Abstract
The potential of both plasma and nanotechnology in producing slow-release fertilizer is immense. These technologies, when combined, may offer green and inexpensive nitrogen fertilizers, from rich renewable resources available in local areas. Together, these technologies may overcome some limitations of conventional synthetic fertilizers, which are currently expensive and associated with low nitrogen use efficiency and significant environmental concerns. This review explores the utilization of recent advances in plasma and nanotechnology, which can be leveraged to create new slow-release nitrogen fertilizers. It emphasizes their crucial role in addressing nitrogen depletion and improving crop production. Despite the lack of attempts to develop slow-release nanofertilizers from low-cost liquid nitrate generated by emission-free nonthermal plasma, the effectiveness of plasma nitrate matches that of conventional fertilizer for crop production. We propose a more efficient electrocatalytic conversion of plasma nitrate to ammonium salt, then coating it with plant-based cellulose nanoparticles to create a slow-release form. This set of processes would synchronize nutrient release with the dynamic N requirements of plants. Formulations using agro-based, low-cost cellulose nanomaterials could replace high-cost carrier hydrogels associated with low mechanical strength. This review also highlights the isolation of nanocellulose from various plant materials and its characterization in different formulations of slow-release nanoplasma N fertilizer. Additionally, we discuss mechanisms of N loss, slow-release, and retention in the soil that can contribute to the production and use of efficient, sustainable fertilizers to improve food security and, consequently, the health of our planet.
1. Introduction
Feeding the growing population living on nutrient-depleted soils is increasingly challenging [1]. Attempts to achieve the Sustainable Development Goals (SDGs) of the United Nations [2,3,4] have indicated a direct influence of poor soil fertility on hunger, poverty, poor nutrition, and an interaction with climate change effects due to global warming. These conditions result from unsustainable agricultural practices and technologies such as agrochemicals. Chemical fertilizer application is currently an indispensable and effective option for providing nutrients required for plant growth, especially nitrogen (N). Nevertheless, fertilizers may be scarce, especially in Africa, expensive, and frequently unfeasible. These challenges are especially true with more extensive applications and requirements that are labor-demanding [5]. Moreover, current high-capital-cost fertilizer production technologies result in expensive fertilizers due to their discriminatory use of scarce nonrenewable resources restricted to small regions [6,7,8,9]. Under such circumstances, soil health and crop production have remained the same for the desired potential with applying fertilizers.
Globally, crop production must be increased to curb the shortage of food. However, the increasing depletion and demand for nitrogen nutrients in the soil is the most limiting factor to crop production [10]. Fortunately, nitrogen is abundant in the Earth’s atmosphere [11]. However, the inertness of atmospheric N renders it unavailable for direct fixation and assimilation by plants, especially by nonleguminous crops [12]. As a result, global nitrogen fixation is estimated at 413 Tg/y from both natural and anthropogenic processes. The primary source is biological nitrogen fixation (BNF) [7,11], which is considered an environmentally benign process [12]. Nevertheless, the nitrogen supply by BNF could be slow and can hardly be relied on to cope with the increasing food demand [6]. Thus, the inability of BNF to provide for all N requirements necessitates industrial fixation to meet the needs of crops required to support the growing population.
1.1. Current Industrial Fertilizer Production Process
Industrial nitrogen fixation uses the Haber–Bosch (H–B) process via ammonia synthesis. It has contributed to 44% of the global food increase [11]. The process is, however, energy-intensive [7,11,13], consuming 2% of the total energy produced worldwide. It also requires large capital-intensive infrastructure [8,13]. The H–B process consumes 2–5% of the total natural gas and is responsible for 300 metric tons of CO2 and 42% of industrial greenhouse gas (GHG) emissions [7,11,14]. Consequently, the current NH3 production rate, or NH3 partial current density, is meagre (~0.1 mA cm−2) due to the sluggish dissociation step of the highly stable N≡N bond [13]. Remarkably, this inorganic N fertilizer production process has a low optimum [7,15] to support the ever-increasing demand that exceeds 150 million tons per annum [4,8,16,17]. A combination of the rise in fertilizer demand and production process has produced environmental distress in the manifestation of climate change, resource, and energy depletion. This has led to a worrying duress on the sustainability of continuous food supply.
Moreover, the solubility of industrially fixed N makes it highly mobile for plant root uptake. This also makes it mobile to both surface and downward movement. As a result, repeated and sometimes applications beyond crop requirements of N fertilizers result in a low use efficiency, mostly below 30%, due to increasing N leaching and volatilization [16,18,19,20,21]. This constitutes a massive economic wastage [6,16,18,20,21,22]. Excessive nitrogen loss from agriculture causes ecological issues such as groundwater degradation and can contribute to global warming, leading to significant health disorders. The H–B process, by implication, is, therefore, considered a leading cause of pollution and climate change [4,11,14,18,21,23].
1.2. Greener Nitrogen Fertilizer Production Processes
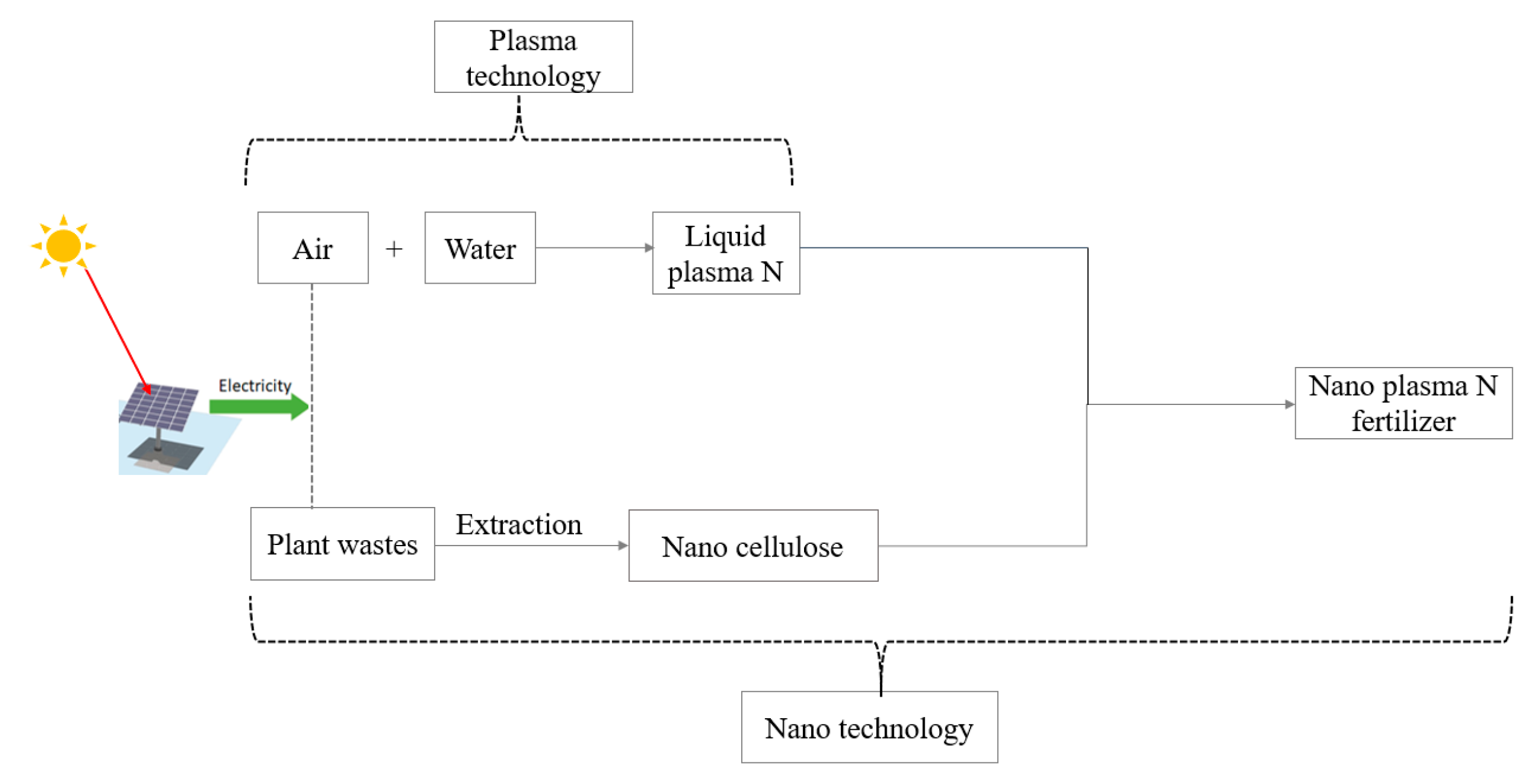
As a mechanism to overcome fertilizer production and use challenges, applying green, renewable, and sustainable materials has recently become significant for the decentralized production of various high-value products [7,13,24,25]. Such materials may be an alternative solution to the ever-depleting nonrenewable resources, energy crisis, environmental pollution, and climate change [7]. In this context, commonly abundant nature-friendly atmospheric air, water, solar energy [7,11,15,26] (Figure 1), and plant cellulose [25,27,28] may be a sustainable replacement for scarce nonrenewable resources used in the H–B process [7]. These materials have the potential to be modified and functionalized for specific industrial uses [29]. Thus, combining technologies that can utilize these materials as feedstocks may facilitate the climate-smart production of higher-value fertilizer products to complement the H–B process [7,11,25,27,30,31,32,33].
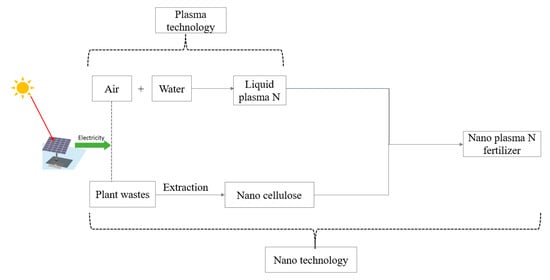
Figure 1.
Proposed model for green production of slow-release nanofertilizer.
Plasma-assisted nitrogen fixation technology can produce liquid nitrate through the electrochemical oxidation of atmospheric air and reduction of water using solar energy available worldwide. This fast, one-step NO3− generation process has a higher partial density and requires less dissociation energy than H–B NH3 production [11,13,34,35]. Plasma-assisted fixation, as an alternative process, can, therefore, supplement H–B and may be domesticated in local areas due to its low capital cost for the establishment, the potential to generate a significant concentration of N [7,11], and the benign environment for nitrate production [7]. If so, plasma N processes may offset some of the challenges associated with unsustainable NH3 fertilizer production by H–B. Additionally, nitrate, a component of plasma, is the most preferred form of N uptake by plants [36] in most aerated plant production environments. Therefore, applying plasma nitrate to crops as a fertilizer promises to improve yields as conventional nitrogenous fertilizers [26,37]. If successful, plasma-generated N may reduce overdependency on expensive traditional fertilizers, which could be especially important for resource-constrained subsistence farmers with the most nutrient-depleted soils in geographically remote regions.
However, despite a vast body of literature on plasma N production techniques, there are few experimental and field studies on its application to crops [7,11,15,26,37]. This limitation hinders nutrient use efficiency (NUE) improvements and reduces environmental footprints associated with plasma nitrogen fertilizer application in crop production. To overcome this challenge and realize the full potential of this technology, it is crucial to improve the release characteristics to reduce nutrient losses and increase NUE in crop production.
One potential solution to this issue is converting plasma N into a slow-release form. This approach may supply nutrients in a more targeted way, potentially reducing losses and increasing nitrogen use efficiency and crop yield. Nanoscale manipulation technology, exceptionally engineered nanotools, shows great promise. Nanotechnology is being explored as a potential solution to improve fertilizer use efficiency using nanofertilizers [22,38,39,40]. By using nutrient-charged porous nanomaterials in the form of polymers like cellulose [32], fertilizers can be applied less frequently [20], with a significant decrease in fertilizer required. This breakthrough has the potential to reduce environmental degradation [16,18,21] and increase NUE, water retention [32,33,38,39,40,41] and crop yields [20,41]. Ideally, nano-N fertilizers have nutrient carrier materials with pores on a nanometer scale of 1–100 nm particles. This helps slowly release essential nutrients to the soil over time, promoting optimal crop growth [38,40]. This innovative technology has the potential for a cleaner and more sustainable agricultural production process by enabling the smart production of greener, slow-release nitrogenous fertilizers with reduced application requirements and environmental footprints, especially in combination with plasma technology [7,16,26,30,42,43].
