Experimental and Statistical Analysis of Iron Powder for Green Heat Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Estimation of the Flame Spectrum
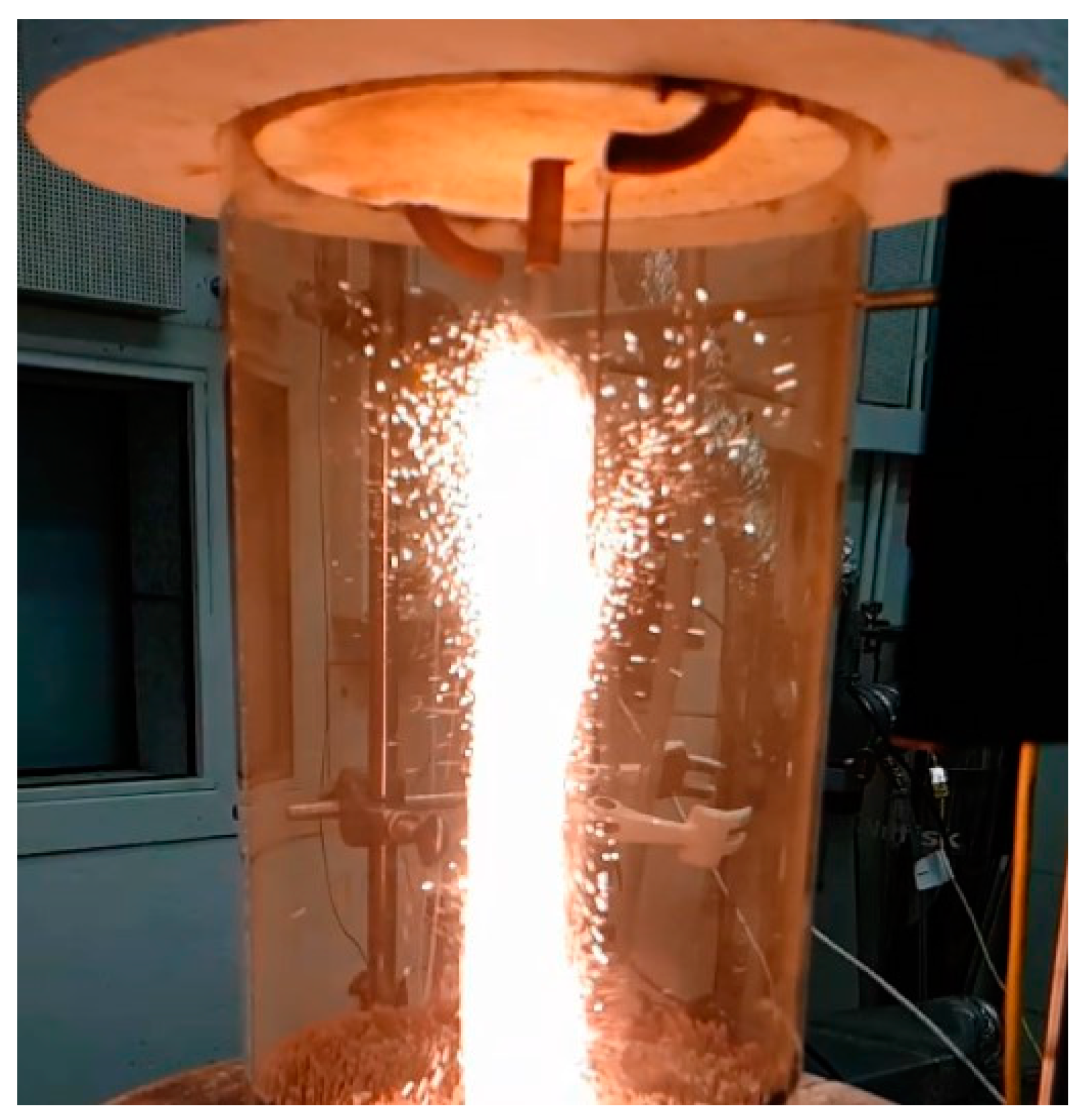
2.2. Iron Powder Mixing and Injection Systems
2.3. Experimental Analysis
2.4. Spectral Preprocessing (MSC, SNV, Derivatives, and Smoothing)
2.5. Partial Least Squares Regression (PLSR) and Principal Component Analysis (PCA)
3. Results and Discussion
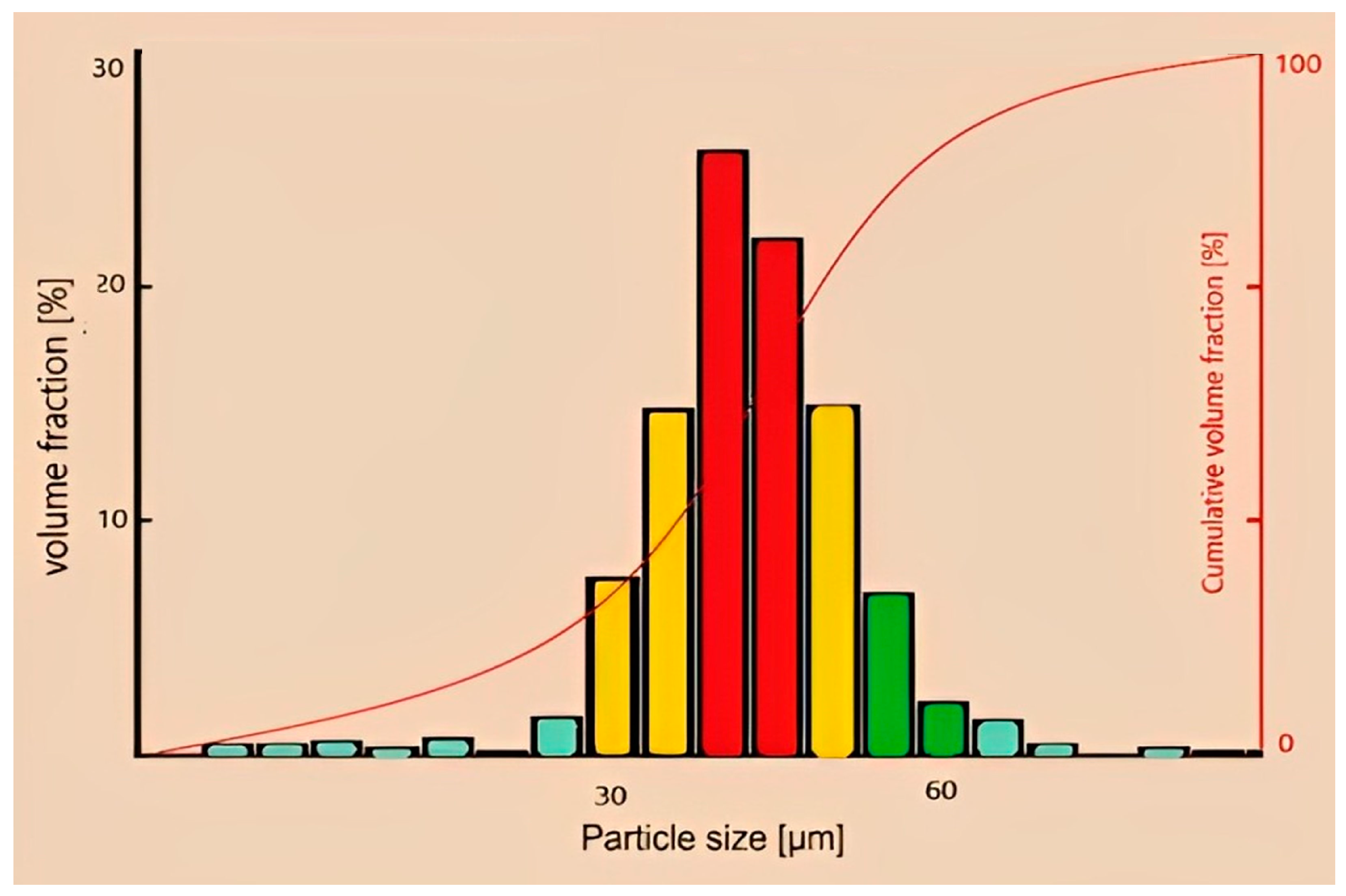
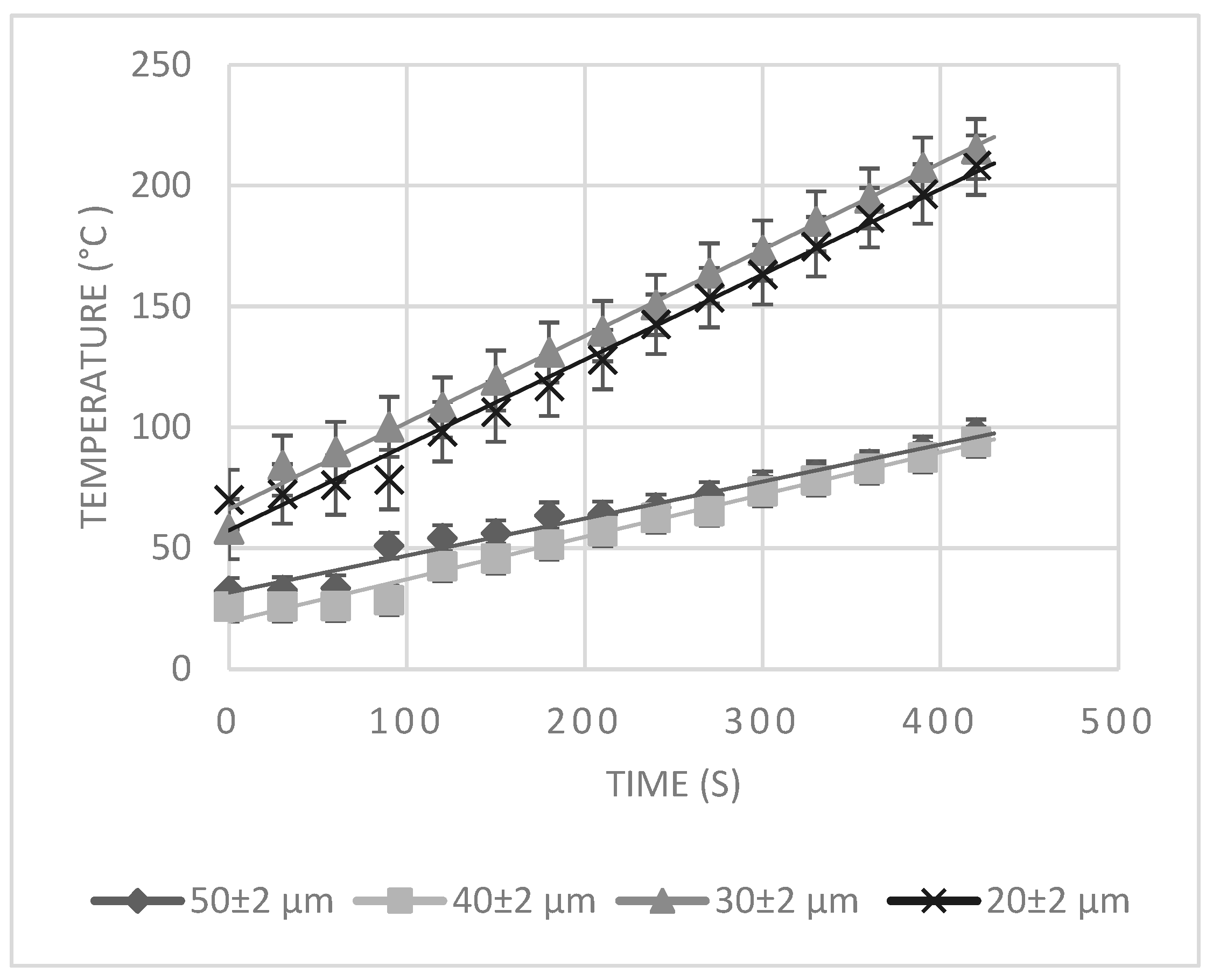
3.1. Experimental Results
3.2. Results of PLS Analysis
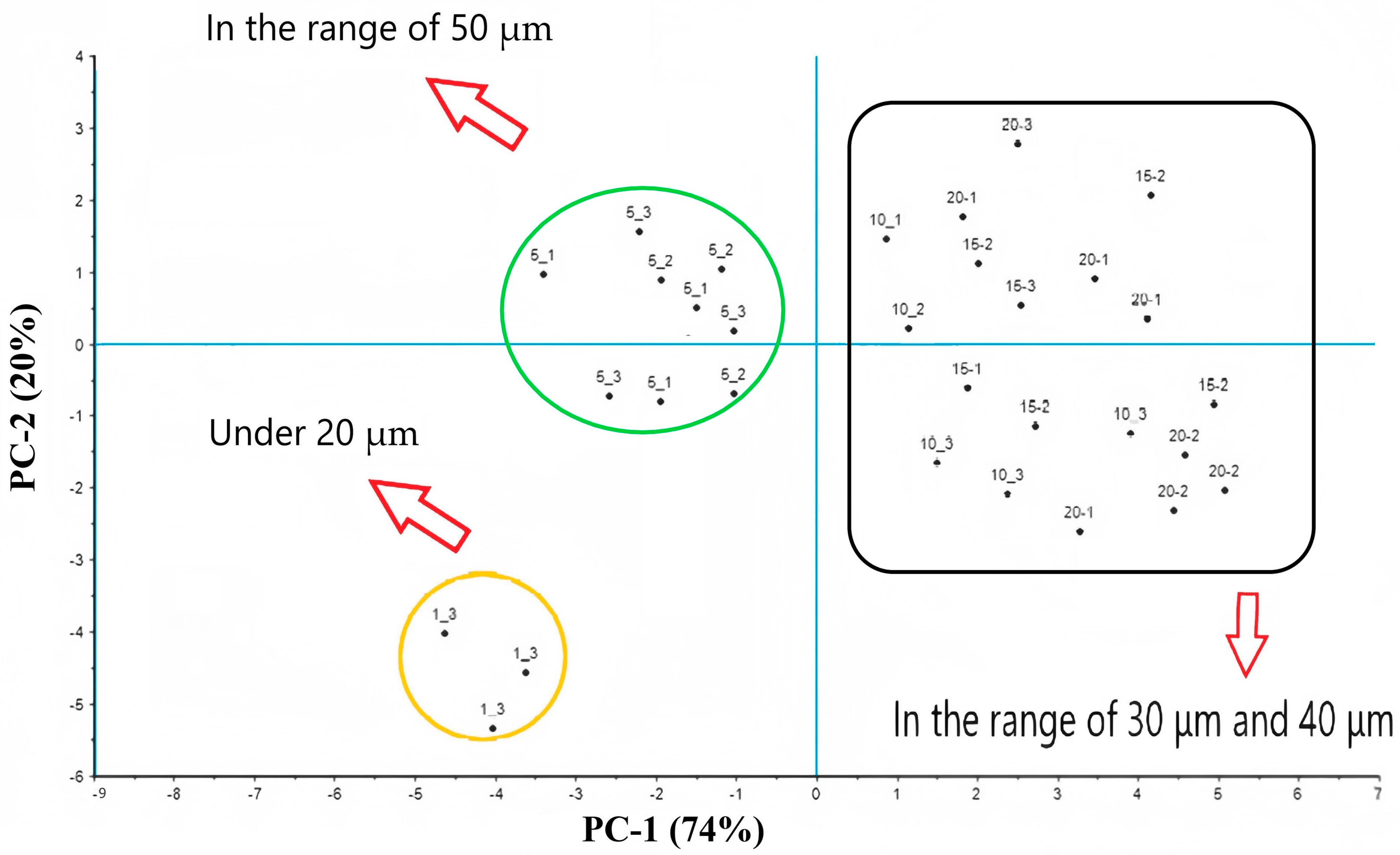
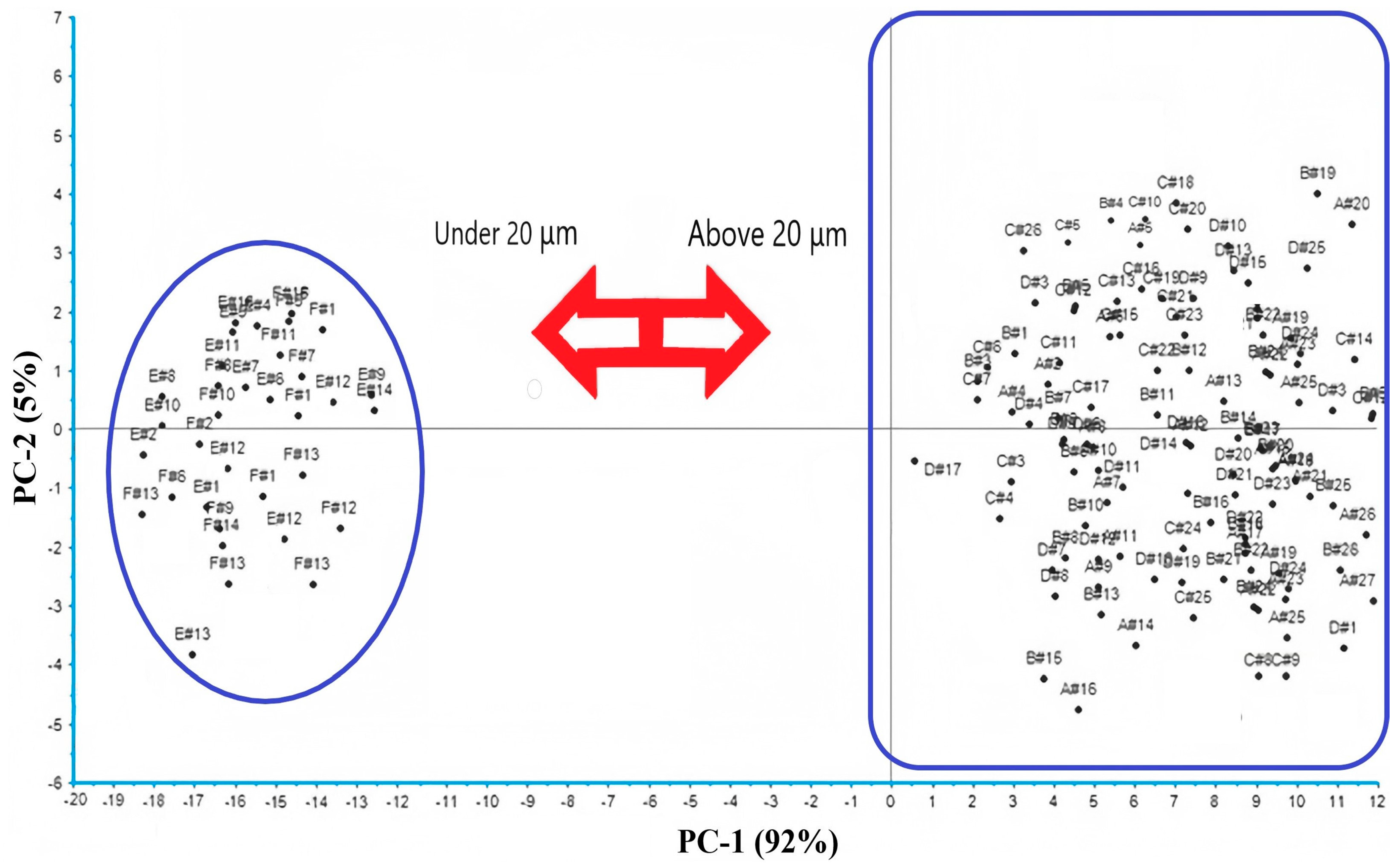
3.3. Results of PCA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergthorson, J.M.; Goroshin, S.; Soo, M.J.; Julien, P.; Palecka, J.; Frost, D.L.; Jarvis, D.J. Direct combustion of recyclable metal fuels for zero-carbon heat and power. Appl. Energy 2015, 160, 368–382. