table S1). Only organic amendments were applied to the organic farming plots (compost, fish fertilizer and potassium sulfate), and only conventional soluble fertilizer was applied to the conventional plots after transplanting.
In both organic and conventional farming plots, macronutrient inputs were calculated for approximate equivalency, and USDA recommended rates were used as a guide. However, organic fertilizer sources are slow-release (mineralization) and contain micronutrients. The total nitrogen added was higher in the organic plots to compensate for the lower efficiency of compost as compared to the soluble N-fertilizer used in the conventional plots (588 and 358 g per tree over three years for organic and conventional, respectively). Phosphorus was applied at 306 and 292 g P2O5 per tree, while potassium was applied at 574 and 430 g K2O per tree, respectively.
Regarding the carbon inputs, sesbania mulch (year 1 only) and banana leaf mulch (after each harvest, unquantified) were applied to the organic plots annually, while the conventional plots remained as bare soil. All plots received 1.6 kg of compost per tree at the time of planting, and then the organic plots received an additional 27.5 kg of compost per tree over the first 3 years, while the conventional plots received no additional carbon. In summary, the carbon budget in Table S1 showed that organic plots received 15.7 kg of carbon, and the conventional plots only 0.9 kg of carbon at the start of the experiment. Only organic pest management practices were applied to both systems as needed to avoid cross-contamination, including Neem Oil for scale insects on mango, and ‘Spinosad’ for leaf miners on citrus.
The experimental design included two organic treatments. One was planted with four citrus trees, the “monoculture,” with a mix of varieties that included grapefruit, orange, lime and lemon, and the other being “diverse” with one citrus, two mangoes (var. ‘Saffron’ and ‘Ros’) and one banana (3–5 leads of local var. ‘Nagal’). Two conventional treatments with the same species mix were also planted, and each of the four treatments was replicated three times in a randomized complete block design. The plot size was 4 m × 4 m, with 2 m between trees allowing for root intermingling.
Trees were measured annually for height, stem diameter (50 cm above the ground, or above the site of the graft) and rated for vigor and pest pressure. The banana harvest began in December 2018, with the weight and number in each bunch continuously recorded, since bananas fruit year-round. Soil samples (0–20 cm) were collected annually in October for soil fertility analysis from a composite sample of 4 subsamples per plot. The pH and EC were determined in 1:2 soil to distilled water. The same soil water extract was used for sodium and potassium quantification using Horiba hand-held sensors and available phosphorus extracts were determined colormetrically using the Olsen method [29]. Soil organic matter content was estimated by the loss of mass during ignition in a muffle furnace at 550 °C for 2 h. For the quantification of the soil water holding capacity, 30 mL of fresh soil was saturated in a funnel, and the gravimetric soil moisture content was assayed after 4 h of free drainage.
2.2. Enumeration of Culturable Heterotrophic Bacteria, Actinomycetes and Fungi
For microbial analysis, fresh soil samples were collected during March 2020, at a distance of 20–30 cm from the base of each of the four plants (at two opposing points) from each plot, at a depth of 0–5 cm. Therefore, each of the 12 plot samples corresponded to a composite of eight subsamples from within each plot.
Soil culturable bacterial, actinomycetes and fungal colony enumeration were assayed from soil matrices by suspending 10 g of freshly collected soil samples in 95 mL of saline solution (0.85% NaCl2). Samples were shaken for 10 min at 100 rpm. Each soil suspension was then subjected to five sequential 10× dilutions. Soil suspensions at increasing dilutions were plated in the following agar growth media: Peptone yeast agar (for general bacteria), Casein glycerol agar (for actinomycetes) and Rose Bengal with streptomycin (for fungi) media. Agar plates were incubated at room temperature (20 to 25 °C) for 72 h. For each sample, six analytical replicates were averaged to obtain the number of colony-forming units (CFUs) per gram of soil on a dry weight basis.
2.3. Microbial Respiration
Microbial respiration was measured using the MicroResp™ system [30]. Each well of the deep-well microplate (96 wells, 1.2 mL per well) was filled with 0.46 ± 0.02 g of fresh soil samples, with four replicate samples/wells for each soil sample and each carbon substrate. The gravimetric moisture content for each soil sample was independently identified via oven drying at 105 °C. Before incubation, soils were moistened with either 25 µL sterile deionized water (for respiration) or a glucose solution (30 µg per well) to assess the substrate-induced respiration (SIR). A second microplate holding a CO2 detection gel (12.5 mg kg−1 cresol red, 150 mM KCl, and 2.5 mM NaHCO3 in 1% purified agar) was assembled on top of the soil microplate using an air-tight sealing system provided by the manufacturer. The system was then incubated in the dark at room temperature, and detection plates were read after 20 hrs. The absorbance of the indicator plates was measured at 570 nm using a Microplate reader before and after incubation with soils. The CO2 released from soils (%CO2) was converted to respiration rate (ug CO2-C g−1 dry soil h−1) [30].
2.4. Microbial Diversity
The DNA from the freshly collected soil samples was extracted using a PurelinkTM microbiome DNA purification kit (Thermo Fisher Scientific, Waltham, MA, USA). The DNA extracts were then sent to Molecular Research (MR-DNA, Shallowater, TX, USA) for V4 16S rRNA and ITS NGS diversity analysis. The 16S rRNA gene V4 variable region, and the ITS 1–2 PCR primers 515/806 were used with a barcode on the forward primer in a 35 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, USA) under the following conditions: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, after which a final elongation step at 72 °C for 10 min was performed. After amplification, PCR products were checked in 2% agarose gel to determine the amplification’s success and the bands’ relative intensity. Samples were purified using calibrated SPRI beads. The pooled and purified PCR products were used to prepare the Illumina DNA library. The DNA sequencing was performed at MR DNA (https://www.mrdnalab.com/, accessed on 1 June 2020, Shallowater, TX, USA) on a MiSeq following the manufacturer’s guidelines.
The sequence data derived from the sequencing process were processed using the MR DNA ribosomal and functional gene analysis pipeline and in-house built software (http://www.mrdnafreesoftware.com, accessed on 1 June 2020, https://www.mrdnalab.com/, accessed on 1 June 2020, MR DNA, Shallowater, TX). In this process, sequences were joined, depleted of barcodes and primers and then sequences with <150 bp or ambiguous base calls were removed. The sequences were filtered for quality using a maximum expected error threshold of 1.0 and dereplicated. Sequences were then denoised, and operational taxonomic units were defined as clustering at 3% divergence (97% similarity) followed by removal of singleton sequences and chimaeras. The final OTUs were taxonomically classified using BLASTn against a curated database derived from NCBI (www.ncbi.nlm.nih.gov, accessed on 1 June 2020).