In conclusion, this review highlights the potential of plasma and nanotechnology to revolutionize the production of eco-friendly nitrogenous slow-release fertilizers. We are paving the way towards achieving multiple Sustainable Development Goals by developing fertilizers with cutting-edge nanoplasma N-based solutions. This approach addresses food insecurity and effectively tackles environmental concerns associated with crop production. By synthesizing a low-cost, slow-release nanofertilizer that is free of GHG emissions and allows for reduced amount and frequency of application, we can contribute to sustainable agriculture and boost crop productivity. These technologies have the potential to lead us towards a greener future, meeting the growing demand for food while reducing our carbon footprint.
2. Plasma N Fixation and Its Potential as a Fertilizer
Although the atmospheric air consists of 78% nitrogen gas, the triple bonds of N (N≡N) make it chemically inert [13] and unable to be directly assimilated by plants. Research in the last century focused on developing alternative, greener methods of nitrogen fixation [11,12]. Plasma processes are currently a priority for intensification and energy savings [7,11,14]. This is an attractive path towards developing a zero-natural gas consumptive, energy-efficient, fast, and environmentally benign nitrogen fixation process, especially in combination with catalysis [43]. The potential environmental benefits of this approach are inspiring, providing a ray of hope for a more sustainable future.
The production of nitrogen-based fertilizer in a plasma reactor involves a nitrogen fixation process. Plasma, the fourth state of matter, consists of ionized atoms and molecules. Plasma is subdivided into thermal and nonthermal plasma due to differences in reaction mechanisms, the energy efficiency of these processes, and the equipment required [14]. In a thermal plasma using arc and torch, electrons, ions, and background gas are at the same temperature of about 104 K [11]. The high-temperature requirement and low energy efficiency of thermal plasma have limited its implementation in nitrogen fixation. In contrast, nonthermal plasma using discharges such as pulsed corona, glow discharge, and dielectric barrier discharge (DBD), among others [7,11], offers a more energy-efficient solution. The fact that electrons are usually at high temperatures of between 104 and 105 K is made possible due to the smaller mass of electrons, while ions and background gas can remain at room temperature; hence, the nonequilibrium [14] is a testament to its energy efficiency. The extremely high temperature of the electrons drives the reaction and the relatively cool surrounding gas saves energy. This nonpolluting, nonthermal plasma technology is not just an attractive alternative to thermal plasma and the traditional Haber–Bosch processes that require high temperature, pressure, and energy [13,14], but is also a promising outlook for sustainable agriculture.
2.1. Nonthermal Plasma N Fertilizer Production
Nitrogen-based fertilizer in nonthermal plasma is produced through nitrogen fixation in a plasma reactor, involving converting N2 from the air into NOx. Nonthermal nitrogen fixation occurs after introducing an electrical charge to the gliding arc to activate the N2 molecule, which is later broken by vibration, creating a plasma (Equations (1) and (2)) [7,14,15]. The unstable N reacts with free oxygen molecules to form nitrogen oxide (Equations (3) [7,11,14,15] and (4)) [11,14,15], which undergoes further reactions (Equations (5) [11,15] and (6) [11,15]). The NO equilibrates by reacting with hydrogen ions (H+) in water to form plasma nitrate (Equation (7)) [11,15]. An attempt to supply H+ using H2 gas from hydrogen evolution reactions (HERs) may be an attractive option following large-scale generation without greenhouse gas emission [44]. If this can be confirmed, exploring the economics of producing H2 gas from cheap sources like water may enable the direct synthesis of ammonia in a plasma reactor [11,35]. Nevertheless, the potential practical application of HER still needs to be explored despite its critical importance in the scientific and industrial production of NH3 in plasma reactors. It is high time we recognized the significance of HER and conducted an extensive analysis to make the most of this resource.
Commonly available plasma reactors can produce 0.5 L of plasma nitrate per minute. Not only is this process energy efficient, consuming only 7.7 kWh/kg with a mass flow controller, but it may also promise a brighter potential to revolutionize greener pathways to produce NH3 sustainably. These energy-saving reactors can sustain a varying concentration range of nitrate in the solution at just 0.2 MJ/mol of NO [11,15]. Moreover, a catalyst can reduce the energy demand by 35% [14] by accelerating the reaction rate, making the process more efficient. The energy reach is far less than the 0.48 MJ/mol of NH3 of the energy-intensive Haber–Bosch process [11,15,35]. Such advantages make nonthermal plasma economically attractive [7]. Equations (1)–(7) summarize the different reaction mechanisms for nitrate synthesis in a plasma reactor.
e− + N2 → N2*(V) (Plasma)
e− + O2 → O2* (Plasma)
O + N2* → NO + N*
O2 + N* → NO + O
NO + O* ↔ NO2
N* + NO ↔ N2 + O*
3NO2 + H2O → 2HNO3 + NO
The dinitrogen species N2*(V), the plasma obtained by collisions with highly energetic electrons (Equation (1)), is vital in keeping the reaction temperature and energy consumption low. Upon forming plasma nitrogen, excessive energy stored in vibrational mode is efficiently delivered to carry out the chemical reactions to overcome the high activation barriers of dinitrogen reactions, as in Equations (1)–(7) [7,11,14,15]. Patil et al. [11] and Li et al. [7] showed much higher NO yield using catalysts such as MoO3 and WO3, which reduced the energy consumption of the process. Alternatively, VIVEX Engineering Ltd. (UK) [45] used high-voltage plasma reactors and a series of wet reactors as absorbers to produce HNO3 with a 15–35% concentration from the air. This method uses 20% less energy than other processes [11]. Thus, combining catalysts and reactor series can significantly reduce the energy requirement and increase the nitrate concentration. Hence, the process can reduce costs and facilitate easier acceptance on an industrial scale. In addition, the instant control and fast, one-step reaction make the system suitable for decentralized small-scale production of nitrogen fertilizer, for example, when using widely available solar energy.
2.2. The Flow Rate of NO from the Plasma Reactor
The plasma flow from production reactors greatly influences its consumptive demands as a fertilizer. Many studies have shown that plasma nitrogen flow rate and concentrations vary with the energy consumption during nonthermal plasma production of NO. For example, a study by Wu et al. [46] indicated that nitrate production at 2.0 L/minute is energy efficient. Generating polluting wastes is impossible, especially when the concentration is at or above 10% (Equation (6)). According to Patil et al. [11], targeting a nitrate concentration of 10% can effectively halve the energy needed for production to 33 GJ/ton N. However, concentrations above 15% require an energy input of 169 GJ/ton N. Therefore, reducing energy requirements is crucial for increasing nitrate concentration. Alternative ways to reduce energy requirements include optimizing flow rates using a series of efficient energy reactors.
On the other hand, studies have suggested that an enhanced plasma flow rate with the application of catalysts can reduce energy consumption [43]. Therefore, identifying a specific combination of catalysts and reactor series may improve the nitrate flow rate and concentration while maintaining the energy flow into the system. The increased concentration and flow would reduce the production cost of plasma nitrogen fertilizer.
2.3. Value Addition to Plasma N
The selective reduction of nitrates to desirable nitrogen compounds during the chemical process uses cathode metals such as Cu, Ru, Al, Zn, and alloys. These metals may effectively work in the presence of catalysts like Rh, Ir, Pd, Pt, Ag, and Au in the electrolysis process [47]. This process requires heat and electrical energy. It uses electrons as reductants, operates in ambient conditions, is fast, has high product turnover, and does not require secondary treatment [13,34]. Therefore, the electrochemical reduction of nitrate is currently the most promising among all the suggested processes for crystallizing plasma nitrate and turning it into higher-value products. These processes are beneficial in converting nitrate into recyclable ammonia, a valuable resource in the form of fertilizers, energy carriers, and chemical precursors [13,34,35]. This strategy’s benefits include the conversion of nitrates into valuable products, using renewable energy sources, and the potential for distributed application [13,34].
2.3.1. Electrosynthesis of Ammonia from Plasma Nitrate
Efficient chemical mechanisms require understanding nitrate-containing reactive intermediates, active species of catalysts, interactions between the two, and kinetic analysis. An example of turning ammonia into a treasure follows a reaction involving a reversible hydrogen electrode (RHE), shown below (Equation (8)). It involves eight electron transfers and many possible pathways [47,48,49], where EO is relative to the standard hydrogen electrode (SHE).
NO3− + 9H+ + 8e− → NH3 + 3H2 EO = −0.12 V vs. SHE
The energy for the RHE is far below the potentials for nitrate reduction reactions (NO3RR) directly through the half-cell reaction of Equation (9).
NO3− + 6H2O + 8e− → NH3 + 9OH− (0.69 V)
The electroreduction of nitrate effectively controls the adsorption of hydrogen atoms, with electron reduction occurring at the cathode. Initially, electrons reduce the adsorbed water on the cathode surface, forming Hads (H+) in the adsorbed hydrogen–intermediates pathway. The H+ then directly reduces nitrate to NH4+ through steps (Equation (10)) involving intermediates such as NO2−ads, NOads, NHads, NH2ads, and many more [34,47]. Because the energy requirement of Nads (0.75 eV) is higher than Hads (0.10 eV), N-H bond formation is kinetically more favorable than the N-N bond formation. In agreement with Zhen-yu et al. [47], the deprivation of H+ from active neighboring sites for N-N coupling pathways prevents N2 gas formation, promoting selectivity towards NH3. Under appropriate potential, NH2ads are formed directly from NO reduction (Equations (11)–(13)). However, as the potential becomes positive from −0.2 to 0.4 V, the energy range becomes favorable for increased N2 production at the expense of NH3 [13]. Hydrogen absorption weakens at a positive potential, leading to a slow hydrogenation rate in ammonia synthesis [34]. The enhanced adsorption of Hads with the introduction of a catalyst leads to the formation of ammonia.
HNO3(aq) → NO3−ads → NO2−ads → NOads → HNOads → H2NOads → NH2OHads → NH3 (0.10 eV)
NOads → NH2ads (0.1 eV)
NOads → N2Oads → N2 (0.75 eV)
NOads → N2Oads → N2O (0.75 eV)
2.3.2. Impact of Catalysts on Electroreduction of Nitrates to Ammonia
Nitrate electroreduction with catalysis at ambient conditions has consistently shown higher ammonia formation than the H–B process [48]. For instance, ammonia electrosynthesis from nitrate using Fe catalyst yields 20,000 μg h−1 mgcat.−1, as demonstrated in H–B studies [47,48]. Using a single Ru catalyst, an impressive yield of up to 55,000 μg h−1 mgcat.−1 in ammonia electroreduction is achievable [48]. This highly effective and efficient method could be the preferred choice to enhance the chemical conversion of plasma N into a more valuable ammonium product.
2.3.3. Effect of Nitrate Concentration on Ammonia Synthesis
The characteristics of ammonia formation vary with the nitrate concentration in the solution. While most catalysts show high faradaic efficiency (FE) for ammonia production [13,34,48,49,50], there is significant variation in current density, which is mostly low. However, production with high concentrations of NO3− has been the norm, as solutions with low concentrations of NO3− are highly affected by the suppression of the competing hydrogen evolution reaction (HER) [13]. Higher nitrate ion availability suppresses the HER, favoring ammonia formation [35]. For plasma N reactors with NO3− concentration as low as 1.0%, efficient recovery of NH3 could be limited, making the entire fertilizer production process costly (Table 1). Chen et al. [13] combined Ru and Cu catalysts to address low nitrate concentration. The FE increased to 99.9% at a very low potential (−0.135 V) and current density compared to using separate or single catalysts. The ammonia production rate was approximately 80,000 µg h−1 cm−2, significantly higher than nitrate reduction to ammonia on Ru or Fe single catalysts. Ru and Fe catalysts have a maximum FE of 75% and a lower ammonia yield rate [47], achieved at very low partial current density and potential of 100 mA cm−2 and −0.85 V, respectively. The increasing NH3 synthesis with decreasing trend in potential energy was similarly observed by Wang et al. [34] and Chen et al. [13].

Table 1.
Catalysts for the selective electrocatalytic reduction of nitrate to ammonia.
Conversely, a combination of Fe and other catalysts used by Yuhang et al. [49] resulted in a faradaic efficiency (FE) as low as 30% compared to the single use of catalysts such as Cu and Ru, indicating the need for suitable catalysts for a given nitrate concentration. Additionally, it could suggest the essentiality of an appropriate combination of catalysts at varying nitrate concentrations to maintain a high FE. Thus, combining catalysts to achieve high FE at both low and high NO3 concentrations could reduce the burden of the HER.
2.3.4. Crystallization of Liquid Ammonia to Ammonium Salt Fertilizers
The ammonia generated can be acid-trapped with HCl or NO3− followed by rotary evaporation to produce crystals of NH4Cl [13] or NH4NO3, respectively. The ammonium nitrate is suitable for a more economically sustainable fertilizer production, resulting in a higher nitrogen concentration than NH4Cl fertilizer. Due to increasing limitations in soil sulfur, ammonium sulfate can also substitute NH4Cl, given its relatively higher nitrogen composition. Fertilizer storage would pose a challenge to farmers; therefore, the explosion risk associated with NH4NO3 could make NH4Cl a safer choice. Thus, although slightly more costly, the NH4Cl production route would be much more favorable. This results from the economic advantages of internally generated nitrates in the plasma reactor compared to external sourcing of HCl.