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Shkolnikov, E.I.; Bersh, A.V.; Zhuk, A.Z.; Lisicyn, A.V.; Sorokovikov, A.I.; Pankina, Y.V. An experimental aluminum-fueled power plant. J. Power Sources 2011, 196, 8828–8835. [Google Scholar] [CrossRef]
- Bergthorson, J.M. Recyclable metal fuels for clean and compact zero-carbon power. Prog. Energy Combust. Sci. 2018, 68, 169–196. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Yavor, Y.; Palecka, J.; Georges, W.; Soo, M.; Vickery, J.; Goroshin, S.; Frost, D.L.; Higgins, A.J. Metal-water combustion for clean propulsion and power generation. Appl. Energy 2017, 186, 13–27. [Google Scholar] [CrossRef]
- Julien, P.; Bergthorson, J.M. Enabling the metal fuel economy: Green recycling of metal fuels. Sustain. Energy Fuels 2017, 1, 615–625. [Google Scholar] [CrossRef]
- Ning, D.; Hazenberg, T.; Shoshin, Y.; van Oijen, J.A.; Finotello, G.; de Goey, L.P.H. Experimental and theoretical study of single iron particle combustion under low-oxygen dilution conditions. Fuel 2024, 357, 129718. [Google Scholar] [CrossRef]