2.5. Statistical Analyses
Data calculations, manipulation, average, standard deviation and correlation analysis were performed using Microsoft Office Excel 2016. Analysis of variance was performed using Minitab (version 20.0), and the Fisher test was used for mean separation. The Krona Excel template [31] was used to construct HTML hierarchical microbial diversity graphics to visualize and summarize differences in microbial community composition within the Prokaryotes and Eurkarotes. The PAST4 software (version 4.15) [32] was used to calculate the Shannon-H and Equitability microbial diversity indices. Graphia 2.0 software was used for correlation network analysis of OTUs, with Pearson’s correlation coefficients above 0.95 [33], clustered by Markov Cluster Algorithm (MCL, granularity 1.1). The taxa of known functional microbial groups, such as mycorrhiza, diazotrophs, nitrifiers and denitrifiers, were grouped to represent the effect of the treatments on soil functional microbial groups.
3. Results
3.1. Soil Physicochemical Properties, Plant Growth and Yield
Though different levels of total N and C had been added to the experimental plots over the three years of the experiment (Table S1), the results showed nonsignificant differences in the soil pH, concentration of K and Na (Table 1). Soil salinity (EC) was also unaffected by our treatments leading to nonsignificant changes in electrical conductivity. Soil organic matter significantly increased, more than doubling in value from 2.55% in conventional plots to 5.49% in the organic plots. Olsen P and water holding capacity (WHC) also significantly increased in the organic plots, though there was no significant difference comparing monoculture and diverse treatments.

Table 1.
Soil test data from October 2019. Letters denote means separation statistical significance using Fisher pairwise comparison.
By the end of the first three years of experimentation, the mango and citrus trees were still young and not yet fruiting, but the banana plants started producing fruit 12 months after transplanting. The total number of bunches per plant was 11 for organic and 8.3 for conventional. The number of hands per bunch was the only significantly different (p = 0.043) banana yield parameter, and was higher for conventional farming with 5.76, compared to 4.85 for organic farming plots (Table S2). However, the total weight per bunch was similar and was not statistically different between conventional and organic farming treatments. Mango and citrus stem diameter, height, and vigor ratings showed no statistically significant differences (Table S3). Due to pruning management, citrus showed a decline in height in some treatments from 2020 to 2021.
3.2. Enumeration of Culturable Heterotrophs and Soil Microbial Respiration
Of the three-culture media used for microbial enumeration, only the nutrient agar (bacteria) showed statistically significant differences, likely due to the high variability of the data (Table 2). The organic diverse plots had the highest bacteria CFU g−1 soil and was significantly higher than conventional diverse. The enumeration of actinomycetes in glycerol casein agar and fungi in Rose Bengal agar was highly variable and imprecise, preventing conclusions about the effect of organic farming and intercropping. For the culturable fungi, the highest levels were in the organic monoculture, but were not statistically different than the other treatments (Table 3).

Table 2.
Enumeration of culturable heterotrophic bacteria, actinomycetes and fungi. Soil respiration, substrate induced respiration (SIR) and metabolic quotient (MQ) estimation (based on bacteria abundance). Letters designate mean separation statistical significance by Fisher pair-wise comparison.

Table 3.
Number of taxa, sequences, Shannon H and Equitability indices for Prokaryotes (16S) and Eukaryotes (ITS). Letters designate mean separation statistical significance based on 95% confidence interval (CI).
Soil respiration rate was highest in the organic diverse plots, and significantly different from the other three treatments (Table 2). Substrate-induced respiration was also significantly higher in the diverse organic plots compared to the conventional monoculture; and the conventional-diverse and organic-monoculture plots were not significantly different from other treatments.
The metabolic quotient (qCO2) corresponds to the respiration rate per unit of soil microbial biomass. The qCO2 was highest in the conventional-diverse plots, and significantly different than the conventional-monoculture and the organic-diverse (Table 2). For this experiment, the bacteria enumeration was also lowest in the conventional-diverse treatment, explaining its high qCO2. The organic-diverse plots, on the other hand, showed the highest respiration rates, the highest culturable bacteria enumeration and the lowest qCO2.
3.3. Soil Microbial Taxonomic Diversity Using 16S and ITS Gene Sequencing
The number of OTUs, the Shannon diversity index (H) and the equitability (EH) indexes are presented for both the prokaryotes (16S rRNA V4 regions) and fungi (ITS) derived from the NGS microbial diversity analysis are presented in Table 3. Together, these indices represent the number of taxa, abundance, and the evenness of population distribution for both culturable and unculturable soil microbes. This analysis resulted in a more sensitive way to represent the differences between the treatments, with statistically significant differences among all treatments. For the prokaryotes 16S diversity (bacteria and archaea), the number of OTUs was highest in the organic-monoculture, followed by the organic-diverse plots, and the conventional-diverse treatment was the lowest (Table 3). A similar pattern was observed for the Shannon H, with the highest values in both the monoculture and diverse organic treatments, compared to the conventionally farmed plots. Species equitability was highest in the conventional monoculture plots, followed by the organic diverse plots. Overall, when analyzed together, the organic plots showed higher equitability than the conventionally farmed soils. For fungi, the highest number of OTUs were found for both the diverse and monoculture organic plots. The Shannon H and equitability indices were also higher for fungi in both organic plots and for the organic treatments overall, and these differences were highly significant.