2.4. Performance of Plasma N on Crop Production
The application of nitrate from a plasma reactor (Equation (7)) to crops as a fertilizer is still in its infancy. Punith et al. [26] reported enhanced growth with a 61% increase in the weight of tomato plants compared to plots without plasma nitrogen. In a study by Kabiri et al. [37], plasma nitrogen application on cereal crops resulted in growth responses similar to urea and calcium ammonium nitrate (CAN). Current plasma application to crops requires a high volume due to low nitrogen concentrations [37]. However, the large quantity of liquid plasma may be advantageous for fertigation, especially in dry farming when solar energy is at its peak. After identifying the best application method for plasma nitrogen fertilizer methods (i.e., foliar or drenching) leaching into deep soil layers, volatilization is minimized or prevented. In this regard, it may be essential to establish a plasma fertigation schedule suitable for different crops with varying nitrogen demands. Plasma nitrate transformed into ammonium salt can be a highly effective alternative to conventional mineral fertilizers using similar application methods. Therefore, plasma nitrogen production and the resulting ammonium fertilizer product from its electroreduction could be made available to farmers after evaluating its optimal use efficiency and environmental impacts from its application to crops.
2.5. Potential Use of Plasma N as Fertilizer
With the world facing significant challenges in fertilizer production, it is time to explore novel and innovative nonthermal plasma-assisted nitrogen fixation technology to augment nitrogen fertilizer production and mitigate the demand due to the reliance on the H–B process. This technology allows decentralized production to create a more sustainable future [7,26]. Local-scale production of fertilizer could reduce transport costs and its price. By producing fertilizer onsite and reducing transport costs, access to fertilizers could be improved. This flexibility could help meet varying application demands [4,8,17,54,55] for crop nitrogen recommendations, ultimately increasing yield and economic returns [56,57,58]. Plasma nitrogen applied to crops has performed similarly to other nitrogenous fertilizers [37]. If this is the case in many environments where nitrogen is needed, domesticating nonthermal plasma nitrogen production could be a greener alternative for providing the plant nitrogen requirements necessary to support the growing demand for food worldwide.
For example, Uganda aims for 200 kg ha−1 of fertilizer nutrients [55] but only imports 6.4 million tons annually [58]. Additionally, the available fertilizer falls far below the minimum recommended application target of 50 kg ha−1 set by the 2006 Abuja declaration, an African Union initiative to promote sustainable agricultural practices [59]. Through intensified decentralization of plasma technology alone, the government could achieve the goal of reducing the volume and value of imported inorganic fertilizers by 75% [60]. Therefore, local-scale plasma technology would create an opportunity to address other soil fertility challenges related to reported multiple nutrient deficiencies [10,61,62].
2.6. Limitation of Plasma N Fertilizer
The liquid plasma nitrate concentration needs an urgent increase to reduce fertilizer usage and facilitate handling and transport to farms. However, plasma nitrogen is prone to rapid leaching and volatilization, which could result in more severe environmental challenges than conventional fertilizers. Due to the liquid form of plasma nitrogen and its small ionic size of 2.8 Å [5], losses from soil can always be higher than conventional crystalized N fertilizers, even with split application. The nutrient loss is due to the small hydrated ionic radius (0.279 nm) of N compounds, particularly NO3−. Additionally, nitrate mobility in soil is estimated to be between 1–5 mM, higher than that of NH4+ compounds. Therefore, we suggest converting plasma nitrate to ammonium compounds for crop application.
A comprehensive evaluation of plant uptake, losses, and emissions throughout the fertilizer value chain would enable the development of intelligent application strategies. These strategies can help reduce labor-intensive repeated applications and excessive fertilizer use, which have been steadily depleting the natural nutrient balance of the soil [38,62,63]. These losses can also be mitigated using traditional methods such as conservation agriculture [23]. A more recent approach involves converting nutrients into nanoforms [64], which has shown the potential to reduce nutrient application quantities by 50% [38], especially when paired with other effective soil management practices [20]. Converting plasma nitrogen into nanoform could enhance nitrogen use efficiency (NUE) and decrease fertilizer needs [55,59], leading to significant economic benefits [58]. This could encourage smallholder farmers to adopt plasma fertilizer use, not only for its environmental benefits but also for its potential to improve their economic situation. Therefore, further research on nanoplasma nitrogen-based fertilizers is necessary to enhance plant nutrition, increase NUE, and decrease the potential environmental impact of plasma nitrogen compared to traditional fertilizers.
3. Mechanism of Nitrogen (Plasma N) Retention and Loss from Soil
The mechanism of ion–soil interaction involves adding and removing nutrients in the soil solution. Lost nutrients must be continuously replenished through fertilization or by releasing ions retained by soil colloids. Soil colloids, such as clay materials, can absorb nutrients through isomorphic substitution during the recrystallization of silicate minerals [65]. This compositional embedding of nutrients in the clay matrix acts as a lunchbox system for plant growth, reserving nutrients for uptake during optimal soil conditions like temperature, moisture, and pH [66].
Solutes in the soil solution and ions retained on soil particle surfaces are typically the most significant fraction of elements available to plants. Ions have sizes ranging from picometers (PM) 10−12 m to angstroms (Å) 10−10 m [5]. Clay particles have angstrom-sized regions where nutrients can be retained, with the particle’s charge strongly influencing sorption [65]. Specifically, nitrate ions, with a diameter of 2.8 Å, are negatively charged like clay and some organic matter. Depending on soil condition, this low electrostatic attraction to soil colloids can negatively impact leaching or loss.
Negatively charged colloidal surfaces attract more positively charged NH4+ than negatively charged NO3−, making NH4+ less mobile than NO3− [21,65]. Therefore, applying fertilizer in ammonium form can slow the release of nitrogen into the environment. Nitrate ions sorbed onto mobile clay lattice due to the transformation of NO3− fertilizers in the soil [21] may become unavailable to plants and microbes due to their small angstrom size.
Although nitrate composites are protected from losses, they are highly mobile and quickly move within soil pores, especially in sand and silt [5,65], potentially contaminating groundwater [21]. This contamination worsens with high levels of NO3− application, leading to increased hydrolysis and acidification that can sorb anions on a polymeric material larger than angstroms, like in the nanometer range (109). The nanosize can reduce ion sorption in clay lattice spaces and loss from the soil system, underscoring the critical need to understand and mitigate the potential environmental consequences of nitrate contamination.
Mitigation Strategies
Preventing nitrogen loss from soil can be achieved by combining techniques such as deep placement, inhibitors, liming, cover cropping, split application, optimal watering rate, and reduced soil compaction, among other best soil management practices [36]. Among the possible technologies, split applications improve the uptake of nutrients by plants through synchronized sigmoidal nutrient supply [67]. However, this practice needs to more adequately reduce losses due to the low retention of fertilizers in the soil [68]. Additionally, the time- and labor-intensive requirements discourage buy-in for split applications.
Developing slow-release compounds applied at appropriate frequencies might contribute to a more efficient fertilizer use in soil amendment. This approach can be applied to slow the nanodelivery of plasma nitrogen fertilizer. By utilizing nanomaterials with unique physical and chemical properties and a network of channels that retard nutrient solubility [38], nanodelivery systems can efficiently supply enough nutrients without losses throughout the entire growing season to meet plant demand for optimum growth [67].
4. Nanoparticle-Based Delivery System and Nanotechnology in Fertilizer Application
Delivery systems for fertilizers are crucial for reducing application frequency and controlling issues such as overapplication, leaching, soil chelation, runoff, and fertilizer nutrient volatilization. A controlled system ensures that only the necessary amounts of nutrients are released to meet plants’ biological demands without causing significant temporal or spatial environmental effects.
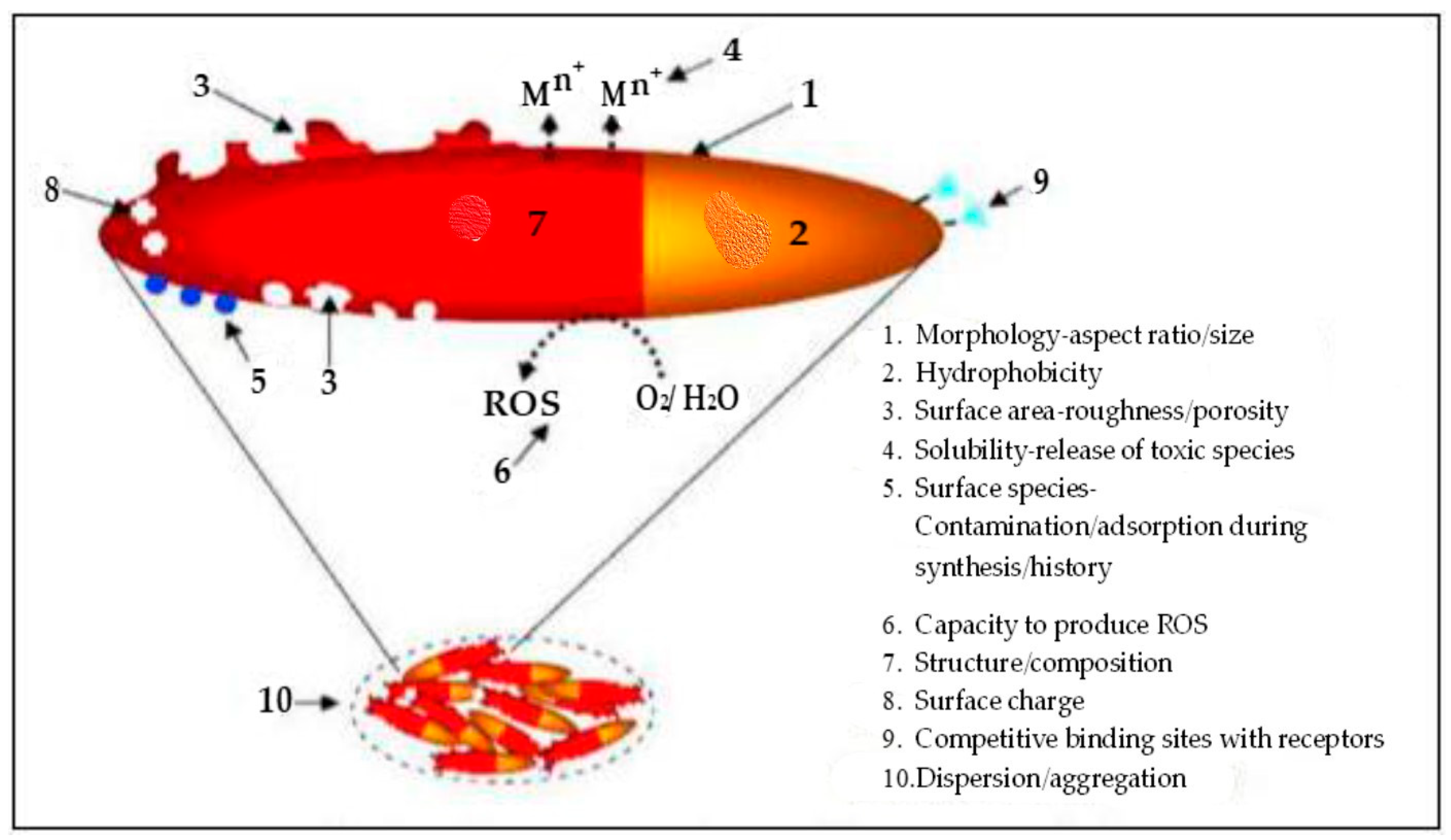
The process begins with producing a suitable adsorbent nanosized barrier with a large surface area [69]. The controlled release, based on the material’s slow-release characteristics design, is tailored to the specific environmental conditions [70]. The controlled release is achieved by strategically bonding nutrients to the nanomaterial [66], increasing the surface-area-to-volume ratio and making nanoparticles highly reactive (Figure 2). These reactions stem from the unique properties of nanoparticles, which differ from their macroscopic bulk counterparts and molecular structures [71].
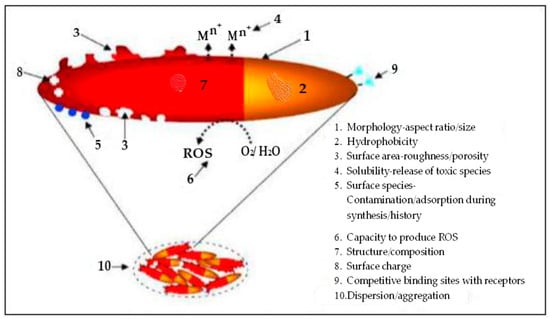
Figure 2.
