Analysis of Mechanisms and Environmental Sustainability in In Situ Shale Oil Conversion Using Steam Heating: A Multiphase Flow Simulation Perspective
Abstract
:1. Introduction
2. Model
2.1. Thermo-Flow–Chemical (TFC) Coupling Model
2.2. Model Verification
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Initial temperature | 350 K | Initial pressure | 20 MPa |
| Steam injection rate | 130 kg/day | Steam temperature | 800 K |
| Reservoir porosity | 0.3 | Vertical heat conductivity | K)−1 |
| Horizontal heat conductivity | K)−1 | Well produce pressure | 10 MPa |
| Horizontal permeability | 3 mD | Vertical permeability | 1 mD |
| Gas constant | K) | Molecular mass of methane | 0.016 kg/mol |
| Density of kerogen [42] | 2590 kg/m3 | Density of heavy oil [43] | 980 kg/m3 |
| Density of light oil [43] | 797.2 kg/m3 | Density of water [35] | 985.8 kg/m3 |
| Density of coke [42] | 1100 kg/m3 | Initial saturation of kerogen [44] | 0.6 |
| Initial saturation of heavy oil [44] | 0.2 | Initial saturation of water [44] | 0.04 |
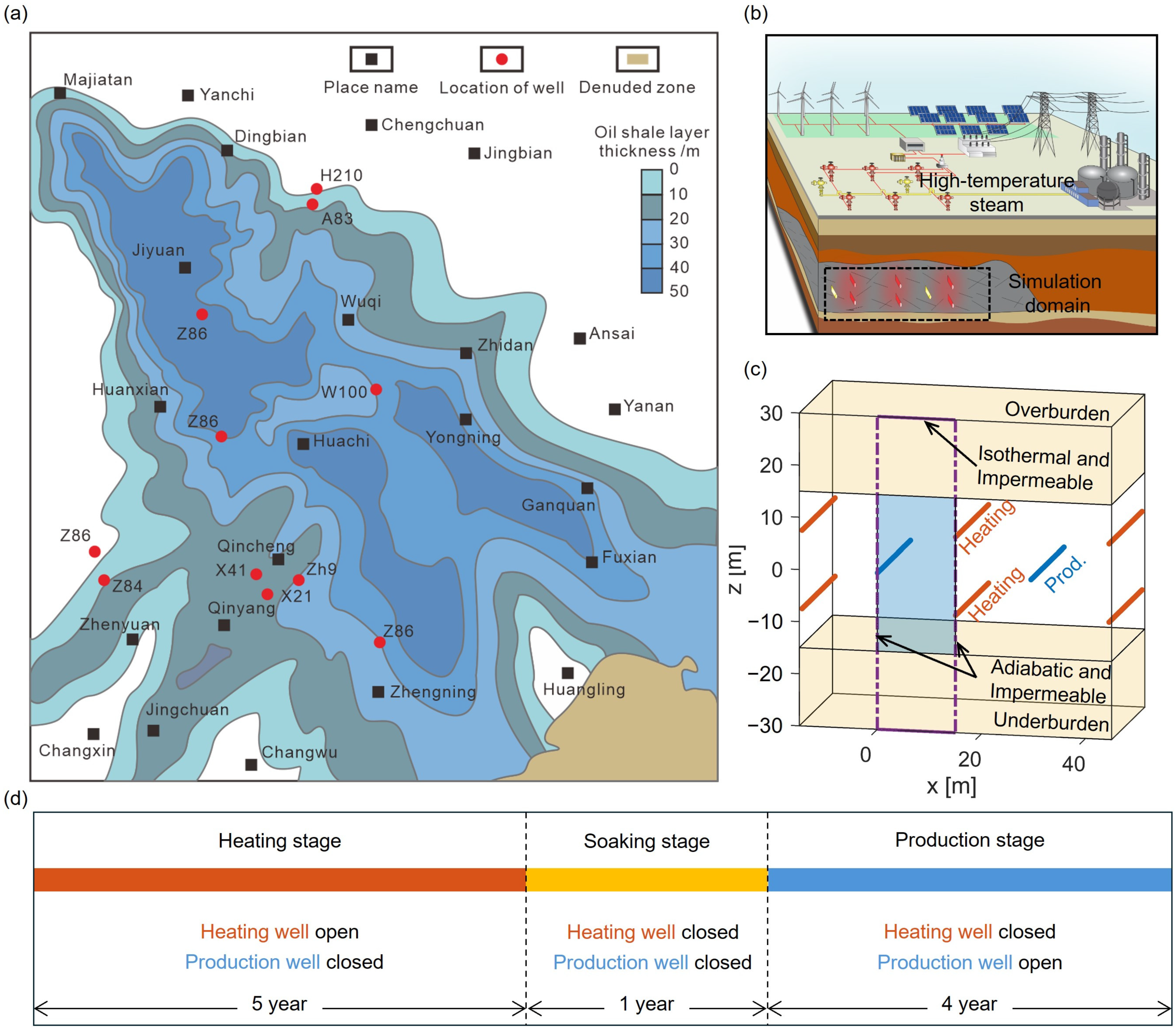
2.3. Model Setup
3. Results
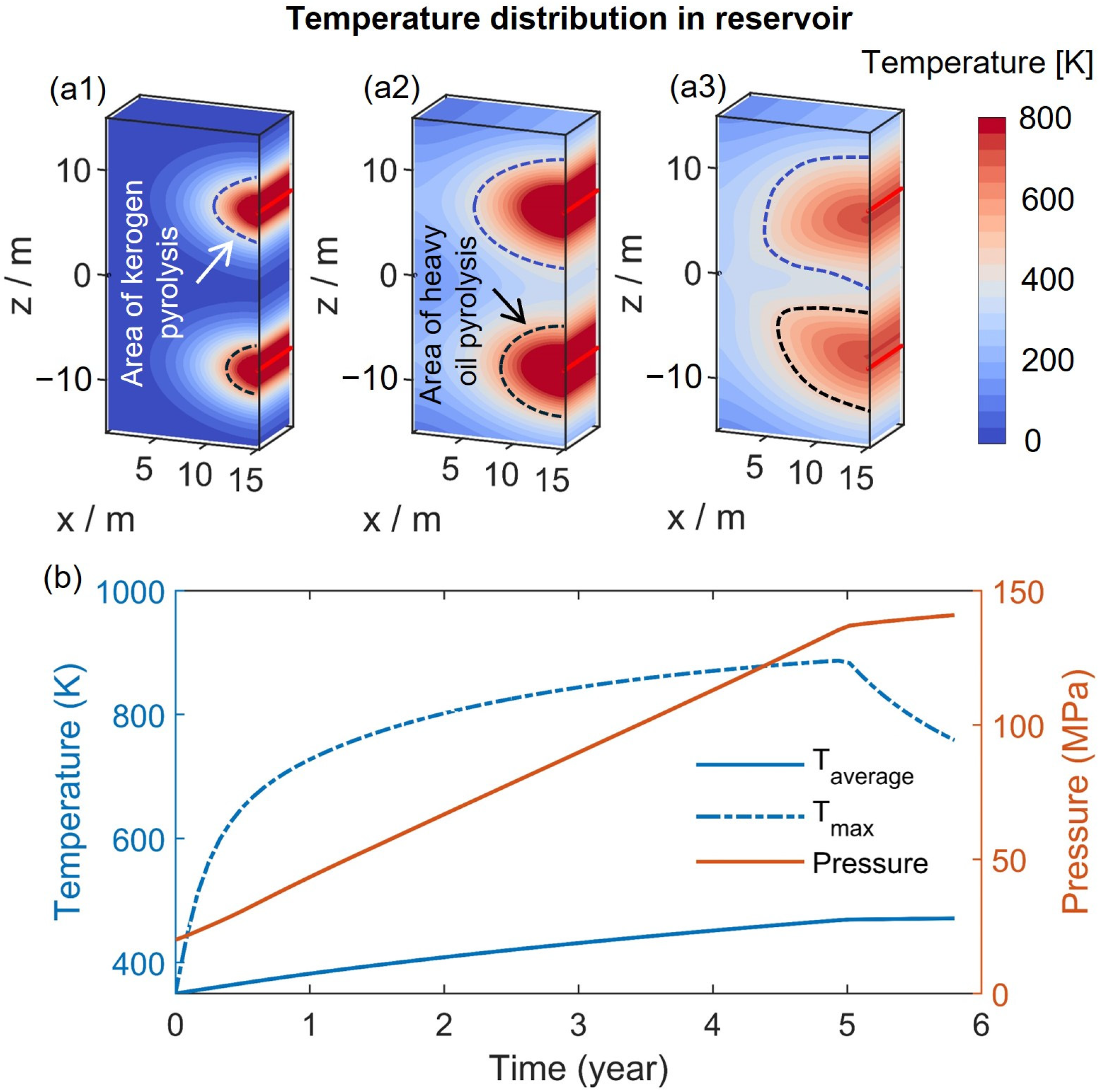
3.1. Evolution of Steam, Liquid Water, and Temperature Fields
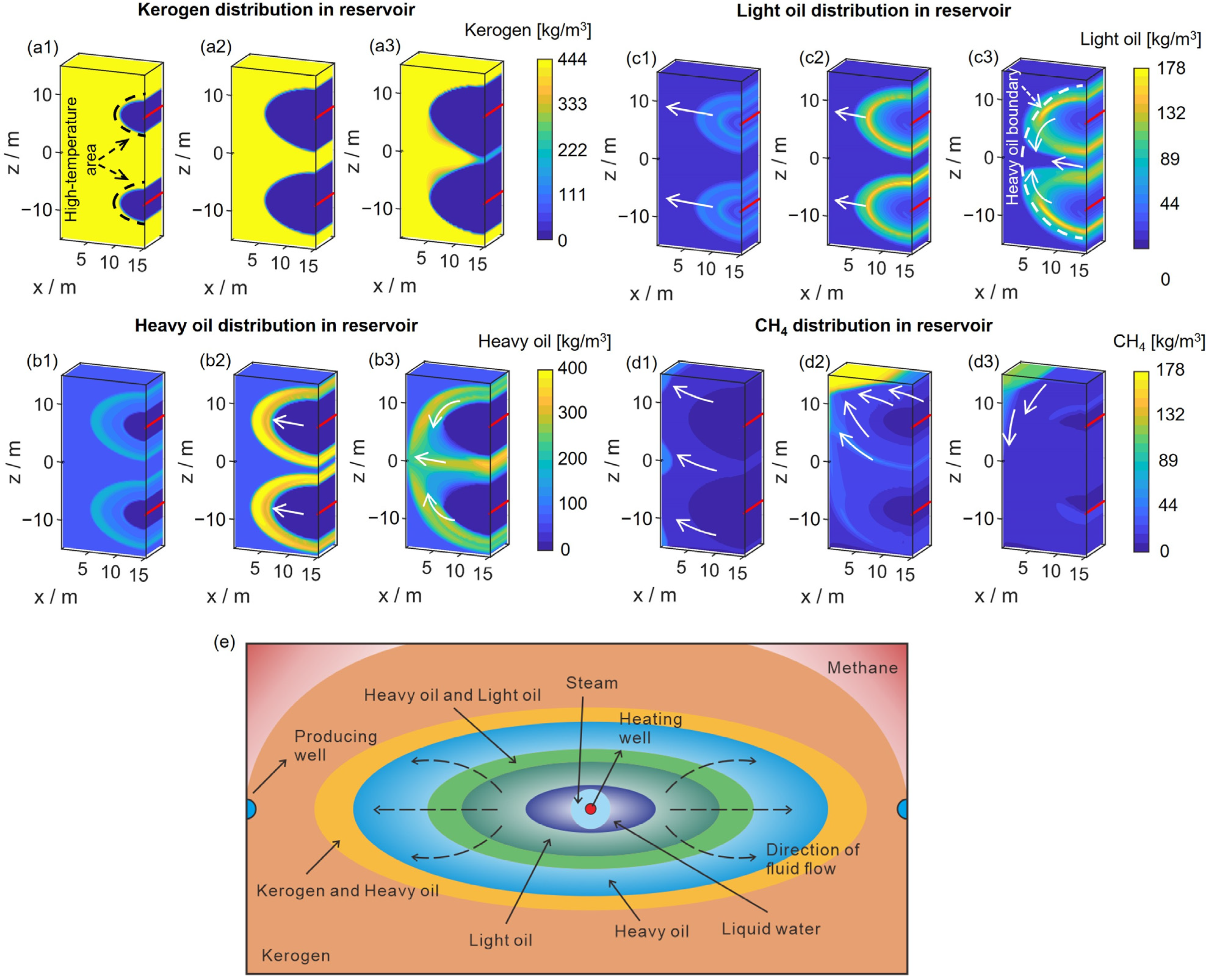