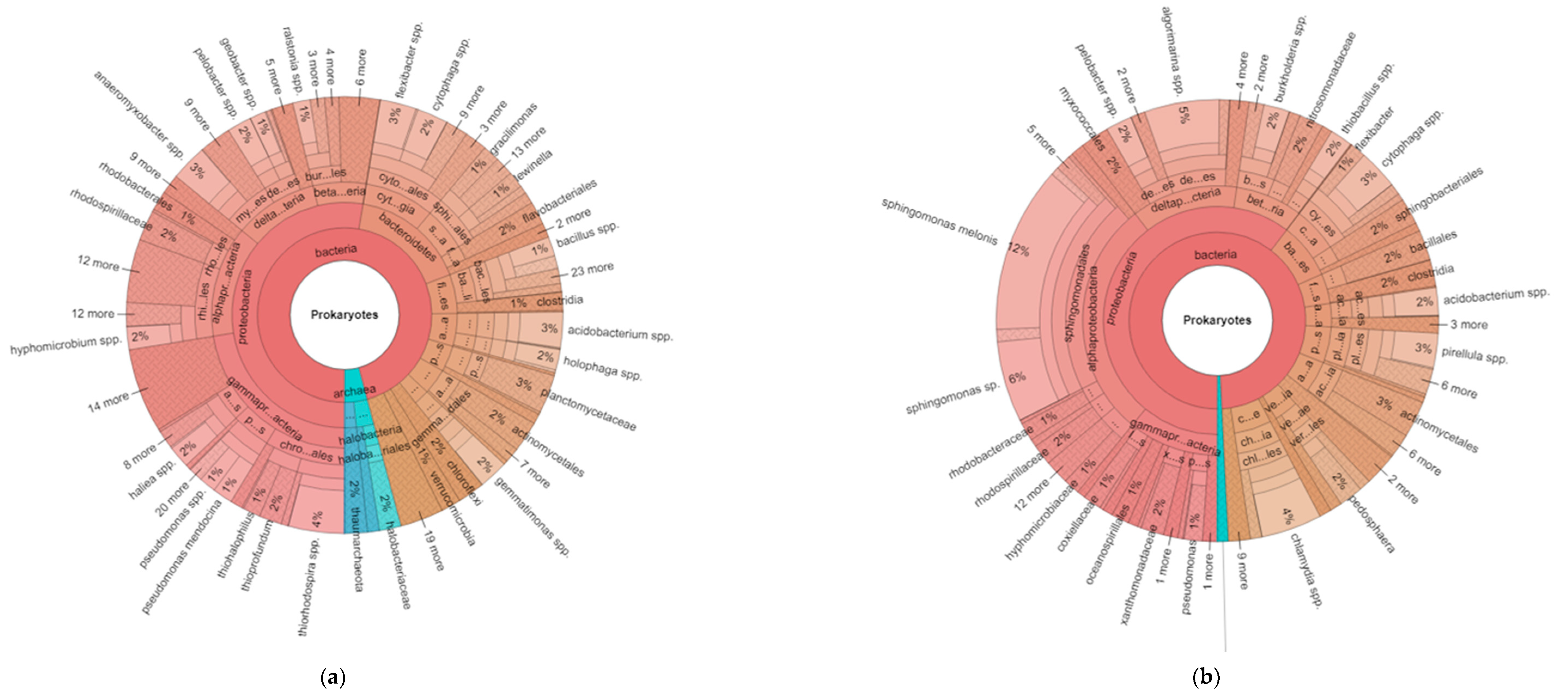
3.4. Functional Microbes and Correlation Networks
The hierarchical taxonomic abundance from kingdom to genus illustrates some of the data described above, plus reveals additional information regarding the abundance of certain specialized taxa. For example, the difference between the prokaryotes for the averaged organic and conventional systems (Figure 1a,b) show the relative proportions of bacteria and archaea, with higher archaea in the organic vs. conventional plots (4% vs. 1% of total prokaryotes, respectively). The ratio of proteobacteria to acidobacteria has been suggested as an indicator of readily available nutrients in soil [19], and was higher in the conventional plots. The proteobacteria made up 55% and 61% of the total bacteria in the organic and conventional systems, respectively, and the acidobacteria were 5% and 3% of bacterial in the two systems, respectively. Thus, the resulting ratio was 11 for the organic systems and 20.3 for the conventional.
Figure 1.
Hierarchical taxonomic abundance (from kingdom to genus) of prokaryotes based on 16S ribosomal RNA for organic (a) and conventional (b) soils (averaged across monoculture and diverse treatments). Bacteria are represented in pink-brown sections of the chart, and archaea in the blue section.
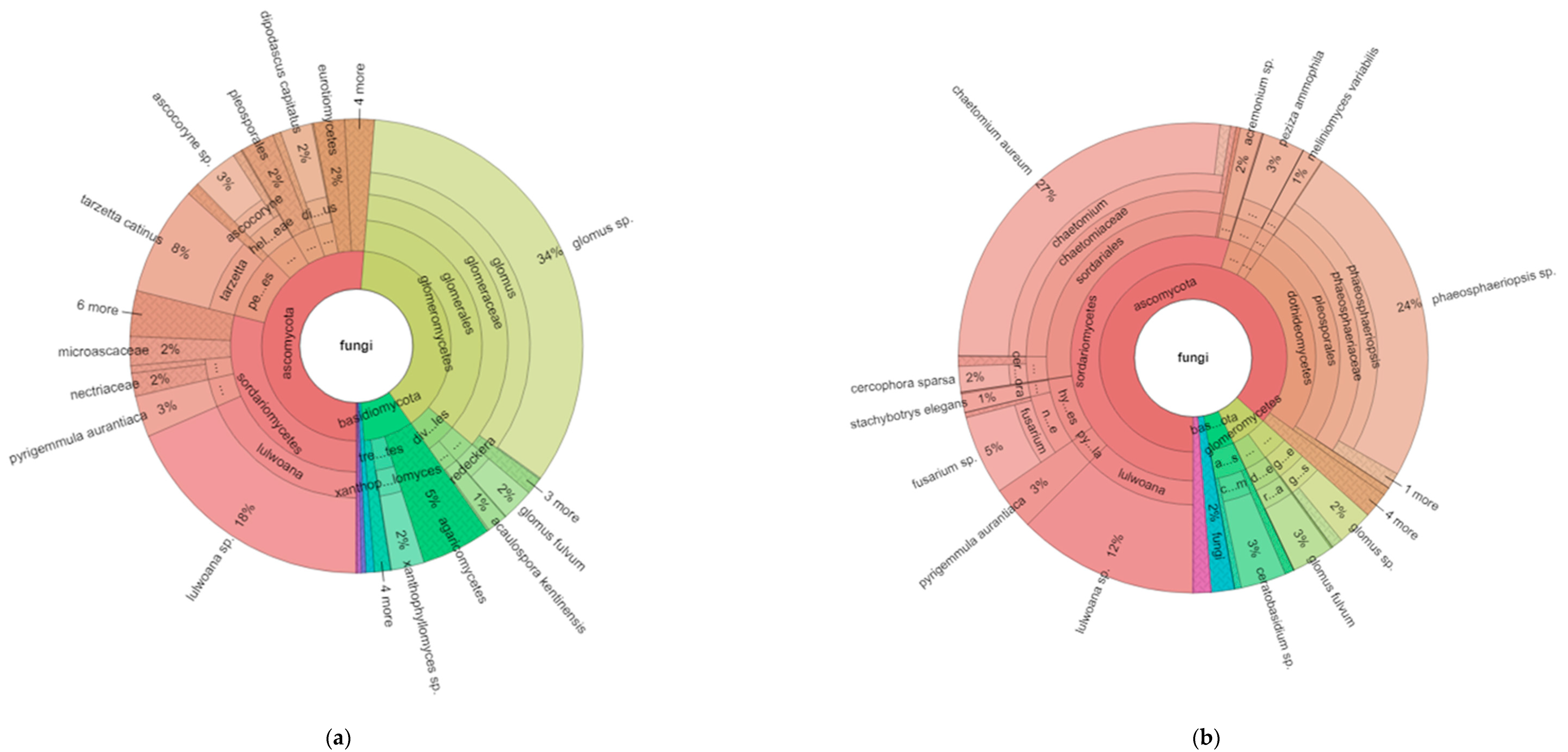
For fungi, the plot of the eukaryotes (Figure 2a,b) clearly shows that the proportion of fungi in the phyla glomeromycota are higher (39% of fungi) in organic as compared to conventional (only 6% of fungi). The relative abundance of ascomycota and basidomycota is also different, with 51% and 9% of these two phyla in organic, and 87% and 4% in the conventional, respectively, resulting in ratios of ascomycota to basidomycota of 5.9 and 20.5 for the organic and conventional systems, respectively.