Properties of nanoparticles. Adapted from Manjunatha et al. [38].
The approach relies on two fundamental properties: increased relative surface area and quantum effects. A nanoparticle is about 10 hydrogen atoms wide or 10–100 times larger than ions of nutrients or clay [38]. Other properties of nanoparticles, such as morphology, aspect ratio, hydrophobicity, adsorption during production, roughness, reactive oxygen species (ROS)-to-water ratio, capacity to produce ROS, high composition, and numerous binding sites (Figure 2), influence the solubility release of active nanoparticle ingredients [38].
Although the nanodelivery of fertilizer nutrients to plants is in its early stages, its application in agriculture for animal drug delivery is successful. The approach can be costly due to the price of materials for encapsulation or grafting nutrients. However, nanotechnology can reduce nutrient loss using nanoparticle capsules that delay delivery. The sustained release can reduce nutrient application rate and frequency, promoting effective plant absorption while minimizing environmental degradation [72].
Nanodelivery systems respond to environmental triggers such as soil texture, temperature, pH, and moisture [70]. When exposed to these external stimuli, crosslinked polymers undergo significant physical and chemical changes [73]. Physical stimuli, like magnetic and electric fields and temperature, directly control the energy level of the solvent–polymer system, prompting a response from the polymer at critical energy levels. Chemical stimuli, including pH and redox potential, also elicit a response from the polymer, altering the molecular interactions between the solvent and polymer. This adjustment can affect the amphiphilic balance or interactions between polymer chains, influencing electrostatic repulsion, crosslink-integrity, or the tendency for hydrophobic association. Therefore, research can tailor the amphiphilic nature of most nanodelivery systems to enhance nutrient release and reduce solubility under extreme soil conditions. This design can target responses to plant exudates during stressful situations [73,74]. A similar slow-release approach for nutrients in nanoparticle material was demonstrated by Stephen et al. [75], showing reduced emission, erodibility, and leaching, increased stress tolerance, higher crop yields, and improved NUE.
Nanodelivery materials can also act as biostimulants, containing hormones such as auxins, gibberellins, cytokinins, and triacontanol. These target primary and secondary pathways in leaves and root tissues to promote plant growth against various stresses [76]. Such diversity presents an opportunity to use nanofertilizers in foliar and soil applications. In a study by Hussein et al. [30], nanotechnology was used to convert banana peel extract into nanoform added value by enhancing its growth and promoting effects. The study demonstrated an increased tomato germination percentage from 14% to 97% by applying nanofertilizers from banana peel. Reviews by Manjunatha et al. [38] and Mejias et al. [20] showed increased yields and tripled NUE when using nanocoated fertilizers on crops. Therefore, there is a need to design a nanofertilizer blend that responds to defense mechanisms against plant nutrient stress. These nanodelivery approaches work against nutrient abiotic stresses and excessive fertilizer and water usage and losses, consistently decreasing environmental pollution from fertilizers [32]. As a result, the growing environmental regulations and unique properties of nanocellulose could promote its use as a valuable material for various agricultural applications, such as carriers for nanofertilizer formulation.
4.1. Nanoparticle Mobility Mechanism for Slow Release of Nitrogen in the Soil
4.1.1. Nanofertilizer–Soil Matrix Transport Mechanism
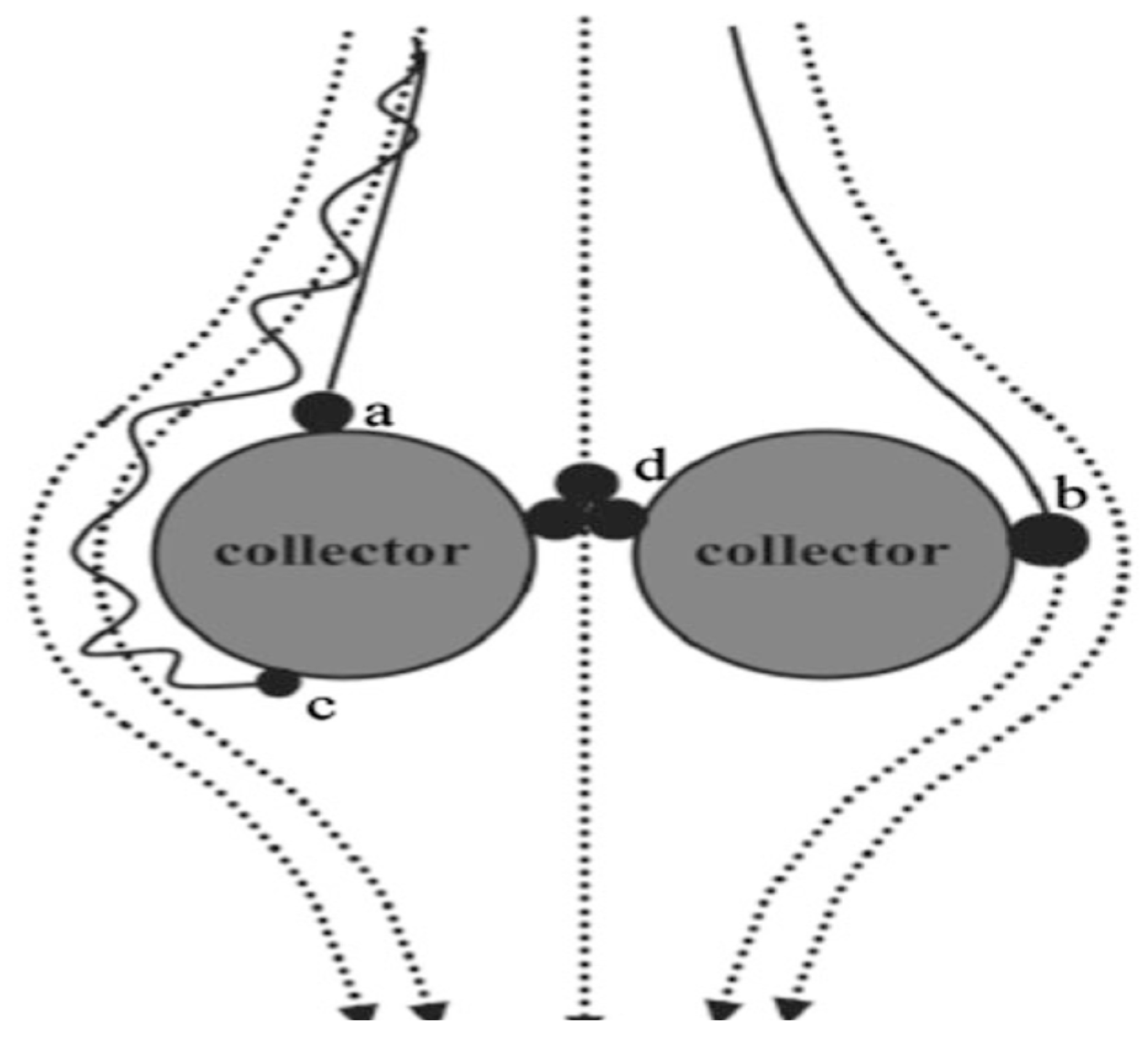
The transport of nutrients encapsulated in nanopolymeric materials depends on environmental factors such as soil pH, temperature, moisture, and texture, among others, which are influenced by the particles [70]. Larger-sized fertilizer particles enclosed in a semipermeable nanopolymer membrane regulate ion or ion-composite illuviation from soil pores. Aggregates of nanoparticles are too large to fit into micropores (Figure 3), allowing them to remain within pores [77]. Additionally, the cohesion of nutrient particles and adhesive forces between nutrients and polymer aggregate surfaces or polymer–soil interfaces increase aggregation at the soil–fertilizer interface, reducing losses (Figure 3). Even the mass of nutrients diffusing from the matrix to the soil is large enough to pass through micropores, leading to clogging and decreased ion mobility [66]. The retention ensures that nutrients are readily available for plant uptake. Aggregating nutrients at the nanoscale and providing a better coating, composite, or additives leverages the quantum effects of the particles. The effect occurs because nanoparticles can agglomerate to form large, clumps difficult to redisperse [75].
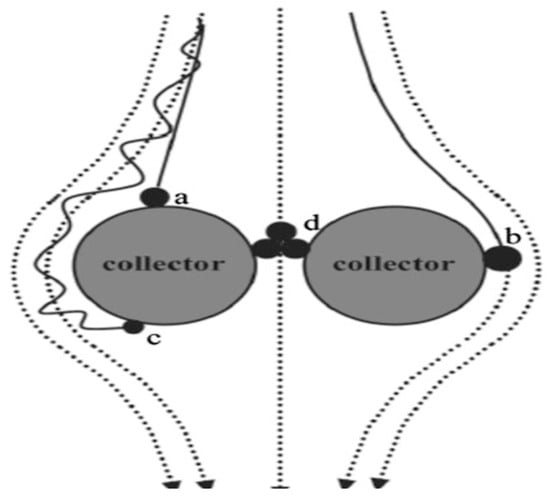
Figure 3.
Schematic diagrams for transport of formulated nanocellulose-coated fertilizer particles through filtration mechanisms. The black spheres, dotted lines, and thick lines stand for nanoparticles, the fluid streamlines, and the particle path (trajectory), respectively. The nanoparticles can attach to the collector through (a) gravitational sedimentation, (b) interception, and (c) Brownian diffusion. (d) Small pores may physically retain large aggregates through cohesive, adhesive, electrostatic, hydrophobic interaction, and van der Waal forces. Adapted from Mani and Mondal [66].
4.1.2. Mechanisms of Soil Moisture: Nanocoating on Nitrogen Release
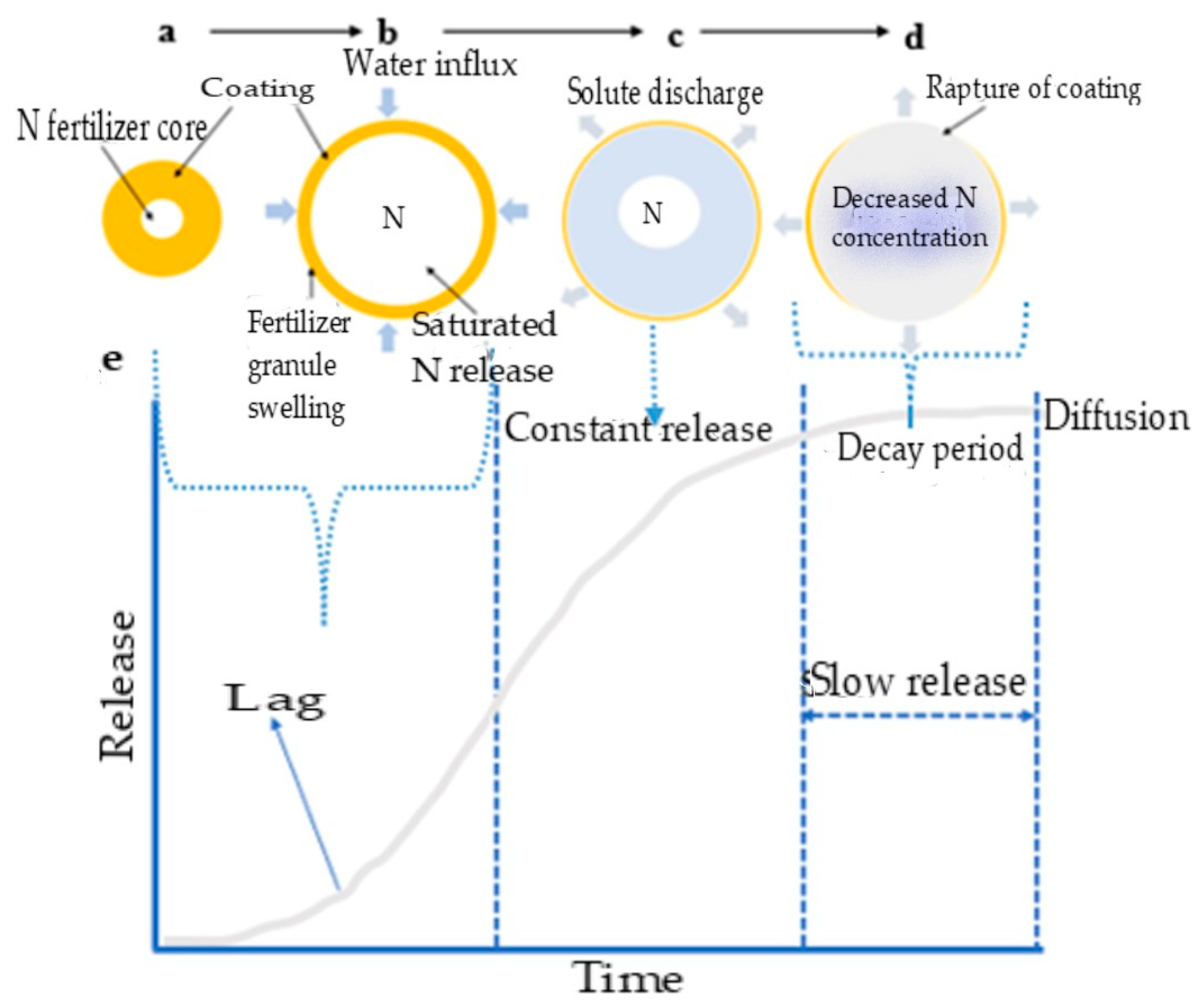
For the slow release of nutrients, soil moisture wets the cracks in the coating and penetrates the capsule (Figure 4a,b). This results in partial nutrient dissolution within the fertilizer core, leading to the buildup of osmotic pressure. This ‘lag’ occurs as filling the internal pores takes time and it establishes a steady state between water influx and solute discharge from the matrix [31,78]. As water penetrates the coating, more fertilizer granules dissolve and swell (Figure 4c). This swelling is due to the osmotic pressure buildup from accumulating a saturated solution to a critical water volume [66]. The concentration of the solution in the granules remains saturated, allowing for a slow, constant release of nutrients from the core fertilizer through diffusion from the capsule-coating membrane [65,66].