- Prasidha, W.; Baigmohammadi, M.; Shoshin, Y.; de Goey, P. Towards an efficient metal energy carrier for zero–emission heating and power: Iron powder combustion. Combust. Flame 2024, 268, 113655. [Google Scholar] [CrossRef]
- McRae, M.; Julien, P.; Salvo, S.; Goroshin, S.; Frost, D.L.; Bergthorson, J.M. Stabilized, flat iron flames on a hot counterflow burner. Proc. Combust. Inst. 2019, 37, 3185–3191. [Google Scholar] [CrossRef]
- Palecka, J.; Sniatowsky, J.; Goroshin, S.; Higgins, A.J.; Bergthorson, J.M. A new kind of flame: Observation of the discrete flame propagation regime in iron particle suspensions in microgravity. Combust. Flame 2019, 209, 180–186. [Google Scholar] [CrossRef]
- Sun, J.-H.; Dobashi, R.; Hirano, T. Combustion behavior of iron particles suspended in air. Combust. Sci. Technol. 1990, 150, 99–114. [Google Scholar] [CrossRef]
- Sun, J.-H.; Dobashi, R.; Hirano, T. Structure of flames propagating through metal particle clouds and behavior of particles. Combust. Sci. Technol. 1998, 27, 2405–2411. [Google Scholar] [CrossRef]