3.2. Pyrolysis Characteristics
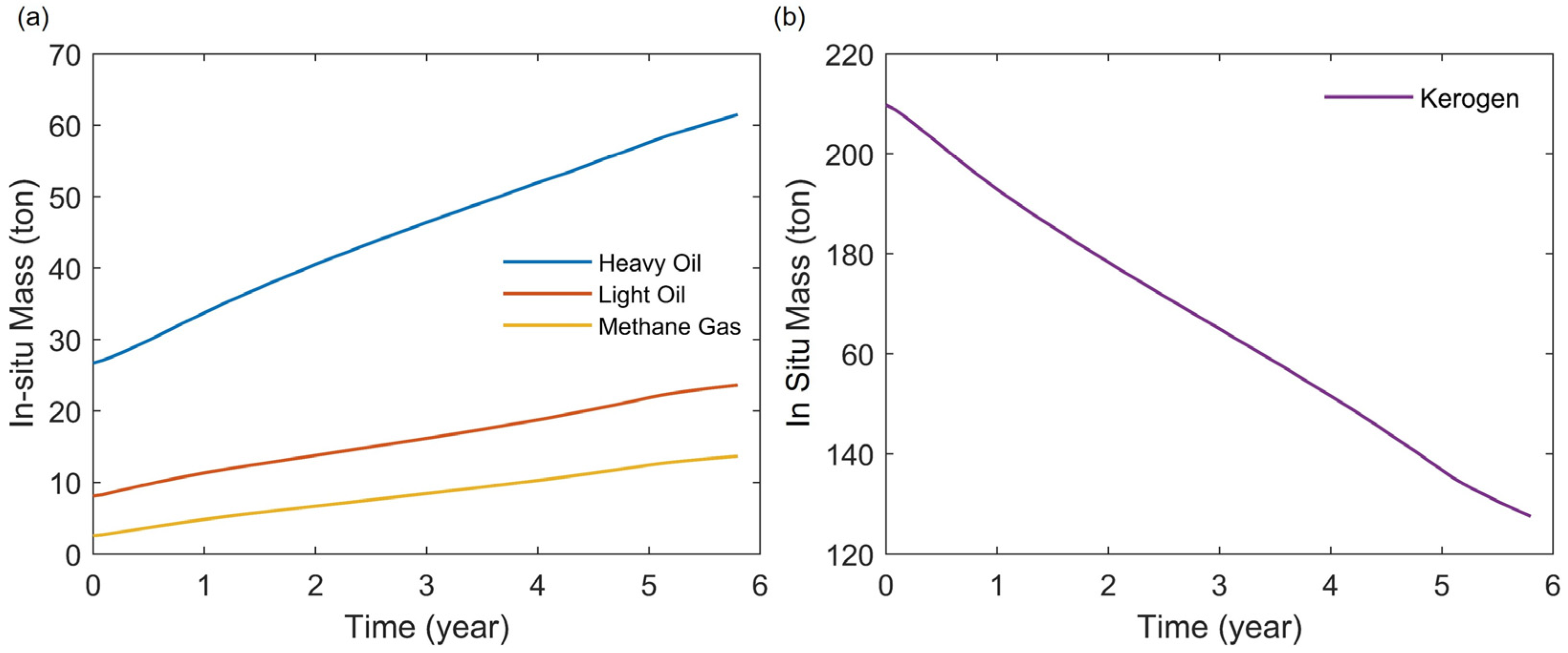
3.3. Production Characteristics
4. Sensitivity Analysis
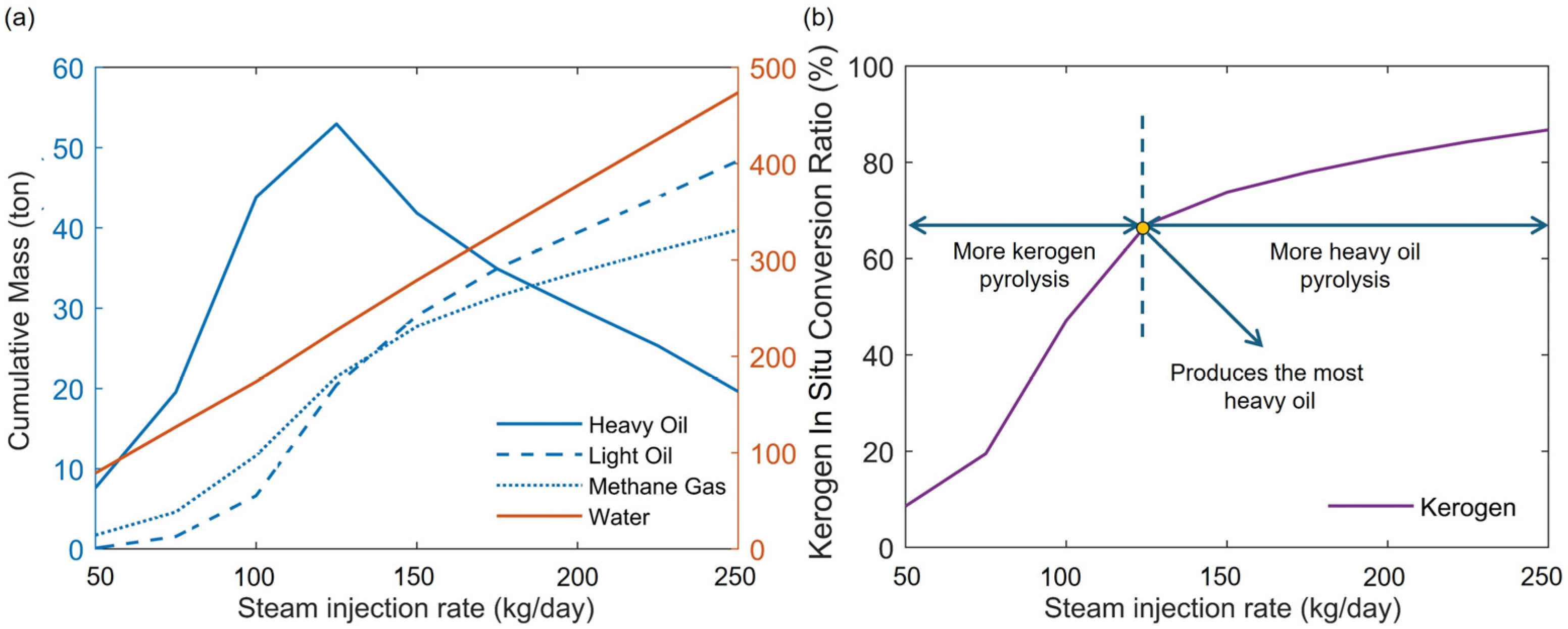
4.1. Steam Injection Rate
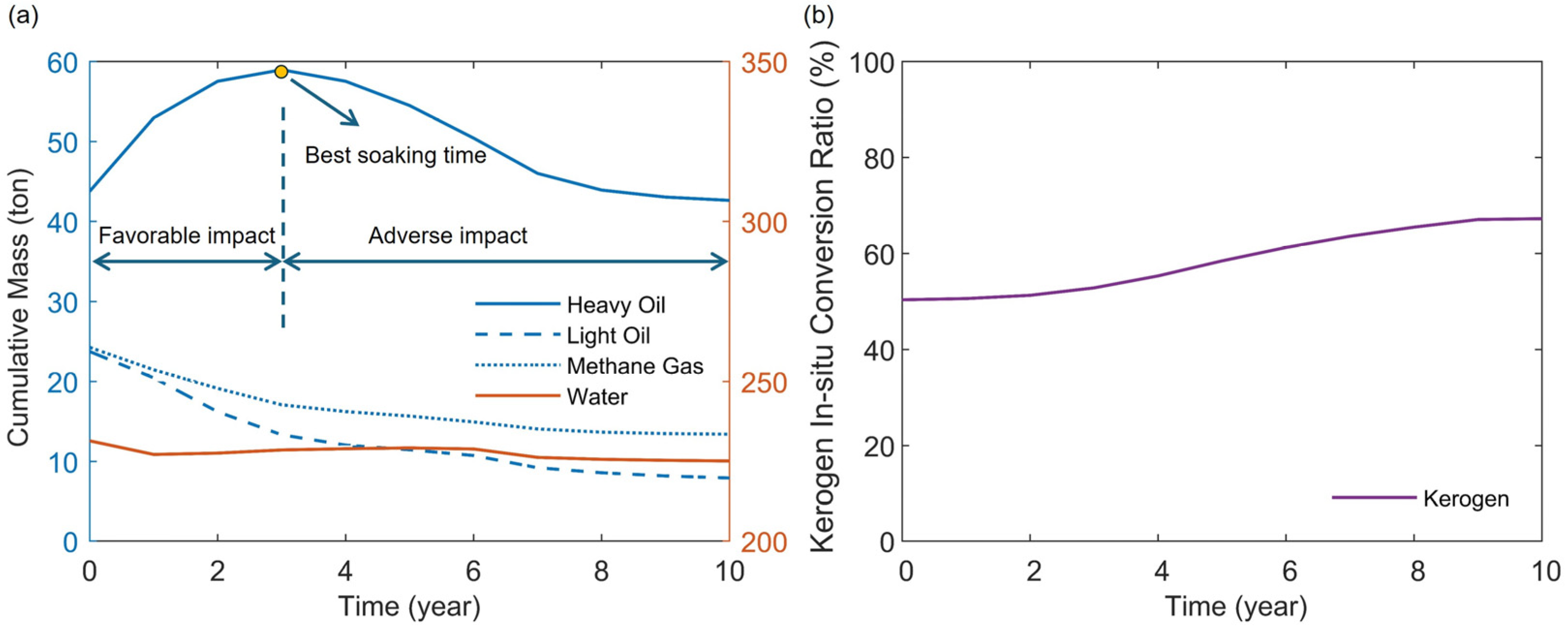
4.2. Soaking Time
5. Discussion
6. Conclusions
- During the heating stage, the range from the heating well to the production well can be divided into five regions: the displacement zone, multiple-reaction zone, kerogen pyrolysis zone, preheating zone, and primitive rock zone. The distribution of different components in the reservoir generally forms approximate elliptical rings centered around the heating well. After the production well is opened, due to differences in the viscosity and distribution of the components, there are differences in the order of production and the production rates of each component.