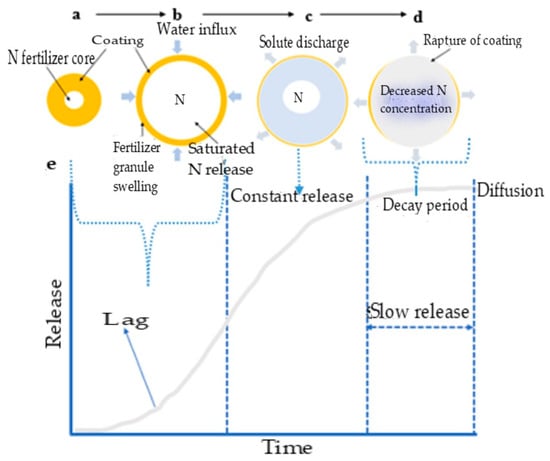
Figure 4.
Diagrammatic presentation of the mechanism of nutrient release in a sigmoid pattern. (a,b) soil moisture wets the cracks in the coating, and (b) penetrates the capsule, (c) water continuously penetrates the coating to dissolve the fertilizer further and swell more (d) the concentration and osmotic pressure decrease, leading to a reduced release rate, the ‘decay period’, and (e) sigmoid release pattern tailored through nano fertilizer formulation.
Once most fertilizer nutrients have been dissolved and released, the concentration and osmotic pressure decrease, leading to a reduced release rate, known as the ‘decay period’ (Figure 4d) [66]. The concentration and pressure gradients control the diffusion mechanism. In essence, this release mechanism follows a pattern of water absorption–swelling (Figure 4b)–diffusion (Figure 4c)–degradation of the polymer coating and dissolution (Figure 4d). This release mechanism allows for a sigmoid release pattern (Figure 4e) that can be tailored through fertilizer formulation to align with plant growth requirements [31]. This process mimics the gradual release of nutrients seen with split fertilizer applications [67]. However, the mechanical strength and modulus of the coating material heavily influence the release rate [25]. Consequently, the polymer obstruction of active ions increases the diffusion distance, resulting in a slower release.
4.1.3. Nanofertilizer Charge Influence towards the Slow-Release Mechanism of Nitrogen
Nanoparticles, such as nanocellulose, possess low sedimentation, degradation, amphiphilic properties, and an enormous specific surface area and quantum effect [66,75]. Nanocellulose, similar to organic matter, contains electronegative hydrophilic functional groups like hydroxyl (−OH), carboxyl (-COOH), phenolic (-C6H4OH), and amine (-NH2) [25,79]. These functional groups create pH-dependent surfaces that allow more electronegatively charged NO3− or N-containing compounds to be attached. Additionally, these materials serve as nanosized N polymeric carriers for nitrate, acting as a means of transporting ions to plants. Enhanced nutrient retention is further by the larger size of the soil solution [65].
Nanoscale fertilizers, additives, and coated nutrients can quickly accumulate in these contact zones, allowing for close interaction with the soil solution and reactive bodies. The free ions strengthen plants against nitrogen deficiency by facilitating free transport through molecular transporters. As a result, there is a reduced likelihood of nitrogen leaching, volatilization, and fixation in clay lattice spaces. Ultimately, nanoform fertilizers increase nitrogen availability and affinity for plants while minimizing environmental loss, making them environmentally friendly and efficient.
5. Materials for Transforming Plasma N Products into Slow-Release Forms
Nowadays, nanomaterials are mainly used to formulate nanofertilizers include carbon-based, polymeric, hydroxyapatite, nanoclays, mesoporous silica, nanoparticles, and materials such as nanocellulose [31,80,81]. Controlled slow-release fertilizers are commonly developed using superabsorbent hydrogels. Their use in fertilizer production is mainly limited by the scarcity of raw materials, high production costs, and low mechanical strength [32]. Nanocellulose is expected to have more significant potential given that plant cellulose is a sustainable feedstock and is the Earth’s most abundant polymeric raw material [25,27]. The renewability and ability to self-assemble into well-defined hard structures at multiple scales from nano- to microsize [25,39,82,83] make cellulose a multifunctional raw material with the potential to replace nonrenewable materials [25,29,83,84]. Therefore, cellulose material may reduce production costs to improve and generate high-value products.
5.1. Production of Cellulose Nanoparticles
Agro-based cellulose has gained attention as a valuable source of nanoparticles for coating fertilizers. Increasing agro-based cellulose use is due to its wide availability and renewability within the biomass economy, low density, cost, and energy biomass, as well as its high specific strength, modulus, and reactive surface [27]. These properties facilitate the grafting of particular groups into valuable and highly profitable nanocellulose products from nonvaluable waste [85]. Significant use of plant-based waste materials can, therefore, improve the ecosystem.
5.1.1. Major Plant Sources of Nanocellulose
The properties of nanoparticles, such as size, depend on the material and extraction methods used during synthesis. Due to their abundance in many local areas, materials commonly utilized include wood, bagasse, banana peel, cereal crop straws, hemp, cotton, and grass [25,27,30,86,87]. These materials contain cellulose as well as undesirable compounds like hemicellulose and lignin.
5.1.2. Extraction of Nanocellulose
Materials with high cellulose content and minimal purification requirements are preferred to prepare nanocellulose (Table 2) [25,27,85]. However, the amount of extractives removed depends on the material. Additionally, extractives decrease with the increased intensity of the chemical extraction process [27]. Therefore, a pretreatment process is necessary to eliminate ashes, waxes, hemicellulose, and lignin to improve cellulose quality [27]. Numerous studies have shown that pretreating plant materials enhances fibrillation and nanofibril extraction [27,31,84,87]. This pretreatment also reduces costs by decreasing the energy needed for nanocellulose production (from 30,000 kWh/ton to 1000 kWh/ton).

Table 2.
Properties and characteristics of nanocellulose substrates reliant on the cellulosic source and defibrillation method.
Among the pretreatment methods, alkaline hydrolysis is the most effective compared to acid hydrolysis, organosolvent treatment, ionic liquid treatment, and enzymatic treatment [24,30]. This is typically followed by bleaching, primarily hydrogen peroxide heated at 80 °C for 30 min [25,31,85]. The acid-bleached holo cellulosic materials are then washed with water or an alkaline solution to achieve ambient pH levels before drying [25,31].
5.1.3. Nanocellulose Isolation Methods
Nanocellulose is extracted from bleached holocellulosic material. Extractions are performed either by mechanical fibrillation into cellulose nanofibrils (CNFs), acid hydrolysis into crystalline nanocellulose (CNC) using HCl or H2SO4, or enzyme hydrolysis into bacterial nanocellulose (BNC) (Table 2) [25,31,85,87]. Although acid hydrolysis is the most common method [27], it is criticized for the wastewater generated during the washing process of nanocellulose. However, enzymatic hydrolysis requires a longer reaction time. The cost-effectiveness, speed, and environmentally friendly nature of mechanical fibrillation make it the preferred method to produce CNF. Several studies have shown superior nanoparticle properties, especially for CNC [25]. These nanoparticles come in various lengths, from 4 to 25 nm in diameter and up to 1000 nm in length [25,85]. Nanofibrils (CNFs) can have a larger diameter (20–90 nm) than CNC, despite being easier to produce [27]. A combination of their flexibility, strength, and aspect ratio offers numerous potential applications for using CNFs. However, no studies have compared CNFs and CNC in terms of the mechanical strength of fertilizers, especially nanoplasma N, against the cost implication of the production process. Nevertheless, given the superior properties of all nanocellulose forms, these two types have great potential to enhance the surface properties of plasma N fertilizer for improved targeted slow release of nutrients to crops.
6. Transformation of Plasma N into Nanoform Using Nanocellulose
Reduction of plasma nitrate to more valuable products could improve its utilization efficiency while reducing environmental risks. Most techniques for nitrate reduction have shown high post-processing costs and rigorous treatment conditions where they have been applied, such as in water treatment [34,40]. The physical and biological techniques are mainly direct processes. Converting plasma N to a slow-release nanoform may involve a series of processes dependent on many factors, including methods, cost, time, nutrient concentrations, availability of resources, efficiency, and general sustainability of the production system. The different processes may directly or indirectly convert the liquid nitrate to a more valuable crystallized nanoform [13,34,69,90,94,95,96]. Nanoparticles have been used in several processes, including chemical and biological controls and physical processes such as ion exchange and reverse osmosis (Table 3), especially for nitrate removal during water treatment [40,94].

Table 3.
Alternative methods for converting conventional inorganic fertilizers into a slow-release nanoform for improved response to crops.
The conventional direct denitrification of nitrate from wastewater produces less valuable dinitrogen [35]. Even the N-rich nanoparticles obtained by absorption from nitrate waste streams are not frequently applied to crops because of the small quantities of N generated [6]. Although rarely applied for fertilizer production, most of these methods have yet to utilize liquid plasma N as a nitrate-rich source for low-cost, high-value nanofertilizer compounds. For example, plasma-N-generated ammonia is a more valuable product that can easily be polymerized or encapsulated in cellulose nanoparticles to release N to crops more slowly (Table 3). Plasma nitrate fertilizer, especially in high concentrations, could, thus, be a cheaper and more efficient source for producing nano-N [34,35]. According to Wang et al. [34], the potential of using plasma N is even more likely broader, given the opportunity for the application of sustainable energy chemistry in the whole fertilizer production process.
6.1. Direct Absorption of Nitrate from Plasma N into Nanoform
6.1.1. Ion Exchange Process of Plasma N Absorption
In ion exchange (IE), chloride ions (Cl−) are commonly used to replace nitrates on resins (such as nanocellulose) in the exchange process. Plant cellulose nanoparticles can, therefore, serve as an efficient and cost-effective resin. Nanoparticles in IE can act as adsorbents (Table 3) to regulate the transport of nutrients and pollutants by controlling organic matter fixation and catalyzing the precipitation of new mineral phases [66,100]. Nanomaterials are also effective adsorbents, capable of removing up to 99.5% of nitrates from a solution [94]. These materials can accommodate 250% of their weight in nitrate, making them highly efficient [111,112]. However, sludge containing nanoparticles with retained nitrates would require secondary treatment.
6.1.2. Reverse Osmosis Process
In reverse osmosis (RO), displacement, rather than elimination, of containing compounds causes N accumulation [34]. Therefore, in RO, for nitrates and other minerals, if any, the semipermeable nanocellulose material absorbs the nitrate solution, allowing only water to pass through. This method has an efficiency as low as 25% and requires high infrastructure, energy, pressure, and maintenance. In this method, nitrate’s removal and absorption rate from the solution is similar to IE [69,113]. A review by El-Lateef et al. [96] showed that removal and absorption are most efficient at a ratio of 1.0 g of cellulose nanoparticles to 60 mL of solvent. With direct methods, such as adsorption methods, the removal rate is low, with minimal consideration of the potential value and hazards of the sludge as fertilizers (Table 3), which would be a major constraint to scaling up both the RO and IE processes in producing nanoplasma nitrogen.