- Sun, J.; Dobashi, R.; Hirano, T. Concentration profile of particles across a flame propagating through an iron particle cloud. Combust. Flame 2003, 134, 381–387. [Google Scholar] [CrossRef]
- Dirven, L.; Deen, N.G.; Golombok, M. Dense energy carrier assessment of four combustible metal powders. Sustain. Energy Technol. Assess. 2018, 30, 52–58. [Google Scholar] [CrossRef]
- Stevens, N.C.; Prasidha, W.; Deen, N.G.; Meeuwsen, L.; Baigmohammadi, M.; Shoshin, Y.; de Goey, R.; Finotello, G. Cyclic reduction of combusted iron powder: A study on the material properties and conversion reaction in the iron fuel cycle. Powder Technol. 2024, 441, 119786. [Google Scholar] [CrossRef]
- Thijs, L.C.; van Gool, C.G.; Ramaekers, W.J.S.; Kuerten, J.G.M.; van Oijen, J.A.; De Goey, L.P.H. Improvement of heat-and mass transfer modeling for single iron particles combustion using resolved simulations. Combust. Sci. Technol. 2024, 196, 572–588. [Google Scholar] [CrossRef]
- Jing, Q.; Li, Y.; Zhang, L.; Wang, D.; Shi, C. Optimization effect of propylene oxide (PO) on evaporation, combustion, and pollutant emissions of high-energy–density JP-10 fuel. Fuel 2024, 361, 130585. [Google Scholar] [CrossRef]
- Helin, R.; Indahl, U.G.; Tomic, O.; Liland, K.H. On the possible benefits of deep learning for spectral preprocessing. J. Chemom. 2022, 36, e3374. [Google Scholar] [CrossRef]
- Yao, Y.; Chang, D.; Panahi, A.; Levendis, Y.A. Spectral emissivities and temperatures of burning iron as single particles or groups of particles. Fuel 2024, 375, 132537. [Google Scholar] [CrossRef]
- Ning, D.; Shoshin, Y.; van Stiphout, M.; van Oijen, J.; Finotello, G.; de Goey, P. Temperature and phase transitions of laser-ignited single iron particle. Combust. Flame 2022, 236, 111801. [Google Scholar] [CrossRef]
- Tang, F.-D.; Goroshin, S.; Higgins, A.J. Modes of particle combustion in iron dust flames. Proc. Combust. Inst. 2011, 33, 1975–1982. [Google Scholar] [CrossRef]