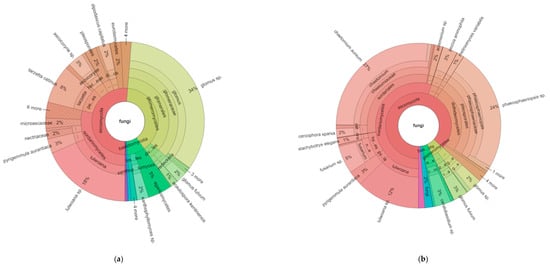
Figure 2.
Hierarchical taxonomic abundance (from kingdom to genus) of eukaryotes based on ITS ribosomal RNA for organic (a) and conventional (b) soils (averaged across monoculture and diverse treatments).
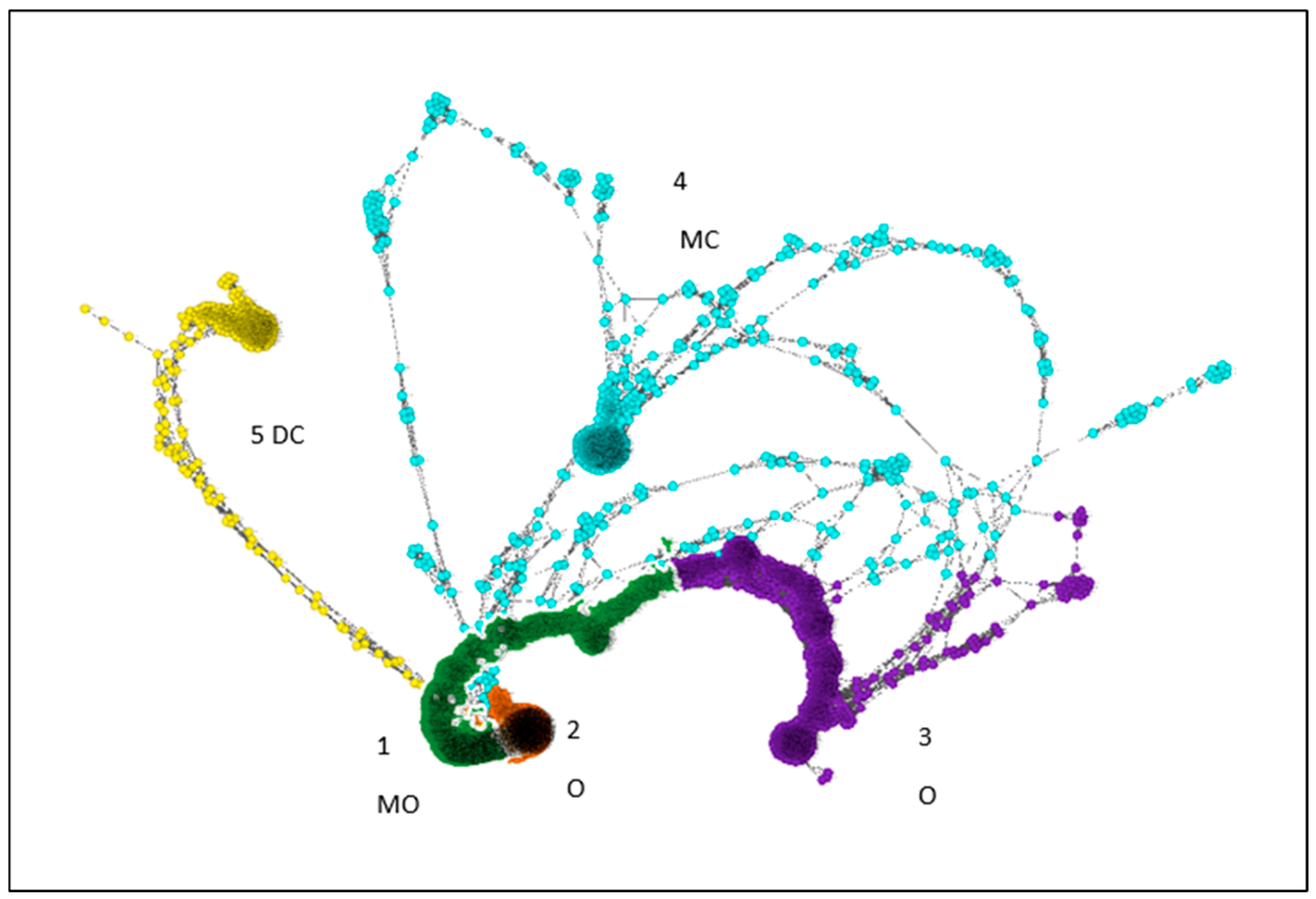
Graphia 2.0 was used to build a correlation network of OTUs (includes both prokaryotes and eukaryotes) with Pearson’s correlation coefficients above 0.95 (Figure 3). Cluster 1 is dominated by OTUs found in monoculture organic (72%), followed by the diverse organic system (15%). Cluster 2 is also dominated by monoculture organic (93%). Cluster 3 is made of 50% diverse organic OTU, followed by monoculture organic with 36%. Cluster 4 is dominated by monoculture conventional (65%) and cluster 5 by diverse conventional (77%). Thus, we see that the microbial communities/associations were different between the two conventional systems, and were also different between the conventional and the organic systems. The first two clusters show differences between organic monoculture and organic diverse cropping systems (Figure 3).