6.1.3. Biological Process
The biological process involves microorganisms such as denitrification bacteria, fungi, and other beneficial organisms. In an appropriate environment where cyanobacteria have been used, nitrogen accumulation has reached 8–12% [6], making it a promising approach. Given the chemical composition of plasma N, under an appropriate bacterial growth environment, it can accumulate nitrogen without any limitation related to generating pathogenic bacteria observed with waste streams currently utilized as N sources [34]. These low-cost, nitrogen-rich materials form the fertilizer. The solids can slowly release the N nutrient more effectively than plasma N (Table 3). Additionally, the solids can be coated with cellulose nanoparticles to form many slower-release nanoforms, enhancing desirable nutrient-release characteristics. Although the biological process may be slower than others for producing large volumes of fertilizer, once biologically transformed into nanoplasma form and applied to soil, farmers may benefit from its enhanced advantages over plasma nitrogen fertilizer due to its likely low retention in the soils.
6.2. Indirect Absorption of Nitrate from Plasma N
Among the indirect processes, the chemical process is promising and can produce some of the safest and most valuable products. It involves crystallizing nitrate into solid compounds, as seen in the subsection above on transforming plasma N into ammonium salt compounds through electrocatalysis. This is followed by graft polymerization, encapsulation into synthesized cellulose nanoparticles, or emulsification (Table 3).
At this stage, the prepared ammonium crystals from plasma N electrocatalysis should be combined with nanocellulose particles. The methods for preparing nanofertilizer may vary depending on the desired release characteristics for a particular crop (Table 4).

Table 4.
Effects of nanofertilizers on crops nutrient use efficiency.
6.2.1. Graft Polymerization
The graft polymerization process involves the covalent bonding of monomers and side chains (the grafts) that are polymerized onto the main polymer chain or the backbone nanocarrier. This polymer grafting technique improves the polymer’s morphology and chemical and physical properties. Nanocellulose linear main chains can be randomly attached to crystal polymeric side chains, which may be of different chemical nature. These interactions generally result in a marked tendency to yield intramolecular phase separation in the bulk polymer [32,119].
Interest in graft copolymers may arise in part from the protection exerted by the grafts on the backbone. This feature has led to several applications such as surface modifying agents, compatibilizers in polymer blends, coating material, and emulsifiers. The grafting of polymers from solid surfaces can be implemented as an effective technique to control interfacial behavior [120]. The ‘grafting from’ method is assumed to result in higher grafting efficiency due to the lower steric hindrance of diffusing small monomers, as compared to the ‘grafting to’ approach, where larger polymer chains have to diffuse to the reactive sites that the already grafted chain may shield [119]. Most grafting studies have reported higher grafting densities due to the ‘grafting from’ method [119,121].
Lohmousavi et al. [32] prepared nanofertilizers from banana peel cellulose via graft polymerization method and found less than 70% N release at neutral pH. The release remained constant at 70% even after two hours. Water retention also significantly improved, reaching at least 20% after 30 days of applying the nanocellulose composite. Fortunately, Olad et al. [122] found that the release behavior of NPK-loaded hydrogel nanocomposite was in good agreement with the standard committee of European Normalization (EN) [123], indicating its slow release property. The EN states that ‘not more than a mass fraction of 15% of a nutrient is released in 24 h, while not more than a mass fraction of 75% of a nutrient is released in 28 days, and at least a mass fraction of 75% of a nutrient released at the stated release time’ [123].
Similarly, when Pereira et al. [124] grafted urea onto montmorillonite clay, the release of urea N in water was slowed by 20%. An analogous process for preparing such slow-release fertilizers can be applied to formulate nanoplasma N with improved slow release. In addition to delaying the N release by increasing the nanocontent of the matrix (Table 3), the mechanical strength of the matrix increased while the cumulative N2O emission was reduced by about 10 times [18]. This shows a significant reduction in GHG fluxes from fertilized agricultural fields [125,126,127]. Mani and Mondal, [66], Preetha and Balakrishnan [41], and Lawrencia et al. [31] reported over a 50% reduction in ammonia volatilization when the nanozeolite content of N fertilizers was increased to at least 6.25%. This increases the diffusion length through the host polymer [38].
Consequently, the polymer’s porosity, which obstructs the diffusion of the active materials for release, is reduced [66]. The slow N release by the nanoparticles is further enhanced through the reduction of ammonium volatilization by sequestering ammonium N on exchange sites [41,66]. This prevents undesirable nutrient loss, water, and air via direct internalization by crops (Table 4). However, despite graft nanofertilizer formulation proving to enhance climate-smart agriculture, validating nanobased plasma N nutrient release could provide a better understanding of synchrony with plant uptake demand.
6.2.2. Coating Method
Another preparation method involves coating fertilizer granules with capsules (Table 3). Pereira et al. [18] reported a nutrient load of 75% by weight of the nanocoated composites, which act as a structural matrix. Uzoh and Odera [101] coated urea with cellulose and found an N release rate of less than 70% even 30 days after application. The release was slower for nanocomposites with a size greater than 4.3 mm in coating thickness, with sigmoidal release observed for sizes between 6.4 and 8.5 mm. The coating thickness may depend on the coating techniques, with rotary drums and fluidized beds being the most common [31,128]. However, some studies [31] suggest that the fertilizer particle size is more influential in inducing the release rate than the coating thickness. They recommend improving the plasticity of the coating materials rather than increasing thickness.
Additionally, a review by Rahman et al. [16] shows that the size of nanocarriers is the most crucial factor. This is because most previous studies focused on the average size of initial nanoparticles. Interestingly, the minimum size of nanoparticles more significantly influences the nutrient uptake than their average size. Stephen et al. [75] emphasize the importance of different nanosize ranges, stating that 0–5 nm provides a catalytic effect. Therefore, the nanosize of a material can be a crucial factor for fertilizer coatings. Various studies indicate coating thicknesses in the range of 1–15% of the fertilizer [82]. However, a higher nanocellulose content of the fertilizer, mainly 40% by weight, is proposed as the maximum for providing the most extended N release duration [31,82,101]. Kassem et al. [82] reported a lower cumulative N release rate with an increasing weight percentage of nanoparticles up to 14.5% of the nanofertilizer composite. These coatings averaged 5.0 mm in thickness and were prepared using a fluidized bed coater. The N release rate, however, exceeded 75% by the 20th day, highlighting the need to optimize nanoparticle concentration for slow-release fertilizer standards [123]. Another study on coated fertilizers by Curtis et al. [129] found less than 80% N release between 28 and 46 days. In a study by Uzoh and Odera [101], nanocellulose-coated N release also followed a sigmoid release pattern, synonymous with nutrient demand for crop growth. This was mainly observed with increasing coating thickness between 4.3 and 8 mm. Therefore, for coating models [31,40], the size and concentration of nanoparticles, coating thickness, and size of the nanofertilizer composite should be considered for improved plasma N release.
However, when nanofertilizers are formulated through either grafting or coating, the application rate should be reduced (Table 4). Crop yields have reportedly improved by up to 30% compared to traditional fertilizers [20]. This is due to improved water and nutrient use efficiency, retention, and reduced losses [18,32,130], particularly with coated fertilizers (Table 4). Therefore, nanoplasma N fertilizer formulated by coating could be a better alternative than those formulated through grafting.
6.2.3. Nanoenabled Emulsions
Amphiphilic graft copolymers can play an essential role as emulsifiers due to differences in the solubility behavior of grafts and the main chain. The nanocarrier’s small size is also crucial for a slow release rate [16], increasing interest in emulsion formulations with nanoscale dimensions. Nanoenabled emulsions applied in foliar form have the potential to be safer and more effective (Table 4). This effect is attributed to preventing nutrient interaction with soil, air, water and microorganisms [38]. For instance, a review by Manjunatha et al. [39] reported that foliar application of 640 mg ha−1 nanophosphorus resulted in an 80 kg ha−1 P fertilizer equivalent yield of cluster beans and millet.
With a proper understanding of translocation mechanisms to various plant parts, nanoenabled plasma N-ammonium salt emulsion can likely be formulated to achieve specific crop growth targets during different periods. Using nanocellulose as a carrier [102] could replace nonrenewable metal oxides for N ion release into plant parts (Table 3), especially considering the lower toxicity of cellulose nanoparticles compared to metal oxides. However, stomata openings can only accommodate particles in the 40 nm size range [16,116]. The particles are distributed to other plant parts through phloem translocation based on the plant’s needs [78]. Nanoscale nitrogen particles, measuring 18–30 nm, enhance assimilation and metabolism by providing a larger surface area, equivalent to 10,000 times that of a 1.0 mm urea prill [116]. There are 55,000 nanonitrogen particles for every 1.0 mm urea prill by mass volume. Nanonitrogen particles with a 20 nm pore size can easily reach the cell wall through the plasma membrane, while particles with pore sizes of 20–50 nm enter the plant through stomata pores. Nanonitrogen particles with 40 nm pores actively traverse phloem cells through plasmodesmata [16], binding to carrier proteins via ion channels. Therefore, selecting the best preparation methods for nanocellulose with a thickness less than that of stomata openings (Table 2) could enhance the use of nanoenabled plasma N-ammonium salt emulsion. Cellulose nanocrystals (CNCs) are highly recommended for emulsion formulations due to their ability to produce smaller particles compared to other types of nanocellulose (Table 2) [25,27]. The process of nanofertilizer preparation significantly influences its properties and costs, which in turn affect the nutrient release rate and uptake by crops (Table 4). A formulation approach with low operating costs, a wide selection of materials, and various nanocellulose preparation methods would be more feasible for scaling up production. Therefore, nanoplasma N emulsion may be restricted by the availability of certain materials and preparation methods in the local environment.
7. Conclusions
Nonthermal plasma nitrogen fixation technology has a green environmental profile, offering a promising alternative, especially when combined with nanotechnology, to complement the Haber–Bosch fertilizer process. Enhancing plasma concentration through a series of reactors and catalysts could make achieving an energy target of less than 33 MJ/ton of nitrogen feasible, mainly when working with nitrate concentrations above 10%. Applying plasma N to crops is just as practical as traditional nitrogen fertilizers. However, low plasma nitrate levels may require high application rates, leading to increased losses and environmental nutrient levels. By applying the right concentration and methods to minimize nitrate losses from the soil, crop yields can be maximized to optimize nutrient use efficiency and minimize production costs by utilizing local-scale production using renewable resources at our disposal. Harnessing appropriate electrocatalytic conversion of plasma N to ammonium salt at high FE, especially with a combination of Ru-Cu catalysts, could enhance crop use efficiency for low and high plasma N concentrations. Further enhancing fertilizer through coating with plant-waste-based cellulose nanoparticles would improve the slow release of plasma nitrogen. The coating creates nanosized reinforcement to encase and form slow-release nanoparticles with superior properties, reducing the mobility of the fertilizer nutrient and creating a slow-release fertilizer with specific release characteristics. The mechanical strength of the fertilizer is also improved compared to coating with high-cost hydrogels. However, the isolation technique and plant material used as a source of nanoparticles determine the type of nanocellulose, thus impacting properties and application state. Nevertheless, the coating method provides higher water holding capacity, soil fertilizer retention, and efficient sigmoid fertilizer slow release, especially for nanofertilizer granules larger than 4.6 mm in diameter. These methods enhance affordability, sustainably reduce plasma nitrogen requirements, decrease the need for repeated applications and losses, and offer a greener approach to combat soil nutrient degradation and climate change.