- Li, Y.-H.; Pangestu, S.; Purwanto, A.; Chen, C.-T. Synergetic combustion behavior of aluminum and coal addition in hybrid iron-methane-air premixed flames. Combust. Flame 2021, 228, 364–374. [Google Scholar] [CrossRef]
- Poletaev, N.I.; Khlebnikova, M.Y. Combustion of iron particles suspension in laminar premixed and diffusion flames. Combust. Sci. Technol. 2022, 194, 1356–1377. [Google Scholar] [CrossRef]
- Wright, A.; Goroshin, S.; Higgins, A. Combustion time and ignition temperature of iron particles in different oxidizing environments. In Proceedings of the 25th International Colloquium on the Dynamics of Explosions and Reactive Systems, Leeds, UK, 2–7 August 2015. [Google Scholar]
- Badiola, C.; Dreizin, E.L. Combustion of micron-sized particles of titanium and zirconium. Proc. Combust. Inst. 2013, 34, 2237–2243. [Google Scholar] [CrossRef]
- Sohrabi, M.; Ghobadian, B.; Najafi, G.; Choisez, L.; Prasidha, W.; Baigmohammadi, M.; de Goey, P. Iron Powder Particles as a Clean and Sustainable Carrier: Investigating Their Impact on Thermal Output. Process Saf. Environ. Prot. 2024, 188, 957–969. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Prasidha, W.; Stevens, N.C.; Shoshyn, Y.L.; Spee, T.; de Goey, P. Towards utilization of iron powders for heating and power. Appl. Energy Combust. Sci. 2023, 13, 100116. [Google Scholar] [CrossRef]
- Sohrabi, M.; Ghobadian, B.; Najafi, G. Toward a sustainable future: Utilizing iron powder as a clean carrier in dry cycle applications. Int. J. Environ. Sci. Technol. 2024, 21, 6891–6910. [Google Scholar] [CrossRef]
- Abrámoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar] [CrossRef]
- Aoki, H.; Kurosaki, Y.; Anzai, H. Study on the tubular pinch effect in a pipe flow: I. Lateral migration of a single particle in laminar Poiseuille flow. Bull. JSME 1979, 22, 206–212. [Google Scholar] [CrossRef]
- Crocker, J.C.; Weeks, E.R. Particle Tracking Using IDL. Available online: http://www.physics.emory.edu/faculty/weeks//idl (accessed on 20 October 2024).