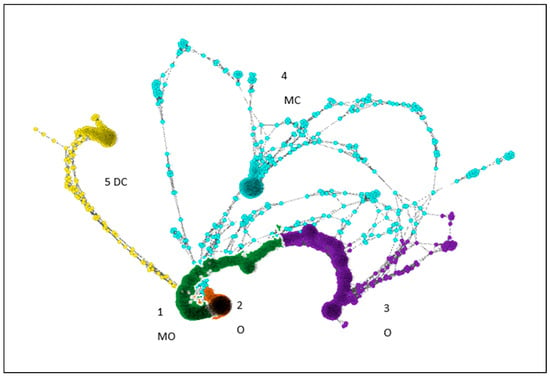
Figure 3.
Graphia 2.0 Pearson correlation network, positive correlation polarity, minimum correlation value 0.95. 1-MO: monoculture organic treatment cluster, 2-O and 3-O: organic (both monoculture and diverse) clusters, 4-MC monoculture conventional cluster, and 5-DC: diverse conventional treatment cluster.
When we look at the correlations of the clusters with specific groups of organisms, clusters 1, 2 and 3 were significantly positively correlated with archaea and bacteria, while cluster 4 was more significantly correlated with fungi. Cluster 5 was negatively correlated with archaea and bacteria, with no significant correlation with fungi (Table S5). Within the kingdom of bacteria, clusters 1, 2 and 3 were strongly and positively correlated with most of the phyla, except tenericutes, which was only positively correlated with cluster 5, thermotogae (only cluster 3), chlamydiae (only cluster 5), cyanobacteria (clusters 1, 2 and 4) and lentisphaerae (only cluster 4) (Table S5). Within the fungi, clusters 1, 2 and 3 were significantly and positively correlated with glomeromycota and cryptomycota. Cluster 4 was significantly and positively correlated with ascomycota, chytridiomycota and basidiomycota, while cluster 5 was either not correlated or negatively correlated with all phyla.
When we look at the soil data, clusters 1, 2 and 3 were significantly and positively correlated with all soil variables except pH, including the soil organic matter, water holding capacity, and the Olsen phosphorus test. However, the correlations between archaea, bacteria and fungi with the soil test data show that only the archaea and bacteria are positively correlated with organic matter, water holding capacity and phosphorus, and fungi does not show a significant correlation. Within the kingdom of fungi, the phyla of glomeromycota and cryptomycota are significantly and positively correlated with organic matter, water holding capacity and phosphorus level; the phyla of ascomycota and chytridiomycota are negatively correlated; and basidiomycota have a weak but positive correlation (Table S5).
When the taxa are sorted by name to indicate their function, the percentage of diazatrophs, denitrifiers and those involved in the sulfur cycle are highest in the organic monoculture system, followed by the organic diverse system (Table S4). The percentage classified as AMF was also higher in the two organic soils/treatments.
4. Discussion
In the present study, we observed an increase in soil organic matter under organic practices as compared to conventional farming. Our findings are consistent with previous studies that have found increases in soil organic matter in organic cropping systems receiving manures, composts, or both, in both tropical and temperate environments [8,9,10,11,14,15,16,24,34]. The organic system also resulted in an increase in soil water holding capacity compared to the conventional farming treatment. The increase in soil water holding capacity can be attributed to several factors associated with organic farming practices, including the addition of organic matter and the promotion of soil micro-organisms [11]. Organic matter improves soil structure [11,24], which enhances water infiltration and retention. Additionally, the presence of beneficial microorganisms in organic soils can enhance soil structure, leading to increased water holding capacity as was evident from the positive correlation of soil organic matter (SOM) with soil microbes in this study (Table S5). While the positive effects of organic matter on soil structure and water holding capacity are clear, our study did not find significant differences in soil EC, pH, K, and Na between organic and conventional farming systems. This finding may be due to the complex and context-specific nature of nutrient levels in soils, as they can vary depending on a variety of factors, including the specific experiment conducted, the duration of the study, the type of cropping system or rotation, and the rates of fertilizers applied [8,35,36]. However, Olsen P was higher in the organic farming compared to the conventional farming system. Organic farming systems receiving organic fertilizer often have higher P level in soils [9,37].
The organic farming system resulted in a higher abundance and diversity of soil bacteria, archaea and fungi in organic farming systems and can be attributed to the presence of organic matter inputs such as manures or composts [11], which were strongly and positively correlated with soil organic matter (Table S5). This is consistent with findings from other studies [8,11,24,34,38,39]. Additionally, the dominance of clusters 1, 2 and 3 by organic farming systems (Figure 3) also contributed to the increased abundance and diversity of soil microorganisms. However, the richness and diversity of species in some systems cannot be solely attributed to whether a system is defined as organic. Factors such as the amount of organic matter inputs and pH can also play significant role [8,10,15,40]; in some cases, soil pH has been negatively correlated with microbial abundance and diversity [8,15]. Some studies have shown different results when comparing microbial diversity in control plots with no fertility amendments, with some finding the lowest diversity in the control [11], others finding the lowest diversity in the fertilizer plot [38], and others finding no significant difference between the no fertilizer and conventional fertilizer treatments, but both being lower than the organic treatments [39].
The composition of bacterial communities in soil can be influenced by agricultural practices. Specifically, the ratio of proteobacteria and acidobacteria has been shown to differ between organic and conventional systems, with higher ratios in conventional systems [10], which is attributed to the more readily available nutrients in conventional systems promoting the rapid growth of proteobacteria, as compared to the slower growing acidobacteria [19]. In our study, the ratio was 11.2 in the organic system, and 18.2 in conventional. These results are consistent with the findings of Suyal et al. [10] in organic and conventional chickpea cropping systems in India, where ratios of 10.3 and 22.2 were observed. In contrast, cowpea and citrus cropping systems in India had ratios of 20.0 and 60.0 for organic and conventional, respectively [24], while a barley–beet crop rotation in Germany had ratios of 2.9 in rotations receiving manure or NPK fertilizer, and 2.2 where no fertilizer or manure was used [34].
Culture-based methods have limitations in detecting soil microbes due to the inability to culture a large percentage of soil microbes. In our study, only the nutrient agar medium for bacteria showed significant differences for the field treatments, likely due to the high variability of the data. This finding is consistent with the limitations of plate count methods for bacteria, fungi and actinomycetes that have been found by others [36]. The differences in results from the NGS methods and plate count methods are likely due to the extremely small percentage of soil microbes that can be cultured, as compared to those that are detected using DNA extraction/genomic methods [36,41]. Our study also found a higher respiration rate and SIR in the organic systems, which is consistent with other studies [13,15,16,36] and has been correlated with the soil organic matter levels in these studies.