Author Contributions
Conceptualization, S.K. (Stewart Kyebogola), S.K. (Stella Kabiri) and R.N.O.; Methodology, S.K. (Stewart Kyebogola) and R.N.O.; Formal analysis, S.K. (Stewart Kyebogola) and R.S.Y.; Investigation, S.K. (Stewart Kyebogola); Writing—original draft preparation, S.K. (Stewart Kyebogola); Writing—review and editing, S.K. (Stewart Kyebogola), S.K. (Stella Kabiri), R.N.O., O.S., R.S.Y. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Government of Uganda funded this research through the CCGS program of the National Agricultural Research Organization (NARO), grant number CCGS/6/VA06/21.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stoorvogel, J.J.; Smaling, E.M.A. Assessment of soil nutrient depletion in sub-Saharan Africa: 1983–2000. In Winand Staring Ctr., for Integrated Land, Soil and Water Research (CSC-DLO); Winand Staring Centre: Wageningen, The Netherlands, 1990; Volume l–4, Report No. 28. [Google Scholar]
- de Bruyn, L.L.; Jenkins, A.; Samson-liebig, S. Lessons Learnt: Sharing Soil Knowledge to Improve Land Management and Sustainable Soil Use. Soil Sci. Soc. Am. J. 2017, 81, 427–438. [Google Scholar] [CrossRef]
- Lal, R.; Bouma, J.; Brevik, E.; Dawson, L.; Field, D.J.; Glaser, B.; Hatano, R.; Hartemink, A.E.; Kosaki, T.; Lascelles, B.; et al. Soils and sustainable development goals of the United Nations: An International Union of Soil Sciences perspective. Geoderma Reg. 2021, 25, e00398. [Google Scholar] [CrossRef]
- FAO. Global Fertilizer Markets and Policies: A Joint FAO/WTO Mapping Exercise; FAO: Rome, Italy, 2023; pp. 1–17. [Google Scholar]
- Bhardwaj, A.K.; Arya, G.; Kumar, R.; Hamed, L.; Anosheh, H.P.; Jasrotia, P. Switching to nanonutrients for sustaining agroecosystems and environment: The challenges and benefits in moving up from ionic to particle feeding. J. Nanobiotechnol. 2022, 9, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Rev. Environ. Sci. Technol. 2014, 45, 37–41. [Google Scholar] [CrossRef]
- Li, S.; Medrano, J.A.; Hessel, V.; Gallucci, F. Recent Progress of Plasma-Assisted Nitrogen Fixation Research: A Review. Processes 2018, 6, 248. [Google Scholar] [CrossRef]
- African Union. Promotion of Fertilizer Production, Cross-Border Trade and Consumption in Africa; African Union: Addis Ababa, Ethiopia, 2019; pp. 1–24. [Google Scholar]
- Alewell, C.; Ringeval, B.; Borrelli, P.; Ballabio, C.; Robinson, D.A.; Panagos, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Kyomuhendo, P.; Tenywa, M.M.; Semalulu, O.; Lenssen, A.W.; Yost, R.S.; Mazur, R.E.; Kyebogola, S.; Goettsch, L.H. Limiting Nutrients for Bean Production on Contrasting Soil Types of Lake Victoria Crescent of Uganda. Afr. Crop Sci. J. 2018, 26, 543–554. [Google Scholar] [CrossRef]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Stahl, D.A.; Clark, D. Brock Biology of Microorganisms, 13th ed.; Pearson Education, Inc.: Wageningen, The Netherlands, 2012. [Google Scholar]
- Chen, F.; Wu, Z.; Gupta, S.; Rivera, D.J.; Lambeets, S.V.; Pecaut, S.; Yoon, J.; Kim, T.; Zhu, P.; Finfrock, Y.Z.; et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 2022, 17, 759–770. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; Li, Z.; Niu, G.; Tang, J. Non-thermal plasma fixing of nitrogen into nitrate: Solution for renewable electricity storage? Front. Optoelectron. 2018, 11, 92–96. [Google Scholar] [CrossRef]
- Rahman, H.; Haque, K.M.S.; Hossain, Z. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- World Bank. Global Market Outlook: Trends in Global Agricultural Commodity Prices Food Price Inflation Dashboard; World Bank: Washington, DC, USA, 2024; Update 30 May 2024. [Google Scholar]
- Pereira, E.I.; Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. Novel Slow-Release Nanocomposite Nitrogen Fertilizers: The Impact of Polymers on Nanocomposite Properties and Function. Ind. Eng. Chem. Res. 2015, 54, 3717–3725. [Google Scholar] [CrossRef]
- Fungo, B.; Chen, Z.; Butterbach-bahl, K.; Lehmannn, J.; Saiz, G.; Braojos, V.; Kolar, A.; Rittl, T.F.; Tenywa, M.; Kalbitz, K.; et al. Nitrogen turnover and N2O/N2 ratio of three contrasting tropical soils amended with biochar. Geoderma 2019, 348, 12–20. [Google Scholar] [CrossRef]
- Mejias, J.H.; Salazar, F.; Amaro, L.P.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Front. Environ. Sci. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Hussain, Z.; Cheng, T.; Irshad, M.; Khattak, R.A.; Yao, C.; Song, D.; Mohiuddin, M. Bentonite clay with different nitrogen sources can effectively reduce nitrate leaching from sandy soil. PLoS ONE 2021, 17, e0278824. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Fungo, B.; Henry, N.; Johannes, L.; Karsten, K. Ammonia and nitrous oxide emissions from a field Ultisol amended with tithonia green manure, urea, and biochar. Biol. Fertil. Soils 2019, 55, 135–148. [Google Scholar] [CrossRef]
- Pandey, J.K.; Takagi, H.; Nakagaito, A.N.; Kim, H. Handbook of Polymer Nanocomposites. Processing, Performance and Application; Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Springer Nature: Dordrecht, The Netherlands, 2015; pp. 15–26. ISBN 978-3-642-45232-1. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Punith, N.; Harsha, R.; Lakshminarayana, R.; Hemanth, M.; Anand, M.S.; Dasappa, S. Plasma Activated Water Generation and its Application in Agriculture. Adv. Mater. Lett. 2019, 10, 700–704. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Das, R.; Gaur, P. Preparation of Chitosan Nanoparticles and their In-vitro Characterization. Int. J. Life-Sci. Sci. Res. 2018, 4, 1713–1720. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Vizireanu, S.; Nicolae, C.A.; Frone, A.N.; Casarica, A.; Carpen, L.G.; Dinescu, G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials 2018, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Shaarawy, H.H.; Hussien, N.H.; Hawash, S.I. Preparation of nano-fertilizer blend from banana peels. Bull. Natl. Res. Cent. 2019, 43, 26. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, K.S.; Low, S.Y.D.; Goh, H.L.; Goh, K.J.; Ruktanonchai, R.U.; Soottitantawat, A.; Lee, H.L.; Tang, Y.S. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Lohmousavi, S.M.; Heidari, H.; Abad, S.; Noormohammadi, G.; Delkhosh, B. Synthesis and characterization of a novel controlled release nitrogen-phosphorus fertilizer hybrid nanocomposite based on banana peel cellulose and layered double hydroxides nanosheets. Arabian J. Chem. 2020, 13, 6977–6985. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Zhang, A.; Yang, Z. Synthesis of a slow-release fertilizer composite derived from waste straw that improves water retention and agricultural yield. Sci. Total Environ. 2021, 768, 144978. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, M.; Yu, Y.; Zhang, B. Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 2012, 50, 6720–6733. [Google Scholar] [CrossRef]
- Van Langevelde, P.H.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Nitrate Reduction for Sustainable Ammonia Production. Joule 2021, 5, 290–294. [Google Scholar] [CrossRef]
- Brady, N.C. The Nature and Properties of Soils, 9th ed.; Macmillan Publishing Company: New York, NY, USA, 1984. [Google Scholar]
- Kabiri, S.; Gallucci, F.; Li, S.; Hessel, V.; Greunen, V.D.; Tetteh, F.; Soares, F.; Lang, J. On-site air- to fertilizer mini-plants relegated by the sensor-based ICT technology to foster African agriculture. Tech. Rep. 2022. Available online: https://library.wur.nl/WebQuery/leap4fnssa-projects/partnership/34 (accessed on 23 February 2023).
- Manjunatha, R.; Naik, D.; Usharani, K.V. Nanotechnology application in agriculture: A review. J. Pharmacogn. Phytochem. 2019, 8, 1073–1083. [Google Scholar]
- Manjunatha, S.; Biradar, D.; Aladakatti, Y. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Wang, Y.; Li, J.; Yang, X. The diffusion model of nutrient release from membrane pore of controlled release fertilizer. Environ. Technol. Innov. 2021, 31301843, 102256. [Google Scholar] [CrossRef]
- Preetha, P.S.; Balakrishnan, N. A Review of Nano Fertilizers and Their Use and Functions in Soil. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3117–3133. [Google Scholar] [CrossRef]
- Kumar, N.; Ram, S.; Venkatesh, K.; Tripathi, S.C. Global trends in use of nano-fertilizers for crop production: Advantages and constraints: A review. Soil Tillage Res. 2023, 228, 105645. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Germano, D.L.; Domenico, L.A.D.; Susana, I.T.C.D. Electrochemistry Plasmon-enhanced electrochemistry: A sustainable path for molecular sensing and energy production. Curr. Opin. Electrochem. 2024, 43, 101422. [Google Scholar] [CrossRef]
- VIVEX Engineering Ltd. Cold Plasma Nitric Oxide Process. 2014. Available online: https://www.vivex.co.uk/ (accessed on 2 April 2023).
- Wu, S.; Thapa, B.; Rivera, C.; Yuan, Y. Nitrate and nitrite fertilizer production from air and water by continuous flow liquid-phase plasma discharge. J. Environ. Chem. Eng. 2020, 9, 104761. [Google Scholar] [CrossRef]
- Zhen-yu, W.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Lei, F.; Li, L.; Chan, A.W.M.; Xu, L.; et al. Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef]
- Yuhang, W.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Luo, M.; Nam, D.; Tan, C.; Ding, Y.; et al. Enhanced nitrate-to-ammonia activity on copper- nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Blair, S.J.; Nielander, A.C.; Schwalbe, J.A.; Koshy, D.M.; Cargnello, M.; Jaramillo, T.F. Electrolyte Engineering for Efficient Electrochemical Nitrate Reduction to Ammonia on a Titanium Electrode. ACS Sustain. Chem. Eng. 2020, 8, 2672–2681. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Y.; Wang, C.; Ling, Y.; Yu, Y.; Zhang, B. Boosting Selective Nitrate Electroreduction to Ammonium by Constructing Oxygen Vacancies in TiO2. ACS Catal. 2020, 10, 3533–3540. [Google Scholar] [CrossRef]
- Chen, G.F.; Yuan, Y.; Jiang, H.; Ren, S.Y.; Ding, L.X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. Engl. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Place, F.; Barrett, C.B.; Freeman, H.A.; Ramisch, J.J.; Vanlauwe, B. Prospects for integrated soil fertility management using organic and inorganic inputs: Evidence from smallholder African agricultural systems. Food Policy 2003, 28, 365–378. [Google Scholar] [CrossRef]
- Ministry of Agriculture Animal Industry and Fisheries, MAAIF. Natl. Fertil. Policy 2016, 4, 1–28. Available online: https://faolex.fao.org/docs/pdf/uga172925.pdf (accessed on 15 April 2023). [CrossRef]
- Kaizzi, K.C.; Byalebeka, J.; Semalulu, O.; Newton, I.; Zimwanguyizza, W.; Nansamba, A.; Odama, E.; Musinguzi, P.; Ebanyat, P.; Hyuha, T.; et al. Optimizing smallholder returns to fertilizer use: Bean, soybean and groundnut. Field Crops Res. 2012, 127, 109–119. [Google Scholar] [CrossRef]
- Kaizzi, K.C.; Angella, N.; Musisi, K.F. Optimizing Fertilizer Use within the Context of Integrated Soil Fertility in Uganda. In Fertilizer Use Optimization in Sub-Saharan Africa; Wortman, S.C., Sones, K., Eds.; CABI: New York, NY, USA, 2017; pp. 193–209. ISBN 978-1-78639-204-6. [Google Scholar] [CrossRef]
- Sommer, R.; Bossio, D.A.; Tamene, L.; Dimes, J.; Kihara, J.; Kaola, S.; Mango, N.; Rodriquez, D.; Thierfelder, C.; Winowiecki, L. Profitable and Sustainable Nutrient Management Systems for East and Southern African Smallholder Farming Systems—Challenges and Opportunities: A Synthesis of the Eastern and Southern Africa Situation in Terms of Past. 2013. Issue January. Available online: https://www.researchgate.net/publication/303798228_Profitable_and_Sustainable_Nutrient_Management_Systems_for_East_and_Southern_African_Smallholder_Farming_Systems_-_Challenges_and_Opportunities?channel=doi&linkId=5752da7208ae02ac1277ff02&showFulltext=true (accessed on 30 September 2024).
- Winnie, N.; Giweta, M.; Gweyi-onyango, J.; Mochoge, B.; Mutegi, J.; Nziguheba, G.; Masso, C. Assessment of the 2006 Abuja Fertilizer Declaration With Emphasis on Nitrogen Use Efficiency to Reduce Yield Gaps in Maize Production. Front. Sustain. Food Syst. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Ministry of Finance Planning and Economic Development, MoFPED. The Republic of Uganda National Budget Framework Paper. 2022. Issue December 2021. Available online: https://budget.finance.go.ug/sites/default/files/National%20Budget%20docs/National%20Budget%20Framework%20Paper%20FY%202021-22.pdf (accessed on 6 April 2023).