- During the heating stage, the injected heat first supplies the pyrolysis of kerogen. Increasing the injected heat can enhance the pyrolysis of more kerogen in the reservoir, thereby increasing the production of heavy oil. However, if the injected heat is sufficient to nearly completely pyrolyze the kerogen in the reservoir, continued injection of heat will primarily affect the pyrolysis of heavy oil. This will reduce the heavy oil content in the products while increasing the light oil and methane content. By maintaining a constant total injected heat while increasing the rate of heat injection, heat loss can be reduced and heat utilization efficiency improved. This results in a more thorough pyrolysis of heavy oil, thereby increasing the content of light oil and methane in the products. Additionally, an excessively long soaking time can negatively impact oil and gas production.
- Most of the superheated steam, after being injected into the reservoir, condenses into liquid water under high pressure and heat exchange condition, which can help displace oil. However, because the energy density of steam is relatively low, a large amount of steam needs to be injected into the reservoir to achieve the desired heating effect. This results in a high proportion of water in the extracted products, which is detrimental to oil and gas production. Future research needs to focus on improving energy utilization efficiency and developing separation and reuse technologies for the water in the products. These advancements may be crucial for the successful implementation of this in situ conversion technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Bai, F.; Liu, B.; Liu, Y.; Guo, M.; Guo, W.; Wang, Q.; Lu, X.; Yang, F.; Yang, Y. Characterization of the oil shale products derived via topochemical reaction method. Fuel 2014, 115, 338–346. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Zhu, Y.; Wang, F.; Wu, H. Multiscale transport mechanism of shale gas in micro/nano-pores. Int. J. Heat Mass Transf. 2017, 111, 1172–1180. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; Yang, D.; Fu, M. Numerical study on the in-situ pyrolysis process of steeply dipping oil shale deposits by injecting superheated water steam: A case study on Jimsar oil shale in Xinjiang, China. Energy 2022, 239, 122182. [Google Scholar] [CrossRef]
- Caineng, Z.; Zhang, G.; Zhi, Y.; Shizhen, T.; Lianhua, H.; Rukai, Z.; Xuanjun, Y.; Qiquan, R.; Denghua, L.; Zhiping, W. Concepts, characteristics, potential and technology of unconventional hydrocarbons: On unconventional petroleum geology. Pet. Explor. Dev. 2013, 40, 413–428. [Google Scholar]
- Soone, J.; Doilov, S. Sustainable utilization of oil shale resources and comparison of contemporary technologies used for oil shale processing. Oil Shale 2003, 20, 311–323. [Google Scholar] [CrossRef]
- Liu, G.; Sun, P.; Ji, Y.; Wan, Y.; Wang, H.; You, X. Current status and energy analysis of oil shale’s retorting process in the world. Pet. Chem. 2021, 61, 123–138. [Google Scholar] [CrossRef]
- Yi, P.; Yang, L.; Min, Y.; Shuangchun, Y.; Jinhui, Z. The environmental impact assessments of oil shale in in-situ mining and surface mining. Int. J. Appl. Environ. Sci. 2012, 7, 403–409. [Google Scholar]
- Yu, H.; Xu, H.; Fan, J.; Zhu, Y.-B.; Wang, F.; Wu, H. Transport of shale gas in microporous/nanoporous media: Molecular to pore-scale simulations. Energy Fuels 2020, 35, 911–943. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, Q.; Dong, Q.; Zhu, J.; Guo, W.; Ye, S.; Liu, R.; Jia, J. Characteristics and resource potential of oil shale in China. Oil Shale 2017, 34, 15–41. [Google Scholar] [CrossRef]
- Yu, H.; Fan, J.; Chen, J.; Zhu, Y.; Wu, H. Pressure-dependent transport characteristic of methane gas in slit nanopores. Int. J. Heat Mass Transf. 2018, 123, 657–667. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Yang, D.; Kang, Z.; Zhao, J. Effect of pyrolysis on oil shale using superheated steam: A case study on the Fushun oil shale, China. Fuel 2019, 253, 1490–1498. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X.; Cui, Z. Studies of the effect of retorting factors on the yield of shale oil for a new comprehensive utilization technology of oil shale. Appl. Energy 2009, 86, 2381–2385. [Google Scholar] [CrossRef]
- Gavrilova, O.; Vilu, R.; Vallner, L. A life cycle environmental impact assessment of oil shale produced and consumed in Estonia. Resour. Conserv. Recycl. 2010, 55, 232–245. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, D.; Kang, Z.; Feng, Z. A micro-CT study of changes in the internal structure of Daqing and Yan’an oil shales at high temperatures. Oil Shale 2012, 29, 357. [Google Scholar] [CrossRef]
- Bientinesi, M.; Petarca, L.; Cerutti, A.; Bandinelli, M.; De Simoni, M.; Manotti, M.; Maddinelli, G. A radiofrequency/microwave heating method for thermal heavy oil recovery based on a novel tight-shell conceptual design. J. Pet. Sci. Eng. 2013, 107, 18–30. [Google Scholar] [CrossRef]