In recent years, few studies have compared the farming systems to “natural” ecosystems, which are either desert, or dryland-grown wild plants in Oman. Khan et al. [42], using Next Generation Sequencing (NGS), found 121 fungal and 3662 unique bacterial OTUs in the rhizosphere of 3 native plants growing in arid conditions in Oman. In comparison, our study found 581 and 413 fungal OTUs in the organic and conventional systems, respectively. Our prokaryote OTUs were 7388 and 2453 in organic and conventional plots, respectively. The higher numbers in our study may be due to the fertility supplements and/or regular watering regime. In Egypt, a desert soil was compared to an organic vegetable farm for rhizosphere bacteria, and found higher diversity in the organic farm, with a Shannon index of 11.21 compared to 7.90 for the desert soil [22]. The study also identified more pathogen antagonists in the organic farm, but more N-fixing bacteria in the desert soil [22].
Kazerooni et al. [17] compared conventional tomato farming and desert soils in Oman, and found similar fungal diversity between the two systems, but with slightly more species in the farmed soil. The Shannon index was slightly higher in the desert soil (1.9 vs. 1.4). In another study, however, that included an organic farming system, the fungal diversity was highest for organic tomato and cucumber, intermediate for date palm and lime farmed with traditional methods that included manures, and lowest in conventional cucumber due to lower use of organic matter inputs plus the use of fungicide to control disease [43]. In experiments that compare organic and conventional farming to other ecosystems in an environment with more rainfall (West Java, Indonesia), the highest organic matter (OM) and % total N were in forests, followed by organic cropping, and lower in conventional. Microbial biomass also followed this same pattern [14].
In our experiment, the organic cropping systems showed a higher percentage of fungi in the Glomeracota, which is similar to the findings of Mullath et al. [26], who reported a higher quantity and diversity of arbuscular mycorrhizal fungi (AMF) in organic cropping systems in the UAE. The desert plants sampled in their study did not contain AMF. All fruit species used in this study are known hosts of AMF, which has been widely documented in citrus [44,45,46,47], including acid lime in Oman [48], mango [49,50] and banana [51]. The benefits of AMF root colonization on fruit tree seedlings have also been documented [47,49,52,53]. It has been reported that fertilizer application may reduce AMF [54,55], that manure and compost application can decrease root colonization by [13], or that higher levels of soil P may reduce the abundance of AMF [55] at low soil Olsen-P (10 mg kg−1); the root colonization and pi transporter genes are significantly upregulated [9]. However, other studies show the positive correlation of AMF colonization with soil properties such as organic matter and available P in citrus orchards [39,56] or no correlation with soil P content [50]. In one study, available phosphorus was negatively correlated with spore density of AMF, but positively correlated with root colonization of papaya [57]. Another study with banana found that P and soil carbon negatively affect AMF colonization, but soil carbon was positively correlated with spore abundance and species richness [51]. In our study, the P levels measured were higher in the organic systems though similar rates were applied, which did not seem to negatively impact AMF.
A study with annual cropping systems demonstrated an increase in diversity of AMF communities in more diverse polycultures as compared to monoculture [58], and it would seem likely to have the same effect in perennial systems as well. In our experiment the monoculture citrus had a higher Shannon H index and higher equitability index for both prokaryotes and eukaryotes than the diverse system that also included banana and mango. When only the glomeromycota are looked at, they were more abundant in the organic systems as compared to conventional, but also more abundant in the monoculture as compared to diverse. This finding should be followed up with more research.
The Pearson correlations and multivariate analysis in our study are consistent with previous research by Liao et al. [59] which reported higher abundance of genera associated with organic farming systems. We observed a positive correlation between archaea and bacteria with clusters 1, 2 and 3 representing organic farming systems (Figure 3), suggesting that these clusters are dominated by prokaryotic organisms, and may have similar ecological roles in the environment. It could also indicate that these clusters share similar environmental preferences or require similar resources to thrive. This positive impact on the microbial community may be attributed to the use of organic fertilizers and soil management practices in organic farming systems [11,59]. In contrast, cluster 4, representing the mono conventional farming system, showed a higher correlation with fungi, indicating a greater abundance of eukaryotic organisms in these soils. This aligns with previous studies indicating a negative impact on soil health and microbial diversity in conventional farming systems [55]. Conversely, the significant correlation between fungi and conventional farming systems suggests that these systems are dominated by eukaryotic organisms, particularly fungi, which may have different ecological roles compared to organic farming systems.
That organic farming systems support a diverse microbial community dominated by prokaryotic organisms is consistent with previous work by Liao et al. [59] who reported a higher abundance of genera associated with organic farming systems. The positive correlation with archaea and bacteria in the present study may indicate that these microorganisms play important roles in nutrient cycling and other ecosystem services in organic farming systems. This suggests that organic farming systems may have a positive impact on the microbial communities in the soil, possibly due to the use of organic fertilizers and reduced tillage practices. Cluster 4, which represents the mono conventional farming system, was more significantly correlated with fungi, indicating a higher abundance of eukaryotic organisms in these soils. This finding is consistent with previous studies that have found that conventional farming systems tend to have a negative impact on soil health and microbial diversity.
The positive correlation between archaea and bacteria with organic farming systems suggests that these clusters are dominated by prokaryotic organisms, and may have similar ecological roles in the environment. It could also indicate that these clusters share similar environmental preferences or require similar resources to thrive. On the other hand, the significant correlation between fungi and conventional farming systems suggests that these systems are dominated by eukaryotic organisms, specifically fungi, and may have a different ecological role compared to organic farming systems. Fungi are known to play important roles in nutrient cycling and decomposition, so this cluster may be more involved in these processes.
5. Conclusions
Organic farming practices can significantly improve soil health and fertility by increasing soil organic matter inputs, soil biodiversity and microbial diversity. The organic farming system resulted in higher soil organic matter content, Olsen P and water holding capacity, but did not affect soil pH, EC, K or Na levels. The enumeration of bacteria, soil respiration and substrate-induced respiration were higher in the organic and diverse plots compared to conventionally-managed and monoculture plots. The genomic analysis of prokaryotes and eukaryotes/fungi revealed a significantly higher number of taxa, Shannon H index, and Equitability index in the organic systems for both prokaryotes and eukaryotes, in comparison to conventional farming. Limitations of this study are that only the first 3 years of what is anticipated to be a longer-term study are included. The study so far shows that organic farming practices and intercropping can increase soil microbial diversity and health, which can have positive impacts on crop yields and overall ecosystem health and sustainability.