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and Environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- Vanlauwe, B.; Giller, K.E. Popular myths around soil fertility management in sub-Saharan Africa. Agric. Ecosyst. Environ. 2006, 116, 34–46. [Google Scholar] [CrossRef]
- Palanisamy, S.; Subramaniam, B.S.; Thangamuthu, S.; Nallusamy, S.; Rengasamy, P. Review on Agro-Based Nanotechnology through Plant-Derived Green Nanoparticles: Synthesis, Application and Challenges. J. Environ. Sci. Public Health 2021, 5, 77–98. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Bohn, H.L.; McNeal, B.L.; O’Connor, G.A. Soil Chemistry, 3rd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Mani, P.K.; Mondal, S. Agri-Nanotechniques for Plant Availability of Nutrients; Springer: Cham, Switzerland, 2016; Volume 11, pp. 263–303. ISBN 9783319421544. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Musyoka, M.W.; Adamtey, N.; Muriuki, A.W.; Bautze, D.; Karanja, E.N.; Komi, M.M. Nitrogen leaching losses and balances in conventional and organic farming systems in Kenya. Nutr. Cycl. Agroecosyst. 2019, 114, 237–260. [Google Scholar] [CrossRef]
- Öztürk, N.; Bektas, T.E. Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. 2004, 112, 155–162. [Google Scholar] [CrossRef]
- Mikkelsen, R. Nanofertilizer and Nanotechnology: A quick look. Better Crop. 2018, 102, 9–11. [Google Scholar] [CrossRef]
- Madkour, L.H. Introduction to Nanotechnology (NT) and Nanomaterials (NMs). In Nanoelectronic Materials; Madkour, Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–47. [Google Scholar]
- Lakkis, J.M. Encapsulation and Controlled Release Technologies in Food Systems, 2nd ed.; Wiley Blackwell: West Sussex, UK, 2016; ISBN 9781118733523. [Google Scholar]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Peng, M.; Hudson, D.; Schofield, A.; Tsao, R.; Yang, R.; Gu, H. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J. Exp. Bot. 2008, 59, 2933–2944. [Google Scholar] [CrossRef]
- Stephen, K.; Helen, J.; Peter, D. Nanoparticle. Encyclopedia Britannica. 14 May 2019. Available online: https://www.britannica.com/science/nanoparticle (accessed on 10 August 2022).
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 2, 18–23. [Google Scholar] [CrossRef]
- Kumar, R.; Rawat, K.S.; Mishra, A.K. Nanoparticles in the soil environment and their behaviour: An overview. J. App. Nat. Sci. 2012, 4, 310–324. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier Ltd.: Landon, UK, 2012; Chapter 2, Issue 1948; pp. 7–47. ISBN 9780123849052. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Liu, X.; Liao, J.; Song, H.; Yang, Y.; Guan, C.A. Biochar-Based Route for Environmentally Friendly Controlled Release of Nitrogen: Urea-Loaded Biochar and Bentonite Composite. Sci. Rep. 2019, 9, 9548. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.; Ablouh, E.; Bouchtaoui, F.E.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Ghalfi, H.; Alami, J.; Achaby, M.E. Cellulose nanocrystals-filled poly (vinyl alcohol) nanocomposites as waterborne coating materials of NPK fertilizer with slow release and water retention properties. Int. J. Biol. Macromol. 2021, 189, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Vicente, A.A.; Menegalli, F.C. Cellulose nano fibers produced from banana peel by chemical and mechanical treatments: Characterization and cytotoxicity assessment. Food Hydrocoll. 2018, 75, 192–201. [Google Scholar] [CrossRef]
- Sun, D.; Onyianta, A.J.; Rourke, D.O.; Perrin, G.; Lip, C.P.; Saw, H.; Cai, Z.; Dorris, M.; Dorris, M. A process for deriving high quality cellulose nanofibrils from water hyacinth invasive species. Cellulose 2020, 27, 3727–3740. [Google Scholar] [CrossRef]
- Gupta, G.K.; Shukla, P. Lignocellulosic Biomass for the Synthesis of Nanocellulose and Its Eco-Friendly. Front. Chem. 2020, 8, 601256. [Google Scholar] [CrossRef]
- Pandey, J.K.; Kim, C.S.; Chu, W.S.; Lee, C.S.; Jang, D.Y.; Ahn, S.H. Evaluation of morphological architecture of cellulose chains in grass during conversion from macro to nano dimensions. e-Polymers 2009, 9, 1221–1235. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Velásquez-Cock, J.; Castro, C.; Gañán, P.; Osorio, M.; Putaux, J.L.; Serpa, A. Influence of the maturation time on the physico-chemical properties of nanocellulose and associated constituents isolated from pseudostems of banana plant c.v. Valery. Ind. Crops Prod. 2016, 83, 551–560. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohyd. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef]
- Yang, X.; Han, F.; Xu, C.; Jiang, S.; Huang, L.; Liu, L. Effects of preparation methods on the morphology and properties of nanocellulose (NC) extracted from corn husk. Ind. Crops Prod. 2017, 109, 241–247. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yaschenko, O.V.; Shniruk, O.M. Preparation and properties of nanocellulose from organosolv straw pulp. Nanoscale Res. Lett. 2017, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Camarero Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Q.; Han, Z.; Li, J.; Liao, B.; Hu, L. Comparison of bacterial nanocellulose produced by different strains under static and agitated culture conditions. Carbohyd. Polym. 2020, 227, 115323. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Gao, W.; Zhang, Y.; Yang, Y. Selective nitrate removal from aqueous solutions by a hydrotalcite-like absorbent FeMgMn-LDH. Sci. Rep. 2020, 10, 12126. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; Al-fengary, A.E.; Elrouby, M. Removal of the Harmful Nitrate Anions from Potable Water Using Different Methods and Materials, including Zero-Valent Iron. Molecules 2022, 27, 2552. [Google Scholar] [CrossRef]
- Khan, H.A.; Naqvi, S.R.; Mehran, M.T.; Khoja, A.H.; Niazi, M.B.K.; Juchelková, D.; Atabani, A. A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture. Chemosphere 2021, 285, 131382. [Google Scholar] [CrossRef]
- Wang, Y.; Shaghaleh, H.; Hamoud, Y.A.; Zhang, S.; Li, P.; Xu, X.; Liu, H. Synthesis of a pH-responsive nano-cellulose/sodium alginate/MOFs hydrogel and its application in the regulation of water and N-fertilizer. Int. J. Biol. Macromol. 2021, 187, 262–271. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, D.; He, L.; Zhong, N.; Yu, M.; Zhang, X.; Wu, Z. Fabrication of a high-performance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustain. Chem. Eng. 2015, 3, 645–653. [Google Scholar] [CrossRef]
- Azadbakht, P.; Pourzamani, H.; Rahman, S.; Petroudy, J.; Bina, B. Removal of nitrate from aqueous solution using nanocrystalline cellulose. Intl. J. Environ. Health Eng. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Uzoh, C.F.; Odera, S.R. Lag, Constant and Decay Release Encapsulated Urea as a Function of Coating Thickness Using Different Empirical Models. In Microencapsulation—Processes, Technologies and Industrial Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J. Colloid Interf. Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef]
- Cai, D.; Wu, Z.; Jiang, J.; Wu, Y.; Feng, H.; Brown, I.G.; Chu, P.K.; Yu, Z. Controlling nitrogen migration through micro-nano networks. Sci. Rep. 2014, 4, 3665. [Google Scholar] [CrossRef]
- Rop, K.; Karuku, G.N.; Mbui, D.; Michira, I.; Njomo, N. Formulation of slow release NPK fertilizer (cellulose-graft-poly (acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agric. Sci. 2018, 63, 163–172. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Liu, X.M.; Feng, Z.B.; Zhang, F.D.; Zhang, S.Q.; He, X.S. Preparation and testing of cementing and coating nano sub nanocomposites of slow/controlled-release fertilizer. Agric. Sci. 2006, 5, 700–706. [Google Scholar] [CrossRef]
- Helal, M.I.; El-Mogy, M.M.; Khater, H.A.; Fathy, M.A.; Ibrahim, F.E.; Li, Y.C.; Tong, Z.; Abdelgawad, K.F. A Controlled-Release Nanofertilizer Improves Tomato Growth and Minimizes Nitrogen Consumption. Plants 2023, 12, 1978. [Google Scholar] [CrossRef]
- AlShamaileh, E.M.; Al-Rawajfeh, A.E.; Alrbaihat, M.R. Solid-state mechanochemical synthesis of Kaolinite-Urea complexes for application as slow release fertilizer. J. Ecol. Eng. 2019, 20, 267–276. [Google Scholar] [CrossRef]
- Rudmin, M.; Makarov, B.; López-Quirós, A.; Maximov, P.; Lokteva, V.; Ibraeva, K.; Kurovsky, A.; Gummer, Y.; Ruban, A. Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate. Materials 2023, 16, 6080. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Tomar, R. Use of surface modified inorganic nano materials as slow release nitrogen fertilizer. In Sustainable Agricultural Development: Recent Approaches in Resources Management and Environmentally-Balanced Production Enhancement; Springer: Berlin/Heidelberg, Germany, 2011; pp. 171–184. [Google Scholar] [CrossRef]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O.; Neamt, C. Preparation and Properties of Novel Slow-Release NPK Agrochemical Formulations Based on Poly (acrylic acid) Hydrogels and Liquid Fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Hosny, A.; Saad-Allah, K. Reducing nitrogen leaching while enhancing growth, yield performance and physiological traits of rice by the application of controlled-release urea fertilizer. Paddy Water Environ. 2021, 19, 173–188. [Google Scholar] [CrossRef]
- Li, J.; Jin, Q.; Liang, Y.; Geng, J.; Xia, J.; Chen, H.; Yun, M. Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method. Int. J. Environ. Res. Public Health 2022, 19, 1890. [Google Scholar] [CrossRef] [PubMed]
- Deo, H.R.; Chandrakar, T.; Srivastava, L.K.; Nag, N.K.; Singh, D.P. Effect of Nano-DAP on yield, nutrient uptake and nutrient use efficiency by rice under Bastar plateau. Pharm. Innov. 2022, 11, 1463–1465. Available online: https://www.thepharmajournal.com/archives/?year=2022&vol=11&issue=9&ArticleId=15550 (accessed on 24 September 2024).
- Al-uthery, H.W.A.; Al-shami, Q.M.N. Impact of fertigation of nano NPK fertilizers, nutrient use efficiency and distribution in soil of potato (Solanum tuberosum L.). Plant Arch. 2019, 19, 1087–1096. [Google Scholar]
- Kumar, Y.; Singh, T.; Raliya, R.; Tiwari, K.N. Nano Fertilizers for Sustainable Crop Production, Higher Nutrient Use Efficiency and Enhanced Profitability. Indian J. Fert. 2021, 17, 1206–1214. [Google Scholar]
- Chhowalla, M. Slow Release Nanofertilizers for Bumper Crops. ACS Cent. Sci. 2017, 3, 156–157. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Coulter, J.A.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Gan, Y. Slow-release fertilizer improves the growth, quality, and nutrient utilization of wintering Chinese chives (Allium tuberosum rottler ex spreng.). Agronomy 2020, 10, 381. [Google Scholar] [CrossRef]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellattif, M.H.; Farghaly, T.A. Polymer Grafting and its chemical reactions. Front. Bioeng. Biotechnol. 2023, 10, 1044927. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Rawlins, J.W.; Ray, P. Polymer Grafting and Crosslinking; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bhattacharjee, C.; Sen, D.; Bengal, W.; Bengal, W. Encyclopedia of Membranes; Springer-Verlag GmbH: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-44324-8. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhani, A. Materials Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. 2018, 90, 333–340. [Google Scholar] [CrossRef]
- EN 13266:2001; Slow-Release Fertilizers—Determination of the Release of the Nutrients—Method for Coated Fertilizers. European Committee for Standardization: Brussels, Belgium, 2002; Volume 3.
- Pereira, E.I.; Minussi, F.B.; Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C. Urea—Montmorillonite-Extruded Nanocomposites: A Novel Slow- Release Material. J. Agric. Food Chem. 2012, 60, 5267–5272. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, S.; Shen, G.; Shaaban, M.; Ju, W.; Cui, Y.; Duan, C.; Fang, L. Effects of inorganic and organic fertilizers on CO2 and CH4 fluxes from tea plantation soil. Elementa 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Mosongo, P.S.; Pelster, D.E.; Li, X.; Gaudel, G.; Wang, Y.; Chen, S.; Li, W.; Mburu, D.; Hu, C. Greenhouse Gas Emissions Response to Fertilizer Application and Soil Moisture in Dry Agricultural Uplands of Central Kenya. Atmosphere 2022, 13, 463. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Pinto, J.C.; Cabral-albuquerque, E.C.D.M. Coating of urea granules by in situ polymerization in fluidized bed reactors. Polímeros Ciência e Tecnologia 2019, 29, e2019004. [Google Scholar] [CrossRef]
- Curtis, J.R.; Jolley, V.D.; Blair, T.A.; Sutton, L.E.; Hopkins, B.G. Nitrogen release rates from slow- and controlled-release fertilizers influenced by placement and temperature. PLoS ONE 2020, 15, e0234544. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient Interaction in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).