- Zema, T.B. A 3-D numerical investigation and parametric CFD analysis of flow through a convergent-divergent nozzle using ANSYS CFX. J. Univ. Shanghai Sci. Technol. 2022, 24, 305–314. [Google Scholar]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, A.M.C. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2000. [Google Scholar]
- Guo, W.; Gu, J.; Liu, D.; Shang, L. Peach variety identification using near-infrared diffuse reflectance spectroscopy. Comput. Electron. Agric. 2016, 123, 297–303. [Google Scholar] [CrossRef]
- Li, X.; He, Y. Non-destructive measurement of acidity of Chinese bayberry using Vis/NIRS techniques. Eur. Food Res. Technol. 2006, 223, 731–736. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Houghton, J.E.; Behnsen, J.; Duller, R.A.; Nichols, T.E.; Worden, R.H. Particle size analysis: A comparison of laboratory-based techniques and their application to geoscience. Sediment. Geol. 2024, 464, 106607. [Google Scholar] [CrossRef]
- Pashchenko, D. Comparative analysis of hydrogen/air combustion CFD-modeling for 3D and 2D computational domain of micro-cylindrical combustor. Int. J. Hydrogen Energy 2017, 42, 29545–29556. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral characterization using scanning electron microscopy (SEM): A review of the fundamentals, advancements, and research directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Buchheiser, S.; Deutschmann, M.P.; Rhein, F.; Allmang, A.; Fedoryk, M.; Stelzner, B.; Nirschl, H. Particle and phase analysis of combusted iron particles for energy storage and release. Materials 2023, 16, 2009. [Google Scholar] [CrossRef]
- Ning, D.; Shoshin, Y.; van Oijen, J.A.; Finotello, G.; De Goey, L.P.H. Burn time and combustion regime of laser-ignited single iron particle. Combust. Flame 2021, 230, 111424. [Google Scholar] [CrossRef]
- Ceroni, M.; Gobber, F.S.; Grande, M.A. Ultraviolet–Visible–Near InfraRed spectroscopy for assessing metal powder cross-contamination: A multivariate approach for a quantitative analysis. Mater. Des. 2024, 242, 113023. [Google Scholar] [CrossRef]
- Chernyshov, D.; Dovgaliuk, I.; Dyadkin, V.; van Beek, W. Principal component analysis (PCA) for powder diffraction data: Towards unblinded applications. Crystals 2020, 10, 581. [Google Scholar] [CrossRef]
- Thomas, K.M.; Ajithaprasad, S.; Mithun, N.; Pavithran, S.; Chidangil, S.; Lukose, J. Raman spectroscopy assisted tear analysis: A label-free, optical approach for noninvasive disease diagnostics. Exp. Eye Res. 2024, 243, 109913. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohrabi, M.; Ghobadian, B.; Najafi, G.; Prasidha, W.; Baigmohammadi, M.; de Goey, P. Experimental and Statistical Analysis of Iron Powder for Green Heat Production. Sustainability 2024, 16, 9416. https://doi.org/10.3390/su16219416
Sohrabi M, Ghobadian B, Najafi G, Prasidha W, Baigmohammadi M, de Goey P. Experimental and Statistical Analysis of Iron Powder for Green Heat Production. Sustainability. 2024; 16(21):9416. https://doi.org/10.3390/su16219416
Chicago/Turabian StyleSohrabi, Mohammadmahdi, Barat Ghobadian, Gholamhassan Najafi, Willie Prasidha, Mohammadreza Baigmohammadi, and Philip de Goey. 2024. "Experimental and Statistical Analysis of Iron Powder for Green Heat Production" Sustainability 16, no. 21: 9416. https://doi.org/10.3390/su16219416
APA StyleSohrabi, M., Ghobadian, B., Najafi, G., Prasidha, W., Baigmohammadi, M., & de Goey, P. (2024). Experimental and Statistical Analysis of Iron Powder for Green Heat Production. Sustainability, 16(21), 9416. https://doi.org/10.3390/su16219416