Supplementary Materials
The following supporting information can be downloaded from the following website: https://www.mdpi.com/article/10.3390/su16219391/s1, Table S1: Soil fertility inputs from the inception of the experiment to fall 2020; Table S2: Banana stem count per plant and yield from 2018 to present (August 2021); Table S3: Citrus and mango stem diameter, height, and vigor ratings for organic and conventional trts; 2020 and 2021; Table S4: Functional microbial taxa; mycorrhiza (AMF), diasotrophs, denitrifying and sulfur-cycle by number and percent of sequences; Table S5: Correlation matrix for soil fertility measurements and selected taxonomic data.
Author Contributions
Conceptualization, D.M.-B. and R.R.J.; methodology, D.M.-B. and R.R.J.; software, D.M.-B. and A.A.H.; validation, D.M.-B., A.A.H. and R.R.J.; formal analysis, A.A.H.; investigation, A.A.H.; resources, R.R.J. and D.M.-B.; data curation, D.M.-B.; writing—original draft preparation, A.A.H.; writing—review and editing, A.R., R.R.J. and D.M.-B.; visualization, D.M.-B.; supervision, D.M.-B. and R.R.J.; project administration, R.R.J.; funding acquisition, R.R.J. and D.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by His Majesty Grant Fund, grant number SR/AGR/CROP/19/02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge in-kind support from the College of Agricultural and Marine Sciences, Sultan Qaboos University, for use of laboratory and greenhouse facilities, and contributions by the support staff.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- Liebhardt, W.C.; Andrews, R.W.; Culik, M.N.; Harwood, R.R.; Janke, R.R.; Radke, J.K.; Riegerschartz, L.L. Crop production during conversion from conventional to low-input methods. Agron. J. 1989, 81, 150–159. [Google Scholar] [CrossRef]
- Barker, A.V. Science and Technology of Organic Farming; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Francis, C. (Ed.) Organic Farming: The Ecological System. Agronomy Monograph; American Society of Agronomy: Madison, WI, USA, 2009; Volume 54. [Google Scholar]
- Lockeretz, W. (Ed.) Organic Farming: An International History; CAB International Press: Wallingford, UK, 2007. [Google Scholar]
- Pacini, C.; Wossink, A.; Giesen, G.; Huirne, R.J. Ecological-economic modelling to support multi-objective policy making: A farming systems approach implemented for Tuscany. Agric. Ecosyst. Environ. Health Perspect. 2004, 102, 349–364. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Chen, X.; Henriksen, T.M.; Svensson, K.; Korsaeth, A. Long-term effects of agricultural production systems on structure and function of the soil microbial community. Appl. Soil Ecol. 2020, 147, 103387. [Google Scholar] [CrossRef]
- Ding, G.-C.; Bai, M.; Han, H.; Li, H.; Ding, X.; Yang, H.; Xu, T.; Li, J. Microbial taxonomic, nitrogen cycling and phosphorus recycling community composition during long-term organic greenhouse farming. Microbiol. Ecol. 2019, 95, fiz042. [Google Scholar] [CrossRef]
- Suyal, D.C.; Soni, R.; Singh, D.K.; Goel, R. Microbiome change of agricultural soil under organic farming practice. Biologia 2021, 76, 1315–1325. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Xiao, X.; Shi, L.; Chen, K.; Wen, L.; Li, W. Effects of short-term manure nitrogen input on soil microbial community structure and diversity in a double-cropping paddy field of southern China. Sci. Rep. 2020, 10, 13540. [Google Scholar] [CrossRef]
- Araújo, A.S.F.; Melo, W.J. Soil microbial biomass in organic farming system. Ciência Rural 2010, 40, 2419–2426. [Google Scholar] [CrossRef]
- Jannoura, R.; Bruns, C.; Joergensen, R.G. Organic fertilizer effects on pea yield, nutrient uptake, microbial root colonization and soil microbial biomass indices in organic farming systems. Eur. J. Agron. 2013, 49, 32–41. [Google Scholar] [CrossRef]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Ge, T.; Chen, X.; Yuan, H.; Li, B.; Zhu, H.; Peng, P.; Li, K.; Jones, D.L.; Wu, J. Microbial biomass, activity, and community structure in horticultural soils under conventional and organic management strategies. Eur. J. Soil Biol. 2013, 58, 122–128. [Google Scholar] [CrossRef]
- Velmourougane, K. Impact of Organic and Conventional Systems of Coffee Farming on Soil Properties and Culturable Microbial Diversity. Scientifica 2016, 2016, 3604026. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Auger, H.; Jaszczyszyn, Y.; Thermes, C. Ten years of next-generation sequencing technology. Trends Genet. 2014, 30, 418–426. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacterial found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Köberl, M.; Müller, H.; Ramadan, E.M.; Berg, G. Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS ONE 2011, 6, e24452. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Aparna, K.; Pasha, M.A.; Rao, D.L.N.; Krishnaraj, P.U. Organic amendments as ecosystem engineers: Microbial, biochemical and genomic evidence of soil health improvement in a tropical arid zone field site. Ecol. Eng. 2014, 71, 268–277. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.N.; Rethinasamy, V.; Al-Mahrouqi, H.; Al-Sadi, A.M. Fungal diversity in tomato rhizosphere soil under conventional and desert farming systems. Front. Microbiol. 2017, 8, 1462. [Google Scholar] [CrossRef] [PubMed]
- Mullath, S.K.; Błaszkowski, J.; Govindan, B.N.; Al Dhaheri, L.; Symanczik, S.; Al-Yahya’ei, M.N. Organic farming practices in a desert habitat increased the abundance, richness and diversity of arbuscular mycorrhizal fungi. Emir. J. Food Agric. 2019, 31, 969–979. [Google Scholar] [CrossRef]
- Symanczik, S.; Błaszkowski, J.; Koegel, S.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Isolation and identification of desert habituated arbuscular mycorrhizal fungi newly reported from the Arabian Peninsula. J. Arid. Land 2014, 6, 488–497. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939.