- Crawford, P.M.; Killen, J.C. New challenges and directions in oil shale development technologies. In Oil Shale: A Solution to the Liquid Fuel Dilemma; American Chemical Society: Washington, DC, USA, 2010; pp. 21–60. [Google Scholar]
- Lu, Z.; Liu, Z.; Zhao, X.; Guo, Y.; Liu, Q. Coupling of methoxy group with organic matter during methanolysis of heavy hydrocarbon using oil shale as an example. J. Anal. Appl. Pyrolysis 2021, 158, 105264. [Google Scholar] [CrossRef]
- Crawford, P.M.; Biglarbigi, K.; Dammer, A.R.; Knaus, E. Advances in world oil shale production technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; p. 116570. [Google Scholar]
- Hou, H.; Du, Q.; Huang, C.; Zhang, L.; Hu, E. An oil shale recovery system powered by solar thermal energy. Energy 2021, 225, 120096. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Razvigorova, M.; Budinova, T.; Petrova, B.; Tsyntsarski, B.; Ekinci, E.; Ferhat, M. Steam pyrolysis of bulgarian oil shale kerogen. Oil Shale 2008, 25, 27. [Google Scholar] [CrossRef]
- Bennouna, C.; Mokhlisse, A.; Lemée, L.; Joffre, J.; Amblès, A. Supercritical fluid extraction of Moroccan (Timahdit) oil shale with water. J. Anal. Appl. Pyrolysis 1999, 50, 163–174. [Google Scholar]
- Saif, T.; Lin, Q.; Bijeljic, B.; Blunt, M.J. Microstructural imaging and characterization of oil shale before and after pyrolysis. Fuel 2017, 197, 562–574. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Zhao, Y.; Kang, Z.; Zhao, J.; Huang, X. Experimental investigation on anisotropic permeability and its relationship with anisotropic thermal cracking of oil shale under high temperature and triaxial stress. Appl. Therm. Eng. 2019, 146, 718–725. [Google Scholar] [CrossRef]
- Lee, K.J.; Moridis, G.J.; Ehlig-Economides, C.A.J.E.E. Exploitation, Compositional simulation of hydrocarbon recovery from oil shale reservoirs with diverse initial saturations of fluid phases by various thermal processes. Energy Explor. Exploit. 2017, 35, 172–193. [Google Scholar] [CrossRef]
- Huang, H.; Yu, H.; Xu, W.; Lyu, C.; Micheal, M.; Xu, H.; Liu, H.; Wu, H. A coupled thermo-hydro-mechanical-chemical model for production performance of oil shale reservoirs during in-situ conversion process. Energy 2023, 268, 126700. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Kang, Z.; Zhao, J.; Lv, Y. Numerical investigation of the in situ oil shale pyrolysis process by superheated steam considering the anisotropy of the thermal, hydraulic, and mechanical characteristics of oil shale. Energy Fuels 2019, 33, 12236–12250. [Google Scholar] [CrossRef]
- Pei, S.; Wang, Y.; Zhang, L.; Huang, L.; Cui, G.; Zhang, P.; Ren, S. An innovative nitrogen injection assisted in-situ conversion process for oil shale recovery: Mechanism and reservoir simulation study. J. Pet. Sci. Eng. 2018, 171, 507–515. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, T.; Li, S.; Li, X.; Montilla, M.J.B.; Lu, C. Comprehensive effects of heat and flow on the methane hydrate dissociation in porous media. Energy 2023, 265, 126425. [Google Scholar] [CrossRef]
- Zhang, Z.; Briceño Montilla, M.J.; Li, S.; Li, X.; Xing, J.; Hu, Y. Numerical evaluations on the fluid production in the in-situ conversion of continental shale oil reservoirs. Pet. Sci. 2024, 21, 2485–2501. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Li, S.; He, J.; Li, X.; Xu, T.; Lu, C.; Qin, X. Optimization of the natural gas hydrate hot water injection production method: Insights from numerical and phase equilibrium analysis. Appl. Energy 2024, 361, 122963. [Google Scholar] [CrossRef]
- Masuda, Y. Modeling and experimental studies on dissociation of methane gas hydrates in Berea sandstone cores. In Proceedings of the Third International Gas Hydrate Conference, Salt Lake City, UT, USA, 18–22 July 1999. [Google Scholar]
- Duan, Z.; Mao, S. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523 K and from 1 to 2000 bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Younglove, B.; Ely, J.F. Thermophysical properties of fluids. II. Methane, ethane, propane, isobutane, and normal butane. J. Phys. Chem. Ref. Data 1987, 16, 577–798. [Google Scholar] [CrossRef]
- Kretzschmar, H.-J.; Wagner, W. International Steam Tables; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Yaws Carl, L. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: Singapore, 2003. [Google Scholar]
- Burnham, A.K.; Braun, R.L. General kinetic model of oil shale pyrolysis. In Lawrence Livermore National Laboratory Report UCRL-89805; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1984. [Google Scholar]
- Braun, R.L.; Burnham, A.K. Chemical Reaction Model for Oil and Gas Generation from Type 1 and Type 2 Kerogen; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1993.