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 2001, 4, 9. [Google Scholar]
- Köhler, J.; Baumbach, J.; Taubert, J.; Specht, M.; Skusa, A.; Rüegg, A.; Rawlings, C.; Verrier, P.; Philippi, S. Graph-based analysis and visualization of experimental results with ONDEX. Bioinformatics 2006, 22, 1383–1390. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Orr, C.H.; Leifert, C.; Cooper, J.M.; Cummings, S.P. The effect of organic and conventional farm management practices on the soil microbial community at the phylum level. Asp. Appl. Biol. 2013, 120, 1–7. [Google Scholar]
- Bettiol, W.; Ghini, R.; Galvao, J.A.H.; Ligo, M.A.V.; Mineiro, J.L.C. Soil organisms in organic and conventional cropping systems. Sci. Agric. 2002, 59, 565–572. [Google Scholar] [CrossRef]
- Norgaard, A.E.; Lewis, D.; Borden, K.A.; Krzic, M.; Carrillo, J.; Smukler, S.M. Trade-offs in organic nutrient management strategies across mixed vegetable farms in Southwest British Columbia. Front. Sustain. Food Syst. 2022, 6, 706271. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Organic farming enhances the diversity and community structure of endophytic archaea and fungi in maize plant: A shotgun approach. J. Soil Sci. Plant Nutr. 2020, 20, 2587–2599. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Shamsi, I.H.; Xu, D.-T.; Wang, G.-H.; Lin, X.-Y.; Jilani, G.; Hussain, N.; Chaudhry, A.N. Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl. Soil Ecol. 2012, 57, 1–8. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. Int. Soc. Microb. Ecol. J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Mazroui, S.S.; Phillips, A.J.L. Evaluation of culture-based techniques and 454 pyrosequencing for the analysis of fungal diversity in potting media and organic fertilizers. J. Appl. Microbiol. 2015, 119, 500–509. [Google Scholar] [CrossRef]
- Khan, A.L.; Asaf, S.; Abed, R.M.M.; Chai, Y.N.; Al-Rawahi, A.N.; Mohanta, T.K.; Al-Rawahi, A.; Schachtman, D.P.; Al-Harrasi, A. Rhizosphere microbiome of arid land medicinal plants and extra cellular enzymes contribute to their abundance. Microorganisms 2020, 8, 213. [Google Scholar] [CrossRef]
- Kazeeroni, E.A.; Al-Sadi, A.M. 454-Pyrosequencing Reveals Variable Fungal Diversity Across Farming Systems. Front. Plant Sci. 2016, 7, 314. [Google Scholar] [CrossRef]
- Nemec, S.; Menge, J.A.; Platt, R.G.; Johson, E.L.V. Vesicular-arbuscular mycorrhizal fungi associated with citrus in Florida and California and notes on their distribution and ecology. Mycologia 1981, 73, 112–127. [Google Scholar] [CrossRef]
- Ortas, I. Mycorrhiza in citrus: Growth and nutrition. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2012; pp. 333–351. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.J.; Shu, B.; Xia, R.X. Arbuscular mycorrhizal fungi associated with citrus orchards under different types of soil management, southern China. Plant Soil Environ. 2012, 58, 302–308. [Google Scholar] [CrossRef]
- Bourazza, M.; Chetto, O.; Talha, A.; Farih, A.; Douira, A.; Benyahia, H. The influence of arbuscular mycorrhizal colonization on key growth parameters of five citrus rootstock cultivars under salt stress. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 125–138. [Google Scholar]
- Al-Sadi, A.M.; Kazerooni, E.A. Illumina-Mi-Seq analysis of fungi in acid lime roots reveals dominance of Fusarium and variation in fungal taxa. Sci. Rep. 2018, 8, 17388. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, S. Arbuscular mycorrhizal fungi benefit mango (Mangifera indica L.) plant growth in the field. Sci. Hortic. 2012, 143, 43–48. [Google Scholar] [CrossRef]
- Govindan, M.; Rajeshkumar, P.P.; Varma, C.K.Y.; Anees, M.M.; Rashmi, C.R.; Nair, A.B. Arbuscular mycorrhizal fungi status of Mango (Mangifera indica) cultivars grown in typic quartzipsamments soil. Agric. Res. 2020, 9, 188–196. [Google Scholar] [CrossRef]
- Jefwa, J.M.; Kahangi, E.; Losenge, T.; Mung’atu, J.; Ngului, W.; Ichami, S.M.; Sanginga, N.; Vanluawe, B. Arbuscular mycorrhizal fungi in the rhizosphere of banana and plantain and the growth of tissue culture cultivars. Agric. Ecosyst. Environ. 2012, 157, 24–31. [Google Scholar] [CrossRef]
- Chebet, D.K.; Wanzala, F.K.; Kariuki, W. Versicular arbuscular mycorrhizal inoculation influences growth, nutrient absorption and hyphae colonization of rough lemon (Citrus limon) seedlings. Afr. J. Educ. Sci. Technol. 2021, 6, 75–85. [Google Scholar]
- Menge, J.A.; Johnson, E.L.V.; Platt, R.G. Mycorrhizal dependency of several citrus cultivars under three nutrient regimes. New Phytol. 1978, 81, 553–559. [Google Scholar] [CrossRef]
- Chen, K.; Liu, W.; Guo, S.; Liu, R.; Li, M. Diversity of arbuscular mycorrhizal fungi in continuous cropping soils used for pepper production. Afr. J. Microbiol. Res. 2012, 6, 2469–2474. [Google Scholar]
- Liao, J.; Xu, Q.; Xu, H.; Huang, D. Natural farming improves soil quality and alters microbial diversity in a cabbage field in Japan. Sustainability 2019, 11, 3131. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Shu, B.; Liu, J.-F.; Xia, R.-X. Relationships between arbuscular mycorrhizal symbiosis and soil fertility factors in citrus orchards along an altitudinal gradient. Pedosphere 2015, 25, 160–168. [Google Scholar] [CrossRef]
- Khade, S.W.; Rodrigues, B.F. Arbuscular mycorrhizal fungi associated with varieties of Carica papaya L. in tropical agro-based ecosystem of Goa, India. Trop. Subtrop. Agroecosystems 2009, 10, 369–381. [Google Scholar]
- Guzman, A.; Montes, M.; Hutchins, L.; DeLaCerda, G.; Yang, P.; Kakouridid, A.; Dahlquist-Willard, R.M.; Firestone, M.K.; Bowles, T.; Kremen, C. Crop diversity enriches arbuscular mycorrhizal fungal communities in an intensive agricultural landscape. New Phytol. 2021, 231, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Liang, Y.; Huang, D. Organic farming improves soil microbial abundance and diversity under greenhouse condition: A case study in Shanghai (Eastern China). Sustainability 2018, 10, 3825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).