- Chen, X.; Rao, X.; Xu, Y.; Liu, Y. An effective numerical simulation method for steam injection assisted in situ recovery of oil shale. Energies 2022, 15, 776. [Google Scholar] [CrossRef]
- Pei, S.; Huang, L.; Zhang, L.; Ren, S. Experimental study on thermal cracking reactions of ultra-heavy oils during air injection assisted in-situ upgrading process. J. Pet. Sci. Eng. 2020, 195, 107850. [Google Scholar] [CrossRef]
- Briceño Montilla, M.J.; Li, S.; Zhang, Z.; Li, X.; Sun, Y.; Ma, S. Theoretical Analysis of the Effect of Electrical Heat In Situ Injection on the Kerogen Decomposition for the Development of Shale Oil Deposits. Energies 2023, 16, 5007. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Huang, W.; Wang, X.; Chen, C. Experimental study on mechanical properties of shale rock and its strength criterion. Arab. J. Geosci. 2021, 14, 264. [Google Scholar] [CrossRef]
- Guanglun, L.; Zi, L.; Chuanjin, Y.; Xin, M.; Dan, W. Numerical simulation on oil shale in-situ upgrading by steam injection. In Proceedings of the 2016 International Field Exploration and Development Conference (IFEDC), Beijing, China, 11–12 August 2016. [Google Scholar]
- Wenzhi, Z.; Suyun, H.; Lianhua, H. Connotation and strategic role of in-situ conversion processing of shale oil underground in the onshore China. Pet. Explor. Dev. 2018, 45, 563–572. [Google Scholar]
- Jin, J.; Jiang, W.; Liu, J.; Shi, J.; Zhang, X.; Cheng, W.; Yu, Z.; Chen, W.; Ye, T. Numerical analysis of in situ conversion process of oil shale formation based on thermo-hydro-chemical coupled modelling. Energies 2023, 16, 2103. [Google Scholar] [CrossRef]
- Lee, K.; Moridis, G.J.; Ehlig-Economides, C.A. A comprehensive simulation model of kerogen pyrolysis for the in-situ upgrading of oil shales. SPE J. 2016, 21, 1612–1630. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xie, Z.; Montilla, M.J.B.; Li, Y.; Xu, T.; Li, S.; Li, X. Analysis of Mechanisms and Environmental Sustainability in In Situ Shale Oil Conversion Using Steam Heating: A Multiphase Flow Simulation Perspective. Sustainability 2024, 16, 9399. https://doi.org/10.3390/su16219399
Zhang Z, Xie Z, Montilla MJB, Li Y, Xu T, Li S, Li X. Analysis of Mechanisms and Environmental Sustainability in In Situ Shale Oil Conversion Using Steam Heating: A Multiphase Flow Simulation Perspective. Sustainability. 2024; 16(21):9399. https://doi.org/10.3390/su16219399
Chicago/Turabian StyleZhang, Zhaobin, Zhuoran Xie, Maryelin Josefina Briceño Montilla, Yuxuan Li, Tao Xu, Shouding Li, and Xiao Li. 2024. "Analysis of Mechanisms and Environmental Sustainability in In Situ Shale Oil Conversion Using Steam Heating: A Multiphase Flow Simulation Perspective" Sustainability 16, no. 21: 9399. https://doi.org/10.3390/su16219399
APA StyleZhang, Z., Xie, Z., Montilla, M. J. B., Li, Y., Xu, T., Li, S., & Li, X. (2024). Analysis of Mechanisms and Environmental Sustainability in In Situ Shale Oil Conversion Using Steam Heating: A Multiphase Flow Simulation Perspective. Sustainability, 16(21), 9399. https://doi.org/10.3390/su16219